Abstract
Respiratory syncytial virus (RSV) causes bronchiolitis, the main cause of infantile hospitalization. Immunity against reinfection is poor, and there is great interest in boosting vaccine responses using live vectors expressing host cytokines. We therefore constructed a recombinant RSV expressing murine interleukin 18 (RSV/IL-18), a cytokine capable of inducing strong antiviral immune responses. In vitro RSV/IL-18 replicated at wild-type levels and produced soluble IL-18. In naïve BALB/c mice, RSV/IL-18 infection significantly increased both IL-18 mRNA and protein and attenuated the peak viral load 3-fold. Despite a reduced viral load, RSV/IL-18 infection caused a biphasic weight loss at days 2 and 6 postinfection that was not seen in wild-type infection. Day 2 disease was associated with enhanced pulmonary natural killer (NK) cell numbers and activity and was prevented by NK cell depletion during infection; day 6 disease was correlated with CD8 T-cell recruitment and was enhanced by NK cell depletion. IL-18 expression during priming also enhanced RSV-specific antibody responses and T-cell responses on secondary RSV infection. Therefore, while IL-18 boosted antiviral immunity and reduced the viral load, its coexpression worsened disease. This is the first recombinant RSV with this property, and these are the first studies to demonstrate that NK cells can induce pathology during pulmonary viral infections.
Human respiratory syncytial virus (RSV) is the major cause of infantile viral bronchiolitis worldwide (27). RSV infection results in lower respiratory tract illness (LRTI) in 25 to 40% of children, with 0.5 to 2% requiring hospitalization. Immunity against RSV is short-lived and incomplete, and reinfection with the same strain can occur regularly throughout life. In elderly persons, RSV causes morbidity and mortality that match those resulting from influenza A virus infection in those vaccinated against seasonal influenza; there is currently no RSV vaccine. The relative roles of the virus and the immune response in causing disease are much debated (9).
The proinflammatory cytokine interleukin 18 (IL-18) is produced by a wide range of cells, including macrophages, neutrophils, and airway epithelial cells, and is a potent promoter of immune responses. It induces gamma interferon (IFN-γ) production from T cells without the requirement for T-cell receptor (TCR) engagement, an effect that is greatly enhanced by the presence of IL-12. Together, these cytokines enhance T helper cell type 1 (Th1) responses (15, 25, 32). IL-18 also directly promotes NK cell activation and proliferation and has been shown to drive antiviral immunity in a number of situations (18, 24, 26). In the presence of IL-12, IL-18 is also capable of preventing IgE production (34), but in the absence of IL-12 (or with an abundance of IL-2 or IL-4), it promotes the differentiation of Th2 cells and induces nonspecific IgE production (33, 35). Increased RSV titers are seen in IL-18 knockout mice (2), and polymorphisms in the IL-18 promoter are associated with increased risk of severe bronchiolitis (23).
To enhance and redirect immune responses upon RSV infection, we inserted various cytokine genes into the RSV genome for coexpression during infection in vitro and in vivo (3-7, 13). In the present study, we used this technique to investigate whether the potent immune-modulating capacity of IL-18 could be used to boost virus-specific immunity as a vaccine candidate; in addition, we aimed to examine how IL-18 expression influenced lung immune responses and disease severity. We found that both innate and adaptive immune responses were boosted by the coexpression of IL-18 from a recombinant RSV during respiratory tract infection of BALB/c mice. This resulted in a reduced primary viral load and enhanced memory responses with enhanced immunity on secondary infection. Unfortunately IL-18 expression also enhanced illness during primary infection, characterized by weight loss and increased pulmonary cellular infiltration. The unexpected and novel pattern of enhanced disease was accompanied by the excess recruitment of NK cells and then CD8 cells into the airways and lungs. Further investigation of this effect led us to identify NK cells as critical mediators of early disease and determinants of later CD8 T-cell responses. These results show that boosting the responses that reduce the viral load can increase disease severity in RSV infection.
MATERIALS AND METHODS
Mice, viral stocks, and infections.
Seven- to 8-week-old female BALB/c mice (Harlan Olac Ltd., Hornby, United Kingdom) were maintained under specific-pathogen-free conditions according to institutional and United Kingdom Home Office guidelines. Recombinant RSV expressing murine interleukin 18 (RSV/IL-18) was constructed as described below. All viruses were grown in HEp-2 cells (ATCC). In vitro viral titers were determined by infectious-focus assay (22). The same assay was used on lung homogenates to determine the in vivo viral load. UV inactivation of RSV was performed using a UV Stratalinker (Stratagene) for 3 min on ice. Mice were inoculated intranasally (i.n.) with 5 × 105 focus-forming units (FFU) of virus in 100 μl under light anesthesia. Rabbit anti-mouse asialo-GM1 polyclonal antibodies (100 μl; Wako chemicals) or control antibodies were administered intravenously (i.v.) on days −1 and +2 of infection.
Construction of RSV/IL-18.
The cDNA including the complete open reading frame (ORF) of murine IL-18 (20) was reverse transcription (RT)-PCR amplified using total RNA isolated from the murine spleen and cloned into the pGEM-T plasmid (Promega Corporation, Madison, WI) using the NdeI and XhoI restriction endonuclease sites according to the manufacturer's recommendations. Thereafter, the IL-18 cDNA was modified by PCR to attach the RSV-specific gene start and gene end transcriptional regulatory sequences upstream and downstream of the open reading frame, respectively, and to flank the resulting transcriptional cassette with XmaI sites. The following primers were used: forward primer, TATACCCGGGATGGGGCAAATATGGCTGCCATGTCAGAAGA, and reverse primer, ATTACCCGGGAATTTTTAATAACTCTAACTTTGATGTAAGTTAG (the gene start and gene end sequences in the forward and reverse primers, respectively, are underlined, and the XmaI sites are in boldface). The XmaI-XmaI fragment of the PCR product, including an IL-18 transcriptional cassette, was inserted into the XmaI site of the plasmid D46/1024, representing the XhoI-BamHI fragment of RSV full-length cDNA with the XmaI site inserted between the G and F genes (5). This RSV backbone had previously been modified to increase stability during bacterial growth by deleting part of the downstream noncoding region of the SH gene and by introducing several silent nucleotide changes to the downstream end of the SH open reading frame (3). These modifications were demonstrated to have no significant effect on viral-protein expression and viral replication in vivo (reference 3 and A. Bukreyev and P. L. Collins, unpublished data). The XhoI-BamHI fragment of the resulting plasmid was used to replace the corresponding fragment of RSV full-length cDNA (10), resulting in full-length cDNA of the RSV anti-genome carrying the IL-18 insert. Recombinant RSV carrying the IL-18 gene as an additional gene was recovered as previously described (10) and propagated in HEp-2 cells. The control recombinant RSV, referred to here as RSV/wt, also had the SH gene modification mentioned above (3) and was therefore identical to RSV/IL-18, except that it lacked the IL-18 transcription cassette.
Collection and analysis of cells.
Collection of bronchoalveolar lavage (BAL) fluid for cells and supernatants and harvesting of lung tissues, for flow cytometry and viral titers were carried out as previously described (22). BAL fluid cells were differentiated by hematoxylin and eosin (H&E) staining. For flow cytometric analysis, cells were initially blocked with Fc block (anti-CD16/32; BD, United Kingdom). Surface staining, polyclonal stimulation, and intracellular-cytokine staining were carried out as previously described (22). RSV-specific CD8+ T cells were analyzed using a major histocompatibility complex (MHC) class I pentamer bearing the immunodominant M2-1 protein epitope SYIGSINNI (Proimmune, United Kingdom). The cells were analyzed on a cyan ADP LX 9 color flow cytometer (Dako, United Kingdom), and the data were analyzed using the Dako Summit v4.3 analysis program.
Histological analysis.
Lung tissue was fixed in 10% formalin and embedded in wax prior to hemotoxylin and eosin staining. Blind scoring was conducted under light microscopy. Each slide was scored for perivascular, peribronchiolar, alveolar, and interstitial inflammation on a scale of 0 to 4, where 0 was none, 1 was slight, 2 was mild, 3 was moderate, and 4 was severe. For each area, the highest score representative of over 25% of the tissue area was taken, and the total inflammatory score was calculated by summing the score for each area.
RSV-specific antibody ELISA.
Serum antibody was assessed by enzyme-linked immunosorbent assay (ELISA). Antigen was prepared by infecting HEp-2 cells with RSV at 1 PFU/cell. Microtiter plates were coated overnight with 100 μl of a 1:500 dilution of either RSV or HEp-2 antigen. After being blocked with 1% bovine serum albumin (BSA) for 1 h, dilutions of test samples were added for a further 1 h. Bound antibody was detected using peroxidase-conjugated rabbit anti-mouse Ig (Dako) and o-phenylenediamine (Sigma-Aldrich, United Kingdom) as a substrate. Color development was blocked with 2 M H2SO4, and the optical density (OD) was read at 490 nm. RSV-specific antibody was determined by subtracting the RSV absorbance from the HEp-2 absorbance for the same sample.
Cytokine quantification.
IL-18 mRNA was quantified using RNase protection assays (RPA) according to the manufacturer's instructions (BD, United Kingdom). IL-4, IFN-γ, and IL-18 ELISA kits were obtained from BD, and the assays were carried out according to the manufacturer's instructions.
Statistical analysis.
Statistical analysis was performed using a nonparametric two-way analysis of variance (ANOVA) statistics program and posttest with GraphPad Prism 4.0 software (GraphPad Software Inc.).
RESULTS
RSV/IL-18 replicates at wild-type levels in vitro while producing significant quantities of soluble IL-18.
To construct the recombinant RSV expressing murine IL-18, the 579-nucleotide-long (including the stop codon) ORF of IL-18 was modified to be flanked by RSV-specific transcriptional gene start and gene end signals, and the resulting transcriptional cassette was inserted between the G and F genes of a cDNA clone of RSV antigenomic RNA. The recombinant RSV, referred to here as RSV/IL-18, was recovered by intracellular coexpression of the viral N, P, L, and M2-1 genes, as described previously (10). Production of recombinant RSV, carrying foreign cDNA, in this manner has been previously demonstrated to have no significant effect on viral-protein expression and viral replication in vitro compared to recombinant wild-type controls (reference 3 and Bukreyev and Collins, unpublished).
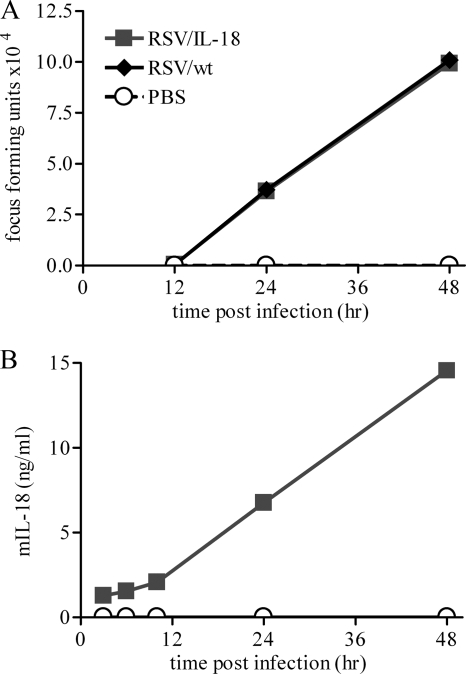
To investigate the IL-18 production and viral replication of this recombinant virus, HEp-2 cells (a human airway cell line) were infected with either RSV/IL-18 or RSV/wt (an identical recombinant backbone lacking an inserted IL-18 gene) at a multiplicity of infection (MOI) of 0.1 FFU per cell or treated with phosphate-buffered saline (PBS). Viral replication was measured by quantitative PCR (data not shown) and infectious-focus-forming assays at various time points postinfection (p.i.). There was no significant difference between the replication rates of RSV/IL-18 and RSV/wt in vitro (Fig. 1A). The supernatant from the infected cells was collected at various time points postinfection and tested for soluble IL-18 protein (Fig. 1B). Approximately 23 ng of IL-18 was produced de novo in the cell culture supernatant over a 2-day period from 106 cells infected with 105 FFU of RSV/IL-18. Infection with RSV/wt or PBS treatment produced no detectable IL-18 (the limit of detection for this assay was 18 pg/ml).
FIG. 1.
RSV/IL-18 viral replication and IL-18 production in vitro. HEp-2 cells were infected at an MOI of 0.1 FFU of either RSV/wt, RSV/IL-18, or nothing. (A) The viral load was measured by infectious-focus assay with a limit of detection of 2 × 102 FFU per ml. (B) IL-18 expression was assessed by ELISA with a limit of detection of 18 pg/ml. mIL-18, murine IL-18.
Coexpression of IL-18 during RSV infection reduces the viral load but worsens disease.
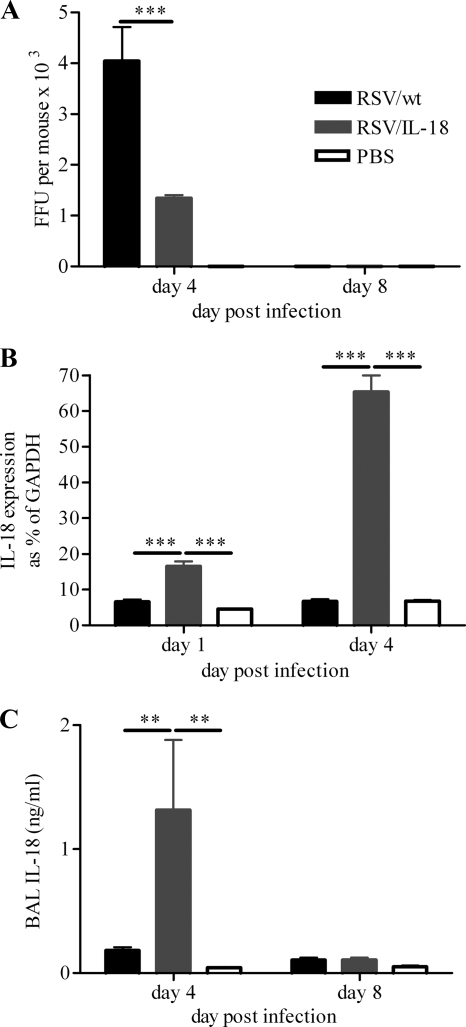
As RSV/IL-18 produced high levels of IL-18 in vitro without affecting the viral load, we wished to see how IL-18 expression would affect the viral load in vivo. Adult BALB/c mice were infected i.n. with 5 × 105 FFU of RSV/wt or RSV/IL-18 or treated with PBS. Mice were sacrificed on days 4 and 8 p.i., and infectious-focus assays were carried out on lung tissue to determine viral titers. Day 4 p.i. was selected as the day of peak viral load and innate immune responses, and day 8 p.i. was selected as the peak of pulmonary adaptive immune responses (22). RSV/IL-18 was modestly (3-fold), but significantly, attenuated compared to the control RSV/wt on day 4 p.i. (P < 0.001) (Fig. 2A). No infectious virus was detected on day 8 p.i. in any group. Lungs were removed from mice on days 1 and 4 p.i., and IL-18 mRNA was quantified using an RPA. There were significantly higher levels of IL-18 mRNA detected in RSV/IL-18-infected mice than in RSV/wt-infected mice or PBS-treated controls (P < 0.001) (Fig. 2B). The significant increase in levels of mRNA was reflected by an increase in the levels of soluble IL-18 protein detected in the airways (P < 0.01) (Fig. 2C).
FIG. 2.
RSV/IL-18 viral replication and IL-18 production in vivo. Adult BALB/c mice were infected i.n. with 5 × 105 FFU of RSV/wt, RSV/IL-18, or PBS. (A) Mice were sacrificed on days 4 and 8 postinfection, and infectious-focus assays were carried out to determine viral titers. (B) IL-18 mRNA in the lungs was determined by RPA on days 1 and 4. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (C) Levels of soluble IL-18 in the airways were determined by ELISA. The error bars and points are means plus standard errors of the mean (SEM); n = 5 mice per group. RPA is representative of 1 experiment; other results are representative of 3 repeated experiments. **, P < 0.01; ***, P < 0.001.
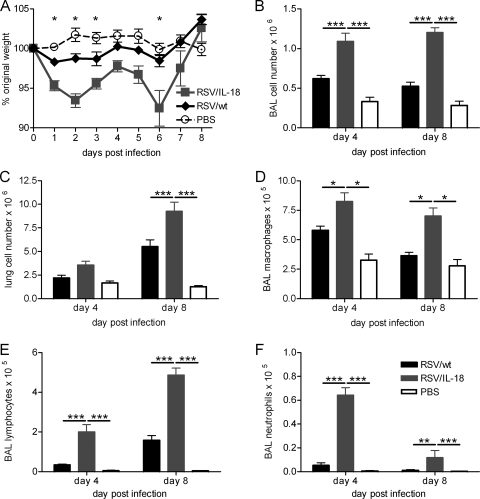
Daily weight change postinfection was used as a measure of disease. This noninvasive measure of disease has previously been shown to correlate well with airway resistance, airway hyperresponsiveness, and cellular inflammation in the murine RSV model (22). Mice infected with RSV/IL-18 lost significantly more weight than control-infected mice (P < 0.05) (Fig. 3A). This weight loss was biphasic, with peaks at day 2 and day 6 postinfection. Concurrent with this weight loss, there were also significantly more viable immune cells in the airways (P < 0.001) (Fig. 3B) in RSV/IL-18-infected mice on both days 4 and 8 p.i. and in the lungs on day 8 p.i. (P < 0.001) (Fig. 3C). Control infection with UV-inactivated RSV/IL-18 caused no significant weight loss (data not shown). Within the airways, there were significant increases in macrophages, lymphocytes, and neutrophils, as assayed by BAL (Fig. 3D to F). The number of macrophages increased in proportion to increased cellular infiltration after both RSV/wt and RSV/IL-18 infection; however, the relative proportions of both lymphocytes and neutrophils were much higher in mice infected with RSV/IL-18 (data not shown).
FIG. 3.
Effect of IL-18 coexpression on disease and the immune response to RSV infection. Adult BALB/c mice were infected i.n. with 5 × 105 FFU of either RSV/wt, RSV/IL-18, or PBS. (A) Weight was monitored daily and plotted as a percentage of weight on the day of infection. (B and C) Counts of viable BAL fluid cells and total lung cells were done by light microscopy. (D to F) Airway cells (BAL fluid) were taken on days 4 and 8 postinfection, and H&E staining was used to carry out differential cell counts for macrophages (D), lymphocytes (E), and neutrophils (F). The error bars and points are means ± SEM; n = 5 mice per group. The results are representative of 3 experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
IL-18 leads to enhanced numbers of natural killer cells and T cells, with increased activity in the lungs.
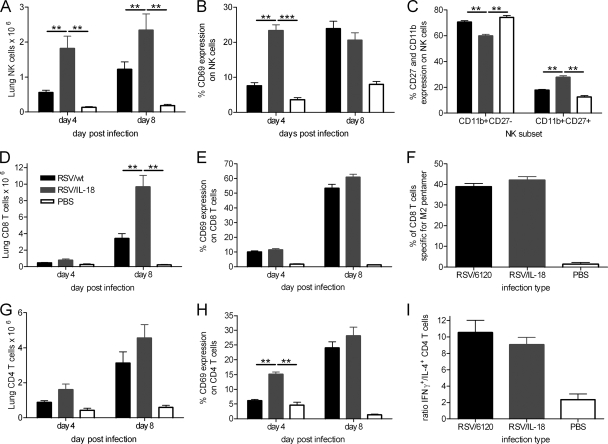
To identify which immune cells might play a role in the enhanced immunity and/or disease induced by RSV/IL-18, lung mononuclear cells were analyzed by flow cytometry on days 4 and 8 postinfection. There were significantly more NK cells in the lungs (P < 0.01) (Fig. 4A) and airways (data not shown) of mice infected with RSV/IL-18 on both days 4 and 8 postinfection. On day 4 p.i., an increased percentage of NK cells were also CD69+ after coexpression of IL-18, indicative of a higher number of activated NK cells (Fig. 4B). NK cells can be divided into two subsets based upon expression of CD11b and CD27 on their surfaces (14). NK cells with a CD11b+ CD27+ phenotype have greater effector function than their CD11b− CD27+ counterparts but are usually located within systemic, rather than peripheral, tissues. There were significantly more CD11b+ CD27+ NK cells on day 4 postinfection in the RSV/IL-18 group than in RSV/wt-infected mice (P < 0.01) (Fig. 4C).
FIG. 4.
Effect of IL-18 coexpression on cellular recruitment to the lung. Adult BALB/c mice were infected i.n. with 5 × 105 FFU of either RSV/wt, RSV/IL-18, or PBS. Total mononuclear lung cells were taken on days 4 and 8 postinfection and analyzed by flow cytometry. (A and B) NK cell (CD3− NKp46+) numbers (A) and activation (CD3− CD69+) (B) were measured on days 4 and 8 postinfection. (C) On day 4, lung NK cell subset recruitment was analyzed by expression of CD11b and CD27 on CD3− DX5+ cells. (D and E) CD8 cell numbers (D) and activation (E) were measured on days 4 and 8 postinfection. (F) On day 8, an RSV-specific MHC class I pentamer was used to enumerate the proportion of RSV-specific CD8 T cells. (G and H) CD4 cell numbers (G) and activation (H) were measured on days 4 and 8 postinfection. (I) On day 8, lung cells were stimulated with phorbol 12-myristate 13-acetate (PMA), and ionomycin and intracellular-cytokine staining were carried out for IL-4 and IFN-γ. The ratio of IFN-γ+ versus IL-4+ CD4 T cells was then calculated. The error bars and points are means plus SEM; n = 5 mice per group. The results are representative of 3 experiments. **, P < 0.01; ***, P < 0.001.
IL-18 expression during RSV infection also altered T-cell recruitment. Significantly more CD8 T cells were recruited to the lungs on day 8 following RSV/IL-18 infection (P < 0.01) (Fig. 4D). However, there was no difference in CD8 T-cell activation on either day 4 p.i. or day 8 p.i. (CD69+) (Fig. 4E). The percentage of RSV-specific CD8 T cells on day 8 postinfection was measured using an MHC class I pentamer bearing the immunodominant epitope, SYIGSINNI, from the M2-1 protein. This showed there was no significant difference between RSV/IL-18 and RSV/wt infections in the recruitment of RSV-specific CD8 T cells (RSV pentamer+) (Fig. 4F). There were no significant differences in the numbers of CD4 T cells after IL-18 coexpression (Fig. 4G). However, on day 4 p.i., RSV/IL-18 infection resulted in a significant increase in the percentage of activated CD4 T cells compared to both RSV/wt- and PBS-treated mice (Fig. 4H) (P < 0.01). By day 8 p.i., the proportions of activated CD4 T cells were similar regardless of the type of infection.
One of the most discussed points in the field of RSV is the importance of Th1 versus Th2 immune responses in determining disease severity (21). Excess IL-4 (Th2) and diminished IFN-γ (Th1) have been associated with increased disease in both clinical cases and murine models (2, 17). As IL-18 is known to enhance the Th1 or Th2 arm of the immune response, depending on the conditions, it might have been thought that its expression during RSV infection would alter or enhance the dominant immune response. On day 8 p.i., we found no significant difference between RSV/wt and RSV/IL-18 infections in the Th1 (IFN-γ+)/Th2 (IL-4+) ratio in the lungs (Fig. 4I). The majority of T cells had a Th1 phenotype in spite of increased disease.
NK cell depletion abrogates early weight loss while enhancing later disease.
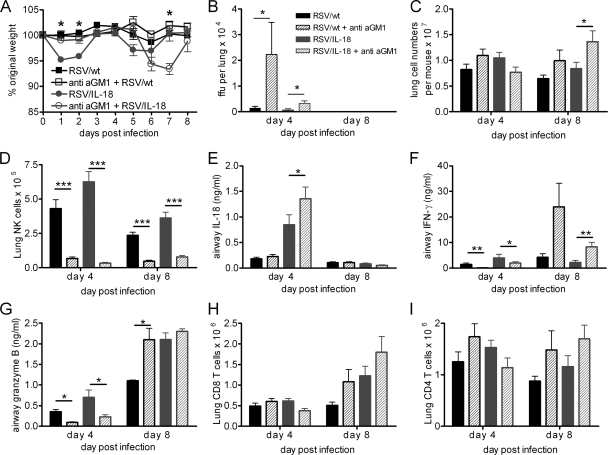
IL-18 expression by recombinant RSV increased NK cell numbers and activity in the lungs and airways. The early weight loss seen during primary infection was of particular interest, as we have not previously observed weight loss at this time point during primary infection; weight loss is normally correlated with CD8 T-cell recruitment (22, 28). We hypothesized that the NK cells might play a role in this increased disease. To test this, anti-mouse asialo-GM1 antibodies were administered on days −1 and +2 of infection to deplete NK cells during infection. Mice were infected with either RSV/wt or RSV/IL-18. As seen previously, mice infected with RSV/wt did not lose any weight, and antibody treatment did not affect this (Fig. 5A). RSV/IL-18 infection led to two peaks of weight loss around days 2 and 6, consistent with previous results. In contrast, NK cell depletion of RSV/IL-18-infected mice eliminated the first weight loss peak on days 1 and 2 p.i. but significantly enhanced weight loss on day 7 p.i. ( P < 0.05).
FIG. 5.
NK cell depletion in RSV/IL-18-infected animals abrogates early weight loss but worsens later disease. Adult BALB/c mice were infected i.n. with 5 × 105 FFU of either RSV/wt or RSV/IL-18 and were given either rabbit anti-mouse asialo-GM1 (aGM1) antibodies or a control serum i.v. on days −1 and +2 of infection. (A) Their weight was monitored daily and plotted against weight on the day of infection. (B) The viral load was assessed by focus-forming assay. (C to I) Lung mononuclear cell numbers (C), total lung NK cells (D), soluble BAL fluid IL-18 (E), IFN-γ (F), BAL fluid soluble granzyme B (G), total lung CD8 T cells (H), and total lung CD4 T cells (I) were measured on days 4 and 8 postinfection. The error bars and points are means ± SEM; n = 5 mice per group. The results are representative of 3 experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Anti-asialo-GM1 treatment of RSV/wt-infected mice increased the peak viral load in the lungs approximately 5-fold (Fig. 5B). As previously seen, the peak viral load in RSV/IL-18-infected mice was significantly reduced compared to RSV/wt-infected mice, but anti-asialo-GM1 treatment of RSV/IL-18-infected mice significantly increased the peak viral load ( P < 0.05) to the same level as RSV/wt infection. By day 8 postinfection, all mice had cleared virus from the lungs.
There was also a slight, but not significant, reduction in lung cell numbers following NK cell depletion of RSV/IL-18-infected mice on day 4 postinfection compared to their untreated counterparts (Fig. 5C). Conversely, on day 8, there were significantly more mononuclear cells in the lungs (P < 0.05) and immune cells in the airways (P < 0.05) (data not shown) of anti-asialo-GM1-treated mice than in those of their undepleted counterparts (Fig. 5C). Anti-asialo-GM1 treatment effectively depleted the NK cell population in the lungs (Fig. 5D) and airways (data not shown) on both days 4 and 8, removing 95% of the NK cells. Anti-asialo-GM1 treatment of RSV/IL-18-infected mice significantly increased the amount of airway IL-18 but had no effect on IL-18 levels in RSV/wt-infected mice (P < 0.05) (Fig. 5E).
On day 4 p.i., mice infected with RSV/IL-18 had significantly elevated levels of IFN-γ in the BAL fluid supernatant compared to the RSV/wt group (P < 0.05), while anti-asialo-GM1 treatment reduced IFN-γ levels to almost nothing in both RSV/wt- and RSV/IL-18-infected mice (P < 0.05) (Fig. 5F). Interestingly, on day 8 p.i., IFN-γ levels were elevated in both anti-asialo-GM1 groups compared to their untreated counterparts. Airway measurements of granzyme B revealed a trend similar to that seen with IFN-γ. Anti-asialo-GM1 treatment reduced the amount of granzyme B detected in the airways at day 4 p.i. but significantly elevated it in the control virus-infected group on day 8 p.i. (Fig. 5G). This indicated that on day 4 p.i. the majority of IFN-γ and granzyme B in the infected tissue was being produced in an NK cell-dependent manner and that coexpression of IL-18 enhanced their production.
Regardless of the infecting virus, anti-GM1 treatment increased the recruitment of both CD8 T cells (Fig. 5H) and CD4 T cells (Fig. 5I) to the lungs on day 8, but there was no significant difference in these numbers. The increased number of CD8 T cells may explain the increased granzyme B and IFN-γ in the airways. There was also no change in the functional characteristics of the CD4 or CD8 T cells, including activation (CD69+), effector memory (CD44+/CD62L−), or Th1/Th2 skewing of CD4 cells (data not shown), IFN-γ production by CD8, or the percentage of CD8 cells that were RSV specific (data not shown).
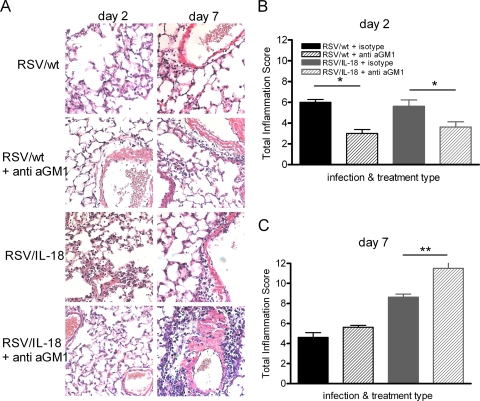
The effects of NK cell depletion on lung pathology were further examined by histological analysis at the peaks of weight loss on days 2 and 7 postinfection (Fig. 6). At day 2 p.i., overall inflammation levels in RSV/wt- and RSV/IL-18-infected mice were similar (Fig. 6B). RSV/IL-18 infection resulted in areas of high interstitial inflammation that were not present after RSV/wt infection; however, these areas represented less than 25% of the tissue area and therefore could not be included in the total score (data not shown). NK cell depletion significantly reduced total inflammation after RSV/wt or RSV/IL-18 infection (P < 0.05) (Fig. 6B). On day 7 postinfection, there was greatly increased inflammation after RSV/IL-18 infection compared to RSV/wt infection (Fig. 6C). At this time point, inflammation was significantly enhanced in RSV/IL-18-infected mice treated with anti-asialo-GM1 (P < 0.01).
FIG. 6.
NK cell depletion lessens early lung inflammation. Adult BALB/c mice were infected with 5 × 105 FFU of either RSV/wt or RSV/IL-18 and were given either rabbit anti-mouse asialo-GM1 antibodies or a control serum i.v. on days −1 and +2 of infection. Lungs were harvest on days 2 and 7 postinfection, fixed, and H&E stained. The slides (×200) (A) were scored blind for inflammation under light microscopy, and total inflammatory scores were determined on day 2 (B) and day 7 (C). The error bars and points are means plus SEM; n = 6 slides per group. *, P < 0.05; **, P < 0.01.
IL-18 expression promotes enhanced immunity to protect against secondary infection.
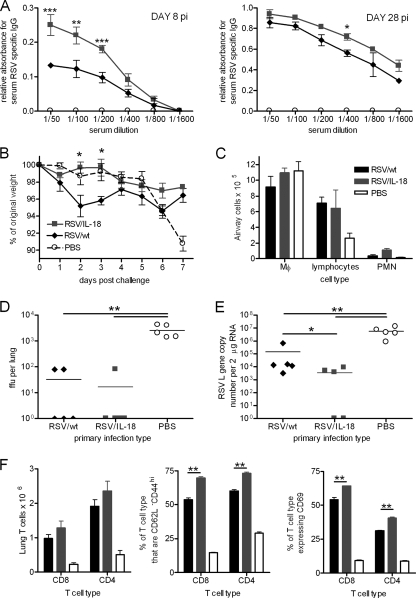
We wished to determine whether RSV/IL-18 increased recall immune responses to subsequent viral infection. On day 8 p.i., RSV/IL-18-infected mice had significantly enhanced antibody compared to RSV/wt infection (P < 0.001) (Fig. 7A). By day 28 p.i., after both infection and inflammation were fully resolved, anti-RSV antibody was detectable in both wild-type RSV- and RSV/IL-18-infected mice, with IL-18 expression causing a slight increase in titers. Twenty-eight days postinfection, the mice were rechallenged i.n. with 1 × 106 FFU of nonrecombinant wild-type RSV. Prior infection prevented the development of the weight loss seen on day 7 p.i. in naïve mice (Fig. 7B). Interestingly, primary infection with RSV/wt resulted in slight weight loss on day 2 p.i., which was prevented by primary infection with RSV/IL-18. Lymphocyte recruitment into the airways was also increased if mice had been previously exposed to RSV, although IL-18 expression did not affect this (Fig. 7C). The viral load was determined at day 4, with 4 out of 5 RSV/IL-18-primed and 3 out of 5 RSV/wt-primed mice found to have viral loads below the level of detection (Fig. 7D). Real-time PCR for the viral polymerase (L) gene was used to increase sensitivity for the viral genome and mRNA (Fig. 7E). While both viruses reduced the L gene copy number compared to naïve animals ( P < 0.01), RSV/IL-18 priming resulted in significantly lower copy numbers than RSV/wt priming (P < 0.05). RSV/IL-18 priming also resulted in significantly more effector (CD44+ CD62L−) and activated (CD69+) CD4 and CD8 T cells than RSV/wt infection (Fig. 7F) (P < 0.01 in all cases).
FIG. 7.
IL-18 enhances recall responses during secondary infection. Adult BALB/c mice were infected i.n. with 5 × 105 FFU of either RSV/6120, RSV/IL-18, or PBS. (A) Serum was taken on days 8 and 28 after primary infection, and total RSV-specific Ig was determined by ELISA. (B) At 28 days postinfection, mice were challenged with 1 × 106 FFU of wild-type RSV A2, and body weight was monitored for 7 days postchallenge. (C) On day 4 postchallenge, airway cells were taken, and cellular infiltration was determined by H&E staining and light microscopy. (D and E) At the same time point, lung tissue was taken to determine the viral load by plaque assay and real-time PCR. (F) Lung T-cell recruitment (CD8 and CD4), effector phenotype (CD44+ CD62L−), and activation (CD69) were determined by flow cytometry. The results are representative of 2 experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Here, we have described a novel recombinant RSV that expresses the cytokine IL-18. In vivo, RSV/IL-18 infection significantly increased the expression of IL-18 and attenuated viral replication. IL-18 coexpression also caused biphasic weight loss, peaking on days 2 and 6. In the early stages of infection, increased NK cell numbers and activation were observed, and their removal using depleting antibodies removed the early weight loss and viral attenuation. In the later stages of infection, the second peak of weight loss was correlated with the recruitment of CD8 T cells and was worsened by NK cell depletion.
The in vivo attenuation seen in this study was similar to the effect we have previously described with recombinant RSV expressing IFN-γ (6). IL-18 was first identified as a potent inducer of IFN-γ, suggesting that a common mechanism may be responsible (20). However, there were significant differences between the effect of IL-18 expression in the present study and that of IFN-γ in previous work (6, 13). First, the level of attenuation was greater following expression of IFN-γ (10- to 100-fold) than following expression of IL-18 (3-fold). This probably reflects the differences in function of each cytokine, as IFN-γ is directly antiviral in infected cells (31), while RSV/IL-18 infection appeared to recruit high numbers of immune cells, a feature not present after RSV/IFN-γ infection (6). In addition, neither RSV/IFN-γ nor any previous cytokine expressed from RSV has been found to cause weight loss during primary infection.
The induction of weight loss during primary infection due to enhancement of host immunity seen in this study was of particular interest. Previous studies using murine models of primary RSV infection have shown that high doses of nonrecombinant, wild-type RSV result in CD8 T-cell-mediated weight loss and disease, peaking around day 7 p.i. (22, 28). Disease can be induced at earlier time points during RSV infection by prior infection as neonates or by vaccination with Formalin-inactivated RSV, single RSV proteins, or recombinant RSV expressing IFN-γ, followed by challenge with wild-type RSV (1, 11, 13). The weight loss in these cases is the result of the biased stimulation of specific memory T-cell subsets. In contrast, the early weight loss after RSV/IL-18 infection correlated well with the early innate immune response (neutrophils and NK cells), with T cells being relatively absent. By the use of depleting antibodies, we showed that the key cells in early IL-18-mediated disease are NK cells. IL-18 is known to be critical in the priming of NK cells to acquire full effector function, such as the secretion of IFN-γ and cytotoxicity (8). In our study, NK cells either proliferated locally or were recruited to the lungs and airways in significantly higher numbers and increased activation states when IL-18 was expressed during viral infection. IL-18 also skewed NK cell recruitment to a CD27+ CD11b+ phenotype. CD27+ CD11b+ NK cells proliferate more readily and have higher cytoxicity and cytokine production, which may partially explain the higher IFN-γ and granzyme B levels in the airways and the reduced viral load (14). NK cells have not previously been associated with increased disease severity in viral infections of the lung. However, the synergistic effect of coadministered IL-18 and IL-2 has been found to cause lethal lung injury in mice as a result of excessive NK cell infiltration into the airways (19). We also found that the enhanced NK cell numbers in the lung after RSV/IL-18 infection is also critical for enhanced clearance, as viral loads were significantly increased on NK cell depletion. In agreement with this, IL-18-deficient animals have recently been found to have increased susceptibility to poxvirus infection (30). The authors found that this was associated with a decreased NK cell response. Interestingly, they also found that in the absence of both IL-12 and IL-18 there were was a greater number of FoxP3+ regulatory T cells (Tregs); however, in our study, Treg recruitment was unaltered by overexpression of IL-18 (data not shown).
We and others have previously shown that the induction of late weight loss during primary RSV infection of mice with nonrecombinant wild-type RSV is correlated with recruitment of CD8 T cells to the sites of infection and is abrogated by their removal (12, 28). Interestingly, however, we have not observed weight loss at this time point with either recombinant wild-type RSV or RSV carrying a foreign gene. The second phase of weight loss seen after infection with RSV/IL-18 correlated well with increased recruitment of CD8 T cells to the lungs compared to wild-type infection, and we hypothesize that during the later phase of RSV/IL-18 infection, CD8 T cells mediate illness. Interestingly, NK cell depletion worsened this late phase of disease, and this was associated with a further increase in CD8 T-cell numbers and their effector molecules, IFN-γ and granzyme B, in the airways. There are a number of potential mechanisms for this effect. After NK cell depletion, the viral load increases approximately 5-fold. An enhanced peak viral load, and therefore antigen burden, in NK cell-depleted mice could drive a stronger CD8 T-cell response. Viral loads in RSV/wt-infected mice, however, also increase by approximately 5-fold with no effect on overall disease. Intriguingly, increased RSV/IL-18 loads also resulted in enhanced IL-18 in the airways, although this was not proportionate to the increase in the viral load, and this could be responsible for the greater activation of CD8 T cells later during infection. It is also possible that during RSV infection NK cells have some immunosuppressive or regulatory effects on adaptive immunity. For instance, NK cells have been shown to kill immature dendritic cells, activated CD4 T cells, and hyperactivated macrophages, the increased presence of all of which could increase CD8 T-cell numbers (29). In addition, NK cells can produce regulatory cytokines, such as IL-10, in response to viral infections, thereby limiting the magnitude of CD8 T-cell responses in a direct manner (16).
We conclude that antiviral immunity is enhanced by expression of IL-18 in a recombinant RSV vector and that this is sufficient to improve immune responses to secondary infection. However, this increased immunity led to enhanced disease by boosting innate immune responses, in particular NK cells, a cell type not previously thought to be detrimental to the outcome of lung disease. Our findings support the concept that host immune responses largely mediate RSV disease, even while restricting virus replication. This study also highlights issues with modulating the immune response, as depleting NK cells prevented early disease but resulted in disease being enhanced at a later stage.
Acknowledgments
Members of the Department of Respiratory Medicine, Imperial College London, were funded by Wellcome Trust Programme Grant 071381/Z/03/Z (United Kingdom). A.B. and P.L.C. were supported by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. J.L.W. and R.R.C. were supported by the Intramural Research Programs of the National Eye Institute, National Institutes of Health.
Footnotes
Published ahead of print on 3 February 2010.
REFERENCES
- 1.Alwan, W. H., W. J. Kozlowska, and P. J. M. Openshaw. 1994. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med. 179:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boelen, A., J. Kwakkel, M. Barends, L. de Rond, J. Dormans, and T. Kimman. 2002. Effect of lack of interleukin-4, interleukin-12, interleukin-18, or the interferon-gamma receptor on virus replication, cytokine response, and lung pathology during respiratory syncytial virus infection in mice. J. Med. Virol. 66:552-560. [DOI] [PubMed] [Google Scholar]
- 3.Bukreyev, A., I. M. Belyakov, J. A. Berzofsky, B. R. Murphy, and P. L. Collins. 2001. Granulocyte-macrophage colony-stimulating factor expressed by recombinant respiratory syncytial virus attenuates viral replication and increases the level of pulmonary antigen-presenting cells. J. Virol. 75:12128-12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukreyev, A., I. M. Belyakov, G. A. Prince, K. C. Yim, K. K. Harris, J. A. Berzofsky, and P. L. Collins. 2005. Expression of interleukin-4 by recombinant respiratory syncytial virus is associated with accelerated inflammation and a nonfunctional cytotoxic T-lymphocyte response following primary infection but not following challenge with wild-type virus. J. Virol. 79:9515-9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukreyev, A., E. Camargo, and P. L. Collins. 1996. Recovery of infectious respiratory syncytial virus expressing an additional, foreign gene. J. Virol. 70:6634-6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukreyev, A., S. S. Whitehead, N. Bukreyeva, B. Murphy, and P. L. Collins. 1999. Interferon gamma expressed by a recombinant respiratory syncytial virus attenuates virus replication in mice without compromising immunogenicity. Proc. Natl. Acad. Sci. U. S. A. 96:2367-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukreyev, A., S. S. Whitehead, C. Prussin, B. R. Murphy, and P. L. Collins. 2000. Effect of coexpression of interleukin-2 by recombinant respiratory syncytial virus on virus replication, immunogenicity, and production of other cytokines. J. Virol. 74:7151-7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaix, J., M. S. Tessmer, K. Hoebe, N. Fuseri, B. Ryffel, M. Dalod, L. Alexopoulou, B. Beutler, L. Brossay, E. Vivier, and T. Walzer. 2008. Cutting edge: priming of NK cells by IL-18. J. Immunol. 181:1627-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, P. L., and B. S. Graham. 2008. Viral and host factors in human respiratory syncytial virus pathogenesis. J. Virol. 82:2040-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins, P. L., M. G. Hill, E. Camargo, H. Grosfeld, R. M. Chanock, and B. R. Murphy. 1995. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc. Natl. Acad. Sci. U. S. A. 92:11563-11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culley, F. J., J. Pollott, and P. J. Openshaw. 2002. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J. Exp. Med. 196:1381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham, B. S., L. A. Bunton, P. F. Wright, and D. T. Karzon. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Investig. 88:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harker, J., A. Bukreyev, P. L. Collins, B. Wang, P. J. Openshaw, and J. S. Tregoning. 2007. Virally delivered cytokines alter the immune response to future lung infections. J. Virol. 81:13105-13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayakawa, Y., and M. J. Smyth. 2006. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J. Immunol. 176:1517-1524. [DOI] [PubMed] [Google Scholar]
- 15.Kohno, K., J. Kataoka, T. Ohtsuki, Y. Suemoto, I. Okamoto, M. Usui, M. Ikeda, and M. Kurimoto. 1997. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J. Immunol. 158:1541-1550. [PubMed] [Google Scholar]
- 16.Lee, S. H., K. S. Kim, N. Fodil-Cornu, S. M. Vidal, and C. A. Biron. 2009. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J. Exp. Med. 206:2235-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legg, J. P., I. R. Hussain, J. A. Warner, S. L. Johnston, and J. O. Warner. 2003. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 168:633-639. [DOI] [PubMed] [Google Scholar]
- 18.Liu, B., I. Mori, M. J. Hossain, L. Dong, K. Takeda, and Y. Kimura. 2004. Interleukin-18 improves the early defence system against influenza virus infection by augmenting natural killer cell-mediated cytotoxicity. J. Gen. Virol. 85:423-428. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto, M., S. Kato, K. Oizumi, M. Kinoshita, Y. Inoue, K. Hoshino, S. Akira, A. N. McKenzie, H. A. Young, and T. Hoshino. 2002. Interleukin 18 (IL-18) in synergy with IL-2 induces lethal lung injury in mice: a potential role for cytokines, chemokines, and natural killer cells in the pathogenesis of interstitial pneumonia. Blood 99:1289-1298. [DOI] [PubMed] [Google Scholar]
- 20.Okamura, H., H. Tsutsi, T. Komatsu, M. Yutsudo, A. Hakura, T. Tanimoto, K. Torigoe, T. Okura, Y. Nukada, K. Hattori, et. al. 1995. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378:88-91. [DOI] [PubMed] [Google Scholar]
- 21.Openshaw, P. J., and J. S. Tregoning. 2005. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin. Microbiol. Rev. 18:541-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pribul, P. K., J. Harker, B. Wang, H. Wang, J. S. Tregoning, J. Schwarze, and P. J. Openshaw. 2008. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J. Virol. 82:4441-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puthothu, B., M. Krueger, J. Forster, J. Heinze, M. Weckmann, and A. Heinzmann. 2007. Interleukin (IL)-18 polymorphism 133C/G is associated with severe respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 26:1094-1098. [DOI] [PubMed] [Google Scholar]
- 24.Reading, P. C., P. G. Whitney, D. P. Barr, M. Wojtasiak, J. D. Mintern, J. Waithman, and A. G. Brooks. 2007. IL-18, but not IL-12, regulates NK cell activity following intranasal herpes simplex virus type 1 infection. J. Immunol. 179:3214-3221. [DOI] [PubMed] [Google Scholar]
- 25.Robinson, D., K. Shibuya, A. Mui, F. Zonin, E. Murphy, T. Sana, S. B. Hartley, S. Menon, R. Kastelein, F. Bazan, and A. O'Garra. 1997. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity 7:571-581. [DOI] [PubMed] [Google Scholar]
- 26.Takeda, K., H. Tsutsui, T. Yoshimoto, O. Adachi, N. Yoshida, T. Kishimoto, H. Okamura, K. Nakanishi, and S. Akira. 1998. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8:383-390. [DOI] [PubMed] [Google Scholar]
- 27.Tregoning, J. S., and J. Schwarze. 2010. Respiratory viral infections in infancy: causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 23:74-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tregoning, J. S., Y. Yamaguchi, J. Harker, B. Wang, and P. J. Openshaw. 2008. The role of T cells in the enhancement of respiratory syncytial virus infection severity during adult reinfection of neonatally sensitized mice. J. Virol. 82:4115-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vivier, E., E. Tomasello, M. Baratin, T. Walzer, and S. Ugolini. 2008. Functions of natural killer cells. Nat. Immunol. 9:503-510. [DOI] [PubMed] [Google Scholar]
- 30.Wang, Y., G. Chaudhri, R. J. Jackson, and G. Karupiah. 2009. IL-12p40 and IL-18 play pivotal roles in orchestrating the cell-mediated immune response to a poxvirus infection. J. Immunol. 183:3324-3331. [DOI] [PubMed] [Google Scholar]
- 31.Wheelock, E. F. 1965. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science 149:310-311. [PubMed] [Google Scholar]
- 32.Xu, D., W. L. Chan, B. P. Leung, D. Hunter, K. Schulz, R. W. Carter, I. B. McInnes, J. H. Robinson, and F. Y. Liew. 1998. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J. Exp. Med. 188:1485-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshimoto, T., H. Mizutani, H. Tsutsui, N. Noben-Trauth, K. Yamanaka, M. Tanaka, S. Izumi, H. Okamura, W. E. Paul, and K. Nakanishi. 2000. IL-18 induction of IgE: dependence on CD4+ T cells, IL-4 and STAT6. Nat. Immunol. 1:132-137. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimoto, T., H. Okamura, Y. I. Tagawa, Y. Iwakura, and K. Nakanishi. 1997. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-gamma production from activated B cells. Proc. Natl. Acad. Sci. U. S. A. 94:3948-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimoto, T., H. Tsutsui, K. Tominaga, K. Hoshino, H. Okamura, S. Akira, W. E. Paul, and K. Nakanishi. 1999. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc. Natl. Acad. Sci. U. S. A. 96:13962-13966. [DOI] [PMC free article] [PubMed] [Google Scholar]