Abstract
A total of 1,991 group B streptococcus (GBS) isolates, collected in 2008 and 2009, were tested for non-penicillin susceptibility by broth microdilution, disk testing, and oxacillin screening agar. No GBS isolates were non-penicillin susceptible. Oxacillin and ceftizoxime disk testing results showed that proposed screening criteria are nonspecific. The oxacillin screening agar was specific but of unknown sensitivity.
Group B streptococcus (GBS) is a normal commensal of the genitourinary tract; however, it can be a major causative organism of invasive infections in neonates and pregnant women (8) and is a significant cause of invasive disease in the elderly and in diabetics (7). Penicillin G is the treatment of choice for both the prophylaxis and treatment of GBS infection. Recently non-penicillin-susceptible GBS (PNSGBS) isolates have been reported in Japan, the United States, and elsewhere, due mainly to a Q557E mutation in pbp2x (2, 3, 6, 7, 9). These reports led us to test a 2007 collection of 200 consecutively collected GBS isolates for penicillin G resistance by broth microdilution testing. One resistant isolate was found (MIC, 0.25 μg/ml) which contained the pbp2x Q557E mutation, suggesting that our PNSGBS prevalence rate ranged between 0.01 and 2.7% (95% confidence interval [CI]). To determine a more accurate estimate of the true incidence of PNSGBS in our hospitals, we extended this study to include 2,000 consecutive GBS isolates, with a secondary aim of refining and determining the specificity of a proposed penicillin resistance screening method (4, 5).
GBS isolates from vaginal-rectal specimens were identified by a positive reaction in the selective chromogenic GBS medium, Carrot Broth (Hardy Diagnostics, Santa Maria, CA). GBS isolates from other sources were identified by their colony morphology, Gram reaction and appearance, catalase reaction, and their positive reaction with GBS antiserum.
Consecutive GBS isolates collected between August 2008 and March 2009 were frozen in Mueller-Hinton 20% glycerol broth at −70°C and, at a later date, subcultured onto tryptic soy 5% sheep blood agar (TSA). Any bacteria not having the characteristic GBS colony morphology and beta-hemolytic pattern and not having a negative catalase test were reidentified. Nine of the 2,000 consecutive isolates were found not to be GBS, leaving 1,991 isolates for susceptibility testing and penicillin resistance screening.
In addition to performing penicillin broth microdilution testing of all GBS isolates, we also tested three different screening methods for detecting PNSGBS—ceftizoxime and oxacillin disk tests and growth on TSA containing oxacillin (0.5 μg/ml). The disk tests were selected based on a report of their utility in detecting PNSGBS (4); the oxacillin screening agar use was based on a report that the oxacillin broth MIC for PNSGBS isolates was >2 μg/ml (3). We found that our 2007 PNSGBS isolate failed to grow on TSA containing 2 μg/ml of oxacillin but that it grew well when the oxacillin concentration was reduced to 0.5 μg/ml.
Penicillin G broth microdilution susceptibility testing was performed using CLSI guidelines (1), with penicillin G concentrations of 0.06 and 0.12 μg/ml. Disk diffusion tests were performed with oxacillin disks (1 μg) and ceftizoxime disks (30 μg) using CLSI guidelines for disk diffusion testing of beta-hemolytic streptococci; Remel Mueller-Hinton agar with 5% sheep blood was used (Lenexa, KS) (1). Zones of inhibition were measured under reflected light by using a certified electronic caliper. TSA-oxacillin (0.5 μg/ml) plates were inoculated (1 μl) with the same bacterial inoculum density used for the disk diffusion tests and incubated at 35 to 37°C in 5 to 10% CO2 for 20 to 24 h before reading; any growth was scored as positive. All bacteriologic media and antibiotic-containing disks were supplied by BD Diagnostics, Franklin Lakes, NJ, except as noted.
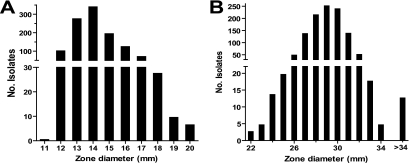
A total of 1,191 GBS strains were collected during the study period. Most of them were from genitourinary specimens (n = 1,074); others were from wounds (n = 52), blood (n = 2), and unspecified sites (n = 63). All of the isolates tested were penicillin susceptible (MIC, ≤0.06 μg/ml), giving a PNSGBS prevalence of 0.00% (99.5% CI, 0.00 to 0.27%). The median oxacillin disk test zone diameter was 14 mm, with a range of 11 to 20 mm (Fig. 1). Fifty-four percent of isolate oxacillin zone sizes were <17 mm, the breakpoint proposed by Kimura and colleagues (5). The median ceftizoxime zone diameter was 29 mm, with a range of 22 mm to 56 mm (Fig. 1). Twenty-three percent of isolates had ceftizoxime zones sizes of <29 mm, the breakpoint proposed by Kimura and colleagues (5). None of the tested GBS strains grew on the oxacillin screening agar.
FIG. 1.
Distribution of zone diameters of oxacillin (A) and ceftizoxime (B) disk tests for 1,991 penicillin-susceptible GBS strains. Note discontinuous axes.
To confirm that GBS isolates with the smallest oxacillin disk zone sizes were not cryptic PNSGBS, despite penicillin MICs of ≤0.06 μg/ml, four isolates with zone sizes of 11, 12, 12, and 12 mm were sequenced in the pbp2x region. None of these isolates contained mutations associated with penicillin resistance.
Our study shows two important findings, that PNSGBS are not emerging in our patient population and that a proposed disk test (5) to detect PNSGBS has an unacceptably high false-positive rate. Since our study was adequately powered to detect a very low prevalence rate of PNSGBS, with a 95% chance of detecting the 0.39% PNSGBS prevalence rate found in a recent survey (2), we can conclude that emerging PNSGBS is extremely unlikely in our patient population.
Kimura and colleagues (5) recently reported that disk diffusion testing with the combination of oxacillin, ceftizoxime, and ceftibuten disks is a useful screening method for the detection of PNSGBS. Although they included 32 PNSGBS isolates in their study, only 16 penicillin-susceptible isolates were tested by them. Using a much larger population of penicillin-susceptible GBS isolates, we found that their proposed method is nonspecific, with true specificities of 77 and 46% for ceftizoxime and oxacillin, respectively. Based on the highest reliable prevalence of PNSGBS reported, 0.7% (6), and an assumption of 100% sensitivity, the positive predictive values of the suggested breakpoints would be 1 and 3%, respectively, for oxacillin and ceftizoxime. Whether the oxacillin screening agar test used by us, which was 100% specific, is of adequate sensitivity requires study with a larger number of PNSGBS strains. Based on the currently very low PNSGBS prevalence and the absence of a known high-performance screening method, routine screening for these strains is not presently indicated.
Acknowledgments
Eric Chen, Matthew Delfiner, Shane Tepper, and Shannon McGettigan provided excellent technical assistance, and the Clinical Microbiology Staff of the Hospital of the University of Pennsylvania kindly provided us with GBS isolates.
Footnotes
Published ahead of print on 27 January 2010.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA.
- 2.Dahesh, S., M. E. Hensler, N. M. Van Sorge, R. E. Gertz, Jr., S. Schrag, V. Nizet, and B. W. Beall. 2008. Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to β-lactam antibiotics. Antimicrob. Agents Chemother. 52:2915-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura, K., S. Suzuki, J. Wachino, H. Kurokawa, K. Yamane, N. Shibata, N. Nagano, H. Kato, K. Shibayama, and Y. Arakawa. 2008. First molecular characterization of group B streptococci with reduced penicillin susceptibility. Antimicrob. Agents Chemother. 52:2890-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura, K., J. Wachino, H. Kurokawa, S. Suzuki, K. Yamane, N. Shibata, and Y. Arakawa. 2008. Practical disk diffusion tests for detecting group B streptococcus with reduced penicillin susceptibility, abstr. D-2248. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother., Washington, DC. [DOI] [PMC free article] [PubMed]
- 5.Kimura, K., J. I. Wachino, H. Kurokawa, S. Suzuki, K. Yamane, N. Shibata, and Y. Arakawa. 2009. Practical disk diffusion test for detecting group B streptococcus with reduced penicillin susceptibility. J. Clin. Microbiol. 47:4154-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Persson, E., S. Berg, H. Bergseng, K. Bergh, R. Valso-Lyng, and B. Trollfors. 2008. Antimicrobial susceptibility of invasive group B streptococcal isolates from south-west Sweden 1988-2001. Scand. J. Infect. Dis. 40:308-313. [DOI] [PubMed] [Google Scholar]
- 7.Phares, C. R., R. Lynfield, M. M. Farley, J. Mohle-Boetani, L. H. Harrison, S. Petit, A. S. Craig, W. Schaffner, S. M. Zansky, K. Gershman, K. R. Stefonek, B. A. Albanese, E. R. Zell, A. Schuchat, and S. J. Schrag. 2008. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA 299:2056-2065. [DOI] [PubMed] [Google Scholar]
- 8.Schrag, S. J., S. Zywicki, M. M. Farley, A. L. Reingold, L. H. Harrison, L. B. Lefkowitz, J. L. Hadler, R. Danila, P. R. Cieslak, and A. Schuchat. 2000. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N. Engl. J. Med. 342:15-20. [DOI] [PubMed] [Google Scholar]
- 9.Simoes, J. A., A. A. Aroutcheva, I. Heimler, and S. Faro. 2004. Antibiotic resistance patterns of group B streptococcal clinical isolates. Infect. Dis. Obstet. Gynecol. 12:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]