Abstract
The ventral horn of the rat spinal cord was investigated with respect to the somatotopic organization of the motor neurons that innervate the lumbar muscles. Neurotracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) was applied to specific sites in lumbar muscles. Spinal cord segments at L1 through L4 levels were cut into 40-μm serial transverse sections. Labeled neurons were located in the ventromedial nucleus (VM) and lateromedial nucleus (LM) nuclei of Rexed’s lamina IX. Motor neurons innervating the m. interspinales lumborum and m. multifidus were without exception present in the VM, whereas all motor neurons innervating the m. rectus abdominis were present in the LM. Forty percent of motor neurons innervating the m. quadratus lumborum were present in the VM and the other 60% were in the LM. Although most of the motor neurons innervating the m. psoas major were present in the LM, a few labeled neurons existed in the VM. These results suggest that the border zone demarcating the areas of innervation of the dorsal and ventral rami of spinal nerves crosses the m. quadratus lumborum.
Keywords: lumbar muscles, motor neurons, rat, somatotopic organization, spinal cord ventral horn
Introduction
The origin of low back pain is difficult to detect in patients without radiculopathic symptoms. In such cases, the pain itself is the only clue for determination of its origin. Previous experimental studies of lumbar referred pain have suggested that disorders of deep somatic tissues and internal organs result in pain in a body surface area specific to the damaged tissue (Kakazu, 1978; McCall et al. 1979; Fortin et al. 1994; Maigne et al. 1996; Fukui et al. 1997). Given that any site on the lumbar spine results in pain in a specific area, the area in which the pain is found should indicate its origin.
To establish a method for the diagnosis of the origin of pain from its location, knowledge of the rostrocaudal and dorsoventral structures of peripheral afferent fibers innervating the lumbar spine is of crucial importance. Detailed maps of peripheral nerve fibers related to the lumbar spine and associated structures are required for the peripheral tissues, dorsal root ganglia, and spinal cord. To this aim, we have reported the rostrocaudal, that is, segmental organization of cutaneous afferent fibers on the body surface (Takahashi et al. 1994; Takahashi & Nakajima, 1996) and that in the dorsal horn (Takahashi et al. 2002, 2003a), dorsal root ganglion neurons innervating the lumbar spine (Takahashi et al. 2003b), and the central field of projection of the spinal nerves (Takahashi et al. 2006).
The segmental organization of spinal motor neurons, ventral nerve roots, or spinal nerves that project efferent fibers to a muscle are termed the ‘myotomes’ of the muscle. Myotomes are indispensable clinically in performing both the manual muscle test and electromyographic examinations. Compared with those of limb muscles, myotomes of spinal muscles are unspecific: Despite an expected metameric pattern of spinal muscles, multisegmental, not specific segmental innervation patterns has been described for the myotomes of spinal muscles.
The dorsoventral organization of the peripheral nerves and nerve fibers has been reported for muscles of the body trunk of vertebrates (Sato et al. 1995). Despite several investigations of the dorsoventral organization of afferent fibers in the spinal cord (Rivero-Melian, 1996; Takahashi et al. 2003a, 2006) and dorsal root ganglia (Burton & McFarlane, 1973; Kausz & Rethelyi, 1985; Peyronnard et al. 1990; Puigdellivol-Sanchez et al. 1998), no reports have yet been published regarding the dorsoventral organization of the afferent fibers innervating the lumbar spine.
We have clarified that the lumbar spine mainly receives afferent fibers from rostral dorsal root ganglia in rats (Takahashi et al. 2003b). Afferent fibers of the lumbar spine showed a non-metameric pattern. In the transverse plane, the further from the intervertebral foramen, the more rostral the dorsal root ganglia innervated. The ventral portion of the lumbar disc and the spinal process of the L5 vertebra were both innervated mainly by the L2 ganglia (Takahashi et al. 2003b). We predict such a non-segmental innervation pattern for myotomes.
Previously, we investigated the central projection field of the afferents from the lumbar intervertebral disc and psoas muscle using the fluorescent neurotracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI). Disappointingly, the projection fields in the dorsal horn were not visible with DiI-labeling (Takahashi et al. 2006). However, motor neurons in the ventral horn were clearly labeled with DiI, suggesting that the dorsoventral organization of the lumbar spine could be represented as a mediolateral organization of motor neurons innervating lumbar spinal muscles. In the present study, we sought to investigate the locations of the motor neurons innervating lumbar spinal muscles in the ventral horn of the spinal cord in rats.
Materials and methods
A total of 49 adult male Sprague Dawley rats were used in the present study. Rats were anesthetized with sodium pentobarbital (40 mg kg−1, i.p.) prior to surgery. Experimental protocols for this study were approved by the Ethical Committee for Animal Experiments of the Chiba University Graduate School of Medicine.
Neurotracer application
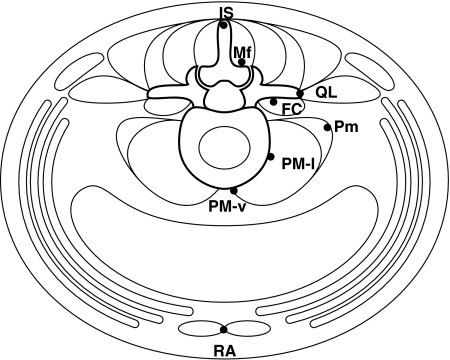
The fluorescent neurotracer DiI was applied to one of the following sites in each rat: m. interspinales lumborum at the spinous process of the L5 vertebra (IS; n = 9), m. multifidus at the lamina of the L5 vertebra (Mf; n = 9), m. quadratus lumborum at the transverse process of the L5 vertebra (QL; n = 5), m. flexor caudae at the dorsolateral surface of the L5–L6 intervertebral disc (FC; n = 8), m. psoas minor at the L5–L6 intervertebral disc level (Pm; n = 5), m. psoas major at the lateral surface of the L5–L6 intervertebral disc (PM-l; n = 9), m. psoas major at the ventromedial surface of the L5–L6 intervertebral disc (PM-v; n = 3), and m. rectus abdominis at the pubic synthesis (RA; n = 3) (Fig. 1). Application sites were chosen from those that are innervated by the L2 and L3 spinal nerves (Takahashi et al. 2003b). Neurotracer was applied dorsally for IS and Mf with the rats in a prone position, and ventrally for the remaining sites with the rats in a supine position. A small amount of DiI crystals (Molecular Probes, Eugene, OR, USA) weighing approximately 10 μg was attached to the tip of a wooden toothpick and applied to a site. The applied DiI was not sealed into the muscle with glue to avoid chemical damage to the fine muscular nerve fibers at the application site. The fissure in muscles caused by the exposure of an application site was closed. Rats were fed ad libitum in a cage in a room with controlled temperature and light for 10 days until removed for tissue fixation.
Fig. 1.
Illustration of the sites to which DiI was applied (closed circles). All the sites are located in the transverse plane through the L5 vertebra and L5–L6 intervertebral disc. IS, m. interspinales lumborum at the spinous process of the L5 vertebra; Mf, m. multifidus at the lamina of the L5 vertebra; QL, m. quadratus lumborum at the transverse process of the L5 vertebra; FC, m. flexor caudae at the dorsolateral surface of the L5–L6 intervertebral disc; Pm, m. psoas minor at the L5–L6 intervertebral disc level; PM-l, m. psoas major at the lateral surface of the L5–L6 intervertebral disc; PM-v, m. psoas major at the ventromedian surface of the L5–L6 intervertebral disc; RA, m. rectus abdominis at the pubic synthesis.
Double labeling
To observe the locations of the two motor neuron groups innervating the Mf and PM-v sites simultaneously, double labeling was conducted in two of the 49 treated rats. In these two rats, DiI and another fluorescent neurotracer, Fluoro-Gold (FG; Fluorochrome, Denver, CO, USA) were applied to the PM-v and Mf sites, respectively. Crystals of FG weighing approximately 10 μg were applied to the Mf site in the same manner as the DiI application.
Fixation and preparation of sections
Rats were terminally anesthetized and transcardially perfused with 500 mL of 4% paraformaldehyde in phosphate buffer (0.1 mol L−1, pH 7.4) at 4 °C. A lumbar laminectomy was performed and a midsagittal incision was made in the dura mater. Spinal cord segments L1 through L4 were determined from a dorsal aspect by identifying the entry of dorsal rootlets under a binocular microscope. Intersegmental points were marked with thick India ink. The spinal cord was removed and immersed in 4% paraformaldehyde in phosphate buffer fixative at 4 °C overnight. DRGs L1 through L4 were removed and immersed in phosphate-buffered saline (PBS, 0.01 mol L−1, pH 7.2) containing 20% sucrose for 48 h at 4 °C. The spinal cord was cut into blocks L1 through L4, followed by separate immersion in PBS containing 20% sucrose for 48 h at 4 °C.
The spinal cord blocks and DRGs were immersed in Tissue-Tek (Sakura, Tokyo, Japan) and frozen in a −80 °C freezer. The spinal cord blocks and DRGs were cut into 40-μm successive transverse and longitudinal sections, respectively, on a cryostat. Sections were mounted on standard glass slides, air dried, and coverslipped with Permaflour (Shandon, Pittsburgh, PA, USA). Spinal cord sections at 160-μm intervals (every fifth section) were mounted. The number of mounted sections ranged between 15 and 25 for each segmental block of the spinal cord.
Plotting and counting of labeled neurons
Sections were viewed immediately using an Eclipse-E600 microscope (Nikon, Tokyo, Japan) equipped with a G-1A filter (excitation wavelength: 546 nm) to observe DiI-labeled neurons. FG-labeled neurons were observed using a UV-1A filter (excitation wavelength: 365 nm). Only neurons with a clearly visible nucleus were counted and considered for analysis.
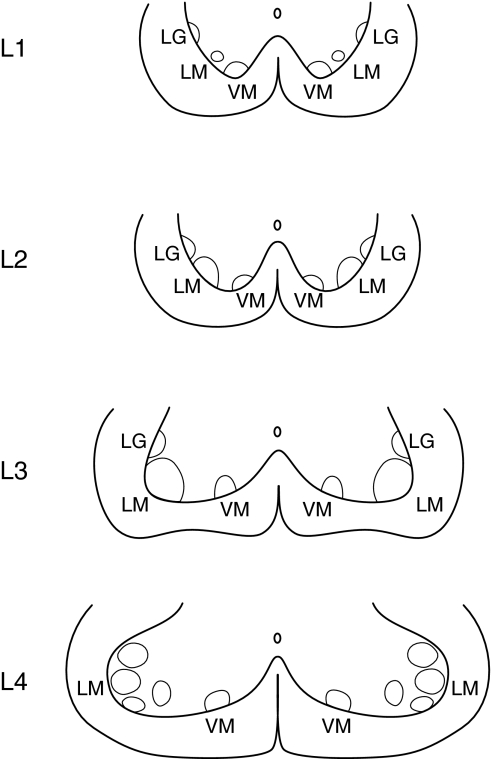
The locations of labeled motor neurons were directly plotted on transverse charts of the spinal cord segments L1 through L4 as reported by Molander et al. (1984) (Fig. 2). After observation, plotted neurons were classified and counted for the nuclei of lamina IX. Two or three nuclei, the ventromedial (VM), lateromedial (LM), and lateral group (LG) neurons were identified in the spinal cord segments (Molander et al. 1984). Because every fifth section was observed, the number of labeled neurons in a spinal segment was multiplied by five to obtain the actual number of labeled neurons in the spinal segment.
Fig. 2.
Three nuclei of Rexed’s lamina IX in the ventral horn of the rat spinal cord L1 through L4 (Molander et al. 1984; Paxinos & Watson, 1998). VM, ventromedial nucleus; LM, lateromedial nucleus; LG, lateral group. Modifications (only the ventral horn is shown) from the original drawings were made by the present authors.
Statistical analysis
Differences in the number of labeled neurons within spinal segments L1–L4 was determined using a one-way analysis of variance. The difference in the number of labeled motor neurons between the VM and LM nuclei was determined using a paired t-test. The level of significance was set at P< 0.05. All data are presented as the mean ± SD.
Results
Neurotracers at fixation stage
After fixation and resection of the spinal cord, we observed application sites under a binocular microscope. An area stained with DiI could be confirmed upon observation of orange-colored tissue. In no rat considered, did the DiI invade adjacent muscles. FG staining could not be verified because it is not detectable after fixation.
Segmental distribution of labeled neurons
Motor neurons labeled with DiI and FG were clearly visible, emitting an intense fluorescence (Fig. 3). In contrast, the central projection fields of muscular afferent fibers were not visible even in tissue sections showing labeled motor neurons.
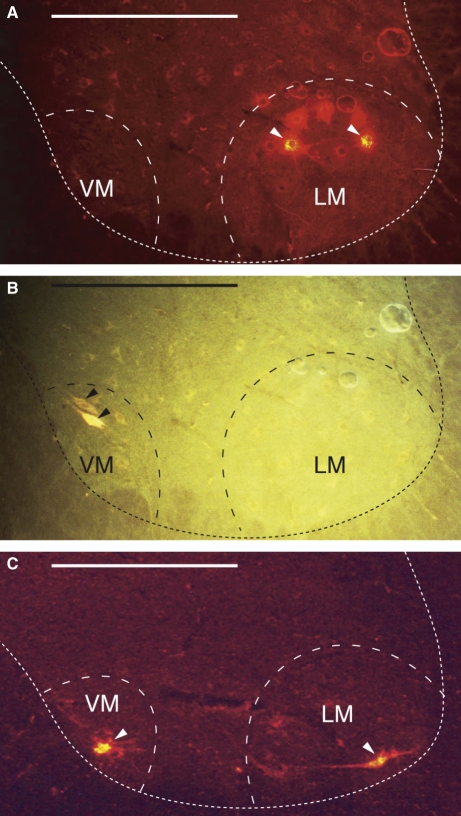
Fig. 3.
Photographic presentation of fluorescent motor neurons in transverse sections of the spinal cord. (A) and (B) are the same section from the spinal cord segment L3 from a rat in which DiI and FG were applied to the m. psoas major (PM-l) and to the m. multifidus (Mf), respectively. (A) Viewed with a G1-A filter. Two DiI-labeled neurons (arrowheads) are identified in the lateromedial nucleus (LM). (B) Viewed with a UV filter. One FG-labeled neuron (arrowheads) is identified in the ventromedial nucleus (VM). (C) A section of the spinal cord segment L3 from a rat in which DiI was applied to the m. quadratus lumborum (QL). DiI-labeled neurons (arrowheads) are present in both the VM and the LM nuclei. The dotted line indicates the contour of the ventral horn. Horizontal bar = 500 μm.
Labeled motor neurons were present in spinal cord segments L1 through L4. The number of labeled motor neurons was greatest in the L2 or L3 spinal cord segments for all the application sites. The difference was statistically significant (P < 0.05 or P < 0.01) except for the RA site (Fig. 4).
Fig. 4.
Number of labeled neurons in the spinal cord segments L1–L4 and DRGs L1–L4. Data are presented as the mean ± SD. *P < 0.05, **P < 0.01. IS, m. interspinales lumborum at the spinous process of the L5 vertebra; Mf, m. multifidus at the lamina of the L5 vertebra; QL, m. quadratus lumborum at the transverse process of the L5 vertebra; FC, m. flexor caudae at the dorsolateral surface of the L5–L6 intervertebral disc; Pm, m. psoas minor at the L5–L6 intervertebral disc level; PM-l, m. psoas major at the lateral surface of the L5–L6 intervertebral disc; PM-v, m. psoas major at the ventromedial surface of the L5–L6 intervertebral disc; RA, m. rectus abdominis at the pubic synthesis.
The distribution of labeled neurons in the DRGs was greatest in L2 or L3 for most of the sites except for the Pm site. The difference was statistically significant (P < 0.01) for IS, Mf, Pm, and PM-l (Fig. 4). Thus, the segmental distribution of motor neurons was almost synchronized with that of DRG neurons. This finding implies that the spinal segments of motor neurons innervating a muscle are identical to those of DRG neurons projecting afferent fibers to the muscle.
Mediolateral locations of labeled motor neurons
Rexed’s lamina IX consists of collections of large motor neurons (Paxinos & Watson, 1998). As stated by Molander et al. (Molander & Grant, 1986), borderlines between the nuclei of lamina IX are very difficult to delineate. However, labeled neurons in the ventromedial corner formed a well-defined neuron group throughout L1 and L4 spinal cord segments in this study. Hence, the VM nucleus could be well delineated. In contrast, labeled neurons outside of the VM nucleus were located in various regions in the ventral horn (Fig. 2). Evidently, no neurons were in the LG nucleus. Therefore, we judged labeled neurons outside of the VM nucleus to be located in the LM nucleus.
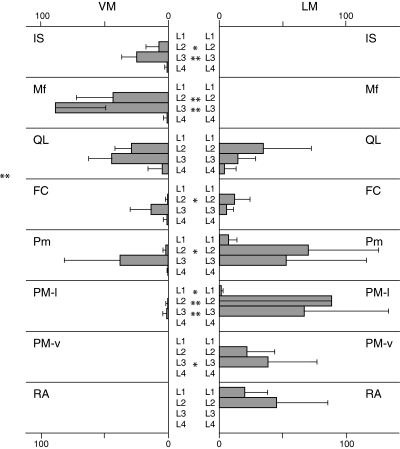
All labeled neurons innervating the IS and Mf sites were present in the VM, whereas all neurons innervating the PM-v and RA sites were present in the LM (Fig. 5). Labeled neurons innervating the QL, FC, Pm, and PM-l were present in both the VM and LM (Fig. 5). In the VM and LM, 46 and 54% of labeled motor neurons were present, respectively, for the QL site, 46 and 54% of labeled motor neurons were present, respectively, for the FC site, 24 and 76%, respectively, for the Pm site; and 1 and 99%, respectively, for the PM-l site. In the QL, FC, Pm, and PM-l sites, the differences in the number of labeled neurons between the VM and LM nuclei were not statistically significant for L2 (QL, Pm) or L3 (QL, FC, Pm), indicating that labeled motor neurons were evenly distributed in the VM and LM nuclei in these DRGs.
Fig. 5.
Number of labeled motor neurons in the ventromedial (VM) and lateromedial (LM) nuclei of lamina IX in the ventral horn of L1–L4 spinal segments. Data are presented as mean ± SD. *P < 0.05, **P < 0.01. IS, m. interspinales lumborum at the spinous process of the L5 vertebra; Mf, m. multifidus at the lamina of the L5 vertebra; QL, m. quadratus lumborum at the transverse process of the L5 vertebra; FC, m. flexor caudae at the dorsolateral surface of the L5–L6 intervertebral disc; Pm, m. psoas minor at the L5–L6 intervertebral disc level; PM-l, m. psoas major at the lateral surface of the L5–L6 intervertebral disc; PM-v, m. psoas major at the ventromedian surface of the L5–L6 intervertebral disc; RA, m. rectus abdominis at the pubic synthesis. Note that application of neurotracer to the QL, FC, Pm, and PM sites produced labeled motor neurons in both the VM or LM nuclei.
Discussion
Central organization of muscular nerve fibers by neurotracing
The central projections of the afferent fibers innervating hindlimb muscles have been investigated using neurotracers including horseradish peroxidase (HRP) (Hoheisel et al. 1989; Rivero-Melian, 1996; Panneton et al. 2005), cholera toxin B subunit (CTB) (Hirakawa et al. 1992), and Phaseolus vulgaris leukoagglutinin (PHA-L) (Ling et al. 2003). These studies indicated that the central projection terminals of hindlimb muscular afferent fibers showed a somatotopic organization in the spinal cord dorsal horn, as demonstrated for the central projections of peripheral nerves (Molander & Grant, 1986; Rivero-Melian, 1996) and cutaneous afferents (Brown & Fuchs, 1975; Koerber & Brown, 1980; Shortland et al. 1989; Rivero-Melian & Grant, 1991; Takahashi et al. 2003a).
Reports regarding the central projection of the afferent fibers innervating the lumbar spine are scarce. Gillette et al. (1993) reported the central projection fields of the lumbar facet joint using HRP conjugated to wheat germ agglutinin (WGA-HRP) in cats (Gillette et al. 1993). We attempted to observe the central projection field of the afferents innervating the lumbar intervertebral disc. Disappointingly, it could barely be observed with DiI labeling (Takahashi et al. 2006). No other reports were found regarding the central projections of the afferent fibers innervating lumbar spine.
Motor neurons have been labeled successfully in the spinal cord ventral horn using various neurotracers including HRP (Crockett et al. 1987; Rivero-Melian, 1996; Gerrits et al. 1997; Vanderhorst & Holstege, 1997; Panneton et al. 2005), CTB (Hirakawa et al. 1992; Tani et al. 1994), PHA-L (Ling et al. 2003), and by DiI (Kobbert & Thanos, 2000).
Myotomes of lumbar spinal muscles
The present study revealed that the myotomes of lumbar spinal muscles differ from the segmental level of the lumbar spinal site from which the muscle originates. It was demonstrated that the segmental innervation patterns of the efferent and afferent nervous system are synchronized, showing a non-metameric pattern. Muscles originating from the L5 vertebra were innervated by motor and DRG neurons predominantly in the L2 and L3 segments (Fig. 4).
Dorsoventral organization
Motor neurons innervating the sites on the dorsal elements of the lumbar spine (IS and Mf) were located in the VM nucleus. Motor neurons innervating the sites on the ventral portion of the lumbar intervertebral disc (PM-v) and on the pubis (RA) were located only in the LM nucleus. For the sites on the lateral elements of the lumbar spine (QL, FC, and PM-l), motor neurons were located both in the VM and LM nuclei (Fig. 5). Thus, motor neurons innervating the sites in the muscles from dorsal and ventral sides of the lumbar spine were located in the VM and LM nucleus, respectively. Motor neurons innervating the muscles from lateral lumbar spine were located either in the VM or in the LM nucleus.
It has been reported that epaxial muscles are innervated by motor neurons in the VM nucleus (Fetcho, 1987; Gutman et al. 1993; Vanderhorst & Holstege, 1997; Sharma & Belmonte, 2001; Schneider & Sulner, 2006; Agalliu et al. 2009). ‘Double nuclei innervation’ that was observed for m. quadratus lumborum, m. flexor caudae, and m. psoas major in the present study has been reported for m. serratus (Tani et al. 1994) and for m. psoas major (Vanderhorst & Holstege, 1997). Macroscopically, Bogduk reported the following regarding the innervation of lumbar spinal muscles through human cadaveric studies (Bogduk et al. 1981; Bogduk, 1983):
Dorsal rami of the spinal nerves innervate supra-axial muscles (m. multifidus, m. longissimus, m. iliocostalis).
Ventral rami of the spinal nerves innervate infra-axial muscles (m. psoas major, m. psoas minor) and limb muscles.
Muscles originating from the transverse process of the spine (m. intertransversarii lumborum, m. quadratus lumborum) are supplied by nerve branches either from the dorsal or ventral rami of spinal nerves (Bogduk, 1997). The present results are consistent with these anatomical observations.
Spatial relationships in the dorsal and ventral horn
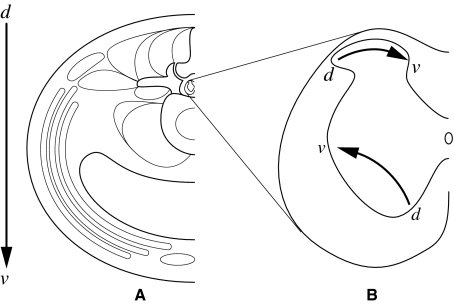
The present study clarified that the dorsoventral organization in lumbar spinal muscles is represented as a mediolateral organization of motor neurons innervating the muscles in the spinal cord ventral horn. Spatially, the dorsoventral axis in the lumbar trunk may be reproduced as the mediolateral axis in the ventral horn of the spinal cord musculoskeletal tissue.
We reported that the dorsoventral axis in the lumbar skin is reproduced as the lateromedial axis in the dorsal horn of the spinal cord (Takahashi et al. 2003a). The dorsoventral organization of thoracic spinal nerve branches is represented as a lateromedial organization of their central projection fields (Ygge & Grant, 1983). Thus, it is likely that the horizontal axes are the same for the cutaneous and muscular afferent projection fields in the dorsal horn. It is assumed that the dorsoventral axis in the lumbar muscular afferent fibers is reproduced as a lateromedial axis in the dorsal horn of the spinal cord. If this is the case, interestingly, the dorsoventral axis of the lumbar muscles is reproduced inversely in the horizontal direction in the dorsal and ventral horns of the spinal cord (Fig. 6).
Fig. 6.
Illustration of how the dorsoventral axis of the lumbar musculoskeletal tissues is reproduced in the dorsal and ventral horn of the spinal cord. Note that the directions are reversed in the dorsal and ventral horns.
References
- Agalliu D, Takada S, Agalliu I, et al. Motor neurons with axial muscle projections specified by Wnt4/5 signaling. Neuron. 2009;61:708–720. doi: 10.1016/j.neuron.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogduk N. The innervation of the lumbar spine. Spine. 1983;8:286–293. doi: 10.1097/00007632-198304000-00009. [DOI] [PubMed] [Google Scholar]
- Bogduk N. Nerves of the lumbar spine. In: Bogduk N, editor. Clinical Anatomy of the Lumbar Spine and Sacrum. New York: Churchill Livingstone; 1997. p. 143. [Google Scholar]
- Bogduk N, Tynan W, Wilson AS. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132:39–56. [PMC free article] [PubMed] [Google Scholar]
- Brown PB, Fuchs JL. Somatotopic representation of hindlimb skin in cat dorsal horn. J Neurophysiol. 1975;38:1–9. doi: 10.1152/jn.1975.38.1.1. [DOI] [PubMed] [Google Scholar]
- Burton H, McFarlane JJ. The organization of the seventh lumbar spinal ganglion of the cat. J Comp Neurol. 1973;149:215–232. doi: 10.1002/cne.901490207. [DOI] [PubMed] [Google Scholar]
- Crockett DP, Harris SL, Egger MD. Plantar motoneuron columns in the rat. J Comp Neurol. 1987;265:109–118. doi: 10.1002/cne.902650108. [DOI] [PubMed] [Google Scholar]
- Fetcho JR. A review of the organization and evolution of motoneurons innervating the axial musculature of vertebrates. Brain Res. 1987;434:243–280. doi: 10.1016/0165-0173(87)90001-4. [DOI] [PubMed] [Google Scholar]
- Fortin JD, Aprill CN, Ponthiux B, et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part II: clinical evaluation. Spine. 1994;19:1483–1489. [PubMed] [Google Scholar]
- Fukui S, Ohseto K, Shiotani M, et al. Distribution of referred pain from the lumbar zygapophyseal joint and dorsal rami. Clin J Pain. 1997;13:303–307. doi: 10.1097/00002508-199712000-00007. [DOI] [PubMed] [Google Scholar]
- Gerrits PO, Boers J, Holstege G. The lumbar cord location of the motoneurons innervating psoas and iliacus muscles: a single and double labeling study in the female Syrian golden hamster. Neurosci Lett. 1997;237:125–128. doi: 10.1016/s0304-3940(97)00842-2. [DOI] [PubMed] [Google Scholar]
- Gillette RG, Kramis RC, Roberts WJ. Spinal projection of cat primary afferent fibers innervating lumbar facet joints and multifidus muscle. Neurosci Lett. 1993;157:67–71. doi: 10.1016/0304-3940(93)90644-z. [DOI] [PubMed] [Google Scholar]
- Gutman CR, Ajmera MK, Hollyday M. Organization of motor pools supplying axial muscles in the chicken. Brain Res. 1993;609:129–136. doi: 10.1016/0006-8993(93)90865-k. [DOI] [PubMed] [Google Scholar]
- Hirakawa M, McCabe JT, Kawata M. Time-related changes in the labeling pattern of motor and sensory neurons innervating the gastrocnemius muscle, as revealed by the retrograde transport of the cholera toxin B subunit. Cell Tissue Res. 1992;267:419–427. doi: 10.1007/BF00319364. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Hehmann-Willenbrock E, Mense S. Termination patterns of identified group II and III afferent fibres from deep tissues in the spinal cord of the cat. Neuroscience. 1989;28:495–507. doi: 10.1016/0306-4522(89)90195-4. [DOI] [PubMed] [Google Scholar]
- Kakazu K. A clinical study on pain arising from lower lumbar intervertebral joints, using a new electrical stimulation method. J Jpn Orthop Assoc. 1978;52:509–520. [Google Scholar]
- Kausz M, Rethelyi M. Lamellar arrangement of neuronal somata in the dorsal root ganglion of the cat. Somatosens Res. 1985;2:193–204. doi: 10.3109/07367228509144563. [DOI] [PubMed] [Google Scholar]
- Kobbert C, Thanos S. Topographic representation of the sciatic nerve motor neurons in the spinal cord of the adult rat correlates to region-specific activation patterns of microglia. J Neurocytol. 2000;29:271–283. doi: 10.1023/a:1026523821261. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Brown PB. Projections of two hindlimb cutaneous nerves to cat dorsal horn. J Neurophysiol. 1980;44:259–269. doi: 10.1152/jn.1980.44.2.259. [DOI] [PubMed] [Google Scholar]
- Ling L-J, Honda T, Shimada Y, et al. Central projection of unmyelinated (C) primary afferent fibers from gastrocnemius muscle in guinea pig. J Comp Neurol. 2003;461:140–150. doi: 10.1002/cne.10619. [DOI] [PubMed] [Google Scholar]
- Maigne J-Y, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation testes in 54 patients with low back pain. Spine. 1996;21:1889–1892. doi: 10.1097/00007632-199608150-00012. [DOI] [PubMed] [Google Scholar]
- McCall IW, Park WM, O’Brien JP. Induced pain referral from posterior lumbar elements in normal subjects. Spine. 1979;4:441–446. doi: 10.1097/00007632-197909000-00009. [DOI] [PubMed] [Google Scholar]
- Molander C, Grant G. Laminar distribution and somatotopic organization of primary afferent fibers from hindlimb nerves in the dorsal horn. A study by transganglionic transport of horseradish peroxidase in the rat. Neuroscience. 1986;19:297–312. doi: 10.1016/0306-4522(86)90023-0. [DOI] [PubMed] [Google Scholar]
- Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol. 1984;230:133–141. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Gan Q, Juric R. The central termination of sensory fibers from nerves to the gastrocnemius muscle of the rat. Neurosci Lett. 2005;134:175–187. doi: 10.1016/j.neuroscience.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinations. San Diego: Academic Press; 1998. [Google Scholar]
- Peyronnard J-M, Messier J-P, Dubreuil M, et al. Three-dimensional computer-aided analysis of the intraganglionic topography of primary muscle afferent neurons in the rat. Anat Rec. 1990;227:405–417. doi: 10.1002/ar.1092270404. [DOI] [PubMed] [Google Scholar]
- Puigdellivol-Sanchez A, Prats-Galio A, Ruano-Gil D, et al. Sciatic and femoral nerve sensory neurons occupy different regions of the L4 dorsal root ganglion in the adult rat. Neurosci Lett. 1998;251:169–172. doi: 10.1016/s0304-3940(98)00518-7. [DOI] [PubMed] [Google Scholar]
- Rivero-Melian C. Organization of hindlimb nerve projections to the rat spinal cord: a choleragenoid horseradish peroxidase study. J Comp Neurol. 1996;364:651–663. doi: 10.1002/(SICI)1096-9861(19960122)364:4<651::AID-CNE4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Rivero-Melian C, Grant G. Choleragenoid horseradish peroxidase used for studying projections of some hindlimb cutaneous nerves and plantar foot afferents in the dorsal horn and Clarke’s column in the rat. Exp Brain Res. 1991;84:125–132. doi: 10.1007/BF00231767. [DOI] [PubMed] [Google Scholar]
- Sato T, Horiguchi M, Kida M, et al. Anatomy of the Peripheral Nervous System. Tokyo: Science Communications International; 1995. [Google Scholar]
- Schneider H, Sulner B. Innervation of dorsal and caudal fin muscles in adult zebrafish Danio rerio. J Comp Neurol. 2006;497:702–716. doi: 10.1002/cne.21038. [DOI] [PubMed] [Google Scholar]
- Sharma K, Belmonte JC. Development of the limb neuromuscular system. Curr Opin Cell Biol. 2001;13:204–210. doi: 10.1016/s0955-0674(00)00198-8. [DOI] [PubMed] [Google Scholar]
- Shortland P, Woolf CJ, Fitzgerald M. Morphology and somatotopic organization of the central terminals of hindlimb hair follicle afferents in the rat lumbar spinal cord. J Comp Neurol. 1989;289:416–433. doi: 10.1002/cne.902890307. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nakajima Y. Dermatomes in the rat limbs as determined by antidromic stimulation of sensory C-fibers in spinal nerves. Pain. 1996;67:197–202. doi: 10.1016/0304-3959(96)03116-8. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nakajima Y, Sakamoto T. Dermatome mapping in the rat hindlimb by electrical stimulation of the spinal nerves. Neurosci Lett. 1994;168:85–88. doi: 10.1016/0304-3940(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Chiba T, Sameda H, et al. Organization of cutaneous ventrodorsal and rostrocaudal axial lines in the rat hindlimb and trunk in the dorsal horn of the spinal cord. J Comp Neurol. 2002;445:133–144. doi: 10.1002/cne.10158. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Chiba T, Kurokawa M, et al. Dermatomes and the central organization of dermatomes and body surface regions in the spinal cord dorsal horn in rats. J Comp Neurol. 2003a;462:29–41. doi: 10.1002/cne.10669. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Chiba T, Kurokawa M, et al. Stereoscopic structure of sensory nerve fibers in the lumbar spine and related tissues. Spine. 2003b;28:871–880. doi: 10.1097/01.BRS.0000058717.43888.B9. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Aoki Y, Douya H, et al. Projection field of primary afferent fibers innervating the ventral portion of the lumbar intervertebral disc in the spinal cord dorsal horn. Anat Sci Int. 2006;81:92–99. doi: 10.1111/j.1447-073X.2006.00137.x. [DOI] [PubMed] [Google Scholar]
- Tani M, Kida MY, Akita K. Relationship between the arrangement of motoneuron pools in the ventral horn and ramification pattern of the spinal nerve innervating trunk muscles in the cat (Felis domestica) Exp Neurol. 1994;128:290–300. doi: 10.1006/exnr.1994.1139. [DOI] [PubMed] [Google Scholar]
- Vanderhorst V, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol. 1997;382:46–76. [PubMed] [Google Scholar]
- Ygge J, Grant G. The organization of the thoracic spinal nerve projection in the rat dorsal horn demonstrated with transganglionic transport of horseradish peroxidase. J Comp Neurol. 1983;216:1–9. doi: 10.1002/cne.902160102. [DOI] [PubMed] [Google Scholar]