Abstract
The fibroblast growth factor receptor (FGFR) signals through adaptors constitutively associated with the receptor. In Drosophila melanogaster, the FGFR-specific adaptor protein Downstream-of-FGFR (Dof) becomes phosphorylated upon receptor activation at several tyrosine residues, one of which recruits Corkscrew (Csw), the Drosophila homolog of SHP2, which provides a molecular link to mitogen-activated protein kinase (MAPK) activation. However, the Csw pathway is not the only link from Dof to MAPK. In this study, we identify a novel phosphotyrosine motif present in four copies in Dof and also found in other insect and vertebrate signaling molecules. We show that these motifs are phosphorylated and contribute to FGF signal transduction. They constitute one of three sets of phosphotyrosines that act redundantly in signal transmission: (i) a Csw binding site, (ii) four consensus Grb2 recognition sites, and (iii) four novel tyrosine motifs. We show that Src64B binds to Dof and that Src kinases contribute to FGFR-dependent MAPK activation. Phosphorylation of the novel tyrosine motifs is required for the interaction of Dof with Src64B. Thus, Src64B recruitment to Dof through the novel phosphosites can provide a new link to MAPK activation and other cellular responses. This may give a molecular explanation for the involvement of Src kinases in FGF-dependent developmental events.
Fibroblast growth factor (FGF) receptors (FGFRs) are highly conserved molecules that belong to the family of receptor tyrosine kinases (RTKs). They form dimers which are activated by autophosphorylation upon ligand binding. Activated RTKs can directly recruit signaling molecules via their phosphorylated tyrosine residues. They can also phosphorylate other signaling molecules, and the phosphosites of those molecules can serve as additional interaction surfaces for downstream signal transducers (8).
RTKs activate conserved intracellular signaling cascades, one of the most frequently activated being the Ras-mitogen-activated protein kinase (MAPK) pathway. Ras activation at the plasma membrane occurs via the recruitment of the phosphotyrosine binding adaptor molecule Grb2 in a complex with the Ras GTP exchange factor (RasGEF) Sos (26). The FGF signal transduction pathway also uses the Ras-MAPK cascade, but while most other RTKs access this cascade by using phosphotyrosines in their intracellular domains to recruit either Grb2 itself or signaling molecules such as Shc or SHP2, which can then recruit Grb2 upon phosphorylation, this is not the case for FGF receptors (8).
Instead, FGF receptors use constitutively bound adaptor proteins, which are tyrosine phosphorylated upon receptor activation at several sites and provide binding surfaces for many signaling molecules, including the known activators of the Ras-MAPK pathway (8). Surprisingly, although the FGF receptor and its downstream signal transducers are conserved between vertebrates and insects, and in both cases an adaptor is required to connect them, the adaptor function is carried out by unrelated molecules in vertebrates and insects. In vertebrates, the adaptor protein FRS2 constitutively binds to the juxtamembrane region of the FGF receptor and becomes tyrosine phosphorylated by the activated receptor (18, 29, 30). The homolog of FRS2 in Drosophila melanogaster is not involved in FGF signaling (A. Michelson, personal communication). Instead, the adaptor molecule Dof/Sms (Downstream-of-FGFR, or Stumps) is the obligate partner of fly FGF receptors (27, 40, 41). Dof shows no sequence similarity to its vertebrate functional correlate FRS2 but is related to two other vertebrate adaptors, BCAP and BANK, which are involved in B-cell receptor signaling (2).
Dof is essential in all FGF signal-mediated processes in the fly, including mesoderm formation and development of the tracheal network during embryogenesis, which we use here as an in vivo system to study signaling through Dof (40). Dof binds to both Drosophila FGF receptors, Heartless and Breathless, via its conserved DBB (Dof-BCAP-BANK) domain and becomes phosphorylated upon receptor activation at several tyrosine residues (32, 41). Corkscrew (Csw), the Drosophila homolog of SHP2, has been shown to bind to one of these phosphosites, providing a molecular link to MAPK activation (32). However, other functional structural studies show that the Csw pathway is not the only one employed by Dof to activate MAPK and indicate that different parts of the molecule might act redundantly (41).
Src family kinases are also known activators of the MAPK pathway in both Drosophila and vertebrates (4, 22, 42). Furthermore, vertebrate FGF receptor signaling has been described to activate Src family kinases in many different systems (4, 5) and genetic studies of Drosophila point to an involvement of Src kinases in FGF-dependent processes (9, 36). However, there is no evidence on whether and how MAPK activation by Src can be driven directly by Drosophila FGF receptors. This prompted us to investigate whether the adaptor protein Dof might contribute to the recruitment of Src to activated FGF receptors as an alternative way of activating MAPK.
Cells are able to fine-tune the strength and duration of signaling, providing a means to generate signal- or cell type-specific readouts in response to different signals, even though the signaling cascades use common components (reviewed in reference 6). For example, in PC12 cells, EGF signaling generates a transient pulse of MAPK activation, inducing cell proliferation, whereas FGF stimulation generates a sustained MAPK activation, which leads to differentiation (25, 44). One way of achieving this modulation is to be able to use several parallel links to a downstream effector. Two different types of parallel signaling connections have been observed. Adaptors can have multiple binding sites for a given signaling molecule, which allows an increase in signaling strength via the recruitment of larger numbers of the same molecule. For example, FRS2α contains four Grb2 binding sites (12), and BCAP has four PI3K interaction sites (17). Adaptor molecules can also display diverse binding sites for multiple different signaling molecules, allowing activation of the same signaling cascade along alternative routes: FRS2α contains both Grb2 and SHP2 binding sites for MAPK activation (12). In Dof we find both cases. It contains a Csw binding site, which has been shown to contribute to its function, and four Grb2 consensus binding sites, for which the relevance for MAPK activation has not been demonstrated so far (32, 41).
The most frequently used binding sites in RTK signaling that allow signal-dependent transient protein-protein interactions are phosphorylatable tyrosine motifs, which provide binding surfaces for signal transducers bearing phosphotyrosine binding domains. Although all proteins containing phosphotyrosine binding domains can bind phosphotyrosines, these domains differ in their specificity for binding sites. The target recognition sites of the two most common phosphotyrosine binding domains, the PTB and SH2 domains, are determined by short stretches of amino acids upstream or downstream of the phosphorylated tyrosine (35). The motif recognized by PTB domains usually contains conserved amino acids N-terminal to a phosphotyrosine (39), whereas conserved residues C-terminal to a phosphotyrosine contribute to the binding specificity of SH2 domains (37, 38). All of the recognizable motifs surrounding phosphotyrosines in Dof suggest interactions with SH2 domains. Although the binding specificity of many SH2 domains is characterized by phosphopeptide library screens and crystallography studies (31), the interaction partners for several known phosphorylated tyrosines in signaling molecules have not yet been identified.
We present here a systematic mapping of FGFR-dependent tyrosine phosphorylation of Dof by mutational analysis and show that a novel phosphotyrosine motif contributes in a redundant manner to FGF signaling by allowing Dof to utilize alternative signal transduction routes to activate the MAPK pathway.
MATERIALS AND METHODS
Sequence analysis.
Alignments were made using ClustalW, and output files were formatted using BOXSHADE. A conserved pattern of the eight novel tyrosine motifs in the D. melanogaster and Anopheles gambiae Dof sequences was generated using Pratt (www.ebi.ac.uk/pratt). ScanProsite (www.expasy.org/tools/scanprosite) was used to find other proteins containing the conserved motif.
In vitro mutagenesis and cloning of the Dof expression constructs.
Mutagenesis was carried out by the pAlter system (Promega) with the exception that we used the Escherichia coli strains JM109 and ES455. The following mutagenesis primers were used: 5′ GATTGCCCACAAGTTTCAGAACACAGC 3′ for Y97F, 5′ ACGGCGGAGTTTATGGAGATGTCCAG 3′ for Y486F, 5′ ATCTCAACTTCATAAGTGTGGAAACCGAGGAC 3′ for Y515F, 5′ CTCTTTCAGACCGACAAAT 3′ for Y592F, 5′ TGACTTCGTACTGCAGCCC 3′ for Y613F, 5′ AACTTTTTGTTCCAGCCATCG 3′ for Y629F, 5′ CTGCAGGCCTCCAACGTTGCGGTGGCCCAG 3′ for 613AA, 5′ TTCCAGGCTTCGAATAGAGCCGTGGAGGAG 3′ for 629AA, 5′ AACTTCATGGTGCCACCGAC 3′ for Y844F, 5′ GCACTTCCAAATGTTCCC 3′ for Y891F, 5′ AGCAACTTCCTGAACACCATC 3′ for Y914F, 5′ CACTTCCAAAACCAGAACGG 3′ for Y930F, 5′ GGTCTTCCAGAATGTGGG 3′ for Y988F, 5′ ACGCCAAACTTCATGAACTGC 3′ for Y1009F, 5′ GTGCCAGCGACACCTAGGGCCGTCTTTACG 3′ for 844AA, and 5′ ATGTTCGCTTCGAACATAGCTGTGTACGGC 3′ for 891AA mutations. The presence of the desired mutation was confirmed by DNA sequence analysis. For Drosophila S2 cell expression and for tissue-specific in vivo expression, mutant Dof coding sequences were cloned into the pRmHa-3 and the pUAST vectors as described earlier (2, 41). Briefly, single-stranded DNA (ssDNA) site-directed mutagenesis was performed on the 5′Flag-Dof.pAlter plasmid using mutagenesis primers alone or in combination. For truncated Dof forms Dof802 and Dof522, 5′Flag-Dof802.pAlter and 5′Flag-Dof522.pAlter plasmids were used as templates. In these constructs, a STOP codon followed by an XbaI site was introduced after amino acids 522 and 802, respectively. Following mutagenesis, EcoRI-SpeI fragments of 5′Flag-Dof.pAlter and EcoRI-XbaI fragments of 5′Flag-Dof802.pAlter and 5′Flag-Dof522.pAlter were inserted into EcoRI-XbaI cut pRmHa-3 or pUAST vectors.
Drosophila genetics.
FlyBase (http://flybase.org/) was used as a source for information throughout this work.
Standard procedures were followed to produce transgenic flies. Insertions were assigned to a particular chromosome based on the segregation of the transgenes from dominant markers on the second and third chromosome balancers and sex linkage for the X chromosome. Two independent transformant lines were analyzed for each construct. The expression of each mutant protein was assayed with anti-Dof antibody. The tracheal, mesoderm, and ectopic MAPK kinase activation assays were carried out essentially as previously described (32, 40, 41).
Immunohistochemistry.
Standard protocols were followed to collect, fix, and stain embryos. Primary antibodies used were rabbit anti-Even-Skipped (1:5,000) (10) (kindly provided by M. Frasch), rabbit anti-Dof (1:200) (40), polyclonal mouse anti-β-galactosidase (1:500) (Sigma), 2A12 (1:20) (kindly provided by Nipam Patel), and anti-double-phosphorylated extracellular signal-regulated kinase (anti-dpErk) (Sigma) antibodies. Weak fluorescent signals of anti-dpErk immunostaining were amplified by the use of biotinylated tyramide (NEN Life Science Product) followed by streptavidin-fluorescein isothiocyanate (FITC) detection.
Drosophila cell culture, immunoprecipitation, and Western blot analysis.
D. melanogaster Schneider line 2 cells were maintained at 27°C in Schneider's Drosophila medium (Invitrogen) supplemented with 10% (vol/vol) fetal calf serum (Sigma). Transient transfections were done as described previously (2). Briefly, 106 cells were used for a transfection. The expression of the pRmHA-3 vector-derived constructs was induced by the addition of CuSO4 to a final concentration of 1 mM. Cells were harvested after 24 h of induction, washed once in ice-cold phosphate-buffered saline, and lysed in 0.5 ml of PLC-TX0.5 buffer (10% [vol/vol] glycerol, 50 mM HEPES [pH 7.5], 150 mM NaCl, 0.5% [vol/vol] Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 200 μM Na3VO4, 10 mM NaF supplemented with protease inhibitor cocktail [Sigma]). For SDS-PAGE, 7.5 μl of lysates were boiled in 1× SDS-PAGE loading buffer and loaded onto the gel. For immunoprecipitation, 90 μl of lysates were incubated overnight at 4°C with anti-FLAG M5 (Sigma), anti-HA 12CA5 (Boehringer Mannheim), or anti-Dof (40) antibodies. Protein G-Sepharose beads (Amersham Pharmacia), preblocked in 5% (wt/vol) bovine serum albumin (BSA) in PLC-TX0.5, were added to the samples and incubated for 2 h at 4°C, followed by three 10-min washes in 0.5 ml of PLC-TX0.5 buffer. Beads were boiled in 30 μl 2× SDS-PAGE loading buffer, and supernatants were fractionated by SDS-PAGE (10% [wt/vol] polyacrylamide gel), loading 10 μl of the sample into one lane, followed by Western blotting according to standard methods using anti-FLAG M5 (1:4,000), anti-HA 3F10 (1:3,000) (Boehringer Mannheim), anti-phosphotyrosine 4G10 (1:1,000) (Upstate Laboratories), and anti-V5 (1:5,000) (Sigma) primary antibodies. Immunoreactive proteins were visualized using a horseradish peroxidase-catalyzed chemiluminescence reaction (Amersham-Pharmacia).
Inhibitor studies of Drosophila cell culture.
For phosphatase inhibitory studies, freshly prepared sodium pervanadate was added to the cells in a final concentration of 100 nM 1.5 h before harvest. In the case of Src inhibitor analysis, cells were cultivated in the presence of 10 μM PP2 (Src inhibitor) for 24 h before harvest.
RESULTS
Mapping of phosphorylated tyrosines in Dof.
Dof contains 26 tyrosines (Fig. 1A), of which 8 lie within consensus recognition sites for Grb2/Drk (five sites), PI3-kinase, SHP2/Csw, and RasGAP or Crk (one site each). The mutation of all of these consensus sites reduces the biological activity of the molecule (41) but does not abolish it completely, indicating that other parts of the molecule are also engaged in linking Dof to downstream effectors. The mutated form also still shows significant levels of FGF-receptor-mediated tyrosine phosphorylation. We therefore determined which of the remaining tyrosines were phosphorylated and important for function.
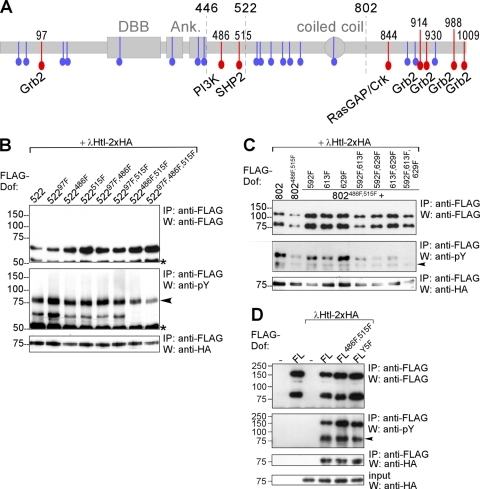
FIG. 1.
Mapping of phosphorylated tyrosines in Dof. (A) Schematic representation of the Dof protein. Two gray boxes and one oval represent the Dof-BCAP-BANK (DBB) domain, the two ankyrin repeats (Ank), and the coiled coil region. All tyrosines are marked in color; red highlights the tyrosines located within consensus motifs for binding sites for the indicated signaling molecules. The amino acid positions of these tyrosines are indicated on top of the scheme. Tyrosines that do not form part of a known consensus motif are marked in blue. Breakpoints of the Dof deletion constructs used or cited in this study—after amino acid positions 446, 522, and 802—are marked with dashed lines. (B to D) FLAG epitope-tagged mutant Dof constructs were expressed together with an activated form of HA-tagged Heartless (λHtl-2xHA) in S2 cells. FL, full-length Dof; 802 and 522, Dof constructs truncated after amino acid positions 802 and 522, respectively. Protein complexes were immunoprecipitated (IP) from whole-cell lysates using an antibody against the FLAG epitope tag of the Dof constructs. The precipitated FLAG-Dof and the coprecipitating λHtl-2xHA proteins were detected on Western blots (W) using antibodies directed against FLAG and HA. In addition to a protein of approximately the size predicted for each of the mutant FLAG-Dof constructs, for the FL and 802 Dof proteins the FLAG antibody also detects a smaller fragment of about 75 kDa representing an N-terminal cleavage product of Dof (see also reference 41). This additional protein band is absent in 522 constructs, because the cleavage site lies C-terminal to the position of truncation. The phosphorylation state of mutant Dof proteins was determined by a phosphotyrosine antibody. This antibody also recognizes the tyrosine-phosphorylated λHtl-2xHA construct, which interacts with Dof and therefore coprecipitates; these bands are marked with a black arrowhead. The asterisk marks the IgG heavy chain of the FLAG antibody. (B) FLAG-Dof constructs containing the first 522 amino acids of Dof with combinations of the Y to F point mutations at positions 97, 486, and 515 were coexpressed with λHtl-2xHA. (C) FLAG-Dof constructs containing the first 802 amino acids with combinations of Y to F point mutations at positions 486, 515, 592, 613, and 629 were coexpressed with λHtl-2xHA. As a positive control for phosphorylation, a Dof construct containing amino acids 1 to 802 without further mutations was used. (D) Full-length FLAG-Dof constructs with combinations of Y to F point mutations at positions 486, 515, 597, 613, and 629 (FLY5F, all of these five tyrosines mutated) were coexpressed with λHtl-2xHA.
We first analyzed a truncated form of Dof consisting of the first 522 amino acids (Dof522). This form retains significant biological function and is phosphorylated when expressed in Schneider S2 cells together with an activated form of the FGF receptor Htl (41). In contrast, this is not the case for a protein consisting only of the first 446 amino acids which is not phosphorylated, although it still retains its ability to bind to the FGF receptor. This shows that there must be phosphorylation sites in the 76 residues between these two breakpoints. Indeed, this region includes two tyrosines, Y486 and Y515, the Corkscrew binding site. If Y515 is mutated in Dof522, the molecule (Dof522515F) is still phosphorylated. However, if in addition Y486 is mutated, phosphorylation is abolished (Fig. 1B), indicating that only Y515 and Y486 can be phosphorylated and that the remaining eight tyrosines in this N-terminal fragment of Dof are not phosphorylation targets upon receptor activation.
We next examined the role of the tyrosines in the remaining part of the protein. In contrast to Dof522486F,515F, a longer form, Dof802, in which tyrosines 486 and 515 are mutated, can be phosphorylated in the presence of the activated FGF receptor (41) (Fig. 1C), pointing to the presence of tyrosines that can be phosphorylated in the middle region of the molecule between residues 522 and 802. Mutational analysis of tyrosines in this middle region identified three further tyrosines (Y592, Y613, and Y629) that become phosphorylated in response to FGF receptor activation (Fig. 1C). It was necessary to make Y-to-F mutations of all five amino acids at positions 486, 515, 592, 613, and 629 to eliminate detectable tyrosine phosphorylation of Dof802. The nonphosphorylated molecule Dof802486F,515F,592F,613F,629F (802Y5F in Fig. 1C) can be reconverted into a phosphorylatable molecule by further addition of C-terminal sequences (the remaining amino acids 803 to 1012) (construct FLY5F in Fig. 1D), showing that further tyrosines in the C terminus can act as substrates for phosphorylation (Fig. 1D). Since all nonphosphorylatable mutant Dof forms were furthermore able to interact with the activated FGF receptor, Htl (Fig. 1B, C, and D), we conclude that the identified tyrosine residues do not influence receptor binding and, thus, Dof phosphorylation in general but indeed represent FGF signaling-dependent phosphorylation sites.
Conserved motifs surrounding phosphotyrosines in Dof and other signaling molecules.
The amino acid environments of tyrosines 613 and 629 do not conform to any consensus sequences for known binding sites for phosphotyrosine binding modules. However, they highly resemble each other and are also very similar to the environments of two tyrosines (Y844 and Y891) in the C-terminal part of Dof. Furthermore, this motif is conserved between the Anopheles and Drosophila Dof genes (Fig. 2A), even though the overall conservation of Dof is not very high. Together these sequences define the consensus motif Y-X3-P-X3-P (Fig. 2B), which has not been described previously as a consensus binding site for signaling molecules.
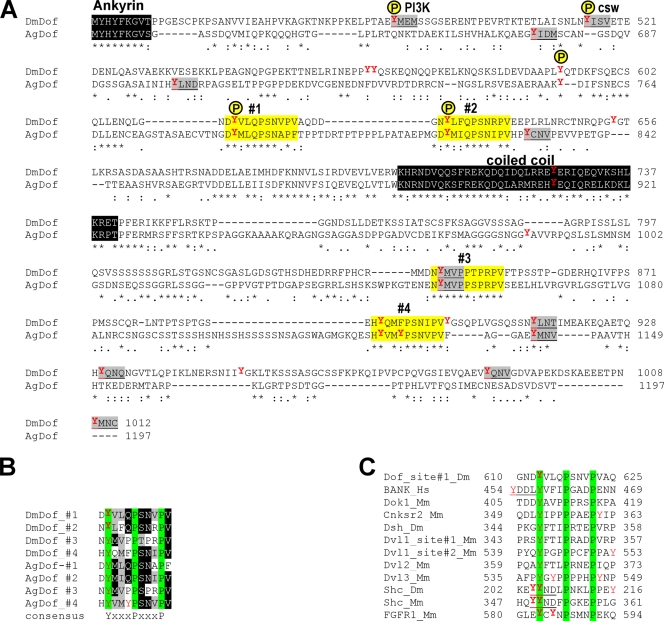
FIG. 2.
Conserved motifs surrounding phosphotyrosines in Dof and other signaling molecules. (A) Protein sequence alignment of the C-terminal half of Drosophila and Anopheles Dof (DmDof and AgDof; UniProtKB accession numbers O96757 and Q8T5J9). Identified tyrosine phosphorylation sites in Drosophila Dof are labeled with a yellow P. Known consensus tyrosine motifs are underlined and shaded gray. Positions of predicted novel tyrosine motifs are shaded yellow and numbered 1 to 4. Note that one consensus tyrosine motif is embedded within one of the novel tyrosine motifs. (B) Alignment of the four novel tyrosine motifs from the two insect Dof sequences. Numbering of the motifs is as in panel A. Residues conserved in all motifs are shaded in green. Four or more identical or similar residues shared by the sequences are shaded black or gray, respectively. The consensus sequence is listed below the alignment. (C) Alignment of the novel tyrosine motifs from Dof, hBANK, and other conserved signaling molecules. Conserved residues are shaded green. Known consensus tyrosine motifs are underlined, and tyrosines known as phosphorylation target sites are marked in bold.
We were curious to see whether this motif also occurred in vertebrate proteins. A database search identified several proteins containing this motif, of which a subset is shown in Fig. 2C. These include BANK, a B-cell signal transduction molecule with similarity to Dof, and the RTK adaptor and signal transducer Shc. In Shc, this site includes the previously identified conserved phosphorylation sites at positions Y239 and Y240, Y239 being the tyrosine of a consensus recognition site for Grb2 (Fig. 2C). Of particular interest is the presence of the motif in one of the mammalian FGF receptors, FGFR-1, where it is exposed on the surface in a variable loop between the small and large lobes of the kinase domain. In this case, the motif includes two conserved autophosphorylation sites (Y583 and Y585) (28) (Fig. 2C). This suggested to us that the novel motif might be functionally significant.
Functional relevance of the novel phosphotyrosine motifs.
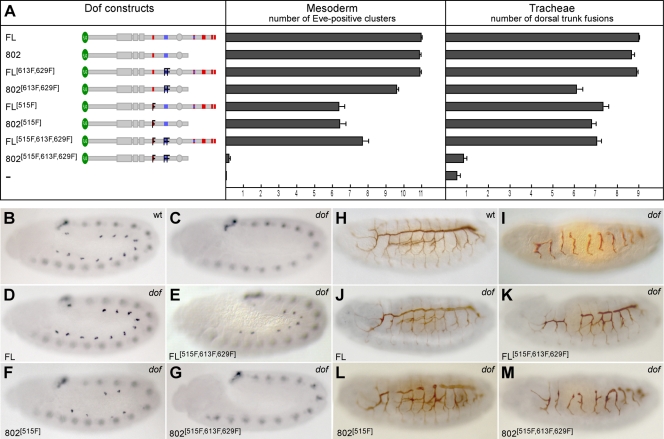
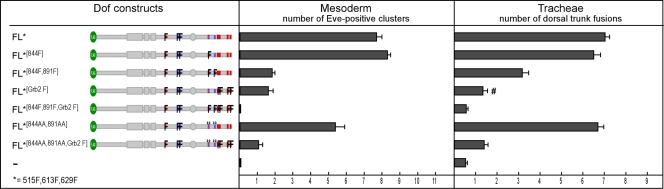
To determine which of the tyrosines were necessary for the biological function of Dof, we tested which of the mutant constructs was able to reconstitute the wild-type function of Dof by expressing them in the mesoderm and the tracheal system of dof mutant embryos. As a quantitative measure for the degree of rescue achieved by the different constructs, we scored the number of pericardial precursor cell (PPC) clusters in the mesoderm, which can be stained with an antibody against the transcription factor Even-skipped (Fig. 3B to G), and the number of correctly established connections in the dorsal trunk of the tracheal system, which we visualized with an antibody against the luminal epitope 2A12 (Fig. 3H to M). Figure 3D and J show that a full-length wild-type version of Dof fully rescues the defects in both systems, as previously reported (40). Neither loss of the C terminus nor mutation of two of the novel tyrosine motifs (613F and 629F) impairs Dof function in these systems (Fig. 3A). Mutation of the Csw binding site Y515 partially reduces Dof function. The combination of any two of these sets of modifications (loss of C terminus, mutation of Csw site, and mutation of two novel sites) does not exacerbate the defects seen with the single mutation of the Csw site (Fig. 3E and F and Fig. 3K and L). However, a Dof construct in which all three sets of mutations are combined, Dof802515F,613F,629F, fails to rescue the defects in the mutant. No PPC clusters are formed in the mesoderm (Fig. 3G), no fusions are seen in the dorsal trunk, and other branches of the tracheal system fail almost completely to grow out (Fig. 3M). Thus, there are three regions in the Dof molecule that can act redundantly in signal transmission: the C terminus, the Csw binding site, and the two novel phosphotyrosine motifs in the middle part of the molecule.
FIG. 3.
Functional redundancy of the novel and Csw-binding phosphotyrosines in Dof. Mutant Dof constructs were tested for their ability to rescue defects in the development of the mesoderm and the tracheae in dof mutant embryos. Anti-Even-skipped antibody (blue signal) was used to visualize the FGF-dependent pericardial precursor cells (PPCs) in the mesoderm of stage 11 embryos (B to G). Anti-2A12 antibody (brown signal) was used to detect the tubular network of the tracheae (H to M). The UAS-Dof transgenes were expressed under the control of twistGal4 (B to G) or breathlessGal4 (H to M). Homozygous mutant embryos were identified by the lack of lacZ-marked Balancer chromosomes. Quantification was performed by counting the number of PPCs and dorsal trunk fusions on both sides of 10 to 15 mutant embryos for each experiment. (A) Summary of the rescue experiments. The left panel shows a schematic representation of mutant Dof constructs used in the assay. Bars in the right panels represent the average number of Even-skipped positive (Eve-positive) clusters in the mesoderm (middle panel) and of dorsal trunk fusions (right panel) ± standard errors of the means. Two independent transgenic fly lines were analyzed for most UAS-Dof constructs. (B to M) Representative examples of antibody-stained embryos from the above rescue experiments.
The ability of the novel phosphotyrosines to activate MAPK.
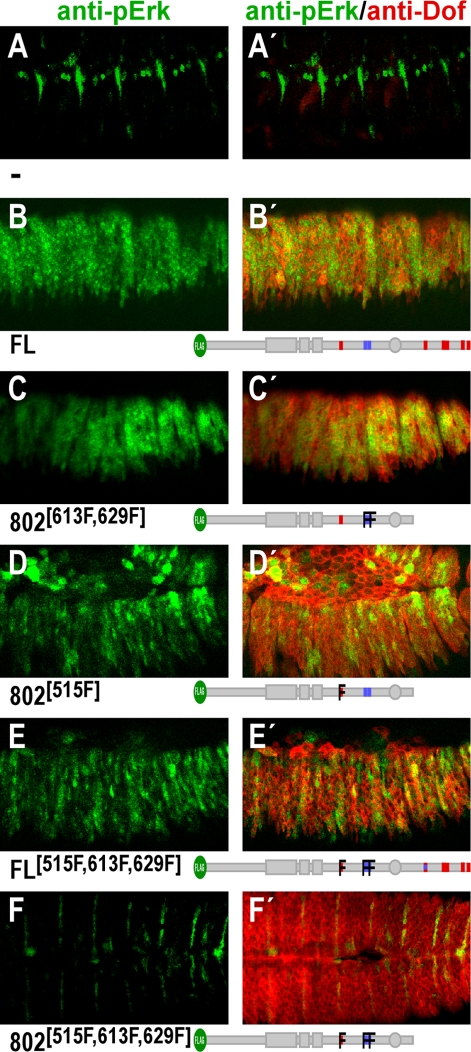
We next tested to what extent the mutant forms of Dof in which the various tyrosines were mutated still had the ability to transmit a signal from the activated receptor to the MAPK cascade. We assayed this in vivo by coexpressing the constitutively active form of the FGF receptor Breathless and the mutant Dof constructs in the pannier expression domain of the epidermis of the embryo and by staining the embryo with an antibody that recognizes the phosphorylated form of ERK. In a stage 15 wild-type embryo, the phospho-ERK antibody stains PPCs, several muscle cells, and ectodermal muscle attachment cells (11) (Fig. 4A and A ′). If wild-type Dof and the constitutively active FGF receptor are coexpressed in the epidermis, activated ERK staining is seen throughout this tissue (32) (Fig. 4B and B′). A similar level of staining is seen with mutant forms of Dof lacking the C terminus (Dof802; not shown) or the C terminus as well as the two tyrosines within the novel motifs Y613 and Y629 (802613F,629F) (Fig. 4C and C′). Mutation of only the Csw site Y515 in Dof802 (802515F) (Fig. 4D and D′) or mutation of the Csw site Y515 and the two novel tyrosine motifs Y613 and Y629 in full-length Dof (FL515F,613F,629F) (Fig. 4E and E′) reduces but does not abolish the ability of Dof to activate MAPK. As was also the case for the rescue of developmental defects, MAPK activation is completely abolished only when Y613, Y629, and the Csw site Y515 are simultaneously mutated and the C terminus is deleted. Thus, the ability of Dof to activate MAPK in this assay mirrors its ability to rescue the developmental defects in dof mutant embryos. These results are in accordance with earlier findings showing that the Csw binding site is sufficient for efficient MAPK activation, but in addition, they show that the novel phosphotyrosine motifs and sites in the C terminus can be used for the activation of the MAPK pathway.
FIG. 4.
Requirement of phosphotyrosines for MAPK activation. The capacity of mutant Dof constructs to activate the MAPK pathway was tested by expressing them in the epidermis under the control of the pannier Gal4 driver together with activated Btl. The expression was monitored using anti-Dof antibody (right panels, red signal). Activation of the MAPK pathway was visualized using anti-dpErk antibody (green signal). Confocal projections are shown for embryos expressing the indicated transgenes (B and B′, full length [FL]; C and C′, to F and F′, various Y-to-F mutations as indicated by the cartoons below each panel) together with UAS-λBtl. Panel A shows a control embryo expressing UAS-λBtl alone. The green staining in this embryo shows the endogenous activation of MAPK induced by Heartless in the pericardial precursor cells and by DER in several muscle and ectodermal muscle-attachment cells. The weak red staining indicates the endogenous expression of Dof in the tracheal system.
Additional redundancy of functional phosphotyrosines in the C terminus of Dof.
We have shown that the C-terminal part of Dof is able to complement the loss of Y613, Y629, and Y515 in the short form Dof802. Since this portion of the molecule contains many additional tyrosines, and most of them lie in consensus tyrosine motifs (4 Grb2 binding sites and 2 novel motifs, one of them overlapping with a recognition site for RasGAP or Crk [see Fig. 1A]), a reasonable assumption is that one or more of these tyrosine motifs act redundantly with respect to Y613, Y629, or Y515. We therefore determined which of the tyrosines in the C terminus might be responsible for this effect by extending our mutational analysis to these tyrosine motifs in the C terminus. The requirement for these tyrosines in the C terminus was assayed in the full-length Dof molecule in which the three tyrosines previously identified in the middle region, Y515, Y613, and Y629, were also mutated, i.e., in a Dof molecule that should be sensitive to the loss of further tyrosines. We modified the C-terminal tyrosines in the following three sets and their combinations: mutation of RasGAP/Crk consensus site Y844 alone (FL*844F), the two novel motifs Y844 and Y891 together (FL*844F,891F), and the four Grb2 binding motifs Y914, Y930, Y988, and Y1009 as a group (FL*Grb2F).
Figure 5 shows that mutation of the RasGAP and Crk binding consensus alone (Y844F, which also changes one of the novel motifs) has no deleterious effects, but if both novel motifs Y844 and Y891 are mutated, only a little biological function remains. This shows that the remaining Grb2 binding sites alone are not fully sufficient to recruit the necessary adaptors to allow signal transmission. If instead of the novel motifs we mutate all four Grb2 binding sites, the molecule is also much impaired in biological activity. Finally, mutating all six tyrosines simultaneously renders Dof completely unfunctional, indicating that the complementing function of the C-terminal portion does indeed rely on these tyrosines. Together, these results show that both the novel tyrosine motifs in the C terminus as well as the Grb2 binding motifs contribute to the function of Dof. Thus, the phosphotyrosines in Dof can be grouped into three redundantly acting functional sets: (i) a Csw binding site at Y515; (ii) four novel tyrosine motifs at Y613, Y629, Y844, and Y891; and (iii) four consensus Grb2 recognition sites at Y914, Y930, Y988, and Y1009.
FIG. 5.
Additional level of redundancy in the C-terminal tail: functional relevance of Grb2 binding and tyrosines of the novel motifs in Dof. Mutant full-length (FL) Dof constructs were tested for their ability to rescue defects in the development of the mesoderm and the tracheae in dof mutant embryos. Grb2F, amino acid exchange of the tyrosines of all four consensus Grb2 recognition sites at positions Y914, Y930, Y988, and Y1009 to phenylalanines. 844AA and 891AA, an amino acid exchange of the two conserved prolines to alanines in the two novel motifs with tyrosine positions 844 and 891. #, reasonable rescue of dorsal and lateral tracheal branches despite a weak rescue of dorsal trunk fusions. For further details, see Fig. 3.
We also wished to investigate the contribution of the amino acids surrounding the tyrosine in the novel consensus motif and therefore mutated the prolines at positions +4 and +8 to alanines. If instead of mutating the tyrosines at Y844 and Y891 (constructs FL*844F,891F and FL*844F,891F,Grb2F), we mutate the prolines at +4 and +8 (constructs FL*844AA,891AA and FL*844AA,891AA,Grb2F), there is a moderate effect on Dof rescue in the mesoderm but only a marginal effect on lateral branching and no effect on the number of dorsal trunk fusions in the tracheae (Fig. 5). This suggests that the prolines are not essential for recognition of the site by signaling molecules.
Signaling molecules recruited by the novel phosphotyrosine motifs in Dof.
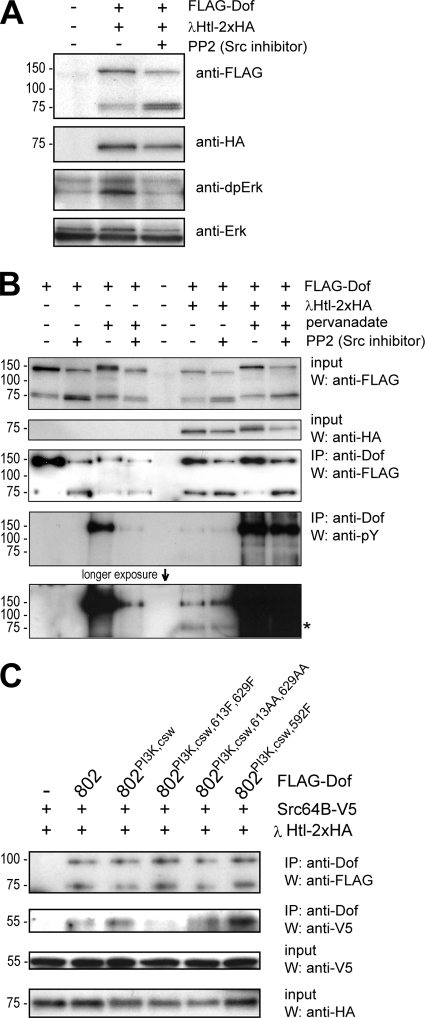
Since the novel phosphotyrosine motifs in Dof contribute to MAPK activation, we were interested which molecules might utilize this phosphosite to transduce the signal to the MAPK. In view of their function in the MAPK signaling pathway and their reported role in tracheal development, one group of likely candidates are the Src kinases. We therefore tested if Src kinases are required for Drosophila FGFR-induced MAPK activation in S2 cells by using the specific Src inhibitor PP2, which has been shown to be active also on Drosophila Src (3). Coexpression of the constitutively active forms of the FGFR Heartless and Dof in S2 cells induced elevated MAPK phosphorylation, as published previously (32). Phosphorylation was sensitive to PP2 treatment (Fig. 6A) indicating that Src participates in FGFR-induced MAPK activation. Since Dof is essential for all aspects of transmitting the signal from an FGFR to downstream effectors, including MAPK (40), it is a reasonable assumption that Src acts through Dof. Two (not necessarily mutually exclusive) scenarios can be envisaged: (i) Src could phosphorylate Dof and thereby generate interaction surfaces for other signaling molecules, or (ii) Src could use Dof as a docking site to become recruited to the signaling complex and phosphorylate other signal transducers. To test the first possibility, we assayed whether FGFR-mediated phosphorylation of Dof also depends on Src activity. We again overexpressed Dof and constitutively active Heartless in S2 cells in the presence or absence of the Src inhibitor PP2. In order not to miss transient phosphorylation events, we also carried out the assays in the presence of the tyrosine phosphatase inhibitor pervanadate. Figure 6B shows that FGFR-mediated phosphorylation of Dof is not affected by Src inhibition. Unexpectedly, however, when expressed in the absence of FGFR but in the presence of pervanadate, Dof was phosphorylated and the phosphorylation was PP2 sensitive, indicating that it depended on Src (Fig. 6B). Individual knockdown of Src42A, Src64B, or Tec29A, the three closest Drosophila homologs of Src, by RNA interference showed that Src64B is the major kinase for Dof in the absence of an activated FGFR (data not shown), indicating that Src64B is able to phosphorylate Dof transiently in S2 cells. However, since Dof phosphorylation was unaffected by PP2 in the presence of a constitutively active FGFR, failure of Dof phosphorylation cannot explain the lack of MAPK phosphorylation in the presence of PP2.
FIG. 6.
Phosphotyrosine-dependent interaction of Dof with Src64B. (A) The relevance of Src kinase activity in FGFR-dependent MAPK activation was tested in S2 cells. FLAG epitope-tagged Dof was expressed together with an activated form of HA-tagged Htl (λHtl-2xHA) in the presence or absence of the Src kinase inhibitor PP2. MAPK activation was detected by Western blotting using an antibody against the diphosphorylated form of Erk. (B) The involvement of Src kinases in Dof phosphorylation was monitored in S2 cells. FLAG-Dof was expressed alone or together with λHtl-2xHA in the presence or absence of tyrosine phosphatase and Src kinase inhibitors pervanadate and PP2. The phosphorylation state of the Dof protein was determined by a phosphotyrosine antibody after immunoprecipitation (IP) from whole-cell lysates using a polyclonal Dof antibody. The asterisk marks the tyrosine-phosphorylated λHtl-2xHA coprecipitating with FLAG-Dof. Note that the apparent molecular weights for the Dof constructs appear not to be identical, although the constructs are the same in all lanes. We assume this is due to phosphorylation, as the migration behavior in the gel correlates with the phosphorylation state. W, West- ern blot analysis. (C) The ability of Src64B to interact with Dof and the requirement of phosphorylated tyrosines and novel tyrosine motifs in Dof for such an interaction were tested in S2 cells. Dof mutants 802, 802[PI3K,csw], 802[PI3K,csw,613F,629F], 802[PI3K,csw,613AA,629AA], and 802[PI3K,csw,597F] were expressed together with λHtl-2xHA and a V5 epitope-tagged form of Src64B (Src64B-V5). Dof protein complexes were immunoprecipitated from whole-cell lysates using an antibody against Dof and the precipitated proteins were detected by Western blotting using antibodies directed against FLAG or V5. Expression levels of Src64B-V5 and λHtl-2xHA were controlled by Western analysis of whole-cell lysates using antibodies against the V5 and HA epitope tags.
We therefore tested the second possibility, i.e., whether Src kinases (specifically, Src64B) might bind to Dof, using it as a docking platform. We performed coimmunoprecipitation on lysates from S2 cells expressing different mutant Dof forms and activated Heartless together with a V5-epitope-tagged form of Src64B. Src64B-V5 coprecipitated with Dof, and efficient binding of Src64B to Dof802 was not changed if the PI3K or Csw consensus motifs were mutated in Dof but was lost if, in addition, the two novel phosphotyrosine motifs of Dof802 were mutated, showing that Src64B can bind to these motifs (Fig. 6C). We also tested the interaction of Src42A and Tec29A with Dof in similar assays. Src42A-V5 failed to interact with Dof, and we had difficulties in expressing Tec29A-V5 to detectable levels in S2 cells (data not shown).
Immunoprecipitates of Dof from lysates of cells cultured in the presence of the phosphatase inhibitor pervanadate also contained a tyrosine-phosphorylated protein of 29 kDa, which, like Src64B, required the novel phosphotyrosine motifs for efficient coprecipitation (data not shown). Thus, these novel phosphotyrosine motifs might be important in the recruitment not only of Src64B but also of an unknown 29-kDa phosphoprotein.
DISCUSSION
Mapping of phosphorylatable tyrosines in Dof.
Our mutational analysis of Dof, which we used as an indirect approach to map tyrosines that are phosphorylated in the presence of an activated FGFR, showed that consensus tyrosine motifs for PI3K and Csw binding at amino acid positions 486 and 515 were substrates of phosphorylation. In addition, three tyrosine residues at positions 592, 613, and 629 were identified as phosphorylation targets, but these do not conform to known conserved tyrosine motifs. Finally, the last 200 amino acids of Dof also contain several phosphorylation target sites. Mutational analyses of this type do not prove that the same residues are phosphorylated in the wild-type situation, but the tyrosine at position 515 is required for the binding of Csw upon FGFR activation (32), and we have shown that mutation of most of the identified sites resulted in impaired activity of the molecule in vivo, supporting the notion that these tyrosines are physiologically relevant phosphorylation targets in Dof.
A novel conserved tyrosine motif in Dof and other signaling molecules.
The three tyrosine-containing motifs at positions 592, 613, and 629 do not resemble known conserved tyrosine motifs, but their positions and their sequences are conserved in Anopheles Dof. In Drosophila the sites are very close together, so that they could act as tandem interaction surfaces, but the fact that they are separated by longer insertions in Anopheles makes this unlikely. The motifs at 613 and 629 resemble each other, and the same sequence motif is present two more times in the C termini of both Drosophila and Anopheles Dof. Protein database searches with the consensus sequence motif Y-X3-P-X3-P, generated from these eight related sites, showed that this motif is present in several signaling molecules, in many cases as known phosphorylation target sites (e.g., in Shc and the mammalian FGFR-1). Mammalian Shc contains two consensus Grb2 binding sites. One, conserved in vertebrate Shc proteins, has been shown to bind Grb2 and activate the Ras-MAPK pathway (13). The other, part of a double-phosphorylation site of two adjacent tyrosines and conserved in all members of the Shc family from insects to vertebrates, does not interact with Grb2, and its function in mitogenic activation and apoptosis protection does not depend on Ras (14, 15, 23). This site is part of the novel tyrosine motif. In vitro kinase assays showed that this pair of adjacent tyrosines can be phosphorylation targets for EGFR and Src as well (34), but no proteins have been identified as binding partners. The highly conserved sequence patch around these two adjacent phosphorylated tyrosines in Shc goes beyond the Grb2 consensus site and outlines exactly the novel Y-X3-P-X3-P motif (24), suggesting that the whole motif is important for an evolutionarily conserved biological function.
In the mammalian FGFR-1, the novel tyrosine motif surrounds the one conserved autophosphorylation site at position Y583 and thus is located in the loop separating the small and large lobes of the kinase domain (28). This is the most variable intracellular sequence part within the FGFR family, and no other mammalian FGFRs share this motif. In spite of the fact that most autophosphorylation sites in the FGFR are conserved (28), many of these tyrosines are dispensable for signal propagation (28, 33) and only a few proteins that associate with these sites have been identified to date (1, 16). Phosphorylated Y583 has no known binding partners, and no signaling function has been linked to it so far.
These findings lead us to speculate that the mammalian FGF-R1 has the ability to recruit a molecule directly that in the case of the Drosophila FGFR is recruited indirectly via Dof and, in the other mammalian FGF receptors, perhaps via other interactors, such as Shc.
Functional relevance of the identified phosphosites.
The rescue experiments we used to assay the functional relevance of the tyrosine mutations that influenced the phosphorylation levels of Dof in vitro yielded two important findings: first, we determined three independent functional units in Dof that contribute to signal propagation, and second, these units act redundantly, in that any one of them is sufficient to provide significant biological activity. However, we cannot rule out the possibility that the overexpression system we used might have masked potential minor qualitative differences and therefore exaggerated the redundancy. Similarly, two of the phosphorylated tyrosines showed no functional relevance in this or previous studies (32, 41). One is the tyrosine of a PI3K consensus site at position 486 for which there was no requirement in any of our assays, employing either full-length Dof or mutant forms retaining only the first 600 amino acids. The other identified phosphotyrosine site without an identified function is located at position 592. Though it is conserved in Anopheles Dof, including several surrounding amino acids, the mutation of this tyrosine alone or in combination with other tyrosines did not affect the biological function of Dof (data not shown). Again, perhaps subtle effects of the loss of these sites might have been missed. Nevertheless, the presence of several copies of docking sites for downstream signaling molecules and the availability of alternative routes to activate the same signaling cascade may provide Dof with options for fine-tuning of signaling strength and duration (6).
Csw and Grb2 binding sites.
Csw has previously been shown to interact with Dof (32). The phosphotyrosine of the Csw consensus site was required for efficient interaction and for MAPK activation in the context of a Dof construct that lacked any of the other phosphorylation sites (32, 41). We now show that the Csw site is indeed important only if other parts of Dof with MAPK activating capacity are deleted or mutated. The same is true for the phosphorylation sites in the C-terminal domain, which we have now shown to be the consensus Grb2 binding sites (41).
Functional relevance of the novel conserved tyrosine motifs.
The four novel phosphotyrosine motifs contributed to the activation of the MAPK cascade, although they were essential only if other parts of the molecule with MAPK activation capability were deleted or mutated. Phosphorylation of these tyrosines was essential for the efficient interaction of Dof with the protein kinase Src64B, and Src activity was in turn required for Dof-dependent MAPK activation in S2 cells. Src kinases can activate mitogenic signaling in many different ways (4). Recently, Drosophila Src64B has been shown to be essential in the regulation of Raf activity by phosphorylating a regulatory tyrosine residue in Raf, which is also conserved in mammalian B-Raf (42). Thus, it is reasonable to postulate that upon FGFR-dependent phosphorylation the novel tyrosine motif in Dof is utilized to recruit Src64B, which can then contribute to MAPK activation via Raf activation.
In addition to Src64B, we found a tyrosine phosphorylated protein of 29 kDa (p29) that coprecipitated with Dof802 and required the phosphorylated tyrosines of the novel motif for this interaction. This raises the possibility that other proteins might use these sites as docking surfaces as well, although we have no evidence that this protein directly binds these phosphosites.
Since the residues surrounding the tyrosines in the novel motifs are conserved, we expected to find them to be important for function. However, while mutating the tyrosines had measurable effects on the function of Dof in vivo, replacing the prolines had only moderate or no effects in the same assays. Similarly, Src64B binding to Dof802 in S2 cells was strongly reduced when the tyrosines of the novel motifs were mutated (in the background of mutated Csw and PI3K sites) but not when the prolines were mutated.
Our finding that the impact of tyrosine mutations in the novel motifs on Dof function was greater than that of the proline mutations agrees with the known general characteristics of the interaction of phosphotyrosine motifs with phosphotyrosine binding domains. The driving force of these interactions is the phosphorylated tyrosine itself, with additional lower-affinity interactions of surrounding residues contributing to specificity for the different phosphotyrosine binding domains (35), as has also been found for dissociation constants when measuring interactions of SH2 domains in phosphopeptide library interaction screens (20). Indeed, it has been previously proposed that the modest selectivity of SH2 domains to phosphotyrosine containing linear peptides (5- to 20-fold) is not sufficient to explain selectivity of signaling pathways in living cells (19). Recent work of Bae et al. identifies additional components of these type of interactions and shows that the selectivity of phospholipase Cγ binding and signaling via activated FGFR-1 are determined by interactions between a secondary binding site on an SH2 domain and a region in the FGFR-1 kinase domain in a phosphorylation-independent manner (1). These data suggest that the mutation of two amino acids in a tyrosine motif might have only mild consequences compared to the loss of the phosphotyrosine site in the context of whole protein-protein interactions, based on the complexity of different binding interfaces and their different affinities of this interaction.
Since SH2 domains preferentially interact with residues C-terminal to the tyrosine, and these are the conserved residues in the novel motif, we expect the motif to interact with SH2-type domains. Why has the motif described here not been found in the extensive searches for SH2 target motifs? The answer may lie in the fact that no motifs with important conserved amino acids at positions +4 and +8 are known at all, and this may be primarily because of the way they have been screened for. The degenerate phosphotyrosine peptide libraries that have been used to determine SH2 domain specificities screened only positions +1 to +3 (35, 38), and the furthest that other studies have gone was up to position +5 (7).
We do not know if the SH2 domain of Src64B is involved in the interaction with the novel phosphomotif of Dof, but if so, it is not clear whether this motif could be accommodated by the same interaction surface as the one that binds to the consensus Src SH2 domain recognition sequence pYEEI, which has been defined by phosphopeptide library screening (38) and structural studies on peptide-bound Src SH2 domains (43).
Finally, little is known about the interaction of Src family kinases with other vertebrate signaling molecules bearing the novel tyrosine motif. For example, the motif in Shc is a phosphorylation target of Src (34), but no interaction studies have been performed, and there are conflicting reports about direct interaction between FGFR-1 and Src (21, 45). The results we describe here suggest that Src might be a good candidate for interacting with mammalian FGFR-1 and other vertebrate signaling molecules via the novel motif. It should be worth probing the general validity of Src binding to this novel phosphotyrosine motif in the future.
Acknowledgments
We thank Silvia Querings for carrying out some of the immunoprecipitations and Western blots in relation to the role of Src. We are grateful to Robert Wilson for fruitful discussions and constructive criticism on the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (LE 546/2-2 and LE 546/3-1).
Footnotes
Published ahead of print on 12 February 2010.
REFERENCES
- 1.Bae, J. H., E. D. Lew, S. Yuzawa, F. Tome, I. Lax, and J. Schlessinger. 2009. The selectivity of receptor tyrosine kinase signaling is controlled by a secondary SH2 domain binding site. Cell 138:514-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battersby, A., A. Csiszar, M. Leptin, and R. Wilson. 2003. Isolation of proteins that interact with the signal transduction molecule Dof and identification of a functional domain conserved between Dof and vertebrate BCAP. J. Mol. Biol. 329:479-493. [DOI] [PubMed] [Google Scholar]
- 3.Biswas, R., D. Stein, and E. R. Stanley. 2006. Drosophila Dok is required for embryonic dorsal closure. Development 133:217-227. [DOI] [PubMed] [Google Scholar]
- 4.Bromann, P. A., H. Korkaya, and S. A. Courtneidge. 2004. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 23:7957-7968. [DOI] [PubMed] [Google Scholar]
- 5.Browaeys-Poly, E., K. Cailliau, and J. P. Vilain. 2000. Signal transduction pathways triggered by fibroblast growth factor receptor 1 expressed in Xenopus laevis oocytes after fibroblast growth factor 1 addition. Role of Grb2, phosphatidylinositol 3-kinase, Src tyrosine kinase, and phospholipase Cgamma. Eur. J. Biochem. 267:6256-6263. [DOI] [PubMed] [Google Scholar]
- 6.Csiszar, A. 2006. Structural and functional diversity of adaptor proteins involved in tyrosine kinase signalling. Bioessays 28:465-479. [DOI] [PubMed] [Google Scholar]
- 7.De Souza, D., L. J. Fabri, A. Nash, D. J. Hilton, N. A. Nicola, and M. Baca. 2002. SH2 domains from suppressor of cytokine signaling-3 and protein tyrosine phosphatase SHP-2 have similar binding specificities. Biochemistry 41:9229-9236. [DOI] [PubMed] [Google Scholar]
- 8.Eswarakumar, V. P., I. Lax, and J. Schlessinger. 2005. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 16:139-149. [DOI] [PubMed] [Google Scholar]
- 9.Firth, L., J. Manchester, J. A. Lorenzen, M. Baron, and L. A. Perkins. 2000. Identification of genomic regions that interact with a viable allele of the Drosophila protein tyrosine phosphatase corkscrew. Genetics 156:733-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frasch, M., T. Hoey, C. Rushlow, H. Doyle, and M. Levine. 1987. Characterization and localization of the even-skipped protein of Drosophila. EMBO J. 6:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabay, L., R. Seger, and B. Z. Shilo. 1997. MAP kinase in situ activation atlas during Drosophila embryogenesis. Development 124:3535-3541. [DOI] [PubMed] [Google Scholar]
- 12.Gotoh, N. 2008. Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins. Cancer Sci. 99:1319-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotoh, N., A. Tojo, K. Muroya, Y. Hashimoto, S. Hattori, S. Nakamura, T. Takenawa, Y. Yazaki, and M. Shibuya. 1994. Epidermal growth factor-receptor mutant lacking the autophosphorylation sites induces phosphorylation of Shc protein and Shc-Grb2/ASH association and retains mitogenic activity. Proc. Natl. Acad. Sci. U. S. A. 91:167-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gotoh, N., A. Tojo, and M. Shibuya. 1996. A novel pathway from phosphorylation of tyrosine residues 239/240 of Shc, contributing to suppress apoptosis by IL-3. EMBO J. 15:6197-6204. [PMC free article] [PubMed] [Google Scholar]
- 15.Gotoh, N., M. Toyoda, and M. Shibuya. 1997. Tyrosine phosphorylation sites at amino acids 239 and 240 of Shc are involved in epidermal growth factor-induced mitogenic signaling that is distinct from Ras/mitogen-activated protein kinase activation. Mol. Cell. Biol. 17:1824-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, J., M. Mohammadi, G. A. Rodrigues, and J. Schlessinger. 1995. Reduced activation of RAF-1 and MAP kinase by a fibroblast growth factor receptor mutant deficient in stimulation of phosphatidylinositol hydrolysis. J. Biol. Chem. 270:5065-5072. [DOI] [PubMed] [Google Scholar]
- 17.Inabe, K., and T. Kurosaki. 2002. Tyrosine phosphorylation of B-cell adaptor for phosphoinositide 3-kinase is required for Akt activation in response to CD19 engagement. Blood 99:584-589. [DOI] [PubMed] [Google Scholar]
- 18.Kouhara, H., Y. R. Hadari, T. Spivak-Kroizman, J. Schilling, D. Bar-Sagi, I. Lax, and J. Schlessinger. 1997. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89:693-702. [DOI] [PubMed] [Google Scholar]
- 19.Ladbury, J. E., and S. Arold. 2000. Searching for specificity in SH domains. Chem. Biol. 7:R3-R8. [DOI] [PubMed] [Google Scholar]
- 20.Ladbury, J. E., M. A. Lemmon, M. Zhou, J. Green, M. C. Botfield, and J. Schlessinger. 1995. Measurement of the binding of tyrosyl phosphopeptides to SH2 domains: a reappraisal. Proc. Natl. Acad. Sci. U. S. A. 92:3199-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landgren, E., P. Blume-Jensen, S. A. Courtneidge, and L. Claesson-Welsh. 1995. Fibroblast growth factor receptor-1 regulation of Src family kinases. Oncogene 10:2027-2035. [PubMed] [Google Scholar]
- 22.Li, W., E. Noll, and N. Perrimon. 2000. Identification of autosomal regions involved in Drosophila Raf function. Genetics 156:763-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luschnig, S., J. Krauss, K. Bohmann, I. Desjeux, and C. Nusslein-Volhard. 2000. The Drosophila SHC adaptor protein is required for signaling by a subset of receptor tyrosine kinases. Mol. Cell 5:231-241. [DOI] [PubMed] [Google Scholar]
- 24.Luzi, L., S. Confalonieri, P. P. Di Fiore, and P. G. Pelicci. 2000. Evolution of Shc functions from nematode to human. Curr. Opin. Genet. Dev. 10:668-674. [DOI] [PubMed] [Google Scholar]
- 25.Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 26.McKay, M. M., and D. K. Morrison. 2007. Integrating signals from RTKs to ERK/MAPK. Oncogene 26:3113-3121. [DOI] [PubMed] [Google Scholar]
- 27.Michelson, A. M., S. Gisselbrecht, E. Buff, and J. B. Skeath. 1998. Heartbroken is a specific downstream mediator of FGF receptor signalling in Drosophila. Development 125:4379-4389. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi, M., I. Dikic, A. Sorokin, W. H. Burgess, M. Jaye, and J. Schlessinger. 1996. Identification of six novel autophosphorylation sites on fibroblast growth factor receptor 1 and elucidation of their importance in receptor activation and signal transduction. Mol. Cell. Biol. 16:977-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong, S. H., G. R. Guy, Y. R. Hadari, S. Laks, N. Gotoh, J. Schlessinger, and I. Lax. 2000. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol. Cell. Biol. 20:979-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong, S. H., Y. R. Hadari, N. Gotoh, G. R. Guy, J. Schlessinger, and I. Lax. 2001. Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins. Proc. Natl. Acad. Sci. U. S. A. 98:6074-6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pawson, T. 2004. Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell 116:191-203. [DOI] [PubMed] [Google Scholar]
- 32.Petit, V., U. Nussbaumer, C. Dossenbach, and M. Affolter. 2004. Downstream-of-FGFR is a fibroblast growth factor-specific scaffolding protein and recruits Corkscrew upon receptor activation. Mol. Cell. Biol. 24:3769-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rusnati, M., P. Dell'Era, C. Urbinati, E. Tanghetti, M. L. Massardi, Y. Nagamine, E. Monti, and M. Presta. 1996. A distinct basic fibroblast growth factor (FGF-2)/FGF receptor interaction distinguishes urokinase-type plasminogen activator induction from mitogenicity in endothelial cells. Mol. Biol. Cell 7:369-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato, K., N. Gotoh, T. Otsuki, M. Kakumoto, M. Aoto, A. A. Tokmakov, M. Shibuya, and Y. Fukami. 1997. Tyrosine residues 239 and 240 of Shc are phosphatidylinositol 4,5-bisphosphate-dependent phosphorylation sites by c-Src. Biochem. Biophys. Res. Commun. 240:399-404. [DOI] [PubMed] [Google Scholar]
- 35.Schlessinger, J., and M. A. Lemmon. 2003. SH2 and PTB domains in tyrosine kinase signaling. Sci. STKE 2003(191):RE12. [DOI] [PubMed]
- 36.Shindo, M., H. Wada, M. Kaido, M. Tateno, T. Aigaki, L. Tsuda, and S. Hayashi. 2008. Dual function of Src in the maintenance of adherens junctions during tracheal epithelial morphogenesis. Development 135:1355-1364. [DOI] [PubMed] [Google Scholar]
- 37.Songyang, Z., and L. C. Cantley. 1995. SH2 domain specificity determination using oriented phosphopeptide library. Methods Enzymol. 254:523-535. [DOI] [PubMed] [Google Scholar]
- 38.Songyang, Z., S. E. Shoelson, M. Chaudhuri, G. Gish, T. Pawson, W. G. Haser, F. King, T. Roberts, S. Ratnofsky, R. J. Lechleider, et al. 1993. SH2 domains recognize specific phosphopeptide sequences. Cell 72:767-778. [DOI] [PubMed] [Google Scholar]
- 39.Uhlik, M. T., B. Temple, S. Bencharit, A. J. Kimple, D. P. Siderovski, and G. L. Johnson. 2005. Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J. Mol. Biol. 345:1-20. [DOI] [PubMed] [Google Scholar]
- 40.Vincent, S., R. Wilson, C. Coelho, M. Affolter, and M. Leptin. 1998. The Drosophila protein Dof is specifically required for FGF signaling. Mol. Cell 2:515-525. [DOI] [PubMed] [Google Scholar]
- 41.Wilson, R., A. Battersby, A. Csiszar, E. Vogelsang, and M. Leptin. 2004. A functional domain of Dof that is required for fibroblast growth factor signaling. Mol. Cell. Biol. 24:2263-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia, F., J. Li, G. W. Hickey, A. Tsurumi, K. Larson, D. Guo, S. J. Yan, L. Silver-Morse, and W. X. Li. 2008. Raf activation is regulated by tyrosine 510 phosphorylation in Drosophila. PLoS Biol. 6:e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu, R. X., J. M. Word, D. G. Davis, M. J. Rink, D. H. Willard, Jr., and R. T. Gampe, Jr. 1995. Solution structure of the human pp60c-src SH2 domain complexed with a phosphorylated tyrosine pentapeptide. Biochemistry 34:2107-2121. [DOI] [PubMed] [Google Scholar]
- 44.Yamada, S., T. Taketomi, and A. Yoshimura. 2004. Model analysis of difference between EGF pathway and FGF pathway. Biochem. Biophys. Res. Commun. 314:1113-1120. [DOI] [PubMed] [Google Scholar]
- 45.Zhan, X., C. Plourde, X. Hu, R. Friesel, and T. Maciag. 1994. Association of fibroblast growth factor receptor-1 with c-Src correlates with association between c-Src and cortactin. J. Biol. Chem. 269:20221-20224. [PubMed] [Google Scholar]