Abstract
Herpesviruses can enter host cells using pH-dependent endocytosis pathways in a cell-specific manner. Envelope glycoprotein B (gB) is conserved among all herpesviruses and is a critical component of the complex that mediates membrane fusion and entry. Here we demonstrate that mildly acidic pH triggers specific conformational changes in herpes simplex virus (HSV) gB. The antigenic structure of gB was specifically altered by exposure to low pH both in vitro and during entry into host cells. The oligomeric conformation of gB was altered at a similar pH range. Exposure to acid pH appeared to convert virion gB into a lower-order oligomer. The detected conformational changes were reversible, similar to those in other class III fusion proteins. Exposure of purified, recombinant gB to mildly acidic pH resulted in similar changes in conformation and caused gB to become more hydrophobic, suggesting that low pH directly affects gB. We propose that intracellular low pH induces alterations in gB conformation that, together with additional triggers such as receptor binding, are essential for virion-cell fusion during herpesviral entry by endocytosis.
Herpes simplex virus (HSV) is an important human pathogen, causing significant morbidity and mortality worldwide. HSV enters host cells by fusion of the viral envelope with either an endosomal membrane (38) or the plasma membrane (63). The entry pathway taken is thought to be determined by both virus (17, 45) and host cell (4, 17, 35, 39, 45) factors. Based on experiments with lysosomotropic agents, which elevate the normally low pH of endosomes, acidic pH has been implicated in the endocytic entry of HSV into several cell types, including human epithelial cells (37). Low pH has also recently been implicated in cell infection by several other human and veterinary herpesviruses (1, 21, 26, 47). The mechanistic role of endosomal pH in herpesvirus entry into cells is not known.
Herpesviruses are a paradigm for membrane fusion mediated by a complex of several glycoproteins. We have proposed that HSV likely encodes machinery to mediate both pH-dependent and pH-independent membrane fusion reactions. Envelope glycoproteins glycoprotein B (gB) and gD and the heterodimer gH-gL are required for both pH-independent and pH-dependent entry pathways (11, 22, 30, 39, 46). Interaction of gD with one of its cognate receptors is an essential trigger for membrane fusion and entry (13, 52), regardless of the cellular pathway. However, engagement of a gD receptor is not sufficient for fusion, and at least one additional unknown trigger involving gB or gH-gL is likely necessary. gB is conserved among all herpesviruses, and in all cases studied to date, it plays roles in viral entry, including receptor binding and membrane fusion. The crystal structure of an ectodomain fragment of HSV type 1 (HSV-1) gB is an elongated, rod-like structure containing hydrophobic internal fusion loops (28). This structure bears striking architectural homology to the low pH, postfusion form of G glycoprotein from vesicular stomatitis virus (VSV-G) (43). Both the gB and G structures have features of class I and class II fusion proteins and are thus designated class III proteins (57).
During entry of the majority of virus families, low pH acts directly on glycoproteins to induce membrane fusion (60). In some cases, the low pH trigger is not sufficient, or it plays an indirect role. For example, host cell proteases, such as cathepsins D and L, require intravesicular low pH to cleave Ebola virus and severe acute respiratory syndrome (SARS) glycoproteins to trigger fusion (14, 51).
We investigated the role of low pH in the molecular mechanism of herpesviral entry. The results suggest that mildly acidic pH, similar to that found within endosomes, triggers a conformational change in gB. We propose that, together with other cellular cues such as receptor interaction, intracellular low pH can play a direct activating role in HSV membrane fusion and entry.
MATERIALS AND METHODS
Cells and viruses.
Vero cells (American Type Culture Collection [ATCC], Rockville, MD) were propagated in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Gemini Bio-Products, West Sacramento, CA). HSV-1 strain KOS (provided by Priscilla Schaffer, Harvard University) and HSV-2 strain 333 (provided by Stephen Straus, National Institutes of Health) were propagated, and the titers of the strains were determined on Vero cells.
Dot blot analysis.
Soluble gB or cell-free preparations of extracellular HSV-1 KOS were diluted in serum-free, bicarbonate-free DMEM with 0.2% bovine serum albumin (BSA) and 5 mM (each) HEPES (Life Technologies), 2-(N-morpholino)ethanesulfonic acid (MES; Sigma), and sodium succinate (Sigma) to achieve final pHs ranging from 7.4 to 4.8. Samples were incubated at 37°C for 10 min. Samples either were blotted directly to nitrocellulose with a Minifold dot blot system (Whatman) or were first neutralized by addition of pretitrated amounts of 0.05 N NaOH. Membranes were blocked and incubated with anti-gB monoclonal antibodies H126 (32), H1359, H1817 (Virusys), DL16, SS10, SS55 (8), SS106, or SS144 (7) or with R69 polyclonal antibody to gB provided by G. Cohen and R. Eisenberg, University of Pennsylvania. After incubation with horseradish peroxidase-conjugated secondary antibodies, enhanced chemiluminescent substrate (Pierce) was added, and blots were exposed to X-ray film (Kodak).
Sucrose density centrifugation.
Extracellular virions were lysed in 1% Triton X-100 for 30 min at room temperature and layered onto a step gradient of 8% to 60% (wt/wt) sucrose in 20 mM (each) MES, sodium succinate, and HEPES, 175 mM NaCl, 2.5 mM EDTA, 2.5 mM EGTA, and 1% Triton X-100. The indicated pHs were obtained with HCl. Samples were sedimented at 40,000 rpm for 16 h at 4°C in a Beckman SW41 rotor. Fractions were collected, immunoprecipitated with monoclonal antibody (MAb) H1817, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by immunoblotting with MAb H1359 to gB. Data were quantitated by densitometry using ImageJ 1.38x (NIH).
Analysis of gB oligomeric structure by PAGE.
HSV-1 KOS, HSV-2 333, or a recombinant soluble form of gB (s-gB) was diluted in medium as described above for dot blotting. s-gB has the transmembrane domain deleted (gB2ΔTM) (61) and was obtained from Stephen Straus. Samples were adjusted to the indicated pHs with pretitrated amounts of 0.05 N HCl and incubated at 37°C for 10 min. SDS (1%) was added, or samples remained untreated. Polyacrylamide gel electrophoresis (PAGE) sample buffer containing 0.2% sodium dodecyl sulfate (SDS) and no reducing agent was added (“native” conditions), and proteins were resolved by PAGE (16). After transfer to nitrocellulose, membranes were blocked and incubated with rabbit polyclonal antibodies specific for gB, gC, gD, or gH-gL (provided by G. Cohen and R. Eisenberg). After incubation with horseradish peroxidase-conjugated secondary antibodies, enhanced chemiluminescent substrate (Pierce) was added, and membranes were exposed to X-ray film (Kodak).
Triton X-114 partitioning.
To increase the association of hydrophobic gB with the amphiphilic (detergent) phase, Triton X-114 (Fisher Scientific) was preconditioned to reduce the amount of the most hydrophilic Triton X-114 molecules and to enrich the amphiphilic molecules (9). The hydrophilic phases from each of three condensation steps were discarded. A total of 200 ng of s-gB or 20 μg of BSA was incubated with preconditioned 2% Triton X-114 in fusion medium buffered to various pHs on ice for 10 min. Samples were incubated at 37°C for 10 min and then centrifuged at 300 × g for 3 min at 25°C. Aqueous and detergent phases were collected and either immunoprecipitated with MAb H1359 to gB or precipitated with trichloroacetic acid. Precipitates were analyzed by SDS-PAGE, followed by either Western blotting with MAb H1359 for gB or Coomassie blue staining for BSA.
Confocal microscopy of virion gB during viral entry.
CHO-nectin-1 cells were mock treated or treated with 25 nM bafilomycin A1 (BFLA) for 15 min. HSV-1 KOS (multiplicity of infection [MOI] of 20) was bound to cells at 4°C for 1 h. Cultures were shifted to 37°C for 1 h in the constant presence of BFLA/vehicle control and 0.5 mM cycloheximide. Cells were fixed with 3% paraformaldehyde (Thomas Scientific) in phosphate-buffered saline (PBS) and permeabilized with 0.2% Triton X-100 (Fisher Scientific). Virion gB was detected with 2 μg/ml MAb H126 or 0.5 μg/ml MAb H1817 (Virusys), followed by Alexa 488-labeled goat anti-mouse antibody. Samples were visualized with a Zeiss LSM 510 Meta microscope equipped with a 63× oil immersion objective lens.
RESULTS
Low-pH treatment alters the exposure of epitopes in the fusion domain of virion gB.
Intracellular pH is required for the cell entry of several herpesviruses, often in a cell type-specific manner. The precise role of low pH in herpesviral entry is not clear. Viral glycoprotein-mediated fusion is accompanied by conformational changes that result in domain rearrangement and exposure of hydrophobic fusion peptide sequences. The hydrophobic peptide then interacts with lipids of the target membrane, which is an essential destabilizing step in the fusion process (60).
To begin to determine whether intracellular low pH directly causes conformational change in gB associated with fusion and entry, we measured the reactivity of mouse monoclonal antibody (MAb) H126. H126 has complement-independent, virus-neutralizing activity and recognizes a linear epitope in the putative fusion domain of gB (domain I) (28, 32, 40, 45). In addition, a fusion-from-without form of virion gB with enhanced fusogenicity was shown to have an H126 epitope with altered accessibility (45).
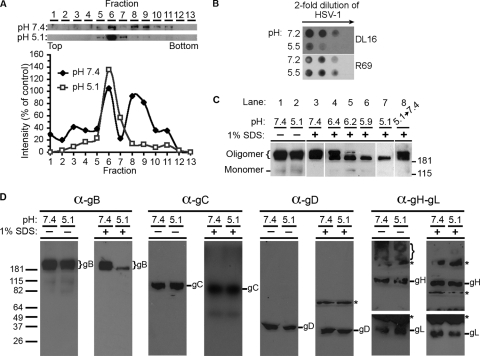
HSV virions were exposed to pHs ranging from 7.4 to 4.8 and were immediately blotted to nitrocellulose membranes. Antibody binding was then assessed at pH 7.4. MAb H126 displayed diminished binding to virions that had been treated at pH <6.2 (Fig. 1). Approximately 50% of H126 reactivity was lost in virions exposed to pH 5.9. Similar results were obtained with an additional MAb to domain I, SS55 (Fig. 1; Table 1). This suggests a specific change in the antigenic structure of the fusion domain. As a control, MAb H1817 to N-terminal residues 31 to 43 (domain VI) of gB displayed unaltered binding to acid-treated virions (Fig. 1). Similarly, MAbs H1359 and SS10, directed to domains III and IV, respectively, and gB-specific polyclonal antibody (PAb) R69, which also bound well to low pH-treated HSV (Fig. 1; Table 1), suggest that the structure of gB is not globally altered and that the detected changes in reactivity are specific. Domains I and V are in close proximity and together form a functional region (7). Interestingly, MAbs to domain V, SS106 and SS144, had reduced binding to virions that had been treated with mildly acidic pH (Fig. 1; Table 1), similar to the domain I-specific antibodies. Together, the results suggest that exposure of HSV to a pH of less than or equal to 6.2 changes the antigenic conformation of the gB functional region 1 that contains fusion loops.
FIG. 1.
Antibody reactivity of low pH-treated virions. Extracellular HSV-1 KOS virions (105 PFU) were treated for 10 min at 37°C with medium buffered to the indicated pHs and were blotted immediately to a nitrocellulose membrane. Blots were probed at neutral pH with the indicated gB-specific antibodies, followed by horseradish peroxidase-conjugated goat secondary antibody. The exposure shown for MAb H126 highlights the pH threshold of conformational change.
TABLE 1.
Summary of monoclonal antibodies to gB used in this studye
| Antibody | Domain | Conformation dependenta | Neutralizingc | Reactive with low pH-treated gBd |
|
|---|---|---|---|---|---|
| HSV-1 KOS | s-gB | ||||
| DL16 | ND | Y (Oligomer specific) | − | − | − |
| H126 | I | N | + | − | − |
| H1359 | III | N | − | + | + |
| H1817 | VI | N | + | + | + |
| SS10 | IV | Yb | + | + | + |
| SS55 | I | Y | + | − | − |
| SS106 | V | N | + | − | − |
| SS144 | V | Yb | + | − | − |
| R69 | Polyclonal | NA | + | + | + |
Y, reactive with HSV-1 gB under native PAGE conditions but not under denaturing conditions; N, reactive under denaturing conditions only.
Reactive with native gB and with a small amount of gB under denaturing conditions (7).
Defined as reducing HSV-1 entry or plaque formation.
gB epitope reactivity was measured by dot blotting, as described in Materials and Methods.
Mildly acidic pH alters the oligomeric structure of gB.
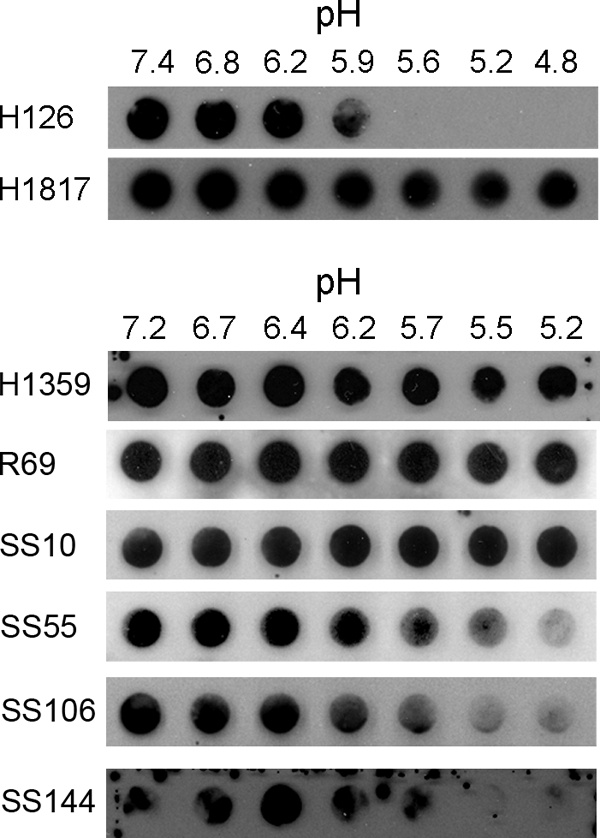
Viral fusion proteins are oligomers (60). HSV gB is oligomeric, and an ectodomain form of HSV-1 gB has a trimeric structure (15, 28). Low pH-triggered membrane fusion can be accompanied by rearrangement of viral glycoprotein oligomer subunits (3, 56). Three approaches were used to determine the effect of acid pH on the quaternary structure of gB. First, HSV was solubilized with the nonionic detergent Triton X-100 and subjected to sucrose gradient centrifugation at pH 7.4 or pH 5.1. Fractions were analyzed by immunoprecipitation, SDS-PAGE, and immunoblotting for gB. At pH 7.4, gB was detected in fractions that corresponded to >181 kDa in molecular mass. This is consistent with gB oligomers and is similar to previous reports (29). At pH 7.4, a peak in fraction 6 and a broad peak from fractions 8 to 11 were observed (Fig. 2A). In contrast, at pH 5.1, gB sedimented predominantly as a single population at fractions 6 and 7, suggesting that low pH caused gB to shift from a higher oligomeric form to a lower-density oligomer. Quantitation confirmed that the sedimentation profile of virion gB at pH 5.1 had shifted to a single, less dense peak (Fig. 2A, bottom).
FIG. 2.
Effect of low-pH treatment on the oligomeric state of gB. (A, top panel) Virions were solubilized with 1% Triton X-100 and subjected to sedimentation through sucrose (8 to 60%) buffered to pH 7.4 or 5.1. gB was immunoprecipitated from each collected fraction with MAb H1817 prior to SDS-PAGE and immunoblotting with MAb H1359 for detection of gB. In parallel experiments, protein standards were employed to approximate the range of molecular weights of proteins in each fraction. (A, bottom panel) Results were quantitated by densitometry. (B) Binding of gB oligomer-specific monoclonal antibody DL16 to low pH-treated virions. As described in the legend to panel A, HSV-1 KOS virions were treated with pH 7.2 or 5.5, and then 2-fold dilutions were blotted to a membrane. (C) Virions were treated with the indicated pH, solubilized with 1% SDS where indicated, and then analyzed by PAGE and immunoblotting for detection of gB. (D) HSV-1 KOS virions were treated at pH 7.4 or 5.1, solubilized with 1% SDS where indicated, and then analyzed by native PAGE and immunoblotting with polyclonal antibodies specific for gB, gC, gD, or gH-gL. Glycoprotein-specific bands are indicated with the name of the protein. Nonspecific bands that were detected in mock-infected, Vero cell-conditioned medium (not shown) are indicated by asterisks. Protein molecular weight standards are indicated on the left. α, anti.
Second, a monoclonal antibody specific for oligomeric gB, DL16 (7), was employed. HSV-1 KOS virions were treated with either pH 7.2 or 5.5 and blotted to membranes, and the reactivity of MAb DL16 was evaluated at neutral pH. After exposure to pH 5.5, there was a decrease in DL16 reactivity (Fig. 2B). As a control, rabbit polyclonal antibody to gB (R69) detected both virion samples to a similar extent.
Lastly, these results were extended by a direct analysis of virion gB by PAGE. Oligomers of HSV gB are comprised of noncovalently associated monomers. gB oligomers are disrupted experimentally by a combination of SDS and heat (15), resulting in monomeric gB that migrates at ∼116 kDa after SDS-PAGE. We tested whether low pH had an effect on the detergent stability of the gB oligomeric structure. Virions were adjusted to pHs ranging from 7.4 to 5.1 and prepared for “native” PAGE in sample buffer containing 0.2% SDS without reducing agent and without heating. Under these conditions, regardless of pH treatment, gB migrated as a range of oligomeric species of >181 kDa (Fig. 2C, lanes 1 and 2). Treatment of HSV with pH 7.4 followed by 1% SDS yielded gB species of similar high molecular weight (Fig. 2C, lane 3), indicating that 1% SDS alone had no detectable effect on the gB oligomer. In contrast, pretreatment with pH <6.4 followed by 1% SDS reduced the number of gB species detected (Fig. 2C, lanes 4 to 7). The highest-molecular-weight forms seemed to disappear, leaving only a single detectable oligomeric species of lower molecular weight (Fig. 2C, lanes 6 and 7). This suggests that low pH alters the oligomeric structure of gB, making it more sensitive to disruption by SDS. With decreasing pH, there was an apparent decrease in detection of gB-reactive species. One explanation is that monomers are detected only weakly relative to oligomers under standard native PAGE analysis (data not shown). Alternately, upon activation by pH, gB may become part of a larger complex that does not enter the native gel. For example, during fusion, gB forms higher-molecular-weight complexes with gD and gH-gL (5, 6). Notably, the total amount of gB detected by dot blot does not change upon exposure to mildly acidic pH (Fig. 1). Together, results from the three approaches (Fig. 2A, B, and C) suggest that low pH alters the oligomeric conformation of virion gB, resulting in a lower-order oligomer.
To address whether the >181 kilodalton gB-reactive bands shown in Fig. 2C were indeed oligomers of gB, we tested whether other HSV entry glycoproteins comigrated with the gB-containing complexes. Virion gC, gD, gH, and gL at pH 7.4 each migrated independently of gB under native PAGE conditions (Fig. 2D, lanes without 1% SDS). gB may associate with other proteins. However, since a purified form of the recombinant gB oligomer has a molecular weight similar to that of virion gB (see Fig. 4A), we favor the interpretation that the high-molecular-weight species shown in Fig. 2C represent gB homo-oligomers.
FIG. 4.
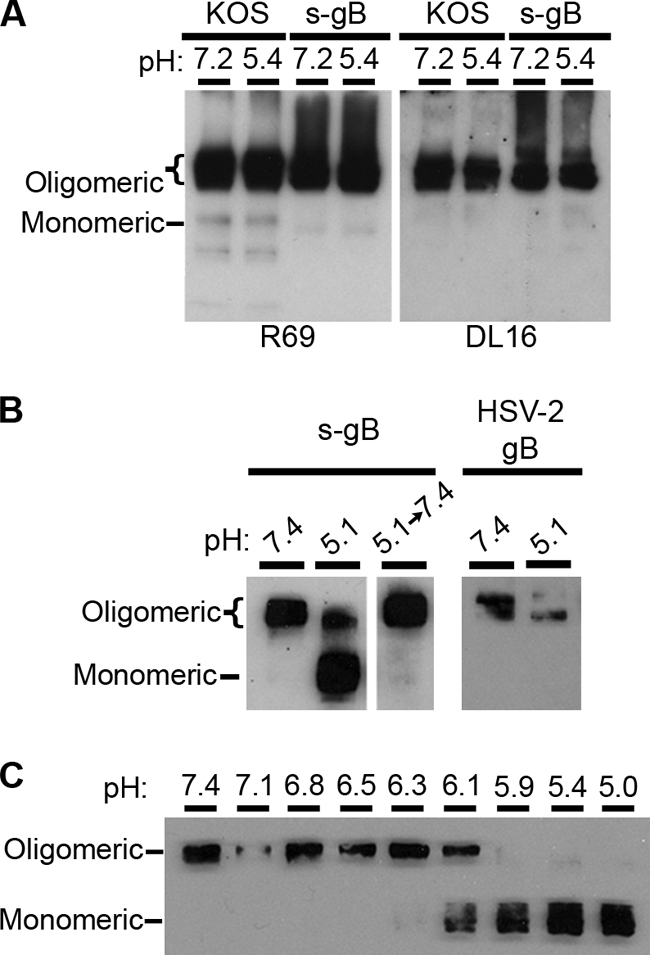
Effect of low-pH treatment on the conformation of purified gB. (A) HSV-1 KOS virions (105 PFU) or s-gB (150 ng) in serum-free medium with 0.2% BSA was kept at pH 7.2 or adjusted to pH 5.5 for 10 min at 37°C with 0.05 N HCl. The pH of acidified samples was reneutralized to 7.2 with 0.05 N NaOH for 10 min at 37°C. Samples were analyzed by native PAGE and immunoblotting with gB-specific PAb R69 or MAb DL16. (B) Soluble gB derived from HSV-2 strain 333 (s-gB) was treated with pH 7.4 or 5.1 and then solubilized with 1% SDS either before or after neutralization of pH (as described in the legend to Fig. 2C). HSV-2 strain 333 virions were also treated at pH 7.4 or 5.1 and solubilized with 1% SDS. (C) s-gB was treated with a range of pH, as indicated, and then solubilized with 1% SDS. Samples were analyzed by PAGE and immunoblotting, with R69 for detection of gB.
Treatment of virions with pH 5.1 did not affect the migration of gC, gD, gH or gL relative to pH 7.4 on native polyacrylamide gels (Fig. 2D, lanes without 1% SDS). In addition, low pH did not reproducibly affect the detection of these glycoproteins, including the gH-gL hetero-oligomer (Fig. 2D, braces). Virion gC, gD, gH, or gL was also not affected when subjected to the conditions that demonstrated the effect of low pH on the detergent stability of gB (Fig. 2D, lanes with 1% SDS). Based on these analyses, gB may be the entry glycoprotein principally affected by pH.
pH-triggered conformational changes are reversible.
Conformational changes in class I and class II fusion proteins are irreversible (60). As an example, the prefusion form of hemagglutinin (HA) (class I) in the influenza viral envelope exists in a metastable state. If it is triggered by low pH in the absence of a target membrane, HA is irreversibly converted to the postfusion form and can no longer mediate fusion with a subsequently presented membrane (59). In contrast, conformational changes in the class III fusion protein VSV-G are reversible, with prefusion and postfusion forms existing in a thermodynamic equilibrium (24, 42). The equilibrium is shifted toward the postfusion state at low pH. To investigate the reversibility of changes in gB, we again assayed the effect of 1% SDS on oligomer stability using native PAGE (Fig. 2C). KOS virions were incubated at pH 5.1, reneutralized to pH 7.4, and then 1% SDS was added (Fig. 2C, lane 8). Oligomeric forms of gB were detected (Fig. 2C, lane 8), indistinguishable from those of gB that had been kept at neutral pH (lane 3). This suggests that low pH-induced changes in the oligomeric structure of gB are reversible.
To extend the findings of reversibility, we tested whether pH-induced antigenic changes were reversible, using a modification of the dot blot approach. pH 5.5-treated HSV that was blotted to nitrocellulose displayed decreased reactivity with MAb H126, relative to pH 7.2 treatment (Fig. 3). Although this is an overexposure, the reduction in H126 reactivity is consistent with the results shown in Fig. 1. However, when virions were treated first at pH 5.5 and then adjusted back to pH 7.2 prior to being blotted, H126 reactivity was partly recovered. Similar results were obtained with the oligomer-specific MAb DL16 (Fig. 3), supporting the notion of reversibility. Control polyclonal antibody to gB R69 reacted similarly with HSV that had been subjected to each of the different pH conditions (Fig. 3). Immobilization of acid-treated virions on nitrocellulose membranes may limit the reversibility of alterations in gB. This would explain why several of the MAbs to gB have diminished reactivity with acid-treated HSV after it is bound to nitrocellulose (Fig. 1 and Table 1).
FIG. 3.
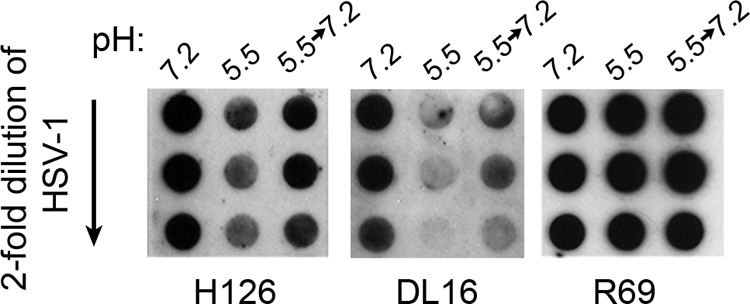
Reversibility of pH-induced conformational changes in gB. Extracellular HSV-1 KOS virions were treated with medium buffered to pH 7.2 or 5.5. For the indicated samples, pH was neutralized back to 7.2 for 10 min at 37°C. Twofold dilutions were blotted immediately to nitrocellulose membranes. Membranes were probed at neutral pH with antibody H126, DL16, or R69, followed by the appropriate horseradish peroxidase-conjugated secondary antibody. The exposures shown document the reversibility of reactivity.
Acid pH alters the conformation of a soluble, recombinant form of gB.
Thus far, pH-triggered changes have been detected in virion gB in the context of the HSV envelope, which includes the other glycoproteins. gB, gD, and gH-gL likely act together during membrane fusion. To address whether changes specific to gB could be observed in the absence of other virion components, purified gB in isolation was evaluated. We utilized a soluble form of gB (s-gB) from HSV-2 strain 333 (61). gB molecules from HSV-1 KOS and HSV-2 333 are 86% identical. In this form of gB, the transmembrane domain has been deleted, and the cytosolic tail is fused directly to the ectodomain. s-gB is secreted and purified from CHO cells (53, 61). It migrates as an oligomer on native PAGE (Fig. 4A), binds to cell surface glycosaminoglycan receptors (61), and reacts with all monoclonal antibodies tested, including the oligomer-specific antibody DL16 (Fig. 4A; Table 1).
Treatment of s-gB with mildly acidic pH followed by 1% SDS affected its oligomeric conformation (Fig. 4B). A pH of 5.1 converted oligomeric s-gB into a monomer (Fig. 4B). Conversion to a monomer began at pH <6.3 and was complete by pH 5.9 (Fig. 4C). Thus, the quaternary structure of purified gB was altered by the same range of mildly acidic pH as that of gB that is present in the viral envelope (compare Fig. 4C and 2C). Acid-induced changes in s-gB were reversible (Fig. 4B, center). Low-pH treatment converted gB present in HSV-2 333 into a lower-molecular-weight oligomeric form (Fig. 4B, right), similar to the result obtained with HSV-1 KOS gB (Fig. 2C). Interestingly, this contrasts with the acid-dependent conversion of the s-gB oligomer into a monomer. The difference may be due to the absence of a membrane anchor or the absence of interaction with gD or gH-gL. Nonetheless, low pH affects the oligomeric structures of both purified gB and virion gB. The reversibility of changes to both forms of gB is further demonstrated in Fig. 4A. Samples that were treated with pH 5.4, reneutralized to pH 7.2, and then analyzed by native PAGE migrated as oligomers, similar to the migration of samples that were not exposed to low pH (Fig. 4A). The change induced by pH 5.4 that was noted using other approaches was likely reversed prior to sample loading. Lastly, s-gB that was treated with acidic pHs and was then bound to nitrocellulose had a pattern of reactivity with MAbs similar to that of pH-treated virion gB (Table 1), indicating that pH directly triggers specific changes in gB antigenic structure. In total, the results suggest that gB need not be present in a membrane, nor does gD, gH, gL, or any other viral component need to be present in order for the observed pH-dependent changes to take place. In the context of the virion interacting with the host cell membrane during fusion, additional changes in gB likely occur that are not apparent in the current experiments.
Low pH increases the hydrophobicity of gB.
Conformational change associated with fusion is often accompanied by transient exposure of hydrophobic regions, namely fusion peptides or fusion loops. To begin to address whether conformational changes have an activating effect on gB, we used the nonionic detergent Triton X-114 and s-gB. Upon centrifugation at room temperature, Triton X-114 can be separated into hydrophilic (aqueous, top) and amphiphilic (detergent, bottom) phases (9). Protein forms that are more hydrophobic associate with hydrophobic Triton X-114 molecules and partition with the amphiphilic phase. This approach was used to define pH-induced changes in alphavirus fusion glycoproteins (31).
Purified s-gB that was treated at pH 7.4 was mixed with Triton X-114 at 37°C. The mixture was centrifuged, and water-soluble gB was recovered from the aqueous phase (Fig. 5), where hydrophilic proteins are expected to partition. Little to no gB was detected in the detergent phase, suggesting little hydrophobic interaction of gB with Triton X-114. In contrast, as a result of treatment with pH 5.9 or 5.1, a fraction of the total gB partitioned in the detergent phase (Fig. 5), suggesting that low pH caused gB to become more hydrophobic. As a control, low-pH treatment of bovine serum albumin did not alter its hydrophobic character (Fig. 5). The results are consistent with the notion that pH results in exposure of hydrophobic regions in gB. This increase in hydrophobicity may correlate with changes in gB conformation, such as alterations in oligomeric structure, and with activation of entry.
FIG. 5.
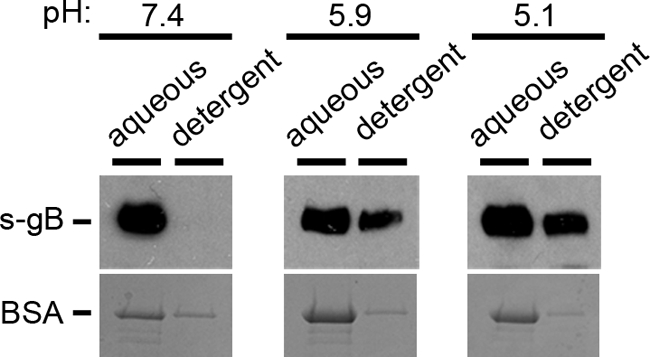
Effect of low-pH treatment on the hydrophobicity of gB. Soluble gB or BSA was added to 2% Triton X-114 that had been adjusted to the indicated pH. Samples were incubated for 10 min at 37°C and were centrifuged at 300 × g for 3 min. The aqueous supernatant phase and detergent phase were collected and diluted 20-fold in PBS. s-gB samples were subjected to immunoprecipitation with antibody to gB, followed by SDS-PAGE and immunoblotting for gB. BSA samples were trichloroacetic acid (TCA) precipitated and analyzed by SDS-PAGE and Coomassie blue staining.
Intracellular low pH affects the H126 epitope of virion gB during viral entry.
The kinetics of the distinct steps in the endocytic entry pathway of HSV have been delineated in CHO-nectin-1 cells (39). Endocytic internalization of HSV from the CHO cell surface has a half-life (t1/2) of ∼9 min. Enveloped virions are trafficked through cellular compartments for up to ∼30 min postinfection (p.i.). Penetration of >50% of infectious virions from a low-pH compartment (virus-cell membrane fusion) occurs by 60 min p.i (39). To probe the exposure of the H126 epitope on gB during endocytic entry of HSV, CHO-nectin-1 cells were infected with HSV-1 for 1 h, and input gB was monitored by immunofluorescence and confocal microscopy. The early time of infection analyzed and the presence of cycloheximide ensured that the signal detected was due to input gB and not to the newly synthesized gB. gB that was brought into the cell with entering virions was detected by MAb H126 as punctate staining (Fig. 6). Bafilomycin A1 (BFLA), a vacuolar H+ ATPase inhibitor that prevents endosome acidification, inhibits HSV entry into CHO-nectin-1 cells (38). When infection proceeded in the presence of BFLA, there was a detectable and reproducible increase in the H126-reactive gB detected (Fig. 6). As a control, MAb H1817 recognized input intracellular gB to a similar extent in the absence or presence of BFLA (Fig. 6). This suggests that the N terminus of gB is accessible to antibody, even after exposure to intravesicular low pH. In contrast, the H126 epitope in domain I appears to be altered during viral entry upon exposure to acidic pH. This change may be detected because all virion gB molecules are exposed to endosomal low pH, regardless of whether they are directly involved in viral entry.
FIG. 6.
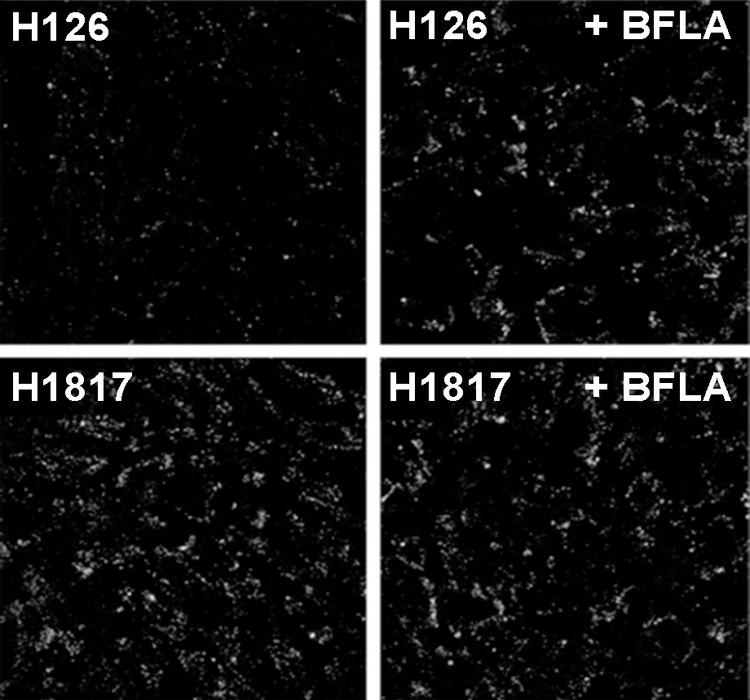
Effect of bafilomycin A1 on the conformation of HSV gB during viral entry. CHO-nectin-1 cells were mock treated (left) or treated with 25 nM bafilomycin A1 (right, +BFLA) for 15 min. HSV-1 KOS (MOI of 20) was bound to cells at 4°C for 1 h. Cultures were shifted to 37°C for 1 h in the constant presence of BFLA and 0.5 mM cycloheximide. Virion gB was visualized with MAb H126 or MAb H1817 followed by Alexa 488-labeled goat anti-mouse antibody. Nuclei were detected with DAPI (4′,6-diamidino-2-phenylindole) (not shown). Samples were visualized by confocal microscopy at 63× magnification. A total of 50 to 70 cells are shown per panel.
DISCUSSION
In this paper, we demonstrate that conformational change in the fusogenic herpesviral glycoprotein gB is triggered by mildly acidic pH that is typically encountered in the endosomal network (pH ∼5.0 to 6.0). Low pH specifically alters the antigenic structure and oligomeric conformation of gB. Both conformational change and an increase in the hydrophobic character of gB occur at a similar pH range. This work describes a critical feature of the complex molecular mechanism of herpesviral entry by pH-dependent endocytosis, a pathway that is employed by HSV in biologically relevant cell types, including epithelial cells.
MAb H126 has HSV-neutralizing activity (32). The H126 epitope in the fusion domain of gB becomes less accessible upon exposure of gB to low pH both in vitro and during viral entry. Thus, H126 may neutralize virus infection by blocking conformational change in gB or by preventing contact of fusion loops with the target membrane. Interestingly, H126 neutralizes HSV entry to a similar extent in cells, regardless of whether the cells support pH-dependent or pH-independent entry (18, 45). HSV-1 strain ANG path has a highly fusogenic form of gB that is responsible for fusion-from-without (FFWO) activity (48). Two distinct mutations in gB are responsible for FFWO. Notably, gB from ANG path has reduced reactivity with MAbs DL16 and H126 (45). Thus, the antigenic conformation of a mutant gB with enhanced fusion activity is similar to the conformation of low pH-treated wild-type gB, supporting the notion that acid pH triggers the fusion activity of gB.
As gB is the most-conserved herpesviral glycoprotein, similar low-pH activation of gB from other herpesviruses may also occur. Cell monolayers transfected with gB or gD alone have been reported previously to undergo pH-dependent cell fusion (2, 10). Low pH has little to no detectable effect on gD's antigenic structure or on its ability to bind to receptors (38; our unpublished data). The present data suggest that gB is a principal target of endosomal pH. However, acid effects on other glycoproteins such as gH, which contains a putative fusion peptide (25), remain to be investigated further.
Host cell triggers that can cause conformational changes in glycoproteins leading to fusion include the low-pH milieu of an endosome, binding to receptors, and cleavage by endosomal proteases (60). HSV likely requires more than a single cellular trigger to mediate membrane fusion and entry. HSV gD binding to one of its cognate receptors causes a displacement of the C terminus of gD (33). This change in gD conformation is thought to initiate the fusion process mediated by gB and gH-gL (5, 23, 54). In the case of HSV entry into cells by acid-dependent endocytosis, we propose that the direct action of endosomal pH on gB is required in addition to engagement of a gD receptor. While we currently have no evidence for the involvement of pH-activated cellular proteases in HSV entry (our unpublished data), other indispensable cellular triggers may be necessary to complete the fusion process.
In addition to endosomal pH, one or more cell factors may serve the redundant function of triggering conformational change in gB. In a similar vein, distinct cell receptors serve a partly redundant receptor-binding role for gD. gB is required for pH-neutral fusion of HSV with the plasma membrane of a subset of cell types, such as Vero cells (11). The recently identified gB receptor PILRα may trigger direct penetration of HSV with the cell surface (4, 50). However, fusion probably cannot occur without a gD receptor and gD, even when PILRα has a role (20, 50). All gB-specific neutralizing antibodies tested have a similar inhibitory effect on HSV entry, regardless of whether entry into the target cell type is dependent on intracellular low pH (18, 45). It is tempting to speculate that in cells that support pH-independent entry, binding to a gB-specific receptor such as PILRα may functionally substitute for endosomal pH and induce a conformational change in gB that leads to pH-independent entry.
Unlike glycoproteins from viruses that mediate fusion exclusively at low pH, HSV cell-cell fusion can occur at physiologic pH (55). In this surrogate assay, transfected cells that express gB, gD, gH, and gL on the cell surface are mixed with untransfected target cells. Comparisons of cell-cell fusion with virus-cell fusion must be drawn cautiously. Results from cell fusion and viral entry assays do not always agree (12, 36, 62). Herpesviral envelopes are derived from internal cellular membranes and not from the plasma membrane. Glycoproteins displayed on the plasma membranes of transfected cells may have distinct roles in fusion (i.e., are activated differently) than glycoproteins that are actually incorporated into virions. We are currently evaluating the effect of pH on glycoprotein-induced cell-cell fusion.
Full-length gB from HSV-2 lacking only the transmembrane region (s-gB) was not sufficiently hydrophobic to associate detectably with micelles of Triton X-114. Only upon treatment with mildly acidic pH did s-gB associate with the detergent phase, suggesting an increase in hydrophobic character. Interestingly, low-pH treatment of virions increases their hydrophobic nature, as measured by binding to liposomes in the presence of soluble receptor (58). gB is a likely candidate for mediating pH-triggered association of virions with membrane. The similar effects of low pH on s-gB and on virion gB suggest that the transmembrane-deleted gB used in this study may resemble the prefusion form found in the virion envelope.
The structure of a truncated form of HSV-1 gB (called gB730) is thought to be the postfusion form (28, 34). Unlike s-gB, which becomes hydrophobic upon exposure to low pH (Fig. 5), gB730 is sufficiently hydrophobic at neutral pH to bind to liposomes (27). The fusion loops are surface exposed in the gB730 structure and are responsible for liposome binding. The absence of N-terminal or C-terminal residues from gB730 may drive it irreversibly to the postfusion conformation, regardless of pH.
HSV gB undergoes a reversible change in structure in response to pH, similar to the change in other class III fusion proteins, VSV-G (19, 44) and baculovirus gp64 (64). Thus, reversibility of conformational change may be a general feature of class III fusion proteins. Interestingly, low-pH treatment of virions irreversibly inactivates viral entry (38). This seeming paradox may be explained by irreversible pH-induced changes in gB, gD, or gH-gL that have yet to be identified.
Our results suggest that low pH may cause a destabilization of the oligomeric conformation of s-gB and virion gB. Similar disruption of soluble gB from HSV-1 was reported previously (49). The observation that the functional region of gB that contains the hydrophobic fusion loops is altered by mildly acidic pH suggests that low pH may facilitate the proper gB-target membrane contact necessary for entry. In the case of VSV, low pH causes a tighter association of G subunits, making them more stable (19). In response to low pH, the bipartite fusion loops of each G monomer are thought to pack as a trimer and contact the target membrane (42). In contrast, virion gB appears to become a lower-order oligomer upon pH activation. This may be a difference in the fusion mechanisms between these two class III fusion proteins. This distinction might reflect the necessity of other HSV glycoproteins to complete the fusion process.
Acknowledgments
This investigation was supported by Public Health Service grants AI-60702, AI-083850, and AI-07617 from the National Institute of Allergy and Infectious Diseases and a grant from the Japan Health Sciences Foundation (grant SAA4832).
We thank Pat Spear for critical reading of the manuscript.
Footnotes
Published ahead of print on 10 February 2010.
REFERENCES
- 1.Akula, S. M., P. P. Naranatt, N. S. Walia, F. Z. Wang, B. Fegley, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J. Virol. 77:7978-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali, M. A., M. Butcher, and H. P. Ghosh. 1987. Expression and nuclear envelope localization of biologically active fusion glycoprotein gB of herpes simplex virus in mammalian cells using cloned DNA. Proc. Natl. Acad. Sci. U. S. A. 84:5675-5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison, S. L., J. Schalich, K. Stiasny, C. W. Mandl, C. Kunz, and F. X. Heinz. 1995. Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J. Virol. 69:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arii, J., M. Uema, T. Morimoto, H. Sagara, H. Akashi, E. Ono, H. Arase, and Y. Kawaguchi. 2009. Entry of herpes simplex virus 1 and other alphaherpesviruses via the paired immunoglobulin-like type 2 receptor alpha. J. Virol. 83:4520-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atanasiu, D., J. C. Whitbeck, T. M. Cairns, B. Reilly, G. H. Cohen, and R. J. Eisenberg. 2007. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc. Natl. Acad. Sci. U. S. A. 104:18718-18723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avitabile, E., C. Forghieri, and G. Campadelli-Fiume. 2007. Complexes between herpes simplex virus glycoproteins gD, gB, and gH detected in cells by complementation of split enhanced green fluorescent protein. J. Virol. 81:11532-11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender, F. C., M. Samanta, E. E. Heldwein, M. P. de Leon, E. Bilman, H. Lou, J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen. 2007. Antigenic and mutational analyses of herpes simplex virus glycoprotein B reveal four functional regions. J. Virol. 81:3827-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender, F. C., J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J. Virol. 79:11588-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604-1607. [PubMed] [Google Scholar]
- 10.Butcher, M., K. Raviprakash, and H. P. Ghosh. 1990. Acid pH-induced fusion of cells by herpes simplex virus glycoproteins gB and gD. J. Biol. Chem. 265:5862-5868. [PubMed] [Google Scholar]
- 11.Cai, W. H., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 62:2596-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cairns, T. M., R. S. Milne, M. Ponce-de-Leon, D. K. Tobin, G. H. Cohen, and R. J. Eisenberg. 2003. Structure-function analysis of herpes simplex virus type 1 gD and gH-gL: clues from gDgH chimeras. J. Virol. 77:6731-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campadelli-Fiume, G., F. Cocchi, L. Menotti, and M. Lopez. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10:305-319. [DOI] [PubMed] [Google Scholar]
- 14.Chandran, K., N. J. Sullivan, U. Felbor, S. P. Whelan, and J. M. Cunningham. 2005. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308:1643-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claesson-Welsh, L., and P. G. Spear. 1986. Oligomerization of herpes simplex virus glycoprotein B. J. Virol. 60:803-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen, G. H., V. J. Isola, J. Kuhns, P. W. Berman, and R. J. Eisenberg. 1986. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J. Virol. 60:157-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delboy, M. G., J. L. Patterson, A. M. Hollander, and A. V. Nicola. 2006. Nectin-2-mediated entry of a syncytial strain of herpes simplex virus via pH-independent fusion with the plasma membrane of Chinese hamster ovary cells. Virol. J. 3:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dollery, S. J., K. D. Lane, M. G. Delboy, D. G. Roller, and A. V. Nicola. 4 January 2010, posting date. Role of the UL45 protein in herpes simplex virus entry via low pH-dependent endocytosis and its relationship to the conformation and function of glycoprotein B. Virus Res. doi: 10.1016/j.virusres. [DOI] [PMC free article] [PubMed]
- 19.Doms, R. W., D. S. Keller, A. Helenius, and W. E. Balch. 1987. Role for adenosine triphosphate in regulating the assembly and transport of vesicular stomatitis virus G protein trimers. J. Cell Biol. 105:1957-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan, Q., E. Lin, T. Satoh, H. Arase, and P. G. Spear. 2009. Differential effects on cell fusion activity of mutations in herpes simplex virus 1 glycoprotein B (gB) dependent on whether a gD receptor or a gB receptor is overexpressed. J. Virol. 83:7384-7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finnen, R. L., K. R. Mizokami, B. W. Banfield, G. Y. Cai, S. A. Simpson, L. I. Pizer, and M. J. Levin. 2006. Postentry events are responsible for restriction of productive varicella-zoster virus infection in Chinese hamster ovary cells. J. Virol. 80:10325-10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fusco, D., C. Forghieri, and G. Campadelli-Fiume. 2005. The pro-fusion domain of herpes simplex virus glycoprotein D (gD) interacts with the gD N terminus and is displaced by soluble forms of viral receptors. Proc. Natl. Acad. Sci. U. S. A. 102:9323-9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaudin, Y. 2000. Reversibility in fusion protein conformational changes. The intriguing case of rhabdovirus-induced membrane fusion. Subcell. Biochem. 34:379-408. [DOI] [PubMed] [Google Scholar]
- 25.Gianni, T., P. L. Martelli, R. Casadio, and G. Campadelli-Fiume. 2005. The ectodomain of herpes simplex virus glycoprotein H contains a membrane alpha-helix with attributes of an internal fusion peptide, positionally conserved in the herpesviridae family. J. Virol. 79:2931-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillet, L., S. Colaco, and P. G. Stevenson. 2008. Glycoprotein B switches conformation during murid herpesvirus 4 entry. J. Gen. Virol. 89:1352-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hannah, B. P., T. M. Cairns, F. C. Bender, J. C. Whitbeck, H. Lou, R. J. Eisenberg, and G. H. Cohen. 2009. Herpes simplex virus glycoprotein B associates with target membranes via its fusion loops. J. Virol. 83:6825-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heldwein, E. E., H. Lou, F. C. Bender, G. H. Cohen, R. J. Eisenberg, and S. C. Harrison. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217-220. [DOI] [PubMed] [Google Scholar]
- 29.Highlander, S. L., W. F. Goins, S. Person, T. C. Holland, M. Levine, and J. C. Glorioso. 1991. Oligomer formation of the gB glycoprotein of herpes simplex virus type 1. J. Virol. 65:4275-4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, D. C., and M. W. Ligas. 1988. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J. Virol. 62:4605-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kielian, M., and A. Helenius. 1985. pH-induced alterations in the fusogenic spike protein of Semliki Forest virus. J. Cell Biol. 101:2284-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kousoulas, K. G., P. E. Pellett, L. Pereira, and B. Roizman. 1984. Mutations affecting conformation or sequence of neutralizing epitopes identified by reactivity of viable plaques segregate from syn and ts domains of HSV-1(F) gB gene. Virology 135:379-394. [DOI] [PubMed] [Google Scholar]
- 33.Krummenacher, C., V. M. Supekar, J. C. Whitbeck, E. Lazear, S. A. Connolly, R. J. Eisenberg, G. H. Cohen, D. C. Wiley, and A. Carfi. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 24:4144-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin, E., and P. G. Spear. 2007. Random linker-insertion mutagenesis to identify functional domains of herpes simplex virus type 1 glycoprotein B. Proc. Natl. Acad. Sci. U. S. A. 104:13140-13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milne, R. S., A. V. Nicola, J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen. 2005. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J. Virol. 79:6655-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muggeridge, M. I. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 81(Pt. 8):2017-2027. [DOI] [PubMed] [Google Scholar]
- 37.Nicola, A. V., J. Hou, E. O. Major, and S. E. Straus. 2005. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J. Virol. 79:7609-7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 77:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicola, A. V., and S. E. Straus. 2004. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J. Virol. 78:7508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pellett, P. E., K. G. Kousoulas, L. Pereira, and B. Roizman. 1985. Anatomy of the herpes simplex virus 1 strain F glycoprotein B gene: primary sequence and predicted protein structure of the wild type and of monoclonal antibody-resistant mutants. J. Virol. 53:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira, L., M. Ali, K. Kousoulas, B. Huo, and T. Banks. 1989. Domain structure of herpes simplex virus 1 glycoprotein B: neutralizing epitopes map in regions of continuous and discontinuous residues. Virology 172:11-24. [DOI] [PubMed] [Google Scholar]
- 42.Roche, S., A. A. Albertini, J. Lepault, S. Bressanelli, and Y. Gaudin. 2008. Structures of vesicular stomatitis virus glycoprotein: membrane fusion revisited. Cell. Mol. Life Sci. 65:1716-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roche, S., S. Bressanelli, F. A. Rey, and Y. Gaudin. 2006. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science 313:187-191. [DOI] [PubMed] [Google Scholar]
- 44.Roche, S., F. A. Rey, Y. Gaudin, and S. Bressanelli. 2007. Structure of the prefusion form of the vesicular stomatitis virus glycoprotein g. Science 315:843-848. [DOI] [PubMed] [Google Scholar]
- 45.Roller, D. G., S. J. Dollery, J. L. Doyle, and A. V. Nicola. 2008. Structure-function analysis of herpes simplex virus glycoprotein B with fusion-from-without activity. Virology 382:207-216. [DOI] [PubMed] [Google Scholar]
- 46.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryckman, B. J., M. A. Jarvis, D. D. Drummond, J. A. Nelson, and D. C. Johnson. 2006. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J. Virol. 80:710-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saharkhiz-Langroodi, A., and T. C. Holland. 1997. Identification of the fusion-from-without determinants of herpes simplex virus type 1 glycoprotein B. Virology 227:153-159. [DOI] [PubMed] [Google Scholar]
- 49.Samanta, S., R. J. Eisenberg, and G. H. Cohen. 1994. Studies of monomeric and oligomeric forms of HSV gB, abstr. 29. Abstr. 19th Int. Herpesvirus Workshop, Vancouver, British Columbia, Canada.
- 50.Satoh, T., J. Arii, T. Suenaga, J. Wang, A. Kogure, J. Uehori, N. Arase, I. Shiratori, S. Tanaka, Y. Kawaguchi, P. G. Spear, L. L. Lanier, and H. Arase. 2008. PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell 132:935-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simmons, G., D. N. Gosalia, A. J. Rennekamp, J. D. Reeves, S. L. Diamond, and P. Bates. 2005. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. U. S. A. 102:11876-11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 53.Stuve, L. L., S. Brown-Shimer, C. Pachl, R. Najarian, D. Dina, and R. L. Burke. 1987. Structure and expression of the herpes simplex virus type 2 glycoprotein gB gene. J. Virol. 61:326-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Subramanian, R. P., and R. J. Geraghty. 2007. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc. Natl. Acad. Sci. U. S. A. 104:2903-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wahlberg, J. M., and H. Garoff. 1992. Membrane fusion process of Semliki Forest virus. I: Low pH-induced rearrangement in spike protein quaternary structure precedes virus penetration into cells. J. Cell Biol. 116:339-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weissenhorn, W., A. Hinz, and Y. Gaudin. 2007. Virus membrane fusion. FEBS Lett. 581:2150-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitbeck, J. C., Y. Zuo, R. S. Milne, G. H. Cohen, and R. J. Eisenberg. 2006. Stable association of herpes simplex virus with target membranes is triggered by low pH in the presence of the gD receptor, HVEM. J. Virol. 80:3773-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White, J., J. Kartenbeck, and A. Helenius. 1982. Membrane fusion activity of influenza virus. EMBO J. 1:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White, J. M., S. E. Delos, M. Brecher, and K. Schornberg. 2008. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 43:189-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams, R. K., and S. E. Straus. 1997. Specificity and affinity of binding of herpes simplex virus type 2 glycoprotein B to glycosaminoglycans. J. Virol. 71:1375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson, D. W., N. Davis-Poynter, and A. C. Minson. 1994. Mutations in the cytoplasmic tail of herpes simplex virus glycoprotein H suppress cell fusion by a syncytial strain. J. Virol. 68:6985-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wittels, M., and P. G. Spear. 1991. Penetration of cells by herpes simplex virus does not require a low pH-dependent endocytic pathway. Virus Res. 18:271-290. [DOI] [PubMed] [Google Scholar]
- 64.Zhou, J., and G. W. Blissard. 2006. Mapping the conformational epitope of a neutralizing antibody (AcV1) directed against the AcMNPV GP64 protein. Virology 352:427-437. [DOI] [PMC free article] [PubMed] [Google Scholar]