Abstract
Newcastle disease virus (NDV), an avian paramyxovirus, is tumor selective and intrinsically oncolytic because of its potent ability to induce apoptosis. Several studies have demonstrated that NDV is selectively cytotoxic to tumor cells but not normal cells due to defects in the interferon (IFN) antiviral responses of tumor cells. Many naturally occurring strains of NDV have an intact IFN-antagonistic function and can still replicate in normal human cells. To avoid potential toxicity issues with NDV, especially in cancer patients with immunosuppression, safe NDV-oncolytic vectors are needed. We compared the cell killing abilities of (i) a recombinant NDV (rNDV) strain, Beaudette C, containing an IFN-antagonistic, wild-type V protein (rBC), (ii) an isogenic recombinant virus with a mutant V protein (rBC-Edit virus) that induces increased IFN in infected cells and whose replication is restricted in normal human cells, and (iii) a recombinant LaSota virus with a virulent F protein cleavage site that is as interferon sensitive as rBC-Edit virus (LaSota V.F. virus). Our results indicated that the tumor-selective replication of rNDV is determined by the differential regulation of IFN-α and downstream antiviral genes induced by IFN-α, especially through the IRF-7 pathway. In a nude mouse model of human fibrosarcoma, we show that the IFN-sensitive NDV variants are as effective as IFN-resistant rBC virus in clearing the tumor burden. In addition, mice treated with rNDV exhibited no signs of toxicity to the viruses. These findings indicate that augmentation of innate immune responses by NDV results in selective oncolysis and offer a novel and safe virotherapy platform.
Several naturally occurring or engineered oncolytic viruses are emerging as novel tools for selective growth in and killing of a variety of tumor cells (1, 21, 34, 41). It has been consistently reported that during tumor evolution, diminished interferon (IFN) responsiveness coevolves as a frequent genetic defect (4, 31, 32, 41). Any defects in responsiveness to interferon will afford permissiveness of tumors for replication of oncolytic viruses by blunting the antiviral innate immune system. Thus, it was suggested that oncolytic viruses could be engineered to induce strong IFN response and/or to be defective in antagonizing the IFN signaling. This would result in virus replication in tumor cells with IFN defects but in reduced or crippled virus replication in normal cells, with the absence of toxicity (42). A variety of oncolytic viruses have been engineered to exploit tumor-specific genetic defects (3, 12, 24, 42, 46) and shown to be potent oncolytic agents.
Newcastle disease virus (NDV), an avian paramyxovirus, is a promising broad-spectrum oncolytic agent (27, 29, 30, 37). Nonengineered, naturally occurring strains of NDV such as 73-T (6), MTH68 (7), PV701 (28, 35), and NDV-HUJ (11) have been successfully employed in several clinical studies for tumor regression. NDV is inherently oncolytic and tumor selective, sparing normal cells (9, 15, 37). The tumor selectivity of NDV is considered to be due to a defective IFN response in tumor cells (10, 23, 37). NDV is a strong inducer of type I IFN in many types of cells (18). In normal cells, a robust IFN-mediated antiviral response limits the replication of NDV (9, 23). This known sensitivity of NDV to cellular antiviral mechanisms affords a wide safety margin for its use in humans.
Recent studies have indicated that improved therapeutic vectors of NDV could be engineered through reverse genetics for enhanced oncolytic efficacy from an increased anti-tumor response and interleukin 2 (IL-2) receptor-mediated targeting (5, 9, 44, 46). Therefore, we reasoned that recombinant NDVs (rNDVs) that are susceptible to cellular innate immune responses would be safer and more effective oncolytic agents. Even though NDV is an avian virus and induces a strong IFN response in normal human cells, it still expresses IFN-antagonizing activity. Ablation of the expression of V protein, which is responsible for this anti-IFN activity, may further reduce the ability of NDV to infect and kill normal human cells without affecting tumor cell infection and lysis. Here, we describe the relative oncolytic efficacies of three rNDV strains differing in IFN antagonism. The rNDV variants with an IFN-sensitive phenotype had parallel therapeutic efficacies in xenotransplanted human fibrosarcoma cells in a nude mouse model and offer great potential as recombinant vectors in therapy of human malignancies.
MATERIALS AND METHODS
Cells and viruses.
DF1 chicken embryo fibroblast, HeLa, HEpG2, CaCo2, SVHUC1, Vero, and HuTu80 cells were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS), 100 μg/ml penicillin, and 0.1 μg/ml streptomycin (Invitrogen, Carlsbad, CA). T84 colon cancer and SH-SY5Y neuroblastoma cells were grown in a 1:1 mixture of DMEM and Ham's F-12 medium with 10% fetal calf serum (FCS) and antibiotics. THP-1, CCRF-CEM, PC3, SW 620, MCF 7, CoLo205, HT29, and HT1080 cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% FBS and antibiotics. The cells were grown at 37°C with 5% CO2 in a humidified incubator. We employed (i) recombinant NDV (rNDV) strain Beaudette C (BC), which contains an IFN-antagonistic, wild-type V protein (rBC), (ii) an isogenic recombinant virus with a mutant V protein (rBC-Edit virus) that induces robust IFN in infected cells, and (iii) a recombinant LaSota virus with a virulent F protein cleavage site that is as interferon sensitive as rBC-Edit virus (rLaSota V.F. virus). The construction and recovery of an infectious clone of a moderately pathogenic NDV strain, Beaudette C, have been described previously (22); this strain was used as a base to construct mutants or viruses with additional transgenes. The construction and recovery of the P gene-editing mutant (rBC-Edit) and rLaSota V.F. viruses have been described in detail elsewhere (16). Recombinant BC-EGFP has an extra cistron-encoding enhanced green fluorescent protein (EGFP) inserted between the P and M gene sequences of the BC strain (9). Viruses were plaque purified, and virus stocks were prepared and titrated in DF1 cells as described previously (9).
ELISA.
IFN-α and IFN-β levels in the supernatants of virus-infected cells were measured using a human IFN-α multisubtype enzyme-linked immunosorbent assay (ELISA) kit (41105-1; PBL Biomedical Laboratories, Piscataway, NJ) and a human IFN-β ELISA kit (41100-1; PBL Biomedical Laboratories, Piscataway, NJ), respectively. Briefly, various cell lines were infected with rNDV at a multiplicity of infection (MOI) of 10. Culture supernatants (100 μl) in triplicate were collected at 48 h postinfection (p.i.), clarified, and assayed. For kinetic assays, HuTu80 cells were infected with rNDVs (rBC and rBC-Edit viruses) at an MOI of 0.01, and the levels of IFN in the supernatants collected at 0, 6, 8, 10, 12, 14, 20, 24, 28, 38, 48, and 72 h p.i. were measured by ELISA as described above. Samples were processed as per the manufacturer's instructions and then read on a Victor multilabel plate reader. Expression of IFN-γ-inducible protein-10 (IP-10) and regulated-on-activation, normal-T cell-expressed and secreted (RANTES) chemokines in cells infected with different strains of rNDV (MOI, 10) at 48 h p.i. were analyzed by using Quantikine immunoassay kits (R&D, Minneapolis, MN) per the manufacturer's instructions.
IFN sensitivity assay.
The relative sensitivities of rBC, rBC-Edit, and rLaSota V.F. viruses to exogenously added human IFN-α (h IFN-α) were measured on SVHUC1 and HuTu80 cells. Briefly, cells in six-well culture dishes at 80% confluence were incubated for 24 h with h IFN-α (PBL Biomedical Laboratories, Piscataway, NJ) and then with rNDV at an MOI of 0.01. Cells were adsorbed with virus for 1 h, the residual virus in the inoculum was removed and washed, and then cells were incubated in medium containing 2% fetal calf serum. The virus yields in culture supernatants were determined by plaque assay in Vero cells.
RT-PCR.
Total RNA was isolated from rNDV- or mock-infected SVHUC1 and HuTu80 cells using the RNeasy mini kit (Qiagen, Valencia, CA) and reverse transcribed with Superscript II reverse transcriptase (RT; Invitrogen, Carlsbad, CA). PCR to detect the message for ISG 6-16, IRF-1, 2′,5′ A synthetase (2′,5′ A), ISG15, and β-actin was performed in the presence of 1.5 mM MgCl2, 1 mM deoxynucleoside triphosphates (dNTPs), 1 μM (each) sense and antisense primers, and 2.5 U of Taq DNA polymerase (Invitrogen, Carlsbad, CA). The primer sequences and their expected sizes (in parentheses) are as follows: ß-actin, 5′-GACTTCGAGCAAGAGATGGCCAC-3′ (sense) and 5′-CAATGCCAGGGTATGGTGGTG-3′ (antisense) (265 bp); ISG 6-16, 5′-CCTGCTGCTCTTCACTTGC-3′ (sense) and 5′-CCTCATCCTCCTCACTATCG-3′ (antisense) (352 bp); ISG15, 5′-CCGTGAAGATGCTGGCG-3′ (sense) and 5′-CGAAGGTCAGCCAGAAC-3′ (antisense) (355 bp); IRF-1, 5′-CGAAGTCCAGAGATG-3′ (sense) and 5′-CCTGGGCTGTCAATTTC-3′ (antisense) (461 bp); and 2′,5′ A, 5′-ATCAACAGTGCCAGA-3′ (sense) and 5′-GTCGTGAAGAGTGGTGC-3′ (antisense; 408 bp). Amplification of reverse-transcribed cDNA was performed for 30 cycles (denaturation at 95°C for 1 min, annealing at 60°C for 2 min, and extension at 72°C for 3 min). PCR products were visualized by electrophoresis on 2% agarose gels and staining with ethidium bromide (2).
Immunoblotting.
DF1 cells or human tumor cells were infected with rNDV at an MOI of 10. Virus-infected cells were harvested at 48 h postinfection, pelleted by centrifugation, washed with ice-cold phosphate-buffered saline, and lysed by sonication in 150 μl of lysis buffer (1% NP-40, 0.15 M NaCl, 5.0 mM EDTA, 0.01 M Tris [pH 8.0], 1.0 mM phenylmethylsulfonyl fluoride [PMSF], 0.02 mg/ml leupeptin, 0.02 mg/ml trypsin inhibitor). Clarified lysates were separated by 4 to 20% SDS-PAGE (Nu-Page; Invitrogen, Carlsbad, CA) and immunoblotted with specific antibodies. The following antibodies were used for blotting: IRF-7 (sc-9083; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), IRF-3 (sc-9082; Santa Cruz Biotechnology, Inc.), Stat-1α (sc-345; Santa Cruz Biotechnology, Inc.), Stat-2 (sc-476; Santa Cruz Biotechnology, Inc.), and NDV-MCA (monoclonal antibody cocktail directed against the NDV HN protein [25]). To ensure equal loadings, all the blots were reprobed with an anti-actin antibody (sc-8432; Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
Animal studies.
All procedures involving animals complied with NIH protocols and were performed according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Virginia Polytechnic Institute and State University. Six-week-old BALB/c nude mice were purchased from Charles River and housed in our enhanced ABSL-2 facility in HEPA-filtered isolators. HT1080 cells (5 × 106) were implanted subcutaneously into the backs of mice. When tumor sizes reached >5 mm in diameter, they were intratumorally inoculated with a single injection of 2 × 107 PFU of rNDV strains in a 50-μl volume. Tumor volumes were monitored every 2 to 3 days using a caliper in three dimensions. Tumor volumes were calculated using the following ellipsoidal formula: tumor volume = 4/3 × π × r3, where “r” is the average diameter of the tumor in all three planes. Animals were killed when tumor sizes reached 20 mm in any plane or at defined experimental time points.
Live imaging.
Live in vivo EGFP imaging was done using a series 200 IVIS imaging system (Xenogen Corp.) with Living Image acquisition and analysis software (version 2.11; Xenogen). Mice were anesthetized with isoflurane-mixed oxygen at 72 h after a single intratumoral injection of rBC-EGFP (2 × 107 PFU) in a 100-μl volume. The overlay of the pseudocolor images represents the spatial distribution of photon counts produced by EGFP. An integration time of 1 min was used for fluorescent image acquisition.
Statistics.
Data are presented as means of results from two independent experiments plus standard deviations. One-way analysis of variance (ANOVA) was used to determine significance among groups. A P value of <0.05 was considered to be significant.
RESULTS
Replication and spread of rNDVs is restricted in normal human cells.
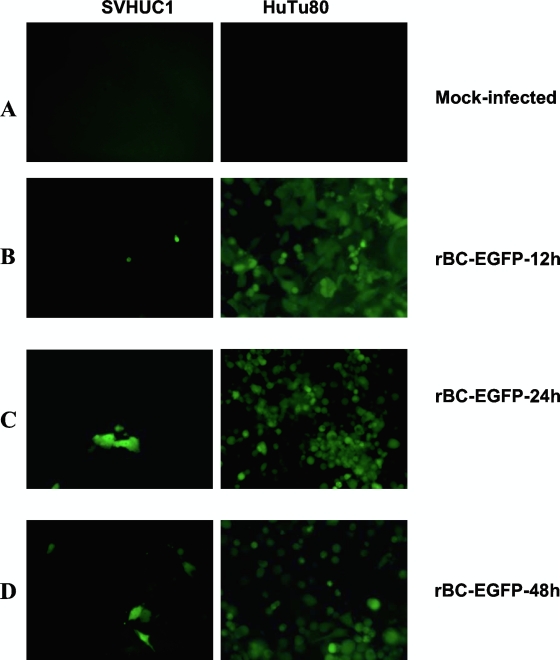
We have earlier reported that the rNDVs used for this study are able to infect and induce cytolysis by caspase-mediated apoptosis in a wide range of tumor cell types, while no cytolysis was observed in normal human cells (9). The IFN-sensitive rBC-Edit virus was compared with the IFN-resistant rBC-EGFP virus. In normal human cells, the rBC-Edit virus is restricted in replication but the rBC-EGFP virus (which differs from the rBC virus only by EGFP expression) replicated to low titers, with limited spread. In most tumor cells, rNDVs replicated to high titers and induced cytotoxicity at 48 h p.i. (Table 1). The virus yields were higher in most tumor cells, except lymphoid tumors and breast cancer cells. In some IFN-α-producing, epithelial tumor cell types (PC3, HuTu80, and CaCo2 cells), the rBC virus produced at least 100- to 1,000-fold more infectious virus than the IFN-sensitive viruses, suggesting a role for type I IFN in restricting virus replication. To study whether levels of virus spread are similar in normal and human tumor cells, we infected cells with rBC-EGFP virus. The infection in infected tumor cells progressed from the foci of a few infected cells to extensive EGFP expression and destruction of the entire monolayer by 48 h p.i., suggesting that cell-to-cell spread of rNDV is more efficient in tumor cells than in normal human cells (Fig. 1A to D).
TABLE 1.
Replication of recombinant Newcastle disease virus strains in normal human and tumor cells
| Cell line | Virus yield (PFU/ml)a |
|
|---|---|---|
| rBC | rBC-Edit | |
| SVHUC1 (normal human uroepithelial) | <10 | <10 |
| PC3 (prostate cancer) | 2.0 × 106 | 2.1 × 104 |
| HT1080 (fibrosarcoma) | 1.0 × 106 | 1.0 × 104 |
| HuTu80 (adenocarcinoma) | 3.0 × 1011 | 1.0 × 108 |
| THP-1 (monocytic leukemia) | 1.0 × 103 | <10 |
| CCRF-CEM (T cell lymphoblast-like) | <10 | <10 |
| HeLa (cervical cancer) | 1.9 × 107 | 1.9 × 107 |
| HEpG2 (hepatocarcinoma) | 1.5 × 107 | 4.8 × 106 |
| CaCo2 (colon cancer) | 7.3 × 1010 | 3.6 × 108 |
| CoLo205 (colon cancer) | 1.5 × 104 | 1.0 × 104 |
| HT29 (colon cancer) | 5.0 × 104 | 1.0 × 104 |
| T84 (colon cancer) | 1.0 × 105 | 1.2 × 105 |
| SH-SY5Y (neuroblastoma) | 7.5 × 105 | 5.0 × 106 |
| MCF-7 (breast cancer) | 1.5 × 103 | 3.0 × 102 |
Cells were infected at an MOI of 0.01 with rNDV, and virus yield was assessed from culture supernatants at 48 h p.i. by plaque assay on DF1 cells.
FIG. 1.
Levels of virus replication and spread of NDV differ in normal human epithelial cells and HuTu80 tumor cells. SVHUC1, normal, immortalized human uroepithelial cells, and HuTu80 intestinal epithelial tumor cells were infected with rBC-EGFP virus at an MOI of 0.01. (A to D) Left panels indicate SVHUC1 cells, and right panels indicate HuTu80 cells. (A) Mock-infected cells; (B) expression of EGFP in a few virus-infected normal cells compared with extensive EGFP fluorescence in virus-infected HuTu80 cells by 12 h postinfection; (C) absence of virus spread and replication in normal cells compared with extensive EGFP and virus spread in tumor cells by 24 h postinfection; (D) expression of EGFP in a few cells, with absence of viral spread in normal human cells at 48 h and complete destruction of a monolayer, with clumps of dead cells expressing EGFP in HuTu80 tumor cells.
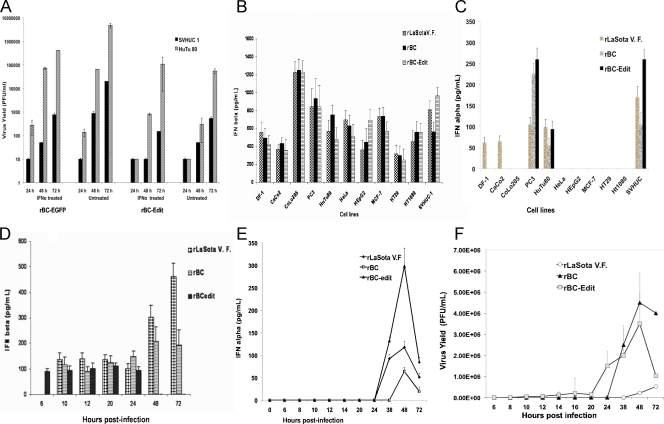
To determine whether IFN pretreatment results in reduced virus growth, normal SVHUC1 and HuTu80 tumor cells were pretreated with h IFN-α. As expected, IFN pretreatment restricted virus growth for all three viruses, but this was more pronounced for the rBC-Edit (Fig. 2A) and rLaSota V.F. (data not shown) viruses. The rLaSota V.F. and rBC-Edit viruses were severely restricted in growth (data not shown) in normal human cells without IFN pretreatment but also had low yields in HuTu80 cells with IFN pretreatment, suggesting that the IFN-mediated antiviral state prevents virus growth in normal cells.
FIG. 2.
Virus replication and type I interferon induction by rNDV in normal and human tumor cells. (A) Interferon sensitivity of rNDV. Normal human (SVHUC1) and tumor (HuTu80) cells in duplicate were treated with 10 pg/ml of human IFN-α for 24 h, and virus yield was examined by plaque assay on Vero cells at 24, 48, and 72 h postinfection with an rNDV multiplicity of infection of 0.01. (B) DF1 chicken embryo fibroblast cells and normal and human tumor cells were either mock infected or infected with rNDV at an MOI of 10 without IFN pretreatment. Culture supernatants were tested for IFN-β and IFN-α by ELISA. IFN-β production in rNDV-infected cells at 48 h postinfection. (C) IFN-α production at 48 h postinfection. (D) Time course analysis of IFN-β production in HuTu80 cells with an MOI of 0.01. (E) Time course analysis of IFN-α production in HuTu80 cells with an MOI of 0.01. (F) Multicycle virus replication of rNDV in normal and HuTu80 cells without IFN pretreatment. Cells were infected with an rNDV MOI of 0.01. Kinetics of the virus yield of rNDV in HuTu80 tumor cells was assessed in Vero cells. Results represent mean values plus standard errors of the means from two independent experiments.
NDV induces production of IFN-α/β in normal cells but only IFN-β in most tumor cells.
To prove that the antiviral effect correlates with type I IFN in NDV-resistant human cells, we measured the quantity of IFN-α/β on NDV-infected (MOI, 10) cell supernatants. As shown in Fig. 2B and C, all three viruses induced IFN-α and IFN-β in SVHUC1 cells, while the IFN-sensitive viruses induced more IFN-β (1.4-fold by rLaSota V.F. virus and 1.7-fold by rBC-Edit virus) than the rBC virus in HuTu80 cells, indicating that V protein of NDV may also block IFN-β induction in human cells. Most of the tested tumor cell types responded with IFN-β upon infection with rNDV (Fig. 2B). In contrast, IFN-α was produced only in PC3 prostate carcinoma cells and HuTu80 intestinal epithelial tumor cells (Fig. 2C) during infection with rNDV. In HuTu80 cells, rLaSota V.F. and rBC-Edit viruses induced higher levels of IFN-α than rBC virus, reinforcing the view that the V protein of NDV antagonizes the induction of IFN-α (16). Time course studies of type I IFN production in HuTu80 tumor cells at a low MOI (0.01) revealed that rBC-Edit virus induced IFN-β as early as 6 h p.i. and for up to 24 h, and by 48 h, no IFN-β was detectable. With the rBC and rLaSota V.F. viruses, there was a delay in the induction of IFN-β (10 h p.i.), with increasing levels of IFN-β produced between 48 and 72 h p.i. (Fig. 2D). At low MOIs, IFN-α, on the other hand, was induced late in the virus replication cycle (38 h p.i.) (Fig. 2E). The rBC-Edit virus induced approximately 6-fold more IFN-α than the rBC virus in HuTu80 cells, while rLaSota V.F. virus induced only 2-fold more IFN-α than the rBC virus (Fig. 2e).
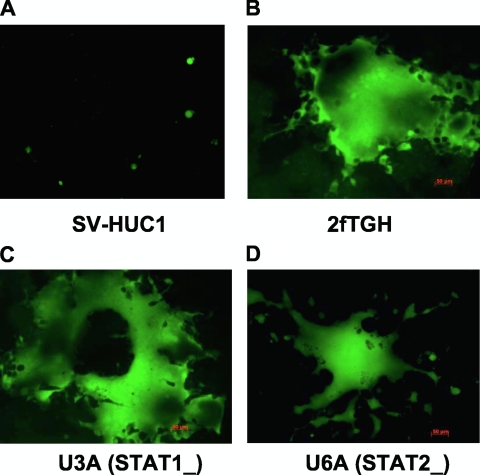
Although both normal and tumor cells infected with rNDV secreted IFN-β, only normal cells responded to its protective effects. This was evident when multicycle virus replication at an MOI of 0.01 in HuTu80 cells was compared with type I IFN secretion. Virus replication and spread were not impaired (Fig. 2F), even though IFN-β was induced early in these cells (Fig. 2C). However, there was a significant reduction in the growth of rLaSota V.F. and rBC-Edit viruses after IFN-α pretreatment of HuTu80 cells compared to the growth of rBC virus (Fig. 2A). Our results suggested that antiviral genes induced by IFN-α probably play a more significant role in preventing rNDV replication and spread in normal or tumor cells than IFN-β. To further confirm that IFN-α defects in tumor cells afford permissiveness for NDV replication, we examined virus growth in IRF-7-hypermethylated 2fTGH human fibrosarcoma cells and their derivatives, namely, U3A (STAT-1-deficient) and U6A (STAT-2-deficient) cells. We found that all three viruses replicated to high titers in these three cell lines (data not shown) and formed extensive syncytia, suggesting unrestricted viral spread (Fig. 3A to D).
FIG. 3.
Failure to activate IRF-7 enhances the replication and spread of NDV. SVHUC1, 2fTGH, U3A, and U6A human fibrosarcoma cells were either mock infected or infected with the rLaSota V.F., rBC, or rBC-Edit strain of NDV at an MOI of 10. Virus replication and spread indicated by EGFP expression in SVHUC1 (IRF-7+) (A) and IRF-7-hypermethylated wild-type and mutant human fibrosarcoma cells (B to D). (B) 2fTGH cells; (C) U3A cells; (D) U6A cells.
Differential regulation of IFN-β- and IFN-α-stimulated genes (ISGs) in normal and tumor cells.
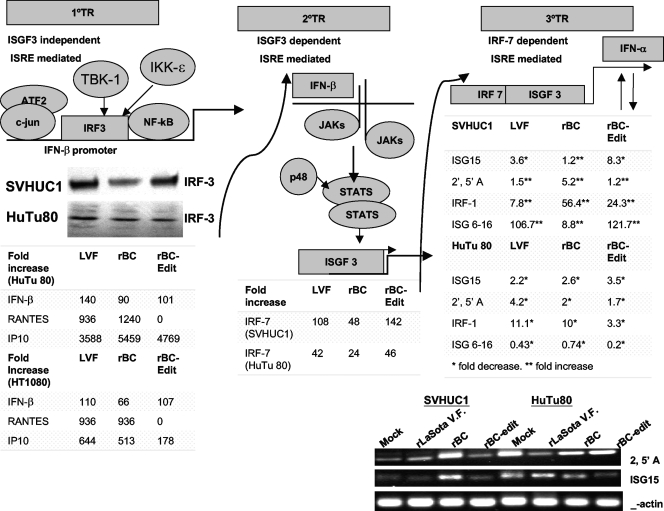
We were able to confirm that in normal and in HuTu80 tumor cells, rNDV induced IRF-3 (Fig. 4), signal transducers and activators of transcription 1 (STAT-1), and IRF-7, depending on the virus strain and cell type (data not shown). Most tumor cells that we employed failed to express IRF-7 after infection with rNDV except PC3, HuTu80, and CaCo2 cells (data not shown). As we found differential regulation of IFN-α and IFN-β in normal and tumor cells, we sought to examine the expression of IFN-responsive genes. RANTES, an IFN-β-inducible gene, was detectable only in HuTu80 and HT1080 cells infected with the rBC and rLaSota V.F. viruses and not in rBC-Edit virus-infected cells (Fig. 4). IP-10, another ISG, was induced only in tumor cell lines of fibroblastic (HT1080) and epithelial (HuTu80) origins (Fig. 4). IP-10 was also produced in HT29, PC3, and CaCO2 cells at various levels, depending on the virus strain (data not shown). To further identify the components of IFN signaling defects that rNDV exploits in tumor cells, we analyzed the induction of several known ISGs in normal SVHUC1 and IFN-responsive HuTu80 cells by quantitative RT-PCR. When the endogenous IFN-α secretion is at its peak after rNDV infection (48 h p.i.), the expression of mRNA for ISGs, including ISG 6-16, and IRF-1, were differentially regulated in normal and tumor cells (Fig. 4). The ISG 6-16 is induced by rBC virus (8.8-fold), rBC-Edit virus (122-fold), and rLaSota V.F. virus (107-fold) in normal SVHUC1 cells, indicating that STAT-1 activation is probably inhibited in rBC virus-infected cells. ISG 6-16 is downstream of STAT-1 activation. In HuTu80 cells, all three viruses had decreased expression of ISG 6-16. In SVHUC1 cells, IRF-1 was induced at very high levels by rBC virus. ISG15 mRNA levels in both normal and tumor cells were approximately 2- to 8-fold lower than in mock-infected cells, depending on the virus strain, except in rBC virus-infected SVHUC1 cells (Fig. 4). The levels of β-actin mRNA in both SVHUC1 and HuTu80 cells were equal. We also found that 2′,5′ A, one of the STAT-independent antiviral mediators, was upregulated by rNDV strains in normal human cells but downregulated in HuTu80 cells (Fig. 4). It appears from our results that ISG 6-16 and 2′,5′ A might play a major role as antiviral effectors in NDV-infected cells. Although HuTu80 cells produced IFN-α, levels of virus replication were comparable with and without IFN pretreatment (Fig. 2A), suggesting that antiviral genes downstream of IFN signaling are probably defective in these cells, as evidenced by the overall downregulation of ISG15, IRF-1, ISG 6-16, and 2′,5′ A (Fig. 4). These results indicated that rNDV triggers the activation of IRF-3 and the subsequent transcription of a cohort of genes to induce the primary antiviral state but that, through coordinated expression of viral gene products (such as V protein), it blunts secondary and tertiary responses in normal cells and exploits the tumor-specific defects in the IFN-α-mediated antiviral signaling pathways for enhanced replication.
FIG. 4.
IFN-α-responsive genes induced after rNDV infection of normal and tumor cells. The failure of secondary and tertiary transcriptional responses (2°TR and 3°TR, respectively) to NDV aids in tumor selectivity and oncolysis. The primary response to viral infection is mediated by IRF-3, leading to stimulation of the IFN-β promoter. IFN-β is then translated and secreted to stimulate, in an autocrine fashion, JAK/STAT signaling to form ISGF3 complexes in the nucleus, which mediate the induction of the secondary transcriptional responses. Without the consequent expression of IRF-7 in cells infected with rNDV, the tertiary transcriptional wave, which includes almost all IFN-α genes and its downstream antiviral mediators, could not take place. In the absence of IFN-α and IFN-α-responsive antiviral genes, virus replication is enabled in tumor cells. In normal human cells, the transcriptional events proceed unhampered, resulting in a robust antiviral state preventing virus replication. IFN-α-responsive antiviral genes were detected by RT-PCR of cell lysates infected with rNDV at 48 h postinfection. Fold increases of different antiviral mediators, such as ISG15, 2′,5′ A, IRF-1, and ISG 6-16 mRNAs, compared to levels in mock-infected cells are shown. Values represent average fold increases over levels in mock-infected cells from two independent experiments. RT-PCR products of 2′,5′ A and ISG15 analyzed in 2% agarose gels are shown, along with β-actin for comparison.
Recombinant NDV effectively cleared tumor burdens in BALB/c nude mice after a single intratumoral treatment.
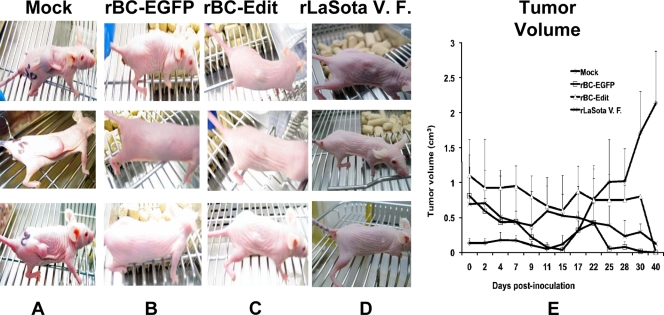
Having shown that rBC-Edit virus selectively replicates and kills tumor cells, we analyzed the toxicity and oncolytic efficacy of the wild-type and interferon-sensitive viruses in athymic nude mice. Toxicity studies were performed by inoculating groups of three BALB/c nude mice subcutaneously with 2 × 107 PFU of rBC-EGFP, rLaSota V.F., or rBC-Edit virus. Over the next 8 weeks, none of the infected animals exhibited any signs of discomfort or illness and continued to gain weight (data not shown). The in vivo therapeutic efficacy of rBC-EGFP virus in comparison with that of the other two viruses against subcutaneously implanted HT1080 tumors in BALB/c nude mice was evaluated after a single intratumoral injection of NDV (2 × 107 PFU) in tumors exceeding 5 mm in diameter in any plane. Three mice from the rBC-EGFP virus, three from the rBC-Edit virus, two from the rLaSota V.F. virus, and four from the PBS treatment groups developed tumors of significant size and had to be euthanized according to the IACUC tumor policy at Virginia Tech. Treatment with wild-type rBC-EGFP virus resulted in a significant reduction in tumor growth (7/7 tumors), leading to complete regression compared to the tumor growth in control mice, whose tumors were treated with PBS (0/14 tumors) (Fig. 5). Treatment with rBC-Edit and rLaSota V.F. viruses had comparable tumor growth inhibitory effects, with 7/7 or 8/8 tumors, respectively, undergoing complete regression. Tumor regression commenced from day 8; by day 31, the rBC virus completely regressed tumors, and by day 40, rBC-Edit and rLaSota V.F. virus-treated tumors regressed completely.
FIG. 5.
Interferon-sensitive NDV strains regress xenotransplanted tumors in nude mice as efficiently as an interferon-antagonistic NDV strain. BALB/c nude mice were subcutaneously implanted with HT1080 human fibrosarcoma tumor cells. When tumors reached a size of >5 mm in any one plane, recombinant NDV strains were administered intratumorally as a single injection (2 × 107 PFU/ml in 50 μl). (A) Mock-infected mice with tumors on day 31; (B) tumor-bearing mice treated with rBC-EGFP virus; (C) mice treated with rBC-Edit virus; (D) mice treated with rLaSota V.F. virus on day 31 posttreatment. (E) Calculated tumor volumes for each virus strain were plotted against days postinoculation. Results are mean values plus standard errors of the means from three BALB/c mice.
Live in vivo imaging of rBC-EGFP virus in BALB/c mice.
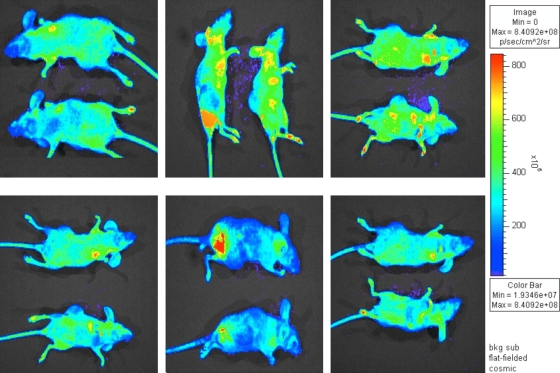
The usefulness of rBC-EGFP virus in measuring gene expression and tissue distribution of virus in vivo was evaluated in BALB/c nude mice. Seventy-two hours postinfection with rBC-EGFP virus (2 × 107 PFU), virus distribution and transgene expression were visualized by IVIS live imaging. As shown in Fig. 6, after a single intratumoral administration of rBC-EGFP virus, the virus was able to spread through the tumor and was visualized in many parts of the BALB/c nude mice, including many of the internal organs. Additional studies in the future are required to determine whether rBC-Edit virus will be tumor restricted in immunocompetent mice.
FIG. 6.
Live imaging of rBC-EGFP virus in tumor-bearing BALB/c nude mice. HT1080 tumor-bearing mice were injected intratumorally with 100 μl of 2.0 × 107-PFU/ml rBC-EGFP virus, and the virus distribution was imaged using an IVIS live imager at 72 h postinoculation. Several views of two tumor-bearing, rBC-EGFP-infected mice are shown. sr, steridian; bkg, background.
DISCUSSION
The genetic malleability, tumor selectivity and high therapeutic index of NDV are the most desirable properties for an oncolytic virus. With the advent of a reverse-genetics system for NDV, it is now possible to refine and optimize oncolytic potency, specificity, and therapeutic efficacy. NDV's tumor specificity is based on cancer-specific defects in the interferon pathway (10, 23, 27, 29, 36). Therefore, it seems that the use of IFN-sensitive viruses would afford an even broader safety margin for oncolytic virotherapy. We have recently reported that NDV exerts oncolysis by direct apoptosis through multiple caspase-dependent pathways, and the IFN-sensitive rNDV caused enhanced apoptosis (9). In this study, we examined whether IFN-sensitive rNDVs would be effective oncolytic agents in a mouse model of xenotransplanted human fibrosarcoma. The rLaSota V.F. virus produces a full-length V protein but has a more fusogenic phenotype than its parental rLaSota virus due to modification of the fusion protein cleavage site with multiple pairs of basic amino acid residues. However, rLaSota V.F. virus functioned similarly to rBC-Edit virus with respect to IFN antagonism and was susceptible to IFN-α. This is probably due to the 12-amino-acid differences in the V proteins of the rLaSota V.F. and rBC viruses.
By using isogenic rNDV strains differing only in their interferon antagonism, we have shown conclusively that IFN-α and IFN-α-responsive antiviral genes limit the spread of NDV in normal cells and that defects in them allow tumor-specific replication and spread. Both normal and human tumor cells produced IFN-β following NDV infection in a rapid manner. However, virus replication progressed in tumors with defects in IFN-α expression, while it was suppressed in normal cells with abundant secretion of IFN-α. Even in tumor cells that are capable of responding with IFN-α expression upon virus infection, such as HuTu80 cells, defects in the downstream signaling of antiviral effectors afford permissiveness for NDV replication. Fortification of the IFN-β-induced antiviral state by the induction of members of the IFN-α family and the IFN-α-responsive downstream antiviral mediators, therefore, seems to be necessary to prevent virus replication in NDV-infected cells.
Differentially regulated IFN-β-mediated antiviral responses were reported to determine the outcome of NDV infection in normal and tumor cells (23). Another study implicated the delay in inducing PKR and MxA proteins as the reason for the tumor selectivity of NDV (10). We were not able to demonstrate that IFN-β-mediated proinflammatory chemokine responses limit NDV replication. Our results imply that it is the concerted effect of defects in the IFN-α signaling cascade through STAT activation and differential regulation of IFN-α-responsive downstream antiviral effectors such as 2′,5′ A and ISG 6-16 which determines the outcome of NDV infection of normal and tumor cells. Dysregulated IFN responses are likely to be one of several mechanisms that NDV exploits for the replication, spread, and cytotoxicity of tumor cells.
It has been argued that an oncolytic virus should possess an ability to replicate in cancer cells that is large enough to cause effective oncolysis to allow for efficient antigen presentation, as tumors have a limited ability to recruit immune cells due to vascular compromise (43). If IFN-sensitive NDV can effectively replicate in tumor cells, it should be able to induce oncolysis. Our hypothesis that a virus which induces a robust IFN response and at the same time remains highly susceptible to the induced IFN (rBC-Edit) would grow well in tumor cells but would be restricted in normal human cells proved to be correct. The V protein-deficient rBC-Edit virus grew to very high titers in many tumor cells lacking an IFN-α response and induced IFN-β earlier but failed to grow and spread in normal cells with a functional IFN system.
IFN-induced IRF-7 plays a critical role in IFN-α gene induction by NDV (39). Studies using IRF-7 knockout mice have demonstrated that transcription of both IFN-α and IFN-β upon NDV infection is dependent on IRF-7 (13, 14), indicating that IRF-7 is a master regulator of type I IFN. We found that most tumor cells were not able to activate IRF-7 after rNDV infection. The promoter region of the IRF-7 gene contains CpG clusters that are methylated in some cancer cells, resulting in the silencing of expression of the IRF-7 gene (26). In normal human 2fTGH cells with hypermethylated IRF-7, all strains of rNDV were able to grow to high titers with extensive syncytia, while in normal SVHUC1 cells that express IRF-7, virus replication and spread was severely restricted, confirming the role of IRF-7 in the type I IFN pathway.
At least 30 genes were transcriptionally activated by type I IFNs (45). Among these, the PKR and 2′,5′ A proteins act as key mediators of intracellular resistance to several viruses (40). PKR undergoes autophosphorylation after binding to double-stranded RNA (dsRNA) or the PKR-activating (PACT) protein. The α-subunit of eukaryotic translation initiation factor 2 (eIF2-α) is phosphorylated by activated PKR, resulting in a block of protein synthesis (33). 2′,5′ A is synthesized by dsRNA-activated oligoadenylate synthetase (OAS) (20), which then bind and activate RNase L, an endoribonuclease that cleaves viral RNA and mRNA, leading to a decrease in protein synthesis and viral replication (8). Recent reports indicate that mitogen-activated protein kinases (MAPK) are also regulated by 2′,5′ A to initiate RNase L-mediated downstream transcription of several antiviral and immune response genes (38). Most of the IFN-α-responsive antiviral ISGs were upregulated, especially ISG 6-16, in normal cells but not in tumor cells after NDV infection. Thus, coordination of the JAK/STAT pathway appears to be required for transcriptional activation of IFN-α-sensitive genes and to achieve an effective antiviral state.
We have shown that rNDV that initiates but does not disable antiviral responses has an oncolytic ability similar to that of viruses that mount a potent antiviral response and exert a significant antagonism toward it. Both rBC and rBC-Edit viruses are replication restricted in normal human cells compared to tumor cells and are comparable in oncolytic efficacy in a nude mouse model of fibrosarcoma. The tumor regression efficiency of rBC virus is better than that of the other two viruses in that rBC virus treated tumors that had regressed 10 days earlier. However, we believe that the V-defective mutant rBC-Edit virus has a number of properties that makes it a suitable oncolytic agent. (i) rBC-Edit virus is highly attenuated in its natural host (16) and more replication restricted in normal human cells. Further, this virus should be well tolerated even in immunocompromised cancer patients (19). (ii) Given that there are multiple serologically defined serotypes of avian paramyxoviruses (serotypes 1 to 9), different recombinant viruses can be constructed by exchanging the antigenic surface glycoproteins of the virus, the hemagglutinin, and the fusion protein (17). The availability of these modified, IFN-sensitive, proapoptotic viruses may allow repeated administration of these oncolytic strains. (iii) The rBC-Edit virus also induced large amounts of proinflammatory chemokine IP-10 in tumor cells such as HuTu80 and PC3 cells. Thus, the V protein-defective oncolytic virus treatment might be associated with an effective immune stimulation in these tumor cells, leading to a specific antitumor response that can function at distal sites. Thus, the tumor specificity and cytotoxicity of this interesting oncolytic virus are governed by multiple, mutually exclusive pathways, depending on the cell type, that can be exploited for enhanced oncolytic efficacy in engineered NDV. The use of this strategy is attractive for improving the safety margin of rNDV without loosing oncolytic efficacy. To our knowledge, this is the first report of the use of an IFN-sensitive rNDV for effective oncolysis in a preclinical model, which we speculate could be seamlessly translated into clinics for treating human malignancies.
Acknowledgments
This research was supported by NIH grant 1 R21 AI070528-01A1.
This work is the sole responsibility of the authors and does not represent the official views of the NIH.
We thank Daniel Rockemann and Pete Jobst for their excellent technical support.
Footnotes
Published ahead of print on 10 February 2010.
REFERENCES
- 1.Advani, A. S., and A. M. Pendergast. 2002. Bcr-Abl variants: biological and clinical aspects. Leukoc. Res. 26:713-720. [DOI] [PubMed] [Google Scholar]
- 2.Arora, T., G. Floyd-Smith, M. J. Espy, and D. F. Jelinek. 1999. Dissociation between IFN-alpha-induced anti-viral and growth signaling pathways. J. Immunol. 162:3289-3297. [PubMed] [Google Scholar]
- 3.Bell, S., S. Hansen, and J. Buchner. 2002. Refolding and structural characterization of the human p53 tumor suppressor protein. Biophys. Chem. 96:243-257. [DOI] [PubMed] [Google Scholar]
- 4.Bello, M. J., J. M. de Campos, M. E. Kusak, J. Vaquero, J. L. Sarasa, A. Pestana, and J. A. Rey. 1994. Allelic loss at 1p is associated with tumor progression of meningiomas. Genes Chromosomes Cancer 9:296-298. [DOI] [PubMed] [Google Scholar]
- 5.Bian, H., P. Fournier, B. Peeters, and V. Schirrmacher. 2005. Tumor-targeted gene transfer in vivo via recombinant Newcastle disease virus modified by a bispecific fusion protein. Int. J. Oncol. 27:377-384. [PubMed] [Google Scholar]
- 6.Cassel, W. A., and D. R. Murray. 1992. A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med. Oncol. Tumor Pharmacother. 9:169-171. [DOI] [PubMed] [Google Scholar]
- 7.Csatary, L. K. 1971. Viruses in the treatment of cancer. Lancet ii:825. [DOI] [PubMed] [Google Scholar]
- 8.Dong, B., and R. H. Silverman. 1997. A bipartite model of 2-5A-dependent RNase L. J. Biol. Chem. 272:22236-22242. [DOI] [PubMed] [Google Scholar]
- 9.Elankumaran, S., D. Rockemann, and S. K. Samal. 2006. Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death. J. Virol. 80:7522-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiola, C., B. Peeters, P. Fournier, A. Arnold, M. Bucur, and V. Schirrmacher. 2006. Tumor selective replication of Newcastle disease virus: association with defects of tumor cells in antiviral defence. Int. J. Cancer 119:328-338. [DOI] [PubMed] [Google Scholar]
- 11.Freeman, A. I., Z. Zakay-Rones, J. M. Gomori, E. Linetsky, L. Rasooly, E. Greenbaum, S. Rozenman-Yair, A. Panet, E. Libson, C. S. Irving, E. Galun, and T. Siegal. 2006. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. 13:221-228. [DOI] [PubMed] [Google Scholar]
- 12.Gromeier, M., and E. Wimmer. 2001. Viruses for the treatment of malignant glioma. Curr. Opin. Mol. Ther. 3:503-508. [PubMed] [Google Scholar]
- 13.Honda, K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772-777. [DOI] [PubMed] [Google Scholar]
- 14.Honda, K., H. Yanai, A. Takaoka, and T. Taniguchi. 2005. Regulation of the type I IFN induction: a current view. Int. Immunol. 17:1367-1378. [DOI] [PubMed] [Google Scholar]
- 15.Hrabak, A., I. Csuka, T. Bajor, and L. K. Csatary. 2006. The cytotoxic anti-tumor effect of MTH-68/H, a live attenuated Newcastle disease virus is mediated by the induction of nitric oxide synthesis in rat peritoneal macrophages in vitro. Cancer Lett. 231:279-289. [DOI] [PubMed] [Google Scholar]
- 16.Huang, Z., S. Krishnamurthy, A. Panda, and S. K. Samal. 2003. Newcastle disease virus V protein is associated with viral pathogenesis and functions as an alpha interferon antagonist. J. Virol. 77:8676-8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, Z., A. Panda, S. Elankumaran, D. Govindarajan, D. D. Rockemann, and S. K. Samal. 2004. The hemagglutinin-neuraminidase protein of Newcastle disease virus determines tropism and virulence. J. Virol. 78:4176-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito, Y., Y. Nagai, and K. Maeno. 1982. Interferon production in mouse spleen cells and mouse fibroblasts (L cells) stimulated by various strains of Newcastle disease virus. J. Gen. Virol. 62:349-352. [DOI] [PubMed] [Google Scholar]
- 19.Kasuya, H., Y. Nishiyama, S. Nomoto, F. Goshima, S. Takeda, I. Watanabe, N. Nomura, T. Shikano, T. Fujii, N. Kanazumi, and A. Nakao. 2007. Suitability of a US3-inactivated HSV mutant (L1BR1) as an oncolytic virus for pancreatic cancer therapy. Cancer Gene Ther. 14:533-542. [DOI] [PubMed] [Google Scholar]
- 20.Kerr, I. M., and R. E. Brown. 1978. pppA2′p5′A2′p5′A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc. Natl. Acad. Sci. U. S. A. 75:256-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khuri, F. R., J. Nemunaitis, I. Ganly, J. Arseneau, I. F. Tannock, L. Romel, M. Gore, J. Ironside, R. H. MacDougall, C. Heise, B. Randlev, A. M. Gillenwater, P. Bruso, S. B. Kaye, W. K. Hong, and D. H. Kirn. 2000. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat. Med. 6:879-885. [DOI] [PubMed] [Google Scholar]
- 22.Krishnamurthy, S., Z. Huang, and S. K. Samal. 2000. Recovery of a virulent strain of Newcastle disease virus from cloned cDNA: expression of a foreign gene results in growth retardation and attenuation. Virology 278:168-182. [DOI] [PubMed] [Google Scholar]
- 23.Krishnamurthy, S., T. Takimoto, R. A. Scroggs, and A. Portner. 2006. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J. Virol. 80:5145-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruyt, F. A., and D. T. Curiel. 2002. Toward a new generation of conditionally replicating adenoviruses: pairing tumor selectivity with maximal oncolysis. Hum. Gene Ther. 13:485-495. [DOI] [PubMed] [Google Scholar]
- 25.Lana, D. P., D. B. Snyder, D. J. King, and W. W. Marquardt. 1988. Characterization of a battery of monoclonal antibodies for differentiation of Newcastle disease virus and pigeon paramyxovirus-1 strains. Avian Dis. 32:273-281. [PubMed] [Google Scholar]
- 26.Li, H. O., Y. F. Zhu, M. Asakawa, H. Kuma, T. Hirata, Y. Ueda, Y. S. Lee, M. Fukumura, A. Iida, A. Kato, Y. Nagai, and M. Hasegawa. 2000. A cytoplasmic RNA vector derived from nontransmissible Sendai virus with efficient gene transfer and expression. J. Virol. 74:6564-6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorence, R. M., B. B. Katubig, K. W. Reichard, H. M. Reyes, A. Phuangsab, M. D. Sassetti, R. J. Walter, and M. E. Peeples. 1994. Complete regression of human fibrosarcoma xenografts after local Newcastle disease virus therapy. Cancer Res. 54:6017-6021. [PubMed] [Google Scholar]
- 28.Lorence, R. M., A. L. Pecora, P. P. Major, S. J. Hotte, S. A. Laurie, M. S. Roberts, W. S. Groene, and M. K. Bamat. 2003. Overview of phase I studies of intravenous administration of PV701, an oncolytic virus. Curr. Opin. Mol. Ther. 5:618-624. [PubMed] [Google Scholar]
- 29.Lorence, R. M., K. W. Reichard, B. B. Katubig, H. M. Reyes, A. Phuangsab, B. R. Mitchell, C. J. Cascino, R. J. Walter, and M. E. Peeples. 1994. Complete regression of human neuroblastoma xenografts in athymic mice after local Newcastle disease virus therapy. J. Natl. Cancer Inst. 86:1228-1233. [DOI] [PubMed] [Google Scholar]
- 30.Lorence, R. M., P. A. Rood, and K. W. Kelley. 1988. Newcastle disease virus as an antineoplastic agent: induction of tumor necrosis factor-alpha and augmentation of its cytotoxicity. J. Natl. Cancer Inst. 80:1305-1312. [DOI] [PubMed] [Google Scholar]
- 31.Lu, X., T. Nikaido, T. Toki, Y. L. Zhai, N. Kita, I. Konishi, and S. Fujii. 2000. Loss of heterozygosity among tumor suppressor genes in invasive and in situ carcinoma of the uterine cervix. Int. J. Gynecol. Cancer 10:452-458. [DOI] [PubMed] [Google Scholar]
- 32.Martin, K. J., E. Graner, Y. Li, L. M. Price, B. M. Kritzman, M. V. Fournier, E. Rhei, and A. B. Pardee. 2001. High-sensitivity array analysis of gene expression for the early detection of disseminated breast tumor cells in peripheral blood. Proc. Natl. Acad. Sci. U. S. A. 98:2646-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meurs, E. F., Y. Watanabe, S. Kadereit, G. N. Barber, M. G. Katze, K. Chong, B. R. Williams, and A. G. Hovanessian. 1992. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J. Virol. 66:5805-5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pecora, A. L., H. M. Lazarus, A. A. Jennis, R. A. Preti, S. L. Goldberg, S. D. Rowley, S. Cantwell, B. W. Cooper, E. A. Copelan, R. H. Herzig, R. Meagher, M. J. Kennedy, L. R. Akard, J. Jansen, A. Ross, M. Prilutskaya, J. Glassco, D. Kahn, and T. J. Moss. 2002. Breast cancer cell contamination of blood stem cell products in patients with metastatic breast cancer: predictors and clinical relevance. Biol. Blood Marrow Transplant. 8:536-543. [DOI] [PubMed] [Google Scholar]
- 35.Pecora, A. L., N. Rizvi, G. I. Cohen, N. J. Meropol, D. Sterman, J. L. Marshall, S. Goldberg, P. Gross, J. D. O'Neil, W. S. Groene, M. S. Roberts, H. Rabin, M. K. Bamat, and R. M. Lorence. 2002. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J. Clin. Oncol. 20:2251-2266. [DOI] [PubMed] [Google Scholar]
- 36.Phuangsab, A., R. M. Lorence, K. W. Reichard, M. E. Peeples, and R. J. Walter. 2001. Newcastle disease virus therapy of human tumor xenografts: antitumor effects of local or systemic administration. Cancer Lett. 172:27-36. [DOI] [PubMed] [Google Scholar]
- 37.Reichard, K. W., R. M. Lorence, C. J. Cascino, M. E. Peeples, R. J. Walter, M. B. Fernando, H. M. Reyes, and J. A. Greager. 1992. Newcastle disease virus selectively kills human tumor cells. J. Surg. Res. 52:448-453. [DOI] [PubMed] [Google Scholar]
- 38.Samuel, M. A., K. Whitby, B. C. Keller, A. Marri, W. Barchet, B. R. Williams, R. H. Silverman, M. Gale, Jr., and M. S. Diamond. 2006. PKR and RNase L contribute to protection against lethal West Nile Virus infection by controlling early viral spread in the periphery and replication in neurons. J. Virol. 80:7009-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato, M., N. Hata, M. Asagiri, T. Nakaya, T. Taniguchi, and N. Tanaka. 1998. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 441:106-110. [DOI] [PubMed] [Google Scholar]
- 40.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 41.Stojdl, D. F., B. Lichty, S. Knowles, R. Marius, H. Atkins, N. Sonenberg, and J. C. Bell. 2000. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6:821-825. [DOI] [PubMed] [Google Scholar]
- 42.Stojdl, D. F., B. D. Lichty, B. R. tenOever, J. M. Paterson, A. T. Power, S. Knowles, R. Marius, J. Reynard, L. Poliquin, H. Atkins, E. G. Brown, R. K. Durbin, J. E. Durbin, J. Hiscott, and J. C. Bell. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263-275. [DOI] [PubMed] [Google Scholar]
- 43.Tikhonov, S. N., K. A. Rotov, N. P. Khrapova, V. V. Alekseev, E. A. Snatenkov, A. A. Zamarin, and N. A. Simakova. 2008. Effect of liposomal tetracycline hydrochloride on enzymatic function of the liver. Bull. Exp. Biol. Med. 145:443-445. [DOI] [PubMed] [Google Scholar]
- 44.Vigil, A., M. S. Park, O. Martinez, M. A. Chua, S. Xiao, J. F. Cros, L. Martinez-Sobrido, S. L. Woo, and A. Garcia-Sastre. 2007. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 67:8285-8292. [DOI] [PubMed] [Google Scholar]
- 45.Williams, B. R. 1991. Transcriptional regulation of interferon-stimulated genes. Eur. J. Biochem. 200:1-11. [DOI] [PubMed] [Google Scholar]
- 46.Zamarin, D., A. Vigil, K. Kelly, A. Garcia-Sastre, and Y. Fong. 2009. Genetically engineered Newcastle disease virus for malignant melanoma therapy. Gene Ther. 16:796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]