Abstract
The scapula is the main skeletal element of the pectoral girdle allowing muscular fixation of the forelimb to the axial skeleton. The vertebrate limb skeleton has traditionally been considered to develop from the lateral plate mesoderm, whereas the musculature originates from the axial somites. However, in birds, the scapular blade has been shown to develop from the somites. We investigated whether a somitic contribution was also present in the mammalian scapula. Using genetic lineage-tracing techniques, we show that the medial border of the mammalian scapula develops from somitic cells. The medial scapula border serves as the attachment site of girdle muscles (serratus anterior, rhomboidei and levator scapulae). We show that the development of these muscles is independent of the mechanism that controls the formation of all other limb muscles. We suggest that these muscles be specifically referred to as medial girdle muscles. Our results establish the avian scapular blade and medial border of the mammalian scapula as homologous structures as they share the same developmental origin.
Keywords: avian, embryo, mouse, Pax3, scapula medial border, somite, Wnt1
Introduction
The pectoral girdle, which in humans is composed of the scapula (shoulder blade) and clavicle (collar bone), attaches the upper limb to the body. The clavicle connects the upper limb to the sternum (breast bone) by a joint, whereas the scapula is attached to the thoracic cage only by skeletal muscles. This organization allows the shoulder girdle a great degree of mobility especially in comparison to the pelvic girdle. In some mammalian species (including the horse and dog), the pectoral girdle lacks the clavicle and therefore there are no joints between the upper limb and thorax. The forelimbs of such species are thus stabilized solely by scapular muscles, an arrangement that is termed synsarcosis. The mammalian scapula is a large triangular flat bone that lies over the dorsolateral region of the thorax. The medial/vertebral border of the human scapula is the longest of the three borders and serves as the insertion point for four muscles connecting the scapula to the axial skeleton: one connects it to the costal surface (serratus anterior) and three to the medial/cranial aspect (major and minor rhomboids, and levator scapulae).
The scapula is of great importance in an evolutionary context not only within the class Mammalia but also for all tetrapods. Its morphology in non-mammalian animals varies considerably (Romer & Parsons, 1986, page 204). For example, in birds, rather than being triangular, the scapula is a long slender bone. There is considerable debate regarding the ontogeny of the scapula as it is traditionally held that all axial/trunk skeleton develops from the somites, whereas limb and limb girdle skeleton originates from lateral plate mesoderm (Chevallier, 1977; Christ & Ordahl, 1995). However, our work (Huang et al. 2000) has shown that this is only partially true for the avian scapula. Using lineage-tracing methods, we have demonstrated that only the head and collum of the avian scapula develop from lateral plate mesoderm. The major part of the avian scapula (the blade) develops from the axial somites. Most remarkably, we were able to show that the blade develops from the dermamyotomal portion of the somite – a compartment that had previously been shown to give rise only to the skeletal muscle and dermis (Christ & Ordahl, 1995). Despite the differences in shape of the avian and mammalian scapulae, we could propose that the blade of the avian scapula and the medial border of the mammalian scapula are homologous structures, based on their muscle attachments. However, for a true homology to be established, the same developmental processes need to be shared during ontogeny.
Unlike the developing chick embryo, the mouse embryo is not readily amenable to traditional lineage-tracing protocols and only the advent of molecular biology and transgenesis permits us to study fate maps and therefore the developmental origin of tissues. In this study we have used Pax3-Cre (Engleka et al. 2005), a genetic manipulation that allows us to follow the distribution of all cells that are expressing or have ever expressed Pax3, a gene found in the dermamyotomal compartment of the somite. We have previously shown (Huang et al. 2000) that the dorsolateral portion of the chick dermamyotome undergoes a molecular and cellular transition in the scapula-forming region. Cells expressing Pax3 in the epithelial layer of the dermamyotome switch off this gene and break away to become mesenchymal in organization and reside under the ectoderm. Simultaneously they initiate the expression of Pax1 as a prelude to the cartilage differentiation programme. Pax3 is, however, also expressed in the dorsal neural tube and the neural crest during embryogenesis. We therefore also utilized Wnt1-Cre, which marks the neural crest derivatives (Jiang et al. 2000; Matsuoka et al. 2005). Subtraction of the Pax3 and Wnt1 lineage derivatives thus allows us to identify tissues that are of somitic origin in the mouse model.
Using this model we show that the medial border of the mammalian scapula develops from somitic cells. Moreover, our fate map studies establish that the avian scapula blade and the medial border of the mammalian scapula are homologous structures.
Materials and methods
Production of transgenic embryos Pax3-Cre, Wnt1-Cre and met-null
The following mice were crossed: (i) Pax3-Cre (Engleka et al. 2005) with R26R-stop-YFP yellow fluorescent protein (Srinivas et al. 2001), (ii) Wnt1-Cre (Danielian et al. 1998) with R26RLacZ (Soriano, 1999) and (iii) met-null (Valasek et al. 2005). All animal experiments were approved by the local animal ethical committees (Weizmann Institue of Science, Rehovot; Charles University, Prague; Developmental Biology Institute of Marseille-Luminy).
Processing of embryos
The Pax3-Cre/R26RYFP embryos [15.5 days post coitum (dpc)] were harvested, fixed overnight in 4% paraformaldehyde (PFA), washed in phosphate-buffered saline (PBS) and gradually transferred and stored in 70% ethanol. Ethanol, xylene and paraplast were used for paraffin embedding (1 h per step). Sections (10 μm) on polylysine slides were boiled for 10 min in 10 mm citrate, pH 6.0. Following blocking with 10% goat serum in PBS, anti-GreenFluorescentProtein-biotin (Abcam, ab6658, 1 : 50) was used overnight. Streptavidin-AlexaFluor 594 (Invitrogen S-11227, 1 : 200) mediated visualization.
The Wnt1-Cre/R26RLacZ embryos were harvested at 13.5 dpc, fixed for 3 h in 4% PFA in PBS, washed in PBS, cryopreserved with sucrose and embedded in O.C.T. compound (Sakura Finetek USA Inc., CA, USA). Sections (7 μm) were washed in PBS, endogenous peroxidase was blocked by 0.6% H2O2 in PBS for 30 min and non-specific antibody binding was blocked by 10% normal goat serum in PBS for 1 h. Rabbit polyclonal anti-beta-galactosidase antibody (1 : 800, kindly provided by Dr Joshua Sanes, Harvard University, Cambridge, MA, USA) was used overnight at 4 °C. Secondary goat anti-rabbit antibody conjugated with horseradish peroxidase (1 : 200, Sigma) was applied for 1.5 h at 22 °C. The immunoreaction was visualized by chromogenic reaction with diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA, USA). Sections were counterstained with haematoxylin.
The Met-null and wild-type 15.0 dpc embryos were cryoembedded, sectioned transversally at 16 μm, rehydrated in PBS, fixed in 4% PFA/PBS for 3 min, rinsed in PBS and preblocked in 10% heat-inactivated goat serum in PBS. Sections were incubated for 2 h with biotinylated mouse monoclonal antibody against myosin heavy chain (DSHB A41025, 1 : 1000), which was developed with ABC streptavidin/peroxidase kit and DAB staining (Vector Laboratories). Sections were counterstained with haematoxylin and alcian blue (0.5% in 0.5% glacial acetic acid).
Skeletal staining
One 4-week-old mouse was killed, skinned, eviscerated, fixed in 95% ethanol and stained with 0.03% alcian blue and 0.01% alizarin red in 1% glacial acetic acid and 95% ethanol. Clearing was performed in 1% KOH for 5 days followed by increasing concentrations of glycerol and finally stored in 100% glycerol.
An 8-month-old mouse scapula was dissected, fixed in 4% PFA/PBS and stained for 2 h in alcian blue/glacial acetic acid (0.5%/0.5%) in water. Alternatively, an 8-month-old hemi-thorax was fixed for 2 h in 4% PFA/PBS, rinsed in water, decalcified for 4 days in 4% formic/4% hydrochloric (orig. 35%) acids, rinsed in water, cryoembedded in TissueTek (Leica), cut at 15 μm and stained with alcian blue/acetic acid (0.5%/0.5%) for 10 min and eosin for 10 s. Dehydration was followed by mounting.
Anatomical terminology and photography
Anatomical terms were used according to Greene (1935). Photographs were taken on a stereomicroscope Nikon SMZ1500 with a digital camera Nikon Coolpix E990. Photographs of sections were taken on a Zeiss Axioskop 2 with AxioCam and an Olympus BX51 microscope with an Olympus DP71 camera. Image processing was carried out using Adobe Photoshop 5.0LE.
Results
Medial border of the mammalian scapula develops from a Pax3-expressing cell population
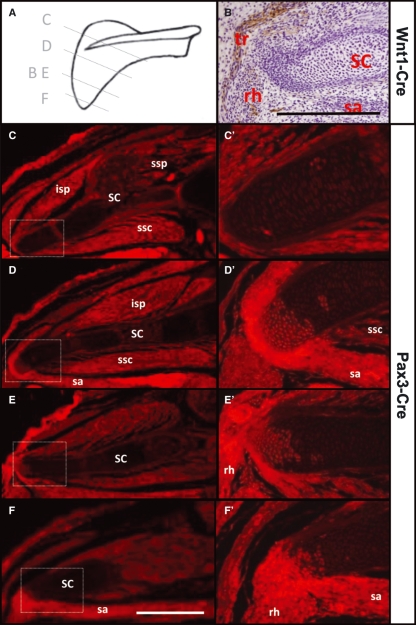
To follow all cells that had ever expressed Pax3, we crossed Pax3-Cre with Rosa-YFP reporter mouse lines and examined the scapula for the presence of YFP-positive cells. Sections from cranial to caudal regions of the scapula revealed YFP-positive cells selectively in the medial border (Fig. 1C–F). In contrast, no YFP expression was found in the body of the scapula. Interestingly, the number of YFP-positive cells within the medial border of the scapula increased in a cranial to caudal direction. Furthermore, the developing tendon tissue joining the YFP-positive medial border cartilage to the inserting muscles was also positive for YFP (Fig. 1D′), indicating that the tendon cells had also expressed Pax3 at some stage in their development. As expected, all skeletal muscle was also positive for YFP.
Fig. 1.
Somitic derivatives in the shoulder region. (A) Sectioning planes in B–E through a scheme of the scapula. (B) Transverse cryosection of the shoulder region of a 13.5 dpc Wnt1-Cre/R26R-LacZ mouse embryo shows the absence of neural crest derivatives in the medial scapular cartilage, whereas they are present in adjacent nerves and connective tissue of the trapezius muscle. Therefore, the Pax3-Cre activity in the scapular cartilage is due to somitic cells and not due to neural crest cells. (C–F) Transverse paraffin sections through the shoulder region of 15.5 dpc Pax3-Cre/R26R-YFP mouse embryo stained with anti-GFP/AlexaFluor 594. Insets are shown at higher magnification in C′–F′ and demonstrate the cranio-caudally increasing contribution of YFP-positive cells to the medial/vertebral (dorsal-most) scapular border. The medial girdle muscles attaching to the medial margin of the scapula are more strongly YFP-positive than other muscles, suggesting that their connective tissue is also somitic in origin. Ossifying regions of scapular cartilage seem falsely positive (spina scapulae in C, centre of scapula in D). Scale bar, 1 mm. Orientation: dorsal to the left, left side to the top. SC, scapula; isp, infraspinatus; ssp, supraspinatus; ssc, subscapularis; sa, serratus anterior; rh, rhomboid major; tr, trapezius muscles.
These results suggest that the medial scapular border does not develop from the lateral plate mesoderm and indicate its possible somitic origin.
Medial border of the mammalian scapula is somitic in origin
As mentioned above, Pax3 is expressed not only in the somitic dermamyotome but also in the neural crest. We therefore wanted to examine whether the cells in the medial border of the scapula derived from the somites rather than from the neural crest. To this end we used the Wnt1-Cre line crossed with the Rosa-LacZ strain (Danielian et al. 1998; Soriano, 1999), which allowed us to label all cells originating from the neural crest. We found that the scapular cartilage was devoid of LacZ-positive cells, although present in the scapular region (e.g. connective tissue of the trapezius muscle in Fig. 1B). These results suggest that neural crest cells do not contribute to the medial border of the mammalian scapula. Assimilating results of both Pax3-Cre and Wnt1-Cre mouse lines we conclude that the medial border of the scapula develops from somitic cells.
Development of the medial scapular border and its differentiation
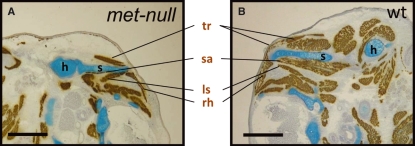
We have previously shown that the development of migratory muscles in the body of vertebrates is under the control of Pax3 (Franz et al.1993), which induces the expression of c-Met (Epstein et al. 1996) – a transmembrane receptor tyrosine kinase that permits the cells of the dermamyotome to undergo an epithelial to mesenchymal transition, which is required for their migration from the somites to the limbs (Bladt et al. 1995; Maina et al. 1996). We therefore examined whether the development of the medial border of the mammalian scapula was dependent on this regulatory cascade. Examination of the Met signalling mutant embryos (Maina et al. 1996) (hereafter, met-null) revealed a normal scapula (Fig. 2). Moreover, the associated muscles (serratus anterior, rhomboidei and levator scapulae) appeared unaffected, in contrast to the limb regions lacking all skeletal muscle (Fig. 2) (Prunotto et al. 2004; Bladt et al. 1995; Maina et al. 1996). These results indicate that, although the medial border of the scapula is formed by cells of somitic origin that expressed Pax3 at some stage of its development, these scapular precursor cells are not reliant on the migratory programme triggered by Met to break away from the dermamyotome. Moreover, the development of the associated muscles does not depend on Met signaling.
Fig. 2.
Medial girdle muscles do not migrate using cMet. (A) Transverse cryosection through shoulder region of 15.0 dpc met-null embryo shows normal skeletal elements, scapula (s) and humerus (h), and the following muscles: trapezius (tr), serratus anterior (sa), rhomboidei (rh) and levator scapulae (ls). (B) Wild-type (wt) littermate shows the presence of normal scapular elements, medial girdle muscles and limb muscles demonstrating that neither the medial scapular border nor its medial girdle muscles require cMet/SF(HGF) for their development. Scale bar, 1 mm.
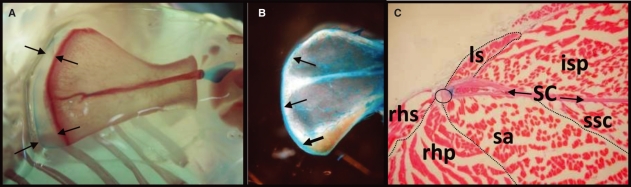
Lastly, we sought to establish the process of differentiation of the scapula in adult animals. The scapula undergoes differing degrees of ossification in a species-specific manner. In humans, the entire scapula ossifies, although some parts only after puberty with the medial border being the last region to do so (Williams et al. 1995, illustration 6.203). We found that, in the mouse, the medial border also remains cartilaginous for a long time (Fig. 3A–C). In particular, the cartilaginous medial border found in 4-week-old mice increased in size in a cranio-caudal direction (Fig. 3A). This margin remained cartilaginous even in 8-month-old mice, although it gradually ossified from the existing bone of the scapular body (Fig. 3B). Moreover, we found that the cartilaginous parts still served as the attachment of the medial girdle muscles (Fig. 3C), although the serratus anterior muscle then attaches to already ossified tissue (Fig. 3B,C).
Fig. 3.
Medial girdle muscles attach in the cartilaginous medial scapular margin. (A) Alizarin red and weak alcian blue staining delineate the ossified and cartilaginous parts of a 4-week-old mouse right scapula. The cartilaginous portion increases in size in a cranio-caudal direction. (B) This margin remains cartilaginous even in 8-month-old mice. (C) Transverse cryosection stained with eosin and alcian blue following demineralization showing the medial margin (circle) serving as the attachment of the medial girdle muscles. Orientation: dorsal to the left, cranial or left side at the top. SC, scapula medial girdle muscles; rhs and rhp, rhomboideus superficialis and profundus; sa, serratus anterior; ls, levator scapulae; isp, infraspinatus; ssc, subscapularis.
Altogether, these results indicate that the somite-derived medial border of the scapula shows delayed ossification and that its development, together with that of its associated muscles, is independent of the migratory mechanism regulated by the Pax3-Met axis.
Discussion
Primaxial somitic origin of the medial girdle
The skeletal elements of the forelimb and its girdle are traditionally thought to develop from the lateral plate mesoderm, whereas all of the musculature and axial skeleton arise from the axial somites. We show that the medial-most part of the mammalian scapula also develops from somites by subtracting the fate maps of Pax3-Cre and Wnt1-Cre mouse lines. This part of the scapula has a delayed ossification in the mouse and in the human it has its own ossification centre, being the last to ossify (Williams et al. 1995, illustration 6.203). Furthermore, it serves as an attachment of four hypaxial muscles (serratus anterior, rhomboideus maior and minor and levator scapulae), which are not migratory as they are not affected in the met-null embryos (our data and Prunotto et al. 2004). These muscles have been described as developing directly from the dermamyotomes (Starck, 1982). This is in contrast to the other forelimb muscles, which develop by single cell migration from the somites, a process that requires Met signalling. We therefore propose to refer to this part of the pectoral girdle as the medial girdle and its associated four muscles as medial girdle muscles.
Our results complement the findings of Durland et al. (2008) who have recently used a Prx1-Cre transgenic approach to identify all tissues that originate from the lateral plate mesoderm during mouse embryogenesis. They showed that most portions of the forelimb skeleton develop from the lateral plate. However, their lateral plate mesoderm identification system did not mark the medial border of the scapula, leading them to propose that it is somitic in origin and primaxial in its classification.
The innervation of medial girdle muscles is distinct from that of the limb muscles
Our data show that the medial scapular border with its medial girdle muscles has a separate developmental programme compared with the rest of the limb. This is also reflected by the innervation whereby the limb muscles are innervated from the brachial plexus, whereas the nerves for the medial girdle muscles branch directly off the cervical spinal nerves.
Homology of the mammalian medial scapular border with the avian scapular blade
Our previous work has shown that the scapular blade of the avian pectoral girdle originates from the dermamyotomal compartment of somites (Huang et al. 2000; Ehehalt et al. 2004) and not the lateral plate mesoderm like the rest of the limb skeleton (Christ & Ordahl, 1995). Furthermore, we showed that the blade was segmental in origin. The skeletal muscle and its connective tissues inserting onto the blade were also derived from the dermamyotome and, moreover, from the same segmental level. This is in keeping with the concept of the syndetome (Brent et al. 2003) where a somite gives rise to tendons from its primaxial compartment. The girdle muscles attaching to the blade are medially the rhomboideus superficialis and profundus, and ventrolaterally the serratus superficialis and profundus. The chick does not have a levator scapulae muscle, and thus for comparative studies the term levator scapulae-rhomboid complex is preferred (Nagashima et al. 2009).
We conclude that the medial border of mouse scapula is homologous to the avian scapular blade. Our finding that the connective tissue of the inserting muscles is also of somitic origin is another feature conserved between mammals and birds.
Model of scapula development
We propose a simple model for the formation of the scapula during vertebrate development. Firstly, the limb field somatopleura adjacent to the cervical/thoracic somites induces migration of the myogenic cells into the forming limb bud. Secondly, the somatopleura together with the ectoderm overlying the somites (Ehehalt et al. 2004) induce the dorsolateral somite quarter, the future ventrolateral dermamyotome (Wang et al. 2005), to form the medial border of the scapula (blade in birds) with its associated medial girdle muscles. Finally, the lateral dermamyotomal material grows laterally (and ventrally) into the final position of the medial scapular border. It is then adjacent to the lateral plate mesoderm, from which the rest of the scapula forms.
Curiously, the basic relationships between the scapula, its medial girdle muscles and vertebrae are preserved to a large extent even in turtle where, however, the whole scapula during its descent translocates ventrally before the growth of the ribs to take up a position inside the thorax (Nagashima et al. 2009).
Apart from the lateral plate mesoderm and the somites there is also a third source of cells for the scapula – from the neural crest. These cells are scattered in the surface of scapular spine, the acromion and coracoid in the mouse where the trapezius muscle attaches as part of its intramuscular connective tissue, which is itself of neural crest origin (Matsuoka et al. 2005).
The model for scapular development is not only of interest to developmental and evolutionary biologists but may also have clinical relevance. Sprengel’s deformity is a rare congenital disorder presenting as failure of the scapula to descend to its correct position from the neck to the thorax. Abnormal ossifying connections with the spine and aberrant development of muscles associated with the scapula are frequently reported. Some of these malformations arise in the trapezius muscle whose connective tissue is of neural crest origin (Matsuoka et al. 2005), whereas others (Baulot et al. 1998) develop in the rhomboid and levator scapulae whose connective tissue is of somitic origin.
Conclusion
Our somitic fate map in mouse showed that somites give rise to the medial scapular border. This confirms the homology of this part of scapula with the avian scapular blade. Furthermore, medial girdle muscles attaching to the medial scapula are non-migratory hypaxial muscles.
Acknowledgments
We thank E. Zelzer for the Pax3-Cre/Rosa-YFP mouse embryos. We thank A.P. McMahon, P. Soriano and H. Sucov for providing the Wnt1-Cre and R26R mouse lines and J.R. Sanes for providing the polyclonal rabbit antibody against Escherichia coli beta-galactosidase. We thank Mohammed I.E. Elashry for discussions on the anatomy. This study was supported by the Wellcome Trust (077750/205/Z, K.P. and P.V.), Ministry of Education of the Czech Republic (MSM 0021620806, E.K. and M.G.) and the German Research Foundation (DFG, Hu729-2, Hu729-5 to R.H.) project grants. We thank M. Pleschnerova for technical assistance.
References
- Baulot E, Trouilloud P, Giroux EA, et al. Ipsilateral omovertebral bones in the levator scapulae muscle and the rhomboid muscle in a Sprengel deformity: case report. Acta Orthop Belg. 1998;64:92–95. [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, et al. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Chevallier PA. Origine des ceintures scapulaires et pelviennes chez l’embryon d’oiseau. J Embryol Exp Morphol. 1977;42:275–292. [Google Scholar]
- Christ B, Ordahl CP. Early stages of chick somite development. Anat Embryol (Berl) 1995;191:381–396. doi: 10.1007/BF00304424. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, et al. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Durland JL, Sferlazzo M, Logan M, et al. Visualizing the lateral somitic frontier in the Prx1Cre transgenic mouse. J Anat. 2008;212:590–602. doi: 10.1111/j.1469-7580.2008.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehehalt F, Wang B, Christ B, et al. Intrinsic cartilage-forming potential of dermomyotomal cells requires ectodermal signals for the development of the scapula blade. Anat Embryol (Berl) 2004;208:431–437. doi: 10.1007/s00429-004-0415-0. [DOI] [PubMed] [Google Scholar]
- Engleka KA, Gitler AD, Zhang M, et al. Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol. 2005;280:396–406. doi: 10.1016/j.ydbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Epstein JA, Shapiro DN, Cheng J, et al. Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc Natl Acad Sci USA. 1996;93:4213–4218. doi: 10.1073/pnas.93.9.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz T, Kothary R, Surani MA, et al. The Splotch mutation interferes with muscle development in the limbs. Anat Embryol (Berl) 1993;187:153–160. doi: 10.1007/BF00171747. [DOI] [PubMed] [Google Scholar]
- Greene EC. Anatomy of the rat. Trans Am Philos Soc. 1935;27:1–370. [Google Scholar]
- Huang R, Zhi Q, Patel K, et al. Dual origin and segmental organisation of the avian scapula. Development. 2000;127:3789–3794. doi: 10.1242/dev.127.17.3789. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, et al. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Maina F, Casagranda F, Audero E, et al. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell. 1996;87:531–542. doi: 10.1016/s0092-8674(00)81372-0. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, et al. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–355. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima H, Sugahara F, Takechi M, et al. Evolution of the turtle body plan by the folding and creation of new muscle connections. Science. 2009;325:193–196. doi: 10.1126/science.1173826. [DOI] [PubMed] [Google Scholar]
- Prunotto C, Crepaldi T, Forni PE, et al. Analysis of Mlc-lacZ Met mutants highlights the essential function of Met for migratory precursors of hypaxial muscles and reveals a role for Met in the development of hyoid arch-derived facial muscles. Dev Dyn. 2004;231:582–591. doi: 10.1002/dvdy.20177. [DOI] [PubMed] [Google Scholar]
- Romer AS, Parsons TS. The Vertebrate Body. 6th ed. Philadelphia: Saunders College Publishing; 1986. [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck D. Vergleichende Anatomie der Wirbeltiere auf evolutionsbiologischer Grundlage. Band 3. Berlin: Springer-Verlag; 1982. [Google Scholar]
- Valasek P, Evans DJ, Maina F, et al. A dual fate of the hindlimb muscle mass: cloacal/perineal musculature develops from leg muscle cells. Development. 2005;132:447–458. doi: 10.1242/dev.01545. [DOI] [PubMed] [Google Scholar]
- Wang B, He L, Ehehalt F, et al. The formation of the avian scapula blade takes place in the hypaxial domain of the somites and requires somatopleure-derived BMP signals. Dev Biol. 2005;287:11–18. doi: 10.1016/j.ydbio.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Williams PL, Bannister LH, Gray H. Gray’s Anatomy: The Anatomical Basis of Medicine and Surgery. 38th edn. New York: Churchill Livingstone; 1995. [Google Scholar]