Abstract
Crocodylians exhibit a fascinating diversity of terrestrial gaits and limb motions that remain poorly described and are of great importance to understanding their natural history and evolution. Their musculoskeletal anatomy is pivotal to this diversity and yet only qualitative studies of muscle-tendon unit anatomy exist. The relative masses and internal architecture (fascicle lengths and physiological cross-sectional areas) of muscles of the pectoral and pelvic limbs of American alligators (Alligator mississippiensis Daudin 1801) were recorded for an ontogenetic series of wild specimens (n = 15, body masses from 0.5 to 60 kg). The data were analysed by reduced major axis regression to determine scaling relationships with body mass. Physiological cross-sectional areas and therefore muscle force-generating capacity were found to be greater in the extensor (anti-gravity) muscles of the pelvic limb than in the pectoral limb, reflecting how crocodylians differ from mammals in having greater loading of the hindlimbs than the forelimbs. Muscle masses and architecture were generally found to scale isometrically with body mass, suggesting an ontogenetic decrease in terrestrial athleticism. This concurs with the findings of previous studies showing ontogenetic decreases in limb bone length and the general scaling principle of a decline of strength : weight ratios with increasing size in animals. Exceptions to isometric scaling found included positive allometry in fascicle length for extensor musculature of both limbs, suggesting an ontogenetic increase in working range interpreted as increasing postural variability – in particular the major hip extensors – the interpretation of which is complicated by previous described ontogenetic increase of moment arms for these muscles.
Keywords: archosaur, crocodylia, functional anatomy, locomotion, muscle architecture, scaling
Introduction
Extant crocodylians are the only known living tetrapods to use nearly the full range of recognized quadrupedal terrestrial locomotion patterns, from highly abducted, laterally undulating ‘sprawling’ gaits to more erect walking, asymmetrical (bounding and galloping) gaits (Cott, 1960; Zug, 1974; Singh & Bustard, 1976; Whitaker, 1978; Brinkman, 1980; Webb & Gans, 1982; Whitaker & Andrews, 1988; Gatesy, 1991; Reilly & Elias, 1998; Blob & Biewener, 1999, 2001; Renous et al. 2002). As one of only two (along with birds) surviving forms of the great Mesozoic Archosauria (crocodylians, pterosaurs, dinosaurs and relatives) crocodylians represent an invaluable and often-used resource to palaeobiologists studying the evolution of locomotion in this diverse group.
Asymmetrical gaits at least superficially convergent with those of mammals have been observed in juveniles and some adults from both Crocodylinae and Gavialidae, but not yet any Alligatoroidea (Bustard & Singh, 1977; Meers, 1999 and references therein). Interpretation of the history and distribution of these gaits is dependent on the controversial phylogenetic position of the Gavialidae. Morphology-based phylogenies have placed Gavialidae basal to the Alligatoroidea/Crocodylidae split, in which case these locomotion patterns may be reconstructed as ancestral for Crocodylia as a whole (e.g. Brochu, 1997; Meers, 1999). However, because molecular data consistently place Gavialidae as sister group to Crocodylinae, this behaviour may be restricted to Crocodylidae (e.g. Gatesy et al. 2003; Roos et al. 2007; Gatesy & Amato, 2008). It seems unintuitive that such an obvious adaptation for rapid terrestrial locomotion would develop within a restricted group of secondarily aquatic crocodylians rather than be inherited from their more terrestrial ancestors (Parrish, 1986, 1987; Sereno, 1991). However, the current controversy over the phylogenetic distribution of bounding and non-bounding species demands that the hypothesis that asymmetrical gaits are ancestral for the whole clade Crocodylia be viewed tentatively.
Unpublished data (John R. Hutchinson) and anecdotal evidence (e.g. Cott, 1960; Singh & Bustard 1976) support the inference that crocodylians experience a strong decline in terrestrial performance (that is the speed, frequency and duration of terrestrial locomotion bouts) across ontogeny. Those crocodylians that use asymmetrical gaits may entirely lose them past a certain size boundary (∼2–4 m length). Although all tetrapods studied to date exhibit qualitatively similar declines in relative performance across ontogeny (e.g. Pennycuick, 1975; Hutchinson et al. 2006), available data and anecdotal evidence support the inference that crocodylians exhibit a markedly steeper decline. Such a dramatic shift and particularly the loss of entire gaits is rare among tetrapods, and thus interesting in its own right – not to mention an excellent case study of scaling (change of biological properties such as shape with body size).
Hypotheses of adult performance decline and gait-loss have yet to be explicitly verified by study of the ontogeny of locomotion in crocodylian species known to bound and gallop. However, support for this inference has been found in the ontogenetic scaling of limb bone stresses and related geometric cross-sectional properties. Observed ontogenetic changes of crocodilian limb bone shape are not sufficient to counteract stress increases caused by increasing body weight (Blob, 2000), and humeral cross-sections (strongly related to bending strength) do not change ontogenetically (Meers, 2002). If bone safety factors are to be maintained in adult crocodylians, this suggests that unless limb postures and/or gait kinematics are altered significantly, overall speed must decrease.
Studies of limb scaling in the American alligator (Alligator mississippiensis Daudin 1801) also provide support for an ontogenetic decrease in performance. Through alligator ontogeny, the limbs become relatively shorter. In particular, distal limb segments became increasingly shorter relative to proximal segments (Dodson, 1975; Livingston et al. 2009). Given the assumptions underlying the theory of dynamic similarity, adult alligators with relatively shorter limbs should move relatively slower [velocity for a given Froude number will be lower with shorter limb lengths where Fr = v2/gl (Fr = Froude number; v= velocity, g= acceleration due to gravity, l= limb length); Alexander & Jayes, 1983]. Furthermore, relative lengthening of proximal segments shifts muscle mass distally on the limb, increasing the moments of inertia that must be overcome to swing the limb and hence either reducing stride frequency or increasing energy expenditure.
The fourth trochanter, a feature of the femoral shaft that marks the insertion of the massive caudofemoral muscles, and the deltopectoral crest, an analogous feature of the humeral shaft that marks the insertion of the large pectoralis and deltoideus muscles, were both found to migrate distally through ontogeny (Dodson, 1975; Livingston et al. 2009). As this increases the effective mechanical advantage of these muscles (Biewener, 1989), a given contraction from either would impart less rotation and speed (but more force) to the limb. Anatomical, biomechanical and EMG data have shown the caudofemoral muscles to be main retractors of the pelvic limb, providing the majority of propulsive power to drive the body forwards (Gatesy, 1990, 1997; Blob & Biewener, 2001), and that the pectoralis performs a similar function in the pectoral limb (Jenkins & Goslow, 1983; Meers, 2003). Ontogenetic increase in their mechanical advantage may therefore indicate slower but more forceful movement for the animal as a whole. However, as alligators have never been known to use bounding or galloping gaits, the relevance of these data to hypotheses of ontogenetic gait loss is limited without suitable comparative data from known bounding species.
These and other studies (e.g. Romer, 1923a; Cong et al. 1998; Blob, 2000; Meers, 2002, 2003) have thoroughly described the basic skeletal scaling of alligators and their qualitative muscular anatomy. However, the quantitative anatomy of crocodylian muscles remains poorly known. Functional biologists since the 1970s have developed simple metrics to describe this quantitative anatomy that are firmly linked to reasonably well-established biomechanical principles of muscle function. Collectively these metrics are termed the muscle architecture (i.e. the geometric properties and internal arrangement of contractile fibres within a muscle) and represent major determinants of how muscles produce force and movement. Fibre length (or fascicle length at the gross anatomical level) has a large effect on the distance over which the muscle may contract (or ‘working range’). Additionally, the total area of muscle fibres contributing to muscle force (or ‘physiological cross-sectional area’, PCSA), determined mainly by the number of fibres present, has a large effect on muscle force-generating capacity. Adding internal tendons and altering the angle that muscle fibres insert onto them (pennation angle) affects the packing of fibres within the muscle volume, and can allow large PCSAs in small volumes while maintaining appropriate direction for the contractile force. If muscle mass is constant, working range and force vary inversely: fewer long fibres could fit in a given muscle volume than short fibres could. Thus greater working ranges come at a cost of reduced forces and vice versa. For a muscle to produce high forces over a large working range (i.e. to do large amounts of work) the muscle volume must be large to allow a large cross-section of long fibres. ‘Powerful’ muscles capable of doing large amounts of work hence have large masses and associated metabolic costs, and tend to be rarer.
Because the limbs of A. mississippiensis become relatively shorter through ontogeny, the length of muscles would be expected to decrease, and considering the available space for packing muscles into the limb, the relative mass of limb muscles would also be expected to decrease. With decreasing muscle mass comes increasing functional constraint, forcing muscles to specialize. Thus ontogenetic decreases in limb length would be expected to be matched by ontogenetic decreases in the ability of muscles specialized for working range to also provide force and vice versa. This would reduce the total forces and range of movement the muscles are able to supply to the limbs. The links between muscle force, working range (i.e. displacement) and power (force × displacement/time) and the specifics of terrestrial locomotion are complex and may depend on kinetic and kinematic factors that are currently unknown for crocodylians. However, the qualitative correlation between these factors is sufficiently strong that we hypothesize that decreasing muscle masses and increasing muscle functional constraint are major factors in ontogenetic decreases of terrestrial ‘performance’, and reduction of the locomotive repertoire in crocodylians.
To investigate this hypothesis our study builds on Dodson’s (1975) and Livingston et al.’s (2009) skeletal scaling analyses through quantification of the architectural properties of muscles relevant to limb function in A. mississippiensis. We use regression analysis to determine the relationship of muscle architectural properties with body mass in an ontogenetic series of specimens, leading to a re-examination of the anatomical implications for significant ontogenetic alteration of terrestrial locomotor patterns in crocodylians. Alligators and relatives are more sedate than other crocodylians and are not known to use the most extreme asymmetrical gaits. Therefore scaling relationships for limb muscles properties of other crocodylian species may not fit within the confidence intervals of scaling relationships determined for alligators, limiting the inferences that may be made for Crocodylia as a whole. However, quantitative anatomical data on one well-known species are a valuable first step toward establishing how much functional diversity exists within extant Crocodylia. Additionally, quantitative data on crocodylian limb muscle properties provide a valuable dataset both for studies of locomotion in this intriguing but poorly understood clade, and for studies seeking to reconstruct function and evolution in extinct archosaurs.
Materials and methods
Dissection
Pectoral and pelvic limb muscles were dissected from A. mississippiensis carcasses (n = 15) in three categories representing an ontogenetic series: juveniles of ∼0.5 kg body mass (n = 5), sub-adults of ∼3–5 kg body mass (n = 4) and ‘adults’ of ∼15–60 kg body mass (n = 6); representing a 120× range in body mass. The specimens were chosen from animals sacrificed for other studies at the Rockefeller Wildlife Refuge, LA, and were all analysed within 24 h of death. Total body mass (kg), total rostro-caudal length (m), pelvic and pectoral limb lengths and limb segment lengths were measured before the cadavers were skinned and individual pectoral and pelvic limb muscles identified and dissected out (see Table 1; following Romer, 1923a; Cong et al. 1998; Meers, 2003).
Table 1.
Specimen lengths and masses.
| Specimen no. | Length snout-tail base (m) | Tail length (m) | Pectoral limb length (m) | Humerus length (m) | Ulna length (m) | Manus length (to 3rd ungual) (m) | Pelvic limb length (m) | Femur length (m) | Tibia length (m) | Pes length (to 3rd ungual) (m) | Mbody (kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 0.27 | 0.33 | 0.104 | 0.038 | 0.028 | 0.037 | 0.131 | 0.04 | 0.037 | 0.052 | 0.52 |
| 15 | 0.29 | 0.33 | 0.104 | 0.042 | 0.028 | 0.035 | 0.135 | 0.044 | 0.039 | 0.05 | 0.58 |
| 11 | 0.29 | 0.35 | 0.109 | 0.042 | 0.031 | 0.04 | 0.14 | 0.044 | 0.037 | 0.054 | 0.67 |
| 13 | 0.31 | 0.38 | 0.119 | 0.044 | 0.033 | 0.041 | 0.156 | 0.049 | 0.042 | 0.061 | 0.75 |
| 14 | 0.32 | 0.38 | 0.122 | 0.044 | 0.033 | 0.04 | 0.152 | 0.048 | 0.041 | 0.054 | 0.86 |
| 8 | 0.47 | 0.55 | 0.167 | 0.068 | 0.047 | 0.062 | 0.219 | 0.075 | 0.06 | 0.087 | 3.20 |
| 6 | 0.51 | 0.56 | 0.148 | 0.072 | 0.055 | 0.061 | 0.233 | 0.078 | 0.022 | 0.096 | 3.50 |
| 9 | 0.53 | 0.65 | 0.187 | 0.072 | 0.052 | 0.063 | 0.244 | 0.083 | 0.071 | 0.089 | 4.40 |
| 7 | 0.56 | 0.70 | 0.202 | – | – | – | 0.26 | 0.089 | 0.076 | – | 5.10 |
| 4 | 0.74 | 0.86 | 0.241 | 0.079 | 0.061 | 0.068 | 0.318 | 0.111 | 0.09 | 0.101 | 12.80 |
| 3 | 0.84 | 0.95 | 0.29 | 0.119 | 0.097 | 0.105 | 0.365 | 0.125 | 0.102 | 0.137 | 15.40 |
| 2 | 0.87 | 0.99 | 0.305 | 0.136 | 0.089 | 0.136 | 0.38 | 0.131 | 0.101 | 0.118 | 17.10 |
| 16 | 1.03 | 1.00 | 0.35 | 0.14 | 0.111 | 0.113 | 0.429 | 0.158 | 0.052 | 0.167 | 36.30 |
| 1 | 1.57 | 1.17 | – | – | – | – | – | 0.152 | 0.126 | – | 40.20 |
| 5 | 1.21 | 1.45 | 0.412 | 0.181 | 0.151 | 0.126 | 0.54 | 0.191 | 0.152 | 0.192 | 57.70 |
| Mean | 0.65 | 0.71 | 0.204 | 0.083 | 0.063 | 0.071 | 0.264 | 0.095 | 0.070 | 0.097 | 13.27 |
| Standard deviation | 0.39 | 0.35 | 0.101 | 0.046 | 0.038 | 0.036 | 0.126 | 0.048 | 0.038 | 0.046 | 17.70 |
A standard protocol for muscle architecture study was followed (e.g. Calow & Alexander, 1973; Sacks & Roy, 1982; Alexander & Ker, 1990; Payne et al. 2005; Smith et al. 2006): Muscle-tendon unit mass (kg) and resting length (m) from origin to insertion along the anatomical line-of-action were recorded. Where a significant external tendon was present it was dissected free and the mass (kg) and resting tendon length (m) from origin to insertion were recorded. The difference between the muscle-tendon unit mass and length and the tendon mass and length were taken to be muscle belly mass and length. Individual fascicles were revealed by sectioning the muscle bellies along the line-of-action, perpendicular to the internal tendon if present. The lengths (Lfasc, m) and angles of insertion (θ, in °) of a representative number of fascicles (n = 5–10) were recorded for each muscle belly (implicit in this method is the assumption made by almost all conventional studies that undisturbed muscle fascicles in severed muscles passively return to their in vivo resting lengths).
Muscle volume (Vmusc) was estimated by dividing muscle belly mass (Mmusc) by estimated vertebrate muscle density (ρmusc = 1.06 g cm−3, Mendez & Keys, 1960) (Eq. 1). Physiological cross-sectional area (PCSA) was estimated to be muscle volume divided by mean muscle fascicle length (Lfasc), multiplied by the cosine of mean muscle fascicle insertion angle (θ) (Eq. 2). Maximum isometric muscle force (Fmax) can be estimated by multiplying physiological cross-sectional area by the estimated maximum isometric stress of vertebrate skeletal muscle [σmax, equal to 300 kNm−2 (Wells, 1965; Woledge et al. 1985; Zajac, 1989; Medler, 2002); approximately equal to measured stress in crocodylian muscles (Seebacher & James, 2008)](Eq. 3).
| (1) |
| (2) |
| (3) |
.
Although Fmax is the more functionally relevant muscle property, its estimation via this method is probably only qualitatively accurate. Individual muscle fibre type populations, the ability of fibres to rotate during contraction (Azizi et al. 2008) and other factors all have large effects on the force a muscle is able to apply, and are all unaccounted for in this study. For this reason we will mostly discuss muscle PCSA below.
Linear regression
Ontogenetic scaling relationships of (non-normalized) muscle properties were analysed using reduced major axis regression (Model II) analysis (using past 1.94 b by Øyvind Hammer, http://folk.uio.no/ohammer/past) for each property vs. body mass. R2 correlation values and upper and lower bounds of the 95% confidence interval were calculated to assess the spread of data points around each regression line.
The scaling model by which we define isometric scaling (lack of change in muscle properties with ontogenetic increases in body mass) is geometric similarity. For two objects to be considered geometrically similar, all of their characteristic lengths must have the same relative proportions, so that transforming one into another involves simply multiplying all length dimensions by a single scaling factor (e.g. Schmidt-Nielsen, 1984). Because characteristic areas will be equal to length2, and volumes to length3, the scaling factor that equally transforms all lengths between two geometrically similar objects will equally transform all areas when squared, and all volumes when cubed.
We are using body mass, a volumetric property, as our predictive value in linear regression, and therefore we define geometric similarity using the inverse of this relationship. We consider muscle properties to scale with geometric similarity if characteristic lengths (i.e. fascicle lengths, tendon lengths) scale with body mass0.33, characteristic area properties (PCSA) scale with body mass0.67, and characteristic masses (muscle mass, tendon mass) scale with body mass1.0. As characteristic angles are not expected to change between geometrically similar objects, pennation angles will be considered isometric if they do not scale (i.e. scale to body mass0.0). Properties scaling with exponents of body mass higher than those for geometric similarity are referred to as displaying positive allometry, and those with exponents lower than geometric similarity as displaying negative allometry.
Results
The 38 pectoral limb and 40 pelvic limb muscles identified and analysed in this study are listed, along with abbreviations, in Tables 2 and 3. Muscle origins, insertions and paths were found to agree with previous descriptions of crocodylian anatomy (Romer, 1923a; Cong et al. 1998; Meers, 2003).
Table 2.
Average muscle properties in the pectoral limb of adult (10+ kg) Alligator mississippiensis.
| Muscle | Abbreviation | Functional group | Mmusc/Mbody(%) | SD Mmusc/Mbody(%) | Lfasc/Mbody0.33(%) | SD Lfasc/Mbody0.33(%) | θ (°) | SD θ (°) | Mtend/Mbody(%) | SD Mtend/Mbody(%) | Ltend/Mbody0.33(%) | SD Ltend/Mbody0.33(%) | PCSA/Mbody0.67(%) | SD PCSA/Mbody0.67(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhomboideus | RHO | Scapular adductors | 0.020 | 0.007 | 5.5 | 1.1 | 3 | 7 | – | – | – | – | 0.363 | 0.158 |
| Serratus ventralis cervicus | SVC | Scapular adductors | 0.050 | 0.007 | 12.3 | 1.3 | – | – | – | – | – | – | 0.384 | 0.062 |
| Serratus ventralis thoracis | SVT | Scapula flexors | 0.132 | 0.008 | 16.7 | 3.5 | 4 | 9 | – | – | – | – | 0.762 | 0.142 |
| Latissimus dorsi | LD | Scapular extensors | 0.082 | 0.004 | 21.0 | 2.1 | – | – | 0.00017 | 0.00041 | – | – | 0.369 | 0.038 |
| Levator scapulae | LS | Scapular extensors | 0.178 | 0.021 | 25.6 | 3.1 | – | – | – | – | – | – | 0.661 | 0.109 |
| Trapezius | TRA | Scapular extensors | 0.054 | 0.016 | 17.7 | 1.7 | – | – | – | – | – | – | 0.293 | 0.088 |
| Costocoracoideus profundus | COCP | Coracoidal retractors | 0.014 | 0.005 | 8.4 | 2.3 | 2 | 6 | – | – | – | – | 0.162 | 0.073 |
| Costocoracoideus superficialis | COCS | Coracoidal retractors | 0.074 | 0.010 | 18.9 | 2.5 | 1 | 3 | – | – | – | – | 0.378 | 0.088 |
| Deltoideus scapularis | DS | Shoulder abductors | 0.032 | 0.002 | 6.5 | 1.8 | 26 | 4 | 0.00049 | 0.00028 | 6.2 | 1.5 | 0.426 | 0.079 |
| Scapulohumeralis caudalis | SHC | Shoulder abductors | 0.027 | 0.005 | 11.0 | 2.2 | – | – | – | – | – | – | 0.236 | 0.046 |
| Teres major | TM | Shoulder abductors | 0.030 | 0.002 | 14.5 | 2.9 | 14 | 21 | 0.00057 | 0.00012 | 5.1 | 1.6 | 0.183 | 0.048 |
| Pectoralis | PEC | Shoulder flexors/adductors | 0.690 | 0.032 | 42.9 | 5.3 | – | – | – | – | – | – | 1.538 | 0.199 |
| Coracobrachialis brevis ventralis | CBV | Shoulder flexors/adductors | 0.052 | 0.007 | 15.3 | 2.4 | – | – | – | – | – | – | 0.328 | 0.079 |
| Subscapularis | SS | Shoulder flexors/adductors | 0.082 | 0.045 | 12.1 | 3.2 | 12 | 18 | – | – | – | – | 0.616 | 0.350 |
| Coracobrachialis brevis dorsalis | CBD | Shoulder extensors | 0.012 | 0.009 | 8.9 | 2.1 | – | – | – | – | – | – | 0.119 | 0.067 |
| Deltoideus clavicularis | DC | Shoulder extensors | 0.085 | 0.011 | 19.8 | 3.3 | – | – | – | – | – | – | 0.414 | 0.091 |
| Supracoracoideus | SC | Shoulder extensors | 0.094 | 0.014 | 17.1 | 1.4 | – | – | – | – | – | – | 0.520 | 0.072 |
| Abductor radialis | AB-R | Elbow flexors | 0.010 | 0.001 | 4.0 | 0.6 | 26 | 2 | 0.000005 | 0.000012 | 0.5 | 1.3 | 0.204 | 0.031 |
| Biceps brachii | BB | Elbow flexors | 0.049 | 0.004 | 18.3 | 3.9 | 9 | 11 | 0.00109 | 0.00018 | 11.8 | 1.1 | 0.253 | 0.033 |
| Brachialis | BR | Elbow flexors | 0.021 | 0.002 | 17.1 | 2.9 | – | – | – | – | – | – | 0.117 | 0.024 |
| Flexor ulnaris | FUL | Elbow flexors | 0.015 | 0.004 | 4.3 | 1.5 | 23 | 3 | 0.00021 | 0.00023 | 2.7 | 2.7 | 0.328 | 0.102 |
| Humeroradialis | HR | Elbow flexors | 0.033 | 0.009 | 18.6 | 1.5 | – | – | 0.00045 | 0.00055 | 3.9 | 3.5 | 0.166 | 0.046 |
| Triceps longus lateralus | TLL | Shoulder flexors/elbow extensors | 0.090 | 0.010 | 11.8 | 1.2 | 24 | 5 | 0.00100 | 0.00073 | 6.7 | 4.9 | 0.669 | 0.124 |
| Triceps longus medialis | TLM | Shoulder flexors/elbow extensors | 0.081 | 0.013 | 10.5 | 1.8 | 24 | 4 | 0.00289 | 0.00180 | 20.7 | 11.2 | 0.680 | 0.139 |
| Triceps brevis caudalis | TBCD | Elbow extensors | 0.053 | 0.009 | 9.5 | 1.5 | 25 | 2 | 0.00068 | 0.00068 | 4.9 | 4.7 | 0.486 | 0.098 |
| Triceps brevis cranialis | TBCR | Elbow extensors | 0.076 | 0.014 | 9.1 | 2.6 | 30 | 5 | 0.00623 | 0.00666 | 9.4 | 2.7 | 0.707 | 0.153 |
| Triceps brevis intermedius | TBI | Elbow extensors | 0.080 | 0.018 | 9.1 | 0.9 | 26 | 6 | 0.00416 | 0.00425 | 4.4 | 3.9 | 0.740 | 0.126 |
| Pronatorquadratus | PQ | Elbow pronators | 0.033 | 0.004 | 4.5 | 0.9 | 30 | 3 | – | – | – | – | 0.600 | 0.098 |
| Pronator teres | PT | Elbow pronators | 0.043 | 0.004 | 6.0 | 0.6 | 27 | 5 | 0.00004 | 0.00010 | 0.3 | 0.7 | 0.608 | 0.074 |
| Supinator | SUP | Elbow supinators | 0.024 | 0.003 | 10.7 | 1.8 | 25 | 2 | 0.00023 | 0.00056 | 1.6 | 3.9 | 0.199 | 0.055 |
| Extensor carpi radialis brevis | ECR-B | Wrist dorsiflexors | 0.012 | 0.002 | 6.0 | 0.9 | 20 | 11 | 0.00013 | 0.00020 | 1.9 | 3.6 | 0.178 | 0.030 |
| Extensor carpi radialis longus | ECR-L | Wrist dorsiflexors | 0.012 | 0.002 | 5.8 | 0.7 | 20 | 2 | 0.00063 | 0.00032 | 8.5 | 3.4 | 0.187 | 0.046 |
| Extensor carpi ulnaris longus | ECU-L | Wrist dorsiflexors | 0.011 | 0.001 | 8.3 | 1.4 | 15 | 8 | 0.00067 | 0.00018 | 15.5 | 1.2 | 0.120 | 0.026 |
| Flexor carpi ulnaris | FCU | Wrist plantarflexors | 0.035 | 0.004 | 3.8 | 0.8 | 29 | 4 | 0.00083 | 0.00054 | 6.1 | 4.5 | 0.799 | 0.176 |
| Flexor digitorum longus 1 (fore) | FDL1 | Wrist plantarflexors | 0.009 | 0.001 | 7.9 | 2.1 | 22 | 3 | 0.00139 | 0.00185 | 13.1 | 8.4 | 0.102 | 0.028 |
| Flexor digitorum longus 2 (fore) | FDL2 | Wrist plantarflexors | 0.025 | 0.005 | 4.3 | 0.7 | 25 | 3 | 0.00271 | 0.00254 | 10.4 | 8.6 | 0.505 | 0.050 |
| Extensor digitorum superficialis | EDS | Digital dorsiflexors | 0.016 | 0.003 | 4.3 | 1.3 | 22 | 12 | 0.00001 | 0.00003 | – | – | 0.328 | 0.087 |
| Flexor digitorum brevis (fore) | FDBF | Digital plantarflexors | 0.010 | 0.002 | 3.3 | 0.7 | 29 | 15 | – | – | – | – | 0.254 | 0.071 |
Table 3.
Average muscle properties in the pelvic limb of adult (10+ kg) Alligator mississippiensis.
| Muscle | Abbreviation | Functional group | Mmusc/Mbody(%) | SD Mmusc/Mbody(%) | Lfasc/Mbody0.33(%) | SD Lfasc/Mbody0.33(%) | θ (°) | SD θ (°) | Mtend/Mbody(%) | SD Mtend/Mbody(%) | Ltend/Mbody0.33(%) | SD Ltend/Mbody0.33(%) | PCSA/Mbody0.67(%) | SD PCSA/Mbody0.67(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caudofemoralisbrevis | CFB | Hip extensors/knee flexors | 0.136 | 0.018 | 26.2 | 2.1 | 4 | 6 | – | – | 1.4 | 3.5 | 0.487 | 0.042 |
| Caudofemoralislongus | CFL | Hip extensors/knee flexors | 1.674 | 0.146 | 65.9 | 7.0 | 20 | 23 | 0.00743 | 0.00126 | 26.8 | 8.0 | 2.084 | 0.478 |
| Flexor tibialisexternus | FTE | Hip extensors/knee flexors | 0.529 | 0.788 | 38.4 | 2.7 | 8 | 19 | 0.00563 | 0.00268 | 30.8 | 13.2 | 1.272 | 1.953 |
| Flexor tibialisinternus 1 | FTI1 | Hip extensors/knee flexors | 0.024 | 0.004 | 35.6 | 3.1 | – | – | 0.00002 | 0.00003 | 0.9 | 1.4 | 0.063 | 0.013 |
| Flexor tibialisinternus 2 | FTI2 | Hip extensors/knee flexors | 0.136 | 0.004 | 44.6 | 11.8 | 2 | 5 | 0.00075 | 0.00045 | 4.2 | 2.8 | 0.311 | 0.119 |
| Flexor tibialisinternus 3 | FTI3 | Hip extensors/knee flexors | 0.069 | 0.004 | 19.9 | 1.8 | 13 | 11 | 0.00043 | 0.00030 | 5.3 | 4.4 | 0.318 | 0.041 |
| Flexor tibialisinternus 4 | FTI4 | Hip extensors/knee flexors | 0.034 | 0.004 | 32.4 | 4.6 | – | – | 0.00084 | 0.00031 | 10.2 | 4.6 | 0.102 | 0.019 |
| Iliofibularis | ILFB | Hip extensors/knee flexors | 0.055 | 0.006 | 35.5 | 5.3 | – | – | 0.00056 | 0.00040 | 10.4 | 1.6 | 0.151 | 0.038 |
| Ischiotrochantericus | ISTR | Hip extensors/knee flexors | 0.033 | 0.016 | 7.9 | 1.0 | 33 | 8 | 0.00201 | 0.00148 | 6.5 | 3.1 | 0.329 | 0.153 |
| Pubo-ischio-tibialis | PIT | Hip extensors/knee flexors | 0.061 | 0.003 | 36.9 | 5.8 | 5 | 7 | 0.00032 | 0.00035 | 2.4 | 2.3 | 0.166 | 0.031 |
| Pubo-ischio-femoralis externus 1 | PIFE1 | Hip flexors | 0.114 | 0.015 | 29.3 | 7.9 | 5 | 11 | 0.00084 | 0.00039 | 6.7 | 3.0 | 0.383 | 0.116 |
| Pubo-ischio-femoralis internus 1 | PIFI1 | Hip flexors | 0.120 | 0.009 | 25.2 | 5.0 | – | – | – | – | – | – | 0.466 | 0.107 |
| Pubo-ischio-femoralis internus 2 | PIFI2 | Hip flexors | 0.500 | 0.063 | 39.0 | 3.4 | – | – | 0.00308 | 0.00091 | 12.1 | 5.1 | 1.218 | 0.201 |
| Iliofemoralis | IF | Hip abductors | 0.095 | 0.006 | 16.3 | 2.2 | 15 | 15 | – | – | – | – | 0.518 | 0.059 |
| Adductor 1 | ADD1 | Hip adductors | 0.098 | 0.018 | 36.6 | 4.4 | – | – | – | – | – | – | 0.261 | 0.077 |
| Adductor 2 | ADD2 | Hip adductors | 0.046 | 0.005 | 40.3 | 5.1 | – | – | 0.00037 | 0.00091 | 0.3 | 0.8 | 0.108 | 0.018 |
| Pubo-ischio-femoralis externus 2 | PIFE2 | Hip adductors | 0.114 | 0.015 | 18.1 | 3.1 | 24 | 15 | 0.00048 | 0.00018 | 4.1 | 2.1 | 0.537 | 0.107 |
| Pubo-ischio-femoralis externus 3 | PIFE3 | Hip adductors | 0.081 | 0.013 | 13.5 | 1.1 | 21 | 12 | 0.00018 | 0.00037 | 1.2 | 2.0 | 0.520 | 0.097 |
| Ambiens 1 | AMB1 | Knee extensors | 0.093 | 0.005 | 19.1 | 2.6 | 8 | 11 | 0.00284 | 0.00038 | 25.5 | 9.0 | 0.449 | 0.054 |
| Ambiens 2 | AMB2 | Knee extensors | 0.015 | 0.006 | 14.5 | 6.3 | 9 | 11 | 0.00041 | 0.00032 | 4.9 | 6.5 | 0.110 | 0.063 |
| Femorotibialisexternus | FMTE | Knee extensors | 0.060 | 0.006 | 8.7 | 0.6 | 30 | 2 | 0.00063 | 0.00059 | 3.8 | 3.5 | 0.561 | 0.086 |
| Femorotibialisinternus | FMTI | Knee extensors | 0.193 | 0.017 | 10.9 | 1.2 | 33 | 5 | 0.01007 | 0.00344 | 7.6 | 2.6 | 1.401 | 0.146 |
| Iliotibialis 1 | IT1 | Knee extensors | 0.028 | 0.003 | 22.9 | 2.5 | – | – | – | – | – | – | 0.119 | 0.025 |
| Iliotibialis 2 | IT2 | Knee extensors | 0.187 | 0.008 | 15.4 | 2.2 | 30 | 4 | 0.00323 | 0.00115 | 18.0 | 7.4 | 1.005 | 0.138 |
| Iliotibialis 3 | IT3 | Knee extensors | 0.053 | 0.006 | 16.0 | 6.8 | 22 | 2 | 0.00148 | 0.00038 | 18.2 | 16.7 | 0.321 | 0.112 |
| Extensor digitorumlongus | EDL | Ankle dorsiflexors | 0.074 | 0.008 | 17.9 | 4.6 | 20 | 10 | 0.00095 | 0.00026 | 10.1 | 1.2 | 0.385 | 0.100 |
| Tibialis anterior | TA | Ankle dorsiflexors | 0.048 | 0.006 | 28.1 | 2.3 | – | – | 0.00024 | 0.00027 | 2.4 | 2.6 | 0.161 | 0.019 |
| Fibularis brevis | FB | Ankle plantarflexors | 0.014 | 0.001 | 6.1 | 1.1 | 25 | 6 | 0.00024 | 0.00025 | 1.4 | 1.6 | 0.200 | 0.040 |
| Flexor digitorumlongus (hind) | FDLH | Ankle plantarflexors | 0.062 | 0.008 | 7.6 | 1.2 | 26 | 4 | 0.00762 | 0.00414 | 34.6 | 4.1 | 0.696 | 0.091 |
| Flexor hallucislongus | FHL | Ankle plantarflexors | 0.019 | 0.003 | 7.3 | 1.6 | 23 | 1 | 0.00318 | 0.00196 | 18.4 | 5.2 | 0.234 | 0.030 |
| Fibularis longus | FL | Ankle plantarflexors | 0.032 | 0.003 | 6.8 | 1.7 | 28 | 2 | 0.00086 | 0.00044 | 4.2 | 2.1 | 0.416 | 0.115 |
| Gastrocnemiusexternus | GE | Ankle plantarflexors | 0.156 | 0.020 | 10.0 | 1.7 | 29 | 6 | 0.01249 | 0.01600 | 14.4 | 15.0 | 1.301 | 0.184 |
| Gastrocnemiusinternus | GI | Ankle plantarflexors | 0.063 | 0.006 | 26.7 | 2.1 | 9 | 10 | 0.00113 | 0.00117 | 5.5 | 4.0 | 0.218 | 0.025 |
| Interosseus cruri | IC | Ankle plantarflexors | 0.056 | 0.004 | 6.8 | 2.2 | 29 | 10 | 0.00254 | 0.00067 | 13.7 | 3.4 | 0.709 | 0.166 |
| Pronator profundus | PP | Ankle plantarflexors | 0.012 | 0.003 | 5.5 | 0.8 | 23 | 14 | – | – | – | – | 0.189 | 0.027 |
| Extensor digitorumbrevis | EDB | Digital dorsiflexors | 0.033 | 0.012 | 6.4 | 1.4 | 29 | 11 | 0.00177 | 0.00231 | 3.6 | 5.1 | 0.445 | 0.218 |
| Extensor hallucisbrevis | EHB | Digital dorsiflexors | 0.009 | 0.002 | 8.4 | 1.4 | 16 | 13 | 0.00026 | 0.00030 | 2.1 | 1.7 | 0.098 | 0.015 |
| Extensor hallucislongus | EHL | Digital dorsiflexors | 0.071 | 0.012 | 3.6 | 0.6 | 36 | 11 | 0.00150 | 0.00068 | 5.0 | 5.2 | 1.527 | 0.546 |
| Flexor digitorumbrevis (hind) | FDBH | Digital plantarflexors | 0.013 | 0.002 | 6.4 | 1.2 | 27 | 6 | 0.00048 | 0.00023 | 5.5 | 2.9 | 0.177 | 0.029 |
| Flexor hallucisbrevis | FHB | Digital plantarflexors | 0.018 | 0.001 | 3.5 | 0.5 | 24 | 13 | 0.00152 | 0.00124 | 4.2 | 3.7 | 0.436 | 0.085 |
Average muscle properties
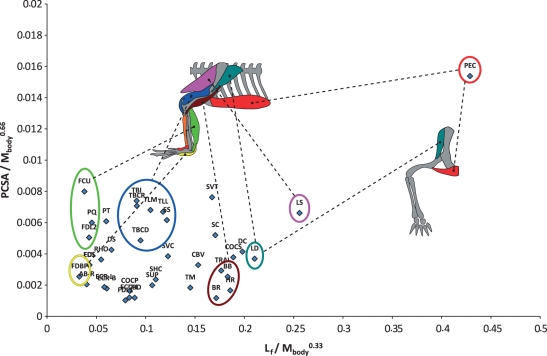
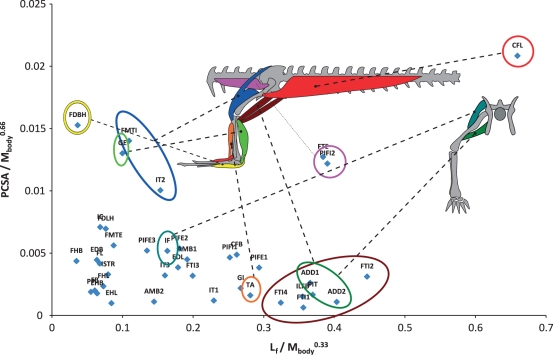
To estimate the distribution of muscle properties in the pectoral and pelvic limbs of a ‘typical’ alligator we used mean data from the five adult specimens (10+ kg body mass). Data were normalized as follows: lengths (fascicle, tendon) were normalized to body mass0.33, areas (PCSA) to body mass0.67, and masses (muscle, tendon) to body mass1.0. Mean normalized data for adult specimens are shown with standard deviations in Tables 2 and 3. Mean fascicle lengths are plotted against mean PCSAs in Figs 1 and 2. Because these properties relate directly to the working range of a muscle and the force it is capable of applying, plotting them reveals a muscle ‘function space’ that allows simple estimation of relative muscle function (e.g. Payne et al. 2005; Sharir et al. 2006). Ranges referred to below (±) are one SD for mean values.
Fig. 1.
Function space plot (fascicle length vs. PCSA, normalized by appropriate exponents of body mass) for muscles of the pectoral limb of Alligator mississippiensis. Schematic anatomy of left pectoral limb is shown in left-lateral view on left, cranial view on right.
Fig. 2.
Function space plot (fascicle length vs. PCSA, normalized by appropriate exponents of body mass) for muscles of the pelvic limb of Alligator mississippiensis. Schematic anatomy of left pelvic limb is shown in left-lateral view on left, cranial view on right.
Mean distribution of muscle mass
As seen in previous studies of limb muscle architecture in cursorial mammals and birds (Alexander, 1977; Alexander et al. 1981; Gans & De Vree, 1987; Gaunt & Gans, 1990; Roberts, 2001; Payne et al. 2005; Smith et al. 2006; Williams et al. 2008a,b;), a proximal-distal gradient in muscle mass was observed for both the pectoral and pelvic limbs. The proximal extrinsic muscles were found to be heaviest, e.g. pectoralis (PEC, 0.69 ± 0.032% of body mass) and caudofemoralis longus (CFL, 1.674 ± 0.146% of body mass). These give way to successively lighter muscles distally; e.g. triceps brachii’s radial head (TBCR, 0.076 ± 0.014% body mass) and femorotibialis internus (FMTI, 0.193 ± 0.017% body mass) on the humeral and femoral segment, respectively, flexor carpi ulnaris (FCU, 0.035 ± 0.004% body mass) and gastrocnemius externus (GE, 0.156 ± 0.02% body mass) on the ulnar and tibial segment, with the muscles of the podial segments being the lightest [e.g. the flexor digitalis brevis of the pectoral (FDBF, 0.01 ± 0.002% body mass) and pelvic limbs (FDBH, 0.08 ± 0.01% body mass)].
Mean distribution of fascicle length, pennation angle and PCSA
Mean fascicle lengths (normalized to body mass0.33), PCSAs (normalized to body weight0.67) and mean pennation angles are displayed in Tables 1 and 2, and Figs 1 and 2. Global mean fascicle lengths in the pelvic limb (∼20% body mass0.33) were nearly twice those in the pectoral limb (∼11% body mass0.33). Global mean PCSAs were also somewhat larger in the pelvic limb (mean 0.511% body mass0.67) than in the pectoral limb (mean 0.421% body mass0.67). Global mean pennation angle was similar (∼15°).
A pattern of proximal-to-distal gradation of architectural properties was observed similar to that previously found in cursorial birds and mammals (see References above). Proximal muscles were found to have generally longer fascicles arranged at lower angles of pennation and small PCSAs, graduating distally towards muscles with larger PCSA and shorter fascicles arranged at higher angles of pennation (Figs 1 and 2). Several massive extrinsic muscles in both the pectoral and pelvic limbs exceed this pattern, possessing long fascicles and high PCSAs. In the pectoral limb this was most notable in the levator scapulae (LS) and pectoralis (PEC, PCSA = 1.538 ± 0.199% body mass0.67, fascicle length = 42.9 ± 5.3% body mass0.33). In the pelvic limb the second head of pubo-ischio-femoralis internus (PIFI2) and caudofemoralis longus (CFL, PCSA = 2.084 ± 0.478% body mass0.67, fascicle length = 65.9 ± 7% body mass0.33) occupy similar regions of function space.
Mean distribution of external tendons, lengths and masses
Mean external tendon lengths (normalized to body mass0.33) and masses (normalized to body mass1.0) are displayed in Tables 2 and 3. External tendons were generally found to be more prevalent in the pelvic limb than in the pectoral limb (33/40 muscles with tendons vs. 16/39). On average they were also longer (7.3% body mass 0.33 vs. 2.9%) and more massive (0.00167% body mass vs. 0.00061%) in the pelvic limb.
The large, extrinsic muscles of the pectoral limb were found to lack the substantial external tendon of their rough pelvic limb functional analogues. The pectoralis lacks an identifiable tendon, whereas the primary and secondary insertion tendons of the caudofemoral muscles sum to give the muscle the longest and most massive tendon analysed (CFL, tendon length = 29 ± 5.4% body mass 0.33, tendon mass = 0.00644 ± 0.0018% body mass). Although substantial tendons (around 0.006% of body mass in the pectoral limb and around 0.01% in the pelvic limb) pass nearly every joint in both the pectoral and pelvic limbs, there were no clear proximal-distal patterns of tendon length or mass observed.
Scaling regression analysis
The slopes of the reduced major axis regression lines for muscle properties vs. body mass are shown in Tables 4 and 5 with R2 and 95% confidence interval values, and Figs 3 and 4. Ranges in slope referred to below are the upper and lower bounds of the 95% confidence intervals (CIs) for regression slopes.
Table 4.
Results of RMA linear regression of muscle properties on body mass in the pectoral limb of Alligator mississippiensis.
| Mmusc vs. Mbody |
Lfasc vs. Mbody |
PCSA vs. Mbody |
θ vs. Mbody |
Mtend vs. Mbody |
Ltend vs. Mbody |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle | Abbreviation | Slope | R2 | Lower 95% CI | Upper 95% CI | Slope | R2 | Lower 95% CI | Upper 95% CI | Slope | R2 | Lower 95% CI | Upper 95% CI | Slope | R2 | Lower 95% CI | Upper 95% CI | Slope | R2 | Lower 95% CI | Upper 95% CI | Slope | R2 | Lower 95% CI | Upper 95% CI |
| Rhomboideus | RHO | 1.119 | 0.966 | 1.014 | 1.172 | 0.401 | 0.808 | 0.314 | 0.485 | 0.793 | 0.866 | 0.671 | 0.913 | – | – | – | – | – | – | – | – | – | – | – | – |
| Serratus ventralis cervicus | SVC | 1.161 | 0.993 | 1.113 | 1.215 | 0.444 | 0.967 | 0.409 | 0.481 | 0.728 | 0.981 | 0.675 | 0.784 | – | – | – | – | – | – | – | – | – | – | – | – |
| Serratus ventralis thoracis | SVT | 1.099 | 0.997 | 1.072 | 1.127 | 0.356 | 0.913 | 0.295 | 0.404 | 0.766 | 0.970 | 0.699 | 0.834 | – | – | – | – | ||||||||
| Latissimus dorsi | LD | 1.045 | 0.997 | 1.024 | 1.069 | 0.301 | 0.974 | 0.274 | 0.326 | 0.751 | 0.989 | 0.718 | 0.787 | – | – | – | – | – | – | – | – | – | – | – | – |
| Levator scapulae | LS | 0.997 | 0.994 | 0.955 | 1.039 | 0.352 | 0.961 | 0.312 | 0.398 | 0.656 | 0.979 | 0.609 | 0.713 | – | – | – | – | – | – | – | – | – | – | – | – |
| Trapezius | TRA | 1.011 | 0.956 | 0.890 | 1.142 | 0.386 | 0.100 | 0.316 | 0.450 | 0.649 | 0.914 | 0.556 | 0.748 | – | – | – | – | – | – | – | – | – | – | – | – |
| Costocoracoideus profundus | COCP | 1.061 | 0.959 | 0.929 | 1.172 | 0.480 | 0.800 | 0.353 | 0.592 | 0.662 | 0.845 | 0.477 | 0.839 | – | – | – | – | – | – | – | – | – | – | – | – |
| Costocoracoideus superficialis | COCS | 1.048 | 0.989 | 0.998 | 1.099 | 0.386 | 0.941 | 0.336 | 0.437 | 0.686 | 0.950 | 0.609 | 0.766 | – | – | – | – | – | – | – | – | – | – | – | – |
| Deltoideus scapularis | DS | 1.039 | 0.993 | 0.996 | 1.091 | 0.492 | 0.898 | 0.412 | 0.568 | 0.603 | 0.957 | 0.531 | 0.678 | −0.067 | 0.312 | −0.126 | −0.008 | 0.949 | 0.965 | 0.836 | 1.062 | 0.474 | 0.867 | 0.365 | 0.583 |
| Scapulohumeralis caudalis | SHC | 1.022 | 0.987 | 0.949 | 1.079 | 0.372 | 0.894 | 0.310 | 0.434 | 0.677 | 0.960 | 0.604 | 0.746 | – | – | – | – | – | – | – | – | – | – | – | – |
| Teres major | TM | 1.055 | 0.993 | 1.019 | 1.097 | 0.355 | 0.889 | 0.280 | 0.427 | 0.705 | 0.963 | 0.636 | 0.776 | – | – | – | – | 0.895 | 0.948 | 0.766 | 1.024 | 0.410 | 0.868 | 0.312 | 0.509 |
| Pectoralis | PEC | 1.012 | 0.996 | 0.982 | 1.040 | 0.329 | 0.923 | 0.286 | 0.377 | 0.703 | 0.975 | 0.656 | 0.754 | – | – | – | – | – | – | – | – | – | – | – | – |
| Coracobrachialis brevis ventralis | CBV | 1.114 | 0.961 | 1.047 | 1.235 | 0.465 | 0.947 | 0.406 | 0.520 | 0.672 | 0.907 | 0.579 | 0.789 | – | – | – | – | – | – | – | – | – | – | – | – |
| Subscapularis | SS | 1.140 | 0.970 | 1.060 | 1.254 | 0.377 | 0.918 | 0.298 | 0.435 | 0.772 | 0.928 | 0.675 | 0.918 | – | – | – | – | – | – | – | – | – | – | – | – |
| Coracobrachialis brevis dorsalis | CBD | 1.314 | 0.903 | 1.101 | 1.558 | 0.497 | 0.911 | 0.406 | 0.591 | 0.895 | 0.748 | 0.625 | 1.165 | – | – | – | – | – | – | – | – | – | – | – | – |
| Deltoideus clavicularis | DC | 1.033 | 0.996 | 0.999 | 1.061 | 0.374 | 0.971 | 0.332 | 0.410 | 0.670 | 0.977 | 0.625 | 0.711 | – | – | – | – | – | – | – | – | – | – | – | – |
| Supracoracoideus | SC | 1.081 | 0.992 | 1.027 | 1.132 | 0.361 | 0.977 | 0.341 | 0.383 | 0.727 | 0.981 | 0.676 | 0.780 | – | – | – | – | – | – | – | – | – | – | – | – |
| Abductor radialis | AB-R | 1.066 | 0.993 | 1.014 | 1.123 | 0.427 | 0.846 | 0.341 | 0.506 | 0.694 | 0.955 | 0.617 | 0.779 | −0.028 | 0.037 | −0.115 | 0.059 | – | – | – | – | – | – | – | – |
| Biceps brachii | BB | 1.014 | 0.993 | 0.969 | 1.050 | 0.304 | 0.829 | 0.226 | 0.373 | 0.739 | 0.969 | 0.653 | 0.808 | −0.272 | 0.344 | −0.890 | 0.347 | 0.944 | 0.842 | 0.713 | 1.174 | 0.399 | 0.938 | 0.338 | 0.460 |
| Brachialis | BR | 0.955 | 0.985 | 0.897 | 1.032 | 0.341 | 0.941 | 0.289 | 0.378 | 0.626 | 0.971 | 0.570 | 0.696 | – | – | – | – | – | – | – | – | – | – | – | – |
| Flexor ulnaris | FUL | 1.133 | 0.990 | 1.063 | 1.203 | 0.415 | 0.832 | 0.332 | 0.489 | 0.796 | 0.954 | 0.720 | 0.899 | −0.103 | 0.516 | −0.162 | −0.044 | 0.892 | 0.870 | 0.290 | 1.495 | 0.230 | 0.405 | −0.250 | 0.711 |
| Humeroradialis | HR | 0.945 | 0.978 | 0.881 | 1.017 | 0.317 | 0.977 | 0.293 | 0.347 | 0.637 | 0.951 | 0.565 | 0.706 | – | – | – | – | 1.039 | 0.625 | −0.192 | 2.269 | 0.651 | 0.828 | 0.132 | 1.171 |
| Triceps longus lateralus | TLL | 1.016 | 0.994 | 0.982 | 1.053 | 0.389 | 0.903 | 0.311 | 0.467 | 0.671 | 0.968 | 0.590 | 0.738 | −0.071 | 0.288 | −0.136 | −0.005 | 0.978 | 0.820 | 0.597 | 1.358 | 0.232 | 0.148 | −0.172 | 0.636 |
| Triceps longus medialis | TLM | 1.052 | 0.993 | 0.999 | 1.097 | 0.461 | 0.952 | 0.417 | 0.516 | 0.630 | 0.941 | 0.559 | 0.689 | −0.057 | 0.222 | −0.121 | 0.006 | 0.988 | 0.884 | 0.783 | 1.194 | 0.416 | 0.691 | 0.258 | 0.573 |
| Triceps brevis caudalis | TBCD | 1.114 | 0.990 | 1.066 | 1.161 | 0.407 | 0.873 | 0.332 | 0.480 | 0.771 | 0.944 | 0.687 | 0.848 | −0.065 | 0.361 | −0.117 | −0.014 | 0.971 | 0.800 | 0.351 | 1.590 | 0.193 | 0.239 | −0.241 | 0.627 |
| Triceps brevis cranialis | TBCR | 1.061 | 0.994 | 1.014 | 1.093 | 0.396 | 0.900 | 0.326 | 0.459 | 0.709 | 0.959 | 0.654 | 0.777 | −0.040 | 0.182 | −0.090 | 0.010 | 1.258 | 0.411 | 0.576 | 1.940 | 0.390 | 0.839 | 0.279 | 0.501 |
| Triceps brevis intermedius | TBI | 1.186 | 0.990 | 1.128 | 1.247 | 0.445 | 0.972 | 0.412 | 0.486 | 0.765 | 0.978 | 0.718 | 0.804 | −0.042 | 0.034 | −0.168 | 0.085 | 1.817 | 0.624 | 0.250 | 3.383 | 0.284 | 0.414 | −0.145 | 0.714 |
| Pronator quadratus | PQ | 1.102 | 0.996 | 1.068 | 1.132 | 0.418 | 0.937 | 0.351 | 0.474 | 0.715 | 0.964 | 0.647 | 0.792 | −0.025 | 0.124 | −0.064 | 0.014 | – | – | – | – | – | – | – | – |
| Pronator teres | PT | 1.126 | 0.984 | 1.049 | 1.228 | 0.501 | 0.973 | 0.457 | 0.552 | 0.660 | 0.920 | 0.542 | 0.778 | −0.036 | 0.131 | −0.091 | 0.019 | – | – | – | – | – | – | – | – |
| Supinator | SUP | 1.049 | 0.994 | 1.016 | 1.091 | 0.409 | 0.921 | 0.347 | 0.459 | 0.668 | 0.954 | 0.611 | 0.743 | 0.018 | 0.035 | −0.038 | 0.073 | – | – | – | – | – | – | – | – |
| Extensor carpi radialis brevis | ECR-B | 1.056 | 0.989 | 1.003 | 1.116 | 0.386 | 0.951 | 0.349 | 0.431 | 0.706 | 0.964 | 0.646 | 0.781 | −0.063 | 0.220 | −0.136 | 0.011 | 0.863 | 0.981 | 0.500 | 1.226 | 0.396 | 0.594 | −0.525 | 1.316 |
| Extensor carpi radialis longus | ECR-L | 1.021 | 0.991 | 0.968 | 1.069 | 0.378 | 0.851 | 0.289 | 0.454 | 0.704 | 0.931 | 0.609 | 0.791 | −0.041 | 0.070 | −0.130 | 0.047 | 1.134 | 0.812 | 0.725 | 1.543 | 0.584 | 0.789 | 0.351 | 0.817 |
| Extensor carpi ulnaris longus | ECU-L | 0.995 | 0.993 | 0.951 | 1.034 | 0.430 | 0.878 | 0.343 | 0.504 | 0.621 | 0.958 | 0.548 | 0.698 | −0.100 | 0.556 | −0.156 | −0.044 | 0.952 | 0.946 | 0.805 | 1.099 | 0.360 | 0.762 | 0.231 | 0.489 |
| Flexor carpi ulnaris | FCU | 1.048 | 0.990 | 0.999 | 1.107 | 0.391 | 0.916 | 0.328 | 0.453 | 0.676 | 0.977 | 0.621 | 0.746 | 0.000 | 0.000 | −0.039 | 0.039 | 0.833 | 0.879 | 0.647 | 1.019 | 0.333 | 0.549 | 0.156 | 0.510 |
| Flexor digitorum longus 1 (fore) | FDL1 | 1.004 | 0.953 | 0.873 | 1.104 | 0.503 | 0.893 | 0.430 | 0.579 | 0.567 | 0.808 | 0.433 | 0.681 | −0.027 | 0.042 | −0.104 | 0.049 | 1.044 | 0.312 | 0.462 | 1.626 | 0.368 | 0.347 | 0.112 | 0.625 |
| Flexor digitorum longus 2 (fore) | FDL2 | 1.077 | 0.991 | 1.017 | 1.135 | 0.379 | 0.854 | 0.293 | 0.473 | 0.747 | 0.964 | 0.666 | 0.800 | −0.049 | 0.457 | −0.081 | −0.017 | 1.019 | 0.905 | 0.759 | 1.278 | 0.289 | 0.788 | 0.158 | 0.421 |
| Extensor digitorum superficialis | EDS | 1.246 | 0.979 | 1.145 | 1.373 | 0.324 | 0.700 | 0.234 | 0.430 | 0.992 | 0.966 | 0.864 | 1.122 | −0.017 | 0.005 | −0.173 | 0.139 | – | – | – | – | – | – | – | – |
| Flexor digitorum brevis (fore) | FDBF | 1.049 | 0.984 | 0.968 | 1.117 | 0.298 | 0.822 | 0.231 | 0.369 | 0.822 | 0.960 | 0.730 | 0.899 | −0.052 | 0.246 | −0.108 | 0.005 | – | – | – | – | – | – | – | – |
Table 5.
Results of RMA linear regression of muscle properties on body mass in the pelvic limb of Alligator mississippiensis.
| Mmusc vs. Mbody |
Lfasc vs. Mbody |
PCSA vs. Mbody |
θ vs. Mbody |
Mtend vs. Mbody |
Ltend vs. Mbody |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle | Abbreviation | Slope | R2 | Lower 95% CI | Upper 95% CI | Slope | R2 | Lower 95% CI | Upper 95% CI | Slope | R2 | Lower 95% CI | Upper 95% CI | Slope | R2 | Lower 95% CI | Upper 95% CI | Slope | R2 | Lower 95% CI | Upper 95% CI | Slope | R2 | Lower 95% CI | Upper 95% CI |
| Caudofemoralis brevis | CFB | 1.104 | 0.998 | 1.080 | 1.123 | 0.465 | 0.985 | 0.430 | 0.495 | 0.644 | 0.990 | 0.607 | 0.683 | 0.006 | 0.025 | −0.106 | 0.118 | – | – | – | – | – | – | – | – |
| Caudofemoralis longus | CFL | 1.023 | 0.998 | 0.997 | 1.062 | 0.438 | 0.966 | 0.402 | 0.476 | 0.594 | 0.959 | 0.548 | 0.663 | 0.071 | 0.205 | −0.033 | 0.175 | 1.132 | 0.981 | 1.038 | 1.227 | 0.289 | 0.785 | 0.200 | 0.378 |
| Flexor tibialis externus | FTE | 1.214 | 0.925 | 0.998 | 1.485 | 0.335 | 0.978 | 0.303 | 0.367 | 0.887 | 0.845 | 0.633 | 1.172 | – | – | – | – | 1.018 | 0.913 | 0.836 | 1.200 | 0.278 | 0.668 | 0.164 | 0.391 |
| Flexor tibialis internus 1 | FTI1 | 0.996 | 0.987 | 0.933 | 1.065 | 0.345 | 0.959 | 0.313 | 0.381 | 0.663 | 0.967 | 0.590 | 0.739 | – | – | – | – | −0.118 | 0.034 | −6.281 | 6.045 | 0.178 | 0.576 | −1.718 | 2.073 |
| Flexor tibialis internus 2 | FTI2 | 1.018 | 0.997 | 0.992 | 1.045 | 0.363 | 0.861 | 0.322 | 0.403 | 0.695 | 0.956 | 0.632 | 0.784 | – | – | – | – | 1.030 | 0.943 | 0.782 | 1.277 | 0.476 | 0.694 | 0.181 | 0.770 |
| Flexor tibialis internus 3 | FTI3 | 1.030 | 0.993 | 0.990 | 1.079 | 0.252 | 0.849 | 0.205 | 0.293 | 0.805 | 0.960 | 0.717 | 0.902 | 0.039 | 0.023 | −0.199 | 0.277 | 0.497 | 0.506 | 0.176 | 0.818 | 0.188 | 0.142 | −0.109 | 0.485 |
| Flexor tibialis internus 4 | FTI4 | 0.991 | 0.987 | 0.944 | 1.046 | 0.367 | 0.950 | 0.326 | 0.409 | 0.652 | 0.943 | 0.591 | 0.734 | – | – | – | – | 1.058 | 0.839 | 0.772 | 1.345 | 0.339 | 0.523 | 0.151 | 0.527 |
| Iliofibularis | ILFB | 1.010 | 0.992 | 0.957 | 1.058 | 0.338 | 0.912 | 0.272 | 0.390 | 0.701 | 0.952 | 0.606 | 0.791 | – | – | – | – | 0.849 | 0.941 | 0.710 | 0.988 | 0.247 | 0.766 | 0.158 | 0.337 |
| Ischiotrochantericus | ISTR | 0.941 | 0.865 | 0.741 | 1.077 | 0.505 | 0.894 | 0.417 | 0.580 | 0.555 | 0.555 | 0.433 | 0.700 | −0.037 | 0.088 | −0.109 | 0.034 | 1.121 | 0.879 | 0.799 | 1.443 | 0.519 | 0.673 | 0.285 | 0.754 |
| Pubo-ischio-tibialis | PIT | 1.015 | 0.997 | 0.981 | 1.046 | 0.360 | 0.956 | 0.318 | 0.397 | 0.681 | 0.969 | 0.615 | 0.747 | −0.013 | 0.062 | −0.647 | 0.622 | 0.686 | 0.760 | 0.275 | 1.097 | 0.111 | 0.109 | −0.223 | 0.444 |
| Pubo-ischio-femoralis externus 1 | PIFE1 | 1.056 | 0.993 | 1.005 | 1.113 | 0.375 | 0.898 | 0.290 | 0.446 | 0.719 | 0.944 | 0.615 | 0.817 | – | – | – | – | 1.096 | 0.772 | 0.717 | 1.475 | 0.405 | 0.701 | 0.238 | 0.571 |
| Pubo-ischio-femoralis internus 1 | PIFI1 | 1.012 | 0.997 | 0.985 | 1.044 | 0.409 | 0.894 | 0.330 | 0.481 | 0.637 | 0.960 | 0.553 | 0.707 | – | – | – | – | – | – | – | – | – | – | – | – |
| Pubo-ischio-femoralis internus 2 | PIFI2 | 1.019 | 0.997 | 0.986 | 1.062 | 0.355 | 0.942 | 0.294 | 0.401 | 0.680 | 0.977 | 0.625 | 0.748 | – | – | – | – | 1.064 | 0.962 | 0.925 | 1.202 | 0.452 | 0.796 | 0.297 | 0.608 |
| Iliofemoralis | IF | 1.034 | 0.999 | 1.015 | 1.049 | 0.504 | 0.903 | 0.401 | 0.607 | 0.569 | 0.948 | 0.497 | 0.629 | 0.018 | 0.003 | −0.185 | 0.222 | – | – | – | – | – | – | – | – |
| Adductor 1 | ADD1 | 0.984 | 0.993 | 0.943 | 1.019 | 0.368 | 0.956 | 0.336 | 0.404 | 0.634 | 0.960 | 0.583 | 0.689 | – | – | – | – | – | – | – | – | – | – | – | – |
| Adductor 2 | ADD2 | 1.044 | 0.995 | 1.005 | 1.081 | 0.415 | 0.953 | 0.363 | 0.463 | 0.649 | 0.962 | 0.584 | 0.714 | – | – | – | – | – | – | – | – | – | – | – | – |
| Pubo-ischio-femoralis externus 2 | PIFE2 | 1.026 | 0.996 | 0.997 | 1.066 | 0.374 | 0.906 | 0.294 | 0.429 | 0.672 | 0.974 | 0.621 | 0.743 | 0.033 | 0.020 | −0.120 | 0.185 | 0.853 | 0.672 | 0.486 | 1.219 | 0.235 | 0.161 | −0.097 | 0.567 |
| Pubo-ischio-femoralis externus 3 | PIFE3 | 1.027 | 0.991 | 0.980 | 1.071 | 0.411 | 0.930 | 0.368 | 0.460 | 0.659 | 0.972 | 0.616 | 0.713 | −0.082 | 0.293 | −0.161 | −0.003 | 1.122 | 0.744 | −0.653 | 2.897 | 0.404 | 0.803 | −0.186 | 0.995 |
| Ambiens 1 | AMB1 | 0.973 | 0.995 | 0.944 | 1.001 | 0.322 | 0.880 | 0.253 | 0.411 | 0.688 | 0.959 | 0.611 | 0.743 | −0.209 | 0.564 | −0.536 | 0.118 | 0.958 | 0.963 | 0.847 | 1.070 | 0.231 | 0.565 | 0.108 | 0.354 |
| Ambiens 2 | AMB2 | 0.939 | 0.956 | 0.825 | 1.072 | 0.416 | 0.665 | 0.260 | 0.557 | 0.641 | 0.567 | 0.428 | 0.913 | 0.152 | 0.259 | −0.127 | 0.430 | 0.892 | 0.821 | 0.619 | 1.166 | −0.007 | 0.000 | −0.336 | 0.323 |
| Femorotibialis externus | FMTE | 0.956 | 0.993 | 0.920 | 1.000 | 0.345 | 0.956 | 0.312 | 0.390 | 0.728 | 0.990 | 0.682 | 0.762 | −0.042 | 0.135 | −0.104 | 0.021 | 0.905 | 0.857 | 0.619 | 1.190 | 0.444 | 0.864 | 0.290 | 0.598 |
| Femorotibialis internus | FMTI | 1.035 | 0.996 | 1.003 | 1.065 | 0.366 | 0.963 | 0.330 | 0.413 | 0.674 | 0.977 | 0.621 | 0.729 | 0.015 | 0.029 | −0.036 | 0.066 | 1.158 | 0.944 | 0.994 | 1.323 | 0.355 | 0.801 | 0.246 | 0.463 |
| Iliotibialis 1 | IT1 | 1.054 | 0.986 | 0.964 | 1.141 | 0.327 | 0.955 | 0.285 | 0.365 | 0.739 | 0.968 | 0.648 | 0.821 | – | – | – | – | – | – | – | – | – | – | – | – |
| Iliotibialis 2 | IT2 | 1.018 | 0.997 | 0.992 | 1.041 | 0.387 | 0.918 | 0.313 | 0.470 | 0.645 | 0.980 | 0.581 | 0.694 | 0.035 | 0.101 | −0.027 | 0.097 | 0.995 | 0.909 | 0.813 | 1.177 | 0.506 | 0.814 | 0.367 | 0.646 |
| Iliotibialis 3 | IT3 | 1.054 | 0.991 | 1.009 | 1.106 | 0.455 | 0.857 | 0.343 | 0.544 | 0.657 | 0.914 | 0.535 | 0.771 | −0.002 | 0.000 | −0.056 | 0.053 | 1.270 | 0.896 | 0.978 | 1.561 | 0.817 | 0.632 | 0.440 | 1.195 |
| Extensor digitorum longus | EDL | 0.977 | 0.998 | 0.952 | 1.005 | 0.324 | 0.623 | 0.203 | 0.440 | 0.738 | 0.961 | 0.652 | 0.818 | −0.040 | 0.021 | −0.248 | 0.168 | 1.043 | 0.916 | 0.841 | 1.245 | 0.490 | 0.918 | 0.400 | 0.581 |
| Tibialis anterior | TA | 0.993 | 0.992 | 0.948 | 1.043 | 0.277 | 0.982 | 0.251 | 0.301 | 0.720 | 0.983 | 0.677 | 0.772 | – | – | – | – | 0.908 | 0.835 | 0.211 | 1.605 | 0.525 | 0.654 | −0.116 | 1.167 |
| Fibularis brevis | FB | 1.086 | 0.987 | 1.009 | 1.162 | 0.375 | 0.847 | 0.302 | 0.442 | 0.764 | 0.955 | 0.679 | 0.866 | −0.053 | 0.163 | −0.125 | 0.018 | 0.867 | 0.723 | 0.476 | 1.258 | 0.120 | 0.033 | −0.282 | 0.522 |
| Flexor digitorum longus (hind) | FDLH | 1.017 | 0.997 | 0.983 | 1.051 | 0.383 | 0.901 | 0.318 | 0.431 | 0.673 | 0.961 | 0.594 | 0.753 | −0.022 | 0.042 | −0.084 | 0.040 | 0.996 | 0.645 | 0.621 | 1.370 | 0.426 | 0.820 | 0.310 | 0.542 |
| Flexor hallucis longus | FHL | 0.981 | 0.994 | 0.937 | 1.019 | 0.378 | 0.928 | 0.325 | 0.429 | 0.637 | 0.980 | 0.606 | 0.678 | −0.066 | 0.338 | −0.120 | −0.011 | 1.354 | 0.963 | 1.190 | 1.517 | 0.651 | 0.872 | 0.500 | 0.802 |
| Fibularis longus | FL | 1.105 | 0.997 | 1.083 | 1.129 | 0.394 | 0.896 | 0.332 | 0.460 | 0.745 | 0.965 | 0.666 | 0.820 | 0.006 | 0.004 | −0.049 | 0.062 | 1.062 | 0.889 | 0.837 | 1.287 | 0.414 | 0.469 | 0.181 | 0.646 |
| Gastrocnemius externus | GE | 0.996 | 0.995 | 0.954 | 1.035 | 0.430 | 0.934 | 0.373 | 0.482 | 0.589 | 0.974 | 0.543 | 0.637 | −0.015 | 0.028 | −0.067 | 0.037 | 1.442 | 0.897 | 1.149 | 1.736 | 0.703 | 0.789 | 0.489 | 0.917 |
| Gastrocnemius internus | GI | 1.028 | 0.993 | 0.996 | 1.063 | 0.461 | 0.930 | 0.379 | 0.532 | 0.606 | 0.952 | 0.532 | 0.666 | −0.072 | 0.279 | −0.175 | 0.030 | 1.285 | 0.837 | 0.856 | 1.714 | 0.710 | 0.610 | 0.310 | 1.109 |
| Interosseus cruri | IC | 0.957 | 0.993 | 0.915 | 0.998 | 0.413 | 0.896 | 0.330 | 0.489 | 0.592 | 0.961 | 0.520 | 0.656 | −0.069 | 0.181 | −0.155 | 0.017 | 1.256 | 0.937 | 1.067 | 1.446 | 0.597 | 0.817 | 0.435 | 0.759 |
| Pronator profundus | PP | 1.098 | 0.989 | 1.022 | 1.172 | 0.454 | 0.867 | 0.387 | 0.531 | 0.707 | 0.935 | 0.599 | 0.802 | −0.110 | 0.355 | −0.240 | 0.020 | – | – | – | – | – | – | – | – |
| Extensor digitorum brevis | EDB | 0.953 | 0.971 | 0.854 | 1.030 | 0.348 | 0.816 | 0.282 | 0.441 | 0.727 | 0.787 | 0.586 | 0.920 | −0.025 | 0.021 | −0.127 | 0.078 | 1.275 | 0.703 | 0.638 | 1.912 | 0.414 | 0.429 | −0.159 | 0.987 |
| Extensor hallucis brevis | EHB | 0.984 | 0.992 | 0.939 | 1.038 | 0.336 | 0.833 | 0.284 | 0.394 | 0.697 | 0.981 | 0.650 | 0.743 | −0.066 | 0.180 | −0.153 | 0.021 | 1.423 | 0.624 | 0.623 | 2.223 | 0.214 | 0.028 | −0.406 | 0.833 |
| Extensor hallucis longus | EHL | 1.056 | 0.968 | 0.931 | 1.168 | 0.273 | 0.857 | 0.221 | 0.330 | 0.793 | 0.936 | 0.663 | 0.911 | 0.030 | 0.051 | −0.205 | 0.265 | 0.726 | 0.749 | 0.282 | 1.170 | 0.275 | 0.335 | −0.070 | 0.621 |
| Flexor digitorum brevis (hind) | FDBH | 0.955 | 0.992 | 0.895 | 1.001 | 0.288 | 0.896 | 0.225 | 0.338 | 0.695 | 0.951 | 0.590 | 0.782 | −0.004 | 0.001 | −0.102 | 0.094 | 0.870 | 0.698 | 0.419 | 1.321 | 0.446 | 0.321 | −0.146 | 1.038 |
| Flexor hallucis brevis | FHB | 1.028 | 0.997 | 0.994 | 1.065 | 0.349 | 0.490 | 0.234 | 0.554 | 0.807 | 0.690 | 0.592 | 1.148 | −0.099 | 0.307 | −0.196 | −0.002 | 1.236 | 0.944 | 0.973 | 1.498 | 0.413 | 0.454 | −0.042 | 0.868 |
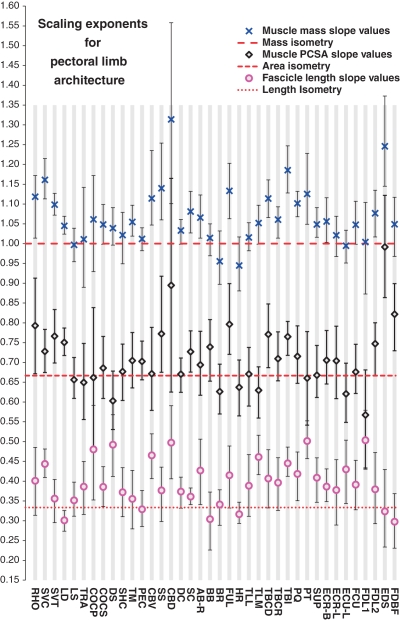
Fig. 3.
Scaling exponents of muscle properties vs. body mass for the pectoral limb of Alligator mississippiensis. Blue crosses indicate regression slopes for body mass, black diamonds indicate regression slopes for PCSA, pink circles indicate regression slopes for fascicle length. Error bars show upper and lower 95% confidence intervals, dashed lines indicates relevant value for isometry.
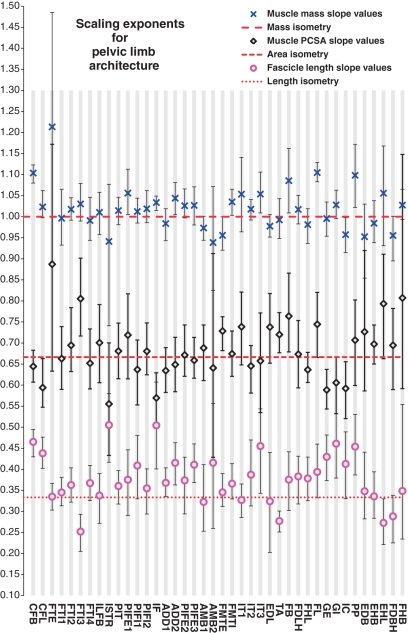
Fig. 4.
Scaling exponents of muscle properties vs. body mass for the pelvic limb of Alligator mississippiensis. Blue crosses indicate regression slopes for body mass, black diamonds indicate regression slopes for PCSA, pink circles indicate regression slopes for fascicle length. Error bars show upper and lower 95% confidence intervals, dashed lines indicates relevant value for isometry.
Scaling of muscle mass
In general, muscle mass and body mass were found to be tightly correlated in both the pectoral and the pelvic limbs. R2 values were > 0.9 for 38/38 and 39/40 muscles in the pectoral and pelvic limbs, respectively. The 95% CIs for regression slope estimates were narrower than 10% of slope values for 34/39 and 36/40 muscles in the pectoral and pelvic limbs, respectively (Figs 3 and 4, Tables 4 and 5).
CIs for muscle mass encompassing a regression slope of 1.0 (scaling with geometric similarity) were found in 18/38 pectoral limb muscles, including the pectoralis (PEC), long heads of triceps (TLL, TLM) and levator scapulae (LS). CIs for muscle mass encompassing a regression slope > 1.0 (positive allometry) were found for 16/38 pectoral limb muscles, notably the thoracic head of serratus ventralis (SVT, slope 1.072–1.127), subscapularis (SS, slope 1.06–1.254), flexor ulnaris (FUL, slope 1.063–1.203), caudal head of triceps brevis (TBCD, slope 1.066–1.203) and pronator quadratus (PQ, slope 1.068–1.132). Strong positive allometry of muscle mass (CIs encompassing regression slopes > 1.1) was observed in only four muscles. These were the cervical head of serratus ventralis (SVC, slope 1.113–1.215), the dorsal head of coracobrachialis brevis (CBD, slope 1.101–1.558), the intermediate head of triceps brevis (TBI, slope 1.128–1.247) and the superficial digital extensors (EDS, slope 1.145–1.373) (Fig. 3, Table 4). No negative allometry of muscle mass was observed in the pectoral limb.
Scaling of muscle mass with geometric similarity was more prevalent in the pelvic limb, with CIs surrounding 1.0 found for 30/40 muscles, including the long head of caudofemoralis (CFL), the ‘hamstrings’ (FTE, ILFB, FTI1-4), the larger two of the three heads of iliotibialis (IT1 and 2) and the gastrocnemii (GE, GI). Weaker positive allometry (CIs surrounding slopes between 1.0 and 1.1) was found in 9/40 muscles, most notably in the short head of caudofemoralis (CFB, slope 1.08–1.123) and the long head of fibularis (FL, slope 1.083–1.129). Weak negative allometry of muscle mass was found only in the interosseus cruri (IC, slope 0.915–0.998) (Fig. 4, Table 5).
Scaling of fascicle length
In general, fascicle length and body mass were less strongly correlated in both the pectoral and pelvic limbs. R2 estimates of > 0.9 were found for 21/38 and 22/40 muscles in the pectoral and pelvic limbs, respectively. 95% CIs were narrower than 10% of the slope value for a minority of slope estimates (8/38 and 8/40, pectoral and pelvic limb), although narrower than 20% for the majority (29/38 and 31/40, pectoral and pelvic limb) (Figs 3 and 4, Tables 4 and 5).
Fascicle length was found to scale with geometric similarity (CIs surrounding 0.33) for 22/38 muscles in the pectoral limb, including the pectoralis (PEC), teres major (TM), lateral head of triceps longus (TLL), parts of the extensor carpi group (ECR-L, ECU-L) and the clavicular deltoid (DC). Weaker positive allometry (CIs encompassing slopes between 0.33 and 0.43) was found in 15/38 muscles, notably including the first head of the long digital flexors (FDL-1, slope 0.43–0.579), scapular deltoid (DS, slope 0.412–0.568), intermediate head of triceps brevis (TBI, slope 0.412–0.486) and the medial head of triceps longus (TLM, slope 0.417–0.516). Strong positive allometry of fascicle length (CIs > 0.43) was found in the pronator teres (PT, slope 0.457–0.552) (Fig. 3, Table 4). No negative allometry was observed.
Fascicle length was found to scale with geometric similarity for 27/40 pelvic limb muscles, including most of the ‘hamstrings’ group (FTE, FTI1, 2 and 4, ILFB), the femorotibialis (FMTE, FMTI) and the short digital flexors (FDB). Weaker positive allometry was found in 11/40 muscles, notably including the long and short caudofemoralis (CFL, slope 0.430–0.495; CFB, slope 0.402–0.476), ischiotrochantericus (slope 0.417–0.580) and iliofemoralis (IF, slope 0.401–0.607). Weaker negative allometry (CIs surrounding slopes between 0.33 and 0.23) of fascicle length was found in the remaining two muscles, the third head of flexor tibialis internus (FTI3, slope 0.205–0.293) and the tibialis anterior (TA, slope 0.251–0.301) (Fig. 4, Table 5).
Scaling of physiological cross-sectional area (PCSA)
In general, PCSA and body mass were found to be correlated in both the pectoral limb and the pelvic limb. R2 values were > 0.9 for 34/38 and 35/40 muscles in the pectoral and pelvic limbs, respectively. CIs were narrower than 10% of the slope value for many muscles (16/38 and 17/40, pectoral and pelvic limb), and narrower than 20% for the majority (35/38 and 36/40, pectoral and pelvic limbs) (Figs 3 and 4, Tables 4 and 5).
Muscle PCSAs were found to scale with geometric similarity (CIs surrounding 0.67) for 27/38 muscles in the pectoral limb, including the levator scapulae (LS), pectoralis (PEC), deltoids (DC, DS) and extensor carpi group (ECR-B, ECR-L, ECU-L). Weak positive allometry (CIs around 0.67–0.77) was found in 11/38 muscles, notably the thoracic head of serratus ventralis (SVT, slope 0.699–0.834), latissimus dorsi (LD, slope 0.718–0.787), flexor ulnaris (FUL, slope 0.713–0.903), intermediate head of triceps brevis (TBI, slope 0.718–0.804) and short digital flexor (FDBF, slope 0.730–0.899). Strong positive allometry (CIs surrounding slopes higher than 0.77) of PCSA was found in the superficial digital extensors (EDS, slope 0.864–1.122) (Fig. 3, Table 4).
Muscle PCSAs were found to scale with geometric similarity for the majority (31/40) of pelvic limb muscles. Weak positive allometry was found for 4/40 muscles, notably the third head of flexor tibialis internus (FTI3, slope 0.717–0.902) and the external femorotibialis (FMTE, slope 0.682–0.762). Weaker negative allometry (CIs surrounding slopes between 0.57 and 0.67) of PCSA was found for 5/40 muscles, notably the long head of caudofemoralis (CFL, slope 0.548–0.663), iliofemoralis (IF, slope 0.497–0.629) and the external gastrocnemius (GE, slope 0.543–0.637) (Fig. 4, Table 5).
Scaling of pennation angles
Pennation was found in 20/38 pectoral limb muscles and 28/40 pelvic limb muscles. In both limbs, pennation angle and body mass were found to be poorly correlated: R2 values were very low (2/20 and 1/28 of R2 values > 0.5, respectively) and CIs were wider than 100% of the slope value for most slope estimates (20/20 in the pectoral limb, 26/28 in the pelvic limb). Slope estimates for the pectoral and pelvic limbs indicate (with poor support) that geometric similarity (CIs around slopes close to 0) is the majority scaling model. Some muscles in the pectoral limb [deltoideus scapularis (DS), flexor ulnaris (FUL) and extensor carpi ulnaris longus (FCU-L)] may scale with negative allometry (slopes less than zero). However, the poor correlation values and wide CIs calculated make these weak assertions (Tables 3 and 4).
Scaling of external tendon mass and length
Consistently identifiable external tendons were found for 16/38 pectoral limb muscles and 33/40 pelvic limb muscles. External tendon mass, length and body mass were found to be somewhat correlated in both limbs. Tendon mass R2 values were > 0.8 for 12/16 pectoral limb and 21/33 pelvic limb muscles, but CIs were wide. Only 7/16 pectoral and 16/33 pelvic limb muscles had CIs < 30% of their slope estimates (Tables 3 and 4). Tendon length R2 values were lower, with only 5/16 pectoral and 8/33 pelvic limb muscles scoring > 0.8. Tendon length CIs were also wider, with only 4/16 pectoral and 5/33 pelvic limb muscles having CIs < 30% of their slope estimates (Tables 3 and 4).
In the pectoral limb, wide CIs make it impossible to state with certainty the scaling model involved in tendon mass scaling for the majority of muscles analysed (Table 4). Those with tighter correlations and better constrained slopes appear to scale close to geometric similarity (slopes near 1): e.g. deltoideus scapularis (DS, slope 0.836–1.062), teres major (TM, slope 0.766–1.024) and flexor carpi ulnaris (FCU, slope 0.647–1.019) (Table 4). In the pelvic limb, CIs and correlation indicators were generally tighter, and it may be stated with reasonable confidence that external tendon mass in the majority of muscles (27/33) scales with geometric similarity (slopes close to 1) (Table 5). Weaker positive allometry (CIs surrounding slopes between 1 and 1.1) was found in two pelvic limb muscles: interosseus cruri (IC, slope 1.067–1.446) and the long head of caudofemoralis (CFL, slope 1.038–1.227). Strong positive allometry (CIs around slopes > 1.1) of tendon mass was found in the flexor hallucis longus (FHL, slope 1.19–1.517) and the external gastrocnemius (GE, slope 1.149–1.736). Negative allometry (slopes lower than 1) was found in the iliofibularis (ILFB, slope 0.710–0.988).
In the pectoral limb, most of those external tendons for which correlation coefficients and CIs are sufficiently tight to support any particular scaling inferences scale their lengths with geometric similarity [slopes close to 0.33, e.g. teres major (TM, slope 0.312–0.509) and the cranial head of triceps brevis (TBCR, slope 0.279–0.501)]. Weak positive allometry for tendon mass (CIs surrounding slopes between 0.33 and 0.43) was found in the scapular deltoid (DS, slope 0.365–0.583), biceps brachii (BB, slope 0.338–0.460) and the long head of extensor carpi radialis (ECR-L, slope 0.351–0.817) (Table 4). In the pelvic limb (Table 5), scaling of external tendon length with geometric similarity can be confidently inferred in the caudofemoralis longus (CFL, slope 0.2–0.378), flexor tibialis externus (FTE, slope 0.164–0.391), the first head of pubo-ischio-femoralis externus (PIFE1, slope 0.238–0.571), the second head of pubo-ischio-femoralis internus (PIFI2, slope 0.297–0.608), the internal and external femorotibialis (FMTI, slope 0.246–0.463; FMTE, slope 0.290–0.598) and the long digital flexor (FDLH, slope 0.310–0.542). Weaker positive allometry of tendon length was found in the second head of iliotibialis (IT2, slope 0.367–0.646) and the long digital extensor (EDL, slope 0.4–0.581). Strong positive allometry (CIs around slopes > 0.43) was found in the flexor hallucis longus (FHL, slope 0.5–0.802), the external gastrocnemius (GE, slope 0.489–0.917) and the interosseus cruri (IC, slope 0.435–0.759) (Table 5).
Discussion
Average distribution of muscle properties
The proximal to distal gradients in muscles properties seen in cursorial birds (Roberts, 2001; Smith et al. 2006) and mammals (Alexander, 1977; Alexander et al. 1981; Payne et al. 2005; Williams et al. 2008a,b;) has been interpreted as an energy-saving adaptation. By placing the more massive muscles required to do the work of locomotion proximally in the limb and replacing them distally with less massive muscles capable of less work but more-or-less equal force, moments of inertia in the distal limb (and hence energy loss in reciprocating limb motion) may be reduced without compromising limb function as a whole (e.g. Roberts, 2001; Biewener et al. 2004; Hutchinson, 2004a; Payne et al. 2005). The repetition of this pattern in our alligator data extends its known occurrence to non-cursorial animals, suggesting that it may be a fundamental aspect of limb design in larger tetrapods (with the caveat that extant Crocodylia has cursorial ancestors; Parrish, 1987).
In both the pectoral and pelvic limbs the heaviest and most powerful muscles are almost entirely extrinsic to the limb, taking their origins from the adjacent vertebral series and inserting proximally on the proximal segment of each limb. This anatomy permits them to be massive enough to have both the long fascicles and large physiological cross-sections to do large amounts of work without contributing problematically to the inertia of the limb, as has been suggested for the roughly analogous extrinsic thoracic limb muscles of horses (Payne et al. 2004).
Architecture and hypotheses of muscle function
The results of this study corroborate previous hypotheses of individual muscle function in crocodylian limbs generated by considerations of functional anatomy (Meers, 2003) and EMG studies (Jenkins & Goslow, 1983; Gatesy, 1997; Reilly & Blob, 2003; Reilly et al. 2005). Muscles in both limbs perform complex three-dimensional roles, but for the purposes of this study we simplify them into categories by their hypothesized primary use in terrestrial locomotion – extensors (generally stance-phase anti-gravity muscles and limb retractors), flexors (generally swing-phase muscles used in limb position control), adductors (generally stance-phase) and abductors (generally swing-phase).
Pectoral limb
Individual muscle function in the crocodylian pectoral limb is less studied than in the hindlimb, with existing hypotheses of function based on anatomy alone (Rodriguez, 2002; Meers, 2003). Varanoids are the closest related group with terrestrial pectoral limbs for which EMG data is available (Jenkins & Goslow, 1983). However, functional equivalence between crocodylians and varanoids is complicated by the differing modes of locomotion (varanoids have a greater tendency to ‘sprawl’, whereas crocodylians vary considerably) and the complex history of locomotion in crocodylian evolution (Parrish, 1986, 1987; Sereno, 1991) which includes secondarily reduced usage of parasagittal gaits.
Of the muscles anatomically placed to primarily effect extension [e.g. the triceps group – longus, brevis and their subdivisions (TBCR, TBI, TBCD, TLL, TLM), flexor digitorum longus 1 and 2 (FDL1 and 2), flexor carpi ulnaris (FCU), flexor digitorum brevis (FDBF)] most occupy a relatively larger PCSA and lower fascicle length area of our function space (upper left, Fig. 1). We interpret these as ‘force specialized’ muscles, able to produce large forces over small working ranges, and so suitable for the primary role of antigravity support. EMG data from varanoids support stance-phase extension activity for the triceps group (Jenkins & Goslow, 1983). PCSA estimates for the distalmost extensors (FDBF) are extremely low (among the lowest of the pectoral limb muscles) compared to their pelvic limb counterparts. This is consistent with at least two explanations: either antigravity support at the interphalangeal joints is controlled by more proximal muscles (FDL2 has the necessary anatomy and suitable properties) in the pectoral limb, or distal limb mechanics differ substantially between the pelvic and pectoral limbs.
Of the muscles anatomically placed to act as flexors [e.g. biceps brachii (BB), abductor radialis (AB-R), brachialis (BR), flexor ulnaris (FUL), humeroradialis (HR), extensor carpi radialis brevis and longus (ECR-B, ECR-L), extensor carpi ulnaris longus (ECR-L) and extensor digitorum superficialis (EDS)], most occupy a relatively longer fascicles, smaller PCSA area of function space (towards the lower right, Fig. 1). We interpret this function space as representing ‘displacement/working range-specialized’ muscles, able to produce relatively smaller forces but to contract over longer distances, and so well-suited to swing-phase limb repositioning. However, EMG and kinematic data from varanoids suggest that biceps brachii act during stance-phase as a stabilizer of the gleno-humeral joint and elbow (Jenkins & Goslow, 1983). If this differing role is maintained in crocodylians, it does not appear to require specialized architecture relative to the other flexors.
Determining function for shoulder muscles from anatomy alone is complicated by their three-dimensional paths over the gleno-humeral joint, which renders lines-of-action and so likely function highly dependent on limb conformation. EMG data from varanoids suggest that the deltoideus scapularis (DS) and, more ambiguously, deltoideus clavicularis (DC) are major swing-phase humeral abductors and protractors, respectively (Jenkins & Goslow, 1983). These suggestions are supported by our architectural data: deltoideus scapularis occupies a somewhat ‘force-specialized’ function space, suitable for lifting and supporting the limb, whereas deltoideus clavicularis is shifted towards a ‘displacement-specialized’ space suitable for unloaded limb protraction. Following Jenkins & Goslow’s (1983) suggestion that latissimus dorsi (LD) is a major swing phase limb abductor, we interpret its occupation of function space as providing a complementary agonist to the deltoideus scapularis. The more ‘displacement-specialized’ latissimus may be able to raise the limb through a larger arc, whereas the more ‘force-specialized’ deltoideus supports the raised limb against gravity and assists the latissimus.
The upper right area of function space defines muscles with long fascicles and large PCSAs, which we interpret as representing ‘powerful’ muscles capable of producing high forces over a large working range. These muscles are of considerably greater mass and hence greater metabolic cost, and are rarer in both limbs. In the pectoral limb these ‘powerful’ muscles are the pectoralis (PEC) and levator scapulae (LS) (Fig. 1). Anatomical and EMG studies suggest that the pectoralis is the major retractor and adductor of the forelimb (Jenkins & Goslow, 1983; Meers, 2003), active during stance to support the limb laterally and move the body forwards over it; a role well supported by its high-powered architecture. The role of levator scapulae in terrestrial locomotion is more ambiguous: as a cranial rotator and protractor of the scapulae, varanoid EMG studies suggest that it is active during swing-phase to protract and stabilize the limb via the scapulocoracoid. Meers (2003) suggests that as the levator scapulae is an effective abductor of the neck, it may play a more significant role in feeding behaviour than in locomotion. This hypothesis which may provide a better explanation for its high-powered architecture. Regardless, more experimental measures of crocodylian forelimb muscle functions are sorely needed.
Pelvic limb
Pelvic limb muscles occupy a wider function space than do pectoral limb muscles (Fig. 2) suggesting pelvic limb muscles are more variable in their functional specializations. Extensor muscles are roughly bimodal in properties. The most massive extensors crossing each joint [iliotibialis 2 (IT2), femorotibialis internus (FMTI), gastrocnemius externus (GE) and flexor digitorum brevis (FDBH)] all occupy ‘force-specialized’ function space (upper left). The functionality that this architecture implies (large forces, little movement) correlates well with EMG data indicating that the primary role of these muscles is to provide stance-phase antigravity support and stabilization (Gatesy, 1997; Reilly et al. 2005). Within these major extensors, PCSA increases and fascicle length decreases with increasingly distal location within the limb (IT2 to FDBH). This supports the inference (implicit in hypotheses of proximal-distal graduation of muscle properties) that static force per unit muscle mass becomes increasingly emphasized over work per unit muscle mass (i.e. muscle length change) in muscles that cross the more distal limb joints. Other limb extensors (see Table 3) occupy the more generalized area of function space (bottom left of Fig. 2), and do not display the same clear proximal to distal graduation, suggesting more varied functions.
PCSA values for pelvic limb extensors are generally greater than for equivalents in the pectoral limb (∼0.006% of body mass0.67 in the pelvic limb vs. ∼0.005% in the pectoral). This fore/hind disparity in available support muscle force, although slight, correlates with data from forceplates (Willey et al. 2004) and centre-of-mass modelling (Henderson, 2003; Allen et al. 2009), indicating that due to the caudal position of their centre of mass, crocodylian pelvic limbs experience greater loads than do their pectoral limbs during terrestrial locomotion. This is opposite to the pattern observed in virtually all quadrupedal mammals, which display roughly 60 : 40 hindlimb : forelimb loading (Alexander, 1985). This may lead to fundamental differences in locomotor patterns that appear superficially similar between mammals and crocodylians, especially extreme behaviour such as bounding and galloping (if this trend extends to those species in which this is observed). The alligator pectoral limb also lacks the highly ‘force specialized’ proximal muscles observed in mammals (Payne et al. 2005; Williams et al. 2008b), which supports significant differences in the roles played by pelvic and pectoral limbs between the two groups. Unfortunately, a more quantitative, experimental study on a wider range of crocodylian locomotor dynamics is needed before this interesting point may be expanded upon.
Pelvic limb flexor muscles also approximate two distinct groups in their occupation of function space: the biarticular ‘hamstring’ flexors and one distal flexor (tibialis anterior, TA), which cluster in ‘displacement-specialized’ function space (lower right), and all other flexors, which occupy the ‘generalized’ area of lower PCSA, lower fascicle length (lower left, Fig. 2). Those ‘hamstring’ muscles for which EMG data are sufficient to allow reasonable hypotheses of function, the flexor tibialis internus group (FTI1-3) and the iliofibularis (ILFB), show differing activity. The iliofibularis is active during swing phase to flex the knee and reposition the limb, whereas the flexor tibialis internus group show more variable activation, perhaps being involved in swing phase flexion as well as stance phase adduction and hip extension (Gatesy, 1997; Blob & Biewener, 2001; Reilly et al. 2005). Swing phase limb positioning involves small loads but large movements, which concurs with our study’s architectural data. Fascicle lengths are longer in these muscles than in equivalent pectoral limb flexors, suggesting that the swing phase involves larger flexion/extension ranges-of-motion in the pelvic limb compared with the pectoral limb. The tibialis anterior (TA, an ankle flexor) clusters with the hamstring muscles in ‘displacement-specialized’ function space. EMG data show that this muscle is active during swing to flex the ankle and re-position the foot, and so has similar requirements to other flexors (Reilly et al. 2005).
Femoral adduction during stance is, in the abducted postures sometimes used by crocodylians (e.g. Reilly & Elias, 1998; Kubo & Ozaki, 2009), equivalent to limb extension in terms of antigravity support (Hutchinson & Gatesy, 2000). However, major muscles responsible for stance-phase adduction [the adductors (ADD1 and 2), pubo-ischio-tibialis (PIT) and perhaps flexor tibialis internus (FTI1-3)] cluster with the ‘hamstrings’ in ‘displacement-specialized’ function space rather than with the extensor muscles in the ‘force-specialized’ area (Fig. 2). Relative small PCSAs for these muscles correlate well with crocodylians’ adoption of adducted postures at the faster speeds that require the greatest support forces (Brinkman, 1980; Gatesy, 1991; Reilly & Elias, 1998; Reilly et al. 2005). We interpret the unexpectedly large working ranges of these muscles as a result of postural variation in alligators. The use of both sprawled and more upright postures requires the adduction muscles to be able to function over a large range of femoral abduction/adduction angles. Muscles responsible for abducting the limb during swing [chiefly iliofemoralis (IF)] occupy the ‘generalized’ area of function space. This indicates that moderate force and moderate working range are of roughly equal importance to their architecture – fitting given the task they perform, raising and holding the limb elevated during swing.
The pelvic limb muscles occupying the ‘powerful’ region of function space (upper right, Fig. 2) are the caudofemoralis longus (CFL – the most ‘powerful’ muscle the alligator possesses by this analysis) and the second pubo-ischio-femoralis internus (PIFI2). Previous studies have amassed considerable evidence that the caudofemoralis longus is the ‘prime mover’ of the crocodylian hindlimb (Gatesy, 1990, 1997). It is active during stance to retract the entire limb from insertions on the femur and tibia and provide the bulk of locomotive power. In contrast, the pubo-ischio-femoralis internus 2 is active during swing to protract the limb (Gatesy, 1997; Reilly & Blob, 2003), corresponding to its lower position in the function space.
Scaling of muscle properties
The overall pattern of ontogenetic scaling for the majority of muscles and muscle properties in alligator limbs suggested by this study is geometric isometry. We predicted that ontogenetic limb shortening would correspond to lower limb volumes and hence smaller muscle mass. However, muscle mass was found to scale predominantly with isometry, suggesting that limbs must also undergo corresponding girth increase. To investigate this possibility, we performed additional regressions on limb segment circumferences for pectoral and pelvic limbs. Limb circumference was recorded where the proximal segment of each limb meets the body, at the elbow and knee joints, at the maximum width of the antebrachium and shank and at the wrist and ankle. Recorded measurements were regressed against body mass with identical techniques to all other data in this study. The results unambiguously show that limb girths scale isometrically (all circumferences displayed slopes at or near body mass 0.33, R2 values of above 0.98 with 95% confidence intervals narrower than 10% of slope estimates), contrary to the interspecific pattern of positive allometry in limb length and girth seen in varanoids (Christian & Garland, 1996). From this we conclude that limb volume must increase in alligators in ways not detectable with simple measurements of circumference, and that appreciable changes in limb shape must be involved.
As relative limb lengths are known to decrease in alligators, geometric similarity in fascicle lengths may indicate a general decrease in stride length for adult A. mississippiensis. All else being equal, positive allometry of fibre length in general could help to maintain stride length by allowing muscles to move the shorter legs through larger arcs. Isometry with respect to PCSA is also consistent with a decline in terrestrial locomotion performance (e.g. maximal Froude number), or at least requires alteration of other parameters to compensate. To support the body against similar forces of locomotion, muscle forces would need to scale 1 : 1 with body mass (as PCSA relates directly to muscle force, we would expect the same relationship). However, significant exceptions to these general patterns of isometry were found, as discussed below.
Positive allometry of length properties with respect to body mass does occur in the major retractors of the pelvic limb. The caudofemoralis longus (CFL) and brevis (CFB) both show significant fascicle length increase, accompanied in the CFL by a slight decrease in PCSA, which we interpret as indicative of a preferential ontogenetic increase in range of motion over force for these major locomotion muscles (Fig. 4, Table 5). This would to some degree offset the distal migration of its attachment and consequent lowered ‘gearing’ of the muscles in adult A. mississippiensis (Dodson, 1975). A larger working range would allow the muscles to retract the pelvic limb through a larger arc, producing longer strides and offsetting the effects of shorter limbs. However, as the muscle’s insertion (the fourth trochanter; Dodson, 1975) migrates distally during ontogeny, the moment arm of the muscles increases and the ratio of caudofemoral length change to limb excursion decreases. Hence any increases of stride length conferred by increased working range would to some degree be counteracted by a larger moment arm. In contrast, as PCSA for the CFB scales isometrically and mass scales positively, positive allometry of the shared moment arm would result in increased maximum joint moments (force × moment arm) and so reciprocally higher forces output to the limb, which has been correlated with faster locomotor speed (Weyand et al. 2000; Hutchinson, 2004a). This may represent a shift in relative importance between these two heads of caudofemoralis, with the short head becoming more important for locomotion in adult alligators. Similar ontogenetic increases of the moment arm for the pectoralis (PEC) due to a distal shift of its insertion on the deltopectoral crest (Livingston et al. 2009) are also correlated with positive allometry in fascicle length, although isometry is observed for the mass and PCSA of the PEC (Fig. 3, Table 4). Ontogenetic interactions between the musculoskeletal geometry of the caudofemoral and pectoral muscles, their internal architecture, and locomotion are obviously complex and require additional data from locomotor biomechanics to interpret fully.
The predicted requirements from extensor muscles, supported by their occupation of function space in typical alligator limbs (Figs 1 and 2), involve large forces and little movement to support the limb against collapse under gravitational loading. Body mass and weight scale to body length3, whereas muscle PCSA scales to body length2; therefore, muscles specifically dealing with weight support would be predicted to scale with positive allometry towards larger PCSAs and greater force production to meet this shortfall, potentially at the expense of other factors. In contrast we find that major stance phase extensors in both the pectoral and pelvic limbs [members of the triceps group (TBI, TLM), long digital flexors (FDL1) and gastrocnemii (GE, GI)] ontogenetically scale towards longer fascicles, some at the expense of force production (Figs 3 and 4). In particular the PCSA of the external head of gastrocnemius (GE), the most important of the pelvic limb ankle extensors because of its relative mass and PCSA (Table 3), scales negatively. As no other ankle extensors appear to be increasing their PCSA to pick up any shortfall, the force-generating capacity of the ankle joint may be lessened in adult alligators; a potential correlate with reduced locomotor performance. These results support the inference that the range of ankle plantar-flexion movement becomes as, if not more, important than static ankle antigravity support (i.e. extensor moment generation) in adult A. mississippiensis, which may in turn indicate usage of a greater range of postures through ontogeny, although a reduction in overall ‘performance’. This speculation requires experimental verification, however.
Previous studies of crocodylian body mass distribution (Allen et al. 2009) indicated that the centre of mass shifts cranially during ontogeny, increasing the relative requirement for supportive force from the extensor muscles of the pectoral limb. As positive allometry for PCSA was observed in only one of the major pectoral limb extensors [triceps brevis intermedius (TBI)], whereas only isometry or negative allometry was observed in the pelvic limb extensors, our findings provide limited support for this hypothesis. However, Allen et al. (2009) based this conclusion on a sample of only one adult and one juvenile Crocodylus johnstoni. Furthermore, ontogenetic reduction of locomotor performance could alleviate the functional constraints imposed by a centre of mass shift. A more inclusive study involving more individuals from a broader range of crocodylians is required to determine correlations between the ontogenetic scaling of centre of mass and of muscle properties.
Blob & Biewener’s (2001) mechanical analysis of muscle force and limb kinematics in various alligator gaits used inverse dynamics to conclude that required extensor muscle force increases dramatically with adoption of more upright postures, to balance the increased leverage the animal’s weight has about its ankle joint. Given an ontogenetic increase in relative weight, our finding of isometry or negative allometry in extensor muscle PCSAs supports the inference that juvenile alligators are more proficient at using erect gaits than adults are, and likewise supports the hypothesis that terrestrial locomotion repertoire reduces ontogenetically in alligators.
Major pectoral and pelvic limb abductors [deltoideus scapularis (DS) and iliofemoralis (IF), respectively] scale positively for fascicle length, indicating potential for larger arcs of abduction during the swing phase. In contrast, major adductors in both limbs appear to scale with geometric similarity for all properties [those for which a relationship can be determined (Tables 4 and 5)]. As stated above, adduction should be under similar demands to extension when using ‘sprawling’ or ‘semi-sprawling’ gaits, so geometric similarity and the reduced forces and motions it entails are again consistent with an ontogenetic reduction of terrestrial locomotor performance in alligators.
The serratus ventralis group [cervicus (SVC) and thoracis (SVT)] displays significant, but divergent scaling relationships. The posterior head (SVT) scales positively for force and the anterior head (SVC) scales positively for fascicle length. As both also scale positively for mass, this represents enhancement of force and working range, respectively, rather than compromise of either. Interpretation of this is hindered by a lack of rigorous functional data – varanoid EMG data indicate that the ‘anterior’ serratus (which may or may not be homologous to the crocodylian SVC) is active during stance to lock the pectoral girdle to the body, a support function, and so scaling towards greater working range is counterintuitive. Perhaps in crocodylians it is the posterior head of serratus ventralis (SVT, which scales positively for force as may be expected of a support muscle) that provides stance phase support, and the anterior part (SVC) that acts to manoeuvre the scapula.
The muscle that shows the strongest scaling relationship between PCSA and body mass is a distal flexor of the pectoral limb, the extensor digitorum superficialis (EDS). Scaling with a slope between slope 0.864 and 1.122, it is the only muscle studied that comes close to the 1 : 1 scaling of force estimates (which are directly proportional to PCSA) with body mass that is theoretically required to maintain relative performance. This is unexpected. As a distal flexor this muscle would be expected merely to lift and position only the phalanges of the pectoral limb during terrestrial locomotion, and so operate under small loads not related directly to the weight of the whole body. Knowledge of this muscle’s function during locomotion is limited to speculation based on its anatomy, and so we hesitate to interpret this result. It is plausible that use of the forelimb during slow-speed swimming would involve flexing the webbed phalanges under loads imposed by drag, and so this unexpected scaling relationship is possibly related to ontogenetic alterations in aquatic locomotion. However, a significant caveat to this hypothesis is that available data on the ontogeny of swimming behaviour in crocodylians (Manter, 1940; Seebacher et al. 2003) support the hypothesis that limb use during swimming decreases strongly with growth.
Conclusions
Here we present the first complete dataset on limb muscle architecture and ontogenetic scaling of muscle properties in an extant crocodylian, the American alligator (A. mississippiensis). Estimates of physiological cross-sectional areas for the pelvic limb of A. mississippiensis are greater than for the pectoral limb, in line with mass modelling and force plate data indicating that crocodylians support more body weight on their hindlimbs than on their forelimbs, contra the mammalian condition. Muscle properties were generally found to be suitable for the major functions (flexors, extensors, etc.) that previous hypotheses of relative muscle function in crocodylian limbs have assigned them (Gatesy, 1997; Meers, 2003; Reilly & Blob, 2003; Reilly et al. 2005).
Contrary to our predictions, relative muscle masses did not decrease despite ontogenetically shorter limbs. The relationship between ontogenetic size increase and changes in muscle properties for the majority of limb muscles in A. mississippiensis was found to correspond to geometric similarity. This indicates that muscle fascicle lengths and PCSAs may not be tightly correlated to segment length across ontogeny. As this isometry would not correct for ontogenetic increases in relative body weight and decreases in relative limb length (Dodson, 1975), these findings support the hypothesis that relative terrestrial locomotion performance decreases in adult alligators.
Previous studies have used inverse dynamics to predict an increase in required extensor muscle force in alligators when using upright gaits (Blob & Biewener, 2001), which would be exacerbated by an ontogenetic increase in relative weight. Our findings indicate either isometry or negative allometry in extensor muscle force production, therefore supporting the hypothesis that adult alligators are less capable of using upright gaits, and that locomotor repertoire is reduced in adult alligators. However, significant positive allometry was observed for fascicle lengths and hence muscle working range in the extensor musculature of both limbs, which may indicate ontogenetic increase in postural variability (and hence suggests that if their locomotor repertoire does become limited, habitual variation within the restricted range that remains may in fact increase). A similar increase was found in the major hindlimb retractor muscles, which could offset some of the negative effects of shorter limbs (reduced stride lengths), although the ontogenetic shift of their insertion sites to more distal locations and concomitant alterations to muscle leverage complicate interpretations.
These findings strengthen the hypothesis that ontogenetic reduction in gait repertoire and terrestrial athleticism is a general trend within extant Crocodylia. However, the implications of our results for Crocodylia as a whole rest on the general scaling relationships observed for alligators being applicable to other crocodylians. Thus, the apparent trend could be contradicted by similar ontogenetic data from a range of species; particularly as alligators tend to be more sedate than other crocodylians. Regardless, this study represents a significant step towards synthesis of anatomical, functional and scaling data to untangle the complex history of terrestrial locomotion in this major tetrapod clade. It is also a valuable resource for future studies seeking to understand the evolution and biomechanics of locomotion in archosaurs and the fundamentals of comparative tetrapod locomotion as a whole.
Acknowledgments
We thank the Rockefeller Wildlife Refuge for providing specimens and dissection facilities for this study, especially Phillip ‘Scooter’ Trosclair III and Dwayne LeJeune for their excellent assistance in this project. We thank colleagues from the Structure and Motion Laboratory and the Bristol Earth Sciences department for discussion and suggestion of scaling methodology, in particular Dr Monica Daley, Dr Andrew Spence, Dr Charlotte Miller and and Dr Manabu Sakamoto. For financial support we thank the Department of Veterinary Basic Sciences of The Royal Veterinary College, and the BBSRC for grant BB/F101169/1; awarded to J.R.H. in 2007.
Supporting Information
Additional Supporting Information may be found in the onlineversion of this article:
Table S1. Raw muscle architecture data, pectoral limb.
Table S2. Raw muscle architecture data, pelvic limb.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Alexander RMcN. Allometry of the limbs of antelopes (Bovidae) J Zool. 1977;183:125–146. [Google Scholar]
- Alexander RMcN. The maximum forces exerted by animals. J Exp Biol. 1985;115:231–238. doi: 10.1242/jeb.115.1.231. [DOI] [PubMed] [Google Scholar]
- Alexander RMcN, Jayes A. A dynamic similarity hypothesis for the gaits of quadrupedal mammals. J Zool (Lond) 1983;201:135–152. [Google Scholar]
- Alexander R, Ker R. The architecture of leg muscles. In: Winters J, Woo S, editors. Multiple Muscle Systems: Biomechanics and Movement Organization. Berlin: Springer; 1990. pp. 568–577. [Google Scholar]
- Alexander RMcN, Jayes AS, Maloiy GMO, et al. Allometry of the leg muscles of mammals. J Zool. 1981;194:539–552. [Google Scholar]
- Allen V, Paxton H, Hutchinson JR. Variation in centre of mass estimates for extant sauropsids, and its importance for reconstructing inertial properties of extinct archosaurs. Anat Rec. 2009;292:535–544. doi: 10.1002/ar.20973. [DOI] [PubMed] [Google Scholar]
- Azizi E, Brainerd EL, Roberts TJ. Variable gearing in pennate muscles. PNAS. 2008;105:1745–1750. doi: 10.1073/pnas.0709212105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewener A. Scaling body support in mammals: limb posture and muscle mechanics. Science. 1989;245:45–48. doi: 10.1126/science.2740914. [DOI] [PubMed] [Google Scholar]
- Biewener A, McGowan C, Card G, et al. Dynamics of leg muscle function in tammar wallabies (M. eugenii) during level versus incline hopping. J Exp Biol. 2004;207:211–223. doi: 10.1242/jeb.00764. [DOI] [PubMed] [Google Scholar]
- Blob RW. Interspecific scaling of the hindlimb skeleton in lizards, crocodilians, felids and canids: does limb bone shape correlate with limb posture? J Zool. 2000;250:507–531. [Google Scholar]
- Blob R, Biewener A. In vivo locomotor strain in the hindlimb bones of Alligator mississippiensis and Iguana iguana: implications for the evolution of limb bone safety factor and non-sprawling limb posture. J Exp Biol. 1999;202:1023–1046. doi: 10.1242/jeb.202.9.1023. [DOI] [PubMed] [Google Scholar]
- Blob R, Biewener A. Mechanics of limb bone loading during terrestrial locomotion in the green iguana (Iguana iguana) and American alligator (Alligator mississippiensis) J Exp Biol. 2001;204:1099–1122. doi: 10.1242/jeb.204.6.1099. [DOI] [PubMed] [Google Scholar]
- Brinkman D. The hind limb step cycle of Caiman sclerops and the mechanics of the crocodile tarsus and metatarsus. Can J Zool. 1980;58:2187–2200. [Google Scholar]
- Brochu CA. Morphology, fossils, divergence timing, and the phylogenetic relationships of Gavialis. Syst Biol. 1997;46:479–522. doi: 10.1093/sysbio/46.3.479. [DOI] [PubMed] [Google Scholar]
- Bustard HR, Singh AK. The Indian gharial Gavialis gangeticus (Gmelin): change in terrestrial locomotory pattern with age. J Bombay Nat Hist Soc. 1977;74:534–536. [Google Scholar]
- Calow L, Alexander R. A mechanical analysis of a hind leg of a frog (Rana temporaria) J Zool. 1973;171:293–321. [Google Scholar]
- Christian A, Garland T., Jr Scaling of limb proportions in monitor lizards (Squamata: Varanidae) J Herpertol. 1996;30:219–230. [Google Scholar]
- Cong L, Hou L-H, Wu XC. The Gross Anatomy of Alligator Sinensis Fauvel. Beijing: CIP; 1998. [Google Scholar]
- Cott HB. Scientific results of an inquiry into the ecology and economic status of the Nile crocodile (Crocodylus niloticus) in Uganda and Northern Rhodesia. Trans Zool Soc Lond. 1960;29:211–340. [Google Scholar]
- Dodson P. Functional and ecological significance of relative growth in Alligator. J Zool (Lond) 1975;175:315–355. [Google Scholar]
- Gans C, De Vree F. Functional bases of fiber length and angulation in muscle. J Morphol. 1987;192:63–85. doi: 10.1002/jmor.1051920106. [DOI] [PubMed] [Google Scholar]
- Gatesy SM. Caudefemoral musculature and the evolution of theropod locomotion. Paleobiology. 1990;16:170–186. [Google Scholar]
- Gatesy SM. Hind limb movements of the American alligator (Alligator mississippiensis) and postural grades. J Zool. 1991;224:577–588. [Google Scholar]
- Gatesy SM. An electromyographic analysis of hindlimb function in Alligator during terrestrial locomotion. J Morphol. 1997;234:197–212. doi: 10.1002/(SICI)1097-4687(199711)234:2<197::AID-JMOR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Gatesy J, Amato G. The rapid accumulation of consistent molecular support for intergeneric crocodylian relationships. Mol Phylogenet Evol. 2008;48:1232–1237. doi: 10.1016/j.ympev.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Gatesy J, Amato G, Norell M, et al. Combined support for wholesale taxic atavism in gavialine crocodylians. Syst Biol. 2003;52:403–422. doi: 10.1080/10635150390197037. [DOI] [PubMed] [Google Scholar]
- Gaunt A, Gans C. Architecture of chicken muscles: short-fibre patterns and their ontogeny. Proc R Soc Lond B. 1990;240:351–362. doi: 10.1098/rspb.1990.0041. [DOI] [PubMed] [Google Scholar]
- Henderson DM. Effects of stomach stones on the buoyancy and equilibrium of a floating crocodilian: a computational analysis. Can J Zool. 2003;81:1346–1357. [Google Scholar]
- Hutchinson JR. Biomechanical modeling and sensitivity analysis of bipedal running ability. I. Extant taxa. J Morphol. 2004a;262:421–440. doi: 10.1002/jmor.10241. [DOI] [PubMed] [Google Scholar]
- Hutchinson JR, Gatesy SM. Adductors, abductors, and the evolution of archosaur locomotion. Paleobiology. 2000;26:734–751. [Google Scholar]
- Hutchinson JR, Schwerda D, Famini D, et al. The locomotor kinematics of African and Asian elephants: changes with speed and size. J Exp Biol. 2006;209:3812–3827. doi: 10.1242/jeb.02443. [DOI] [PubMed] [Google Scholar]
- Jenkins FA, Jr, Goslow G., Jr The functional anatomy of the shoulder of the savannah monitor lizard (Varanus exanthematicus) J Morphol. 1983;175:195–216. doi: 10.1002/jmor.1051750207. [DOI] [PubMed] [Google Scholar]
- Kubo T, Ozaki M. Does pace angulation correlate with limb posture? Palaeogeogr Palaeoclimatol Palaeoecol. 2009;275:54–58. [Google Scholar]
- Livingston VJ, Bonnan MF, Elsey RM, et al. Differential limb scaling in the American Alligator (Alligator mississippiensis) and its implications for archosaur locomotor evolution. Anat Rec. 2009;292:787–797. doi: 10.1002/ar.20912. [DOI] [PubMed] [Google Scholar]
- Manter JT. The mechanics of swimming in the alligator. J Exp Zool. 1940;83:345–358. [Google Scholar]
- Medler S. Comparative trends in shortening velocity and force production in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2002;283:R368–R378. doi: 10.1152/ajpregu.00689.2001. [DOI] [PubMed] [Google Scholar]
- Meers MB. Evolution of the Crocodylian Forelimb: Anatomy, Biomechanics, and Functional Morphology. Maryland: The Johns Hopkins University School of Medicine; 1999. PhD dissertation. [Google Scholar]
- Meers MB. Cross-sectional geometric properties of the crocodylian humerus: an exception to Wolff’s Law? J Zool (Lond) 2002;258:405–418. [Google Scholar]
- Meers MB. Crocodylian forelimb musculature and its relevance to Archosauria. Anat Rec A Discov Mol Cell Evol Biol. 2003;274A:891–916. doi: 10.1002/ar.a.10097. [DOI] [PubMed] [Google Scholar]
- Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- Parrish JM. Locomotor adaptations in the pelvis of the Thecodontia (Reptilia: archosauria) Paleobiology. 1987;13:396–414. [Google Scholar]
- Parrish J. Locomotor evolution in the hindlimb and pelvis of the Thecodontia (Reptilia: archosauria) Hunteria. 1986;1:1–35. [Google Scholar]
- Payne R, Veenman P, Wilson A. The role of the extrinsic thoracic limb muscles in equine locomotion. J Anat. 2004;205:479–490. doi: 10.1111/j.0021-8782.2004.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RC, Hutchinson JR, Robilliard JJ, et al. Functional specialisation of pelvic limb anatomy in horses (Equus caballus) J Anat. 2005;206:557–574. doi: 10.1111/j.1469-7580.2005.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennycuick CJ. On the running of the gnu (Connochaetes taurinus) and other animals. J Exp Biol. 1975;63:775–799. [Google Scholar]
- Reilly SM, Blob RW. Motor control of locomotor hindlimb posture in the American alligator (Alligator mississippiensis) J Exp Biol. 2003;206:4327–4340. doi: 10.1242/jeb.00688. [DOI] [PubMed] [Google Scholar]
- Reilly S, Elias J. Locomotion in Alligator mississippiensis: kinematic effects of speed and posture and their relevance to the sprawling-to-erect paradigm. J Exp Biol. 1998;201:2559–2574. doi: 10.1242/jeb.201.18.2559. [DOI] [PubMed] [Google Scholar]
- Reilly S, Willey J, Biknevicius AR, et al. Hindlimb function in the alligator: integrating movements, motor patterns, ground reaction forces and bone strain of terrestrial locomotion. J Exp Biol. 2005;208:993–1009. doi: 10.1242/jeb.01473. [DOI] [PubMed] [Google Scholar]
- Renous S, Gasc JP, Bels V, et al. Asymmetrical gaits of juvenile Crocodylus johnstoni, galloping Australian crocodiles. J Zool. 2002;256:311–325. [Google Scholar]
- Roberts T. Muscle force and stress during running in dogs and wild turkeys. Bull Mus Comp Zool. 2001;156:283–295. [Google Scholar]
- Rodriguez D. Comparative Myology of the Crocodylian Shoulder and Forelimb. San Marco: Southwest Texas State University; 2002. MSc Thesis. [Google Scholar]
- Romer AS. Crocodilian pelvic muscles and their avian and reptilian homologues. Bull Am Mus Nat Hist. 1923a;48:533–552. [Google Scholar]
- Roos J, Aggarwal RK, Janke A. Extended mitogenomic phylogenetic analyses yield new insight into crocodylian evolution and their survival of the Cretaceous-Tertiary boundary. Mol Phylogenet Evol. 2007;45:663–673. doi: 10.1016/j.ympev.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Sacks RD, Roy RR. Architecture of the hind limb muscles of cats: functional significance. J Morphol. 1982;173:185–195. doi: 10.1002/jmor.1051730206. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Scaling. Cambridge: Cambridge University Press; 1984. [Google Scholar]
- Seebacher F, James RS. Plasticity of muscle function in a thermoregulating ectotherm (Crocodylus porosus): biomechanics and metabolism. Am J Physiol Regul Integr Comp Physiol. 2008;294 doi: 10.1152/ajpregu.00755.2007. [DOI] [PubMed] [Google Scholar]
- Seebacher F, Elsworth PG, Franklin CE. Ontogenetic changes of swimming kinematics in a semi-aquatic reptile (Crocodylus porosus) Aust J Zool. 2003;51:15–24. [Google Scholar]
- Sereno PC. Basal archosaurs: phylogenetic relationships and functional implications. J Vertebr Paleontol. 1991;11(Suppl):1–53. Memoir, 2. [Google Scholar]
- Sharir A, Milgram J, Shahar R. Structural and functional anatomy of the neck musculature of the dog (Canis familiaris) J Anat. 2006;208:331–351. doi: 10.1111/j.1469-7580.2006.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L, Bustard HR. Locomotory behaviour during basking and spoor formation in the gharial (Gavialis gangeticus) Br J Herpertol. 1976;5:673–676. [Google Scholar]
- Smith NC, Wilson AM, Jespers KJ, et al. Muscle architecture and functional anatomy of the pelvic limb of the ostrich (Struthio camelus) J Anat. 2006;209:765–779. doi: 10.1111/j.1469-7580.2006.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb G, Gans C. Galloping in Crocodylus johnstoni– a reflection of terrestrial activity. Rec Aust Mus. 1982;34:607–618. [Google Scholar]
- Wells JB. Comparison of mechanical properties between slow and fast mammalian muscles. J Physiol. 1965;178:252–269. doi: 10.1113/jphysiol.1965.sp007626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand PG, Sternlight DB, Bellizzi MJ, et al. Faster top running speeds are achieved with greater ground forces not more rapid leg movements. J Appl Physiol. 2000;89:1991–1999. doi: 10.1152/jappl.2000.89.5.1991. [DOI] [PubMed] [Google Scholar]
- Whitaker R. Gharial walk. Hamadryad. 1978;3:5. [Google Scholar]
- Whitaker R, Andrews H. Notes on crocodylian locomotion. J Bombay Nat Hist Soc. 1988;853:621–622. [Google Scholar]
- Willey JS, Biknevicius AR, Reilly SM, et al. The tale of the tail: limb function and locomotor mechanics in Alligator mississippiensis. J Exp Biol. 2004;207:553–563. doi: 10.1242/jeb.00774. [DOI] [PubMed] [Google Scholar]
- Williams SB, Wilson AM, Rhodes L, et al. Functional anatomy and muscle moment arms of the pelvic limb of an elite sprinting athlete: the racing greyhound (Canis familiaris) J Anat. 2008a;213:361–372. doi: 10.1111/j.1469-7580.2008.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SB, Wilson AM, Rhodes L, et al. Functional anatomy and muscle moment arms of the thoracic limb of an elite sprinting athlete: the racing greyhound (Canis familiaris) J Anat. 2008b;213:373–382. doi: 10.1111/j.1469-7580.2008.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woledge RC, Curtin NA, Homsher E. Energetic aspects of muscle contraction. Monogr Physiol Soc. 1985;41:1–357. [PubMed] [Google Scholar]
- Zajac FE. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng. 1989;17:359–411. [PubMed] [Google Scholar]
- Zug GR. Crocodilian galloping: an unique gait for reptiles. Copeia. 1974;1974:550–552. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.