Abstract
Canine herpesvirus (CHV; Canid herpesvirus 1) is principally a perinatal pathogen of pregnant bitches and newborn pups and secondarily a respiratory tract pathogen of older pups and dogs. Infectious disease of the canine respiratory tract frequently occurs among dogs in groups, in which it is called “ infectious tracheobronchitis” (ITB). Mortality from ITB is generally negligible, and the clinical importance of CHV as an ITB pathogen is considered to be low. The present report describes a novel ITB outbreak accompanied by death among aged dogs in an animal medical center. Most inpatient dogs had received medications that could induce immunosuppression. CHV was the only pathogen identified, and several CHV isolates were recovered in cell culture. No other viral pathogens or significant bacterial pathogens were found. Molecular and serological analyses revealed that the causative CHV isolates were from a single source but that none was a peculiar strain when the strains were compared with previous CHV strains. The virus had presumably spread among the dogs predisposed to infection in the center. The present results serve as a warning to canine clinics that, under the specific set of circumstances described, such serious CHV outbreaks may be expected wherever canine ITB occurs.
Canine herpesvirus (CHV; Canid herpesvirus 1) is classified in the Varicellovirus genus of the Alphaherpesvirinae subfamily of the Herpesviridae. CHV was first described in 1965 as a pathogen responsible for a fatal generalized hemorrhagic disease of newborn pups (5). The host range of CHV is generally restricted to domestic and wild Canidae (11), and the worldwide distribution of CHV infection in domestic dog populations has been shown by virus isolation (2, 7, 10, 12, 24, 27) and seroepidemiological studies (6, 25, 28, 30, 31, 34). It is now well recognized that the pathogenic potential of CHV is mostly influenced by the age of the host (11). Pups infected in the postnatal period show the typical fatal hemorrhagic syndrome. Older pups manifest less severe clinical syndromes upon infection, and the respiratory disease called “infectious tracheobronchitis” (ITB) or “kennel cough” may be the most frequent clinical disorder in the field. Ocular disorders such as conjunctivitis and keratitis, either with or without ulceration, were also observed in young pups (17). Although the pathogenic potential of CHV for older dogs is apparently low and adult dogs often do not show any clinical signs following CHV infection, CHV may become an important perinatal pathogen for pregnant bitches, causing papulovesicular genital lesions and reproductive disorders such as embryonic resorption, abortion, and stillbirth (11).
CHV is spread mainly by the oronasal and venereal transmission of viruses in the respiratory and genital secretions of acutely infected dogs, and fetuses are infected in utero (13, 14). Following the initial productive infection, in most cases CHV is not fully cleared by acquired immunity so that latency becomes established in several tissues, including the sensory ganglia (4, 19), and may persist for life. Latent viruses are sometimes reactivated by factors that alter immunity, such as stress, immunosuppressive therapy, or pregnancy, with the virus subsequently being excreted.
The significance of CHV in the etiology of canine ITB has been considered to be rather low compared with that of other agents, such as canine parainfluenza virus (CPIV), canine adenovirus type 2 (CAV-2), and Bordetella bronchiseptica (9). Experimental infection of older pups or adult dogs with CHV isolates often resulted in either no clinical signs or mild upper respiratory symptoms (1, 15, 24, 32). Consequently, CHV has not generally been regarded to be a primary cause of canine ITB (9, 11). On the other hand, CHV has repeatedly been detected in dogs with ITB (3, 16, 24, 36), and a longitudinal study on the respiratory diseases of dogs housed in a rehoming kennel in the United Kingdom (8) indicated that CHV should be reevaluated as a significant agent responsible for ITB.
This report is an account of a novel outbreak of canine ITB which was accompanied by deaths among dogs hospitalized in an animal referral medical center near Tokyo, Japan. It was concluded that CHV alone was responsible for the nosocomial outbreak. All of the dogs had been fully vaccinated before they entered the hospital, and during the hospitalization, most of the dogs had received steroid medication, surgery, or radiation therapy. It was considered that such medical treatments, in addition to hospitalization itself, might induce stress in the patients, leading to a condition of low-level immune resistance, and cause the recrudescence of latent CHV. Canine herpesvirus might then have spread contagiously among the dogs in the same clinic. The results of the present study suggest the need for caution, because CHV, which was previously thought to be an agent of low pathogenicity for adult dogs, should not be overlooked in specific circumstances.
MATERIALS AND METHODS
Clinical specimens for virus detection.
Swabs from two populations of dogs were investigated. The first population comprised dogs which were hospitalized in a referral animal medical center. In retrospect, it was considered that an ITB outbreak started among dogs in the center on about 20 May 2008 and was over after 6 weeks, following the application of quarantine measures. At least 15 dogs showed typical signs of respiratory disease, and 5 dogs died during the period. After the first two dogs died on 9 June 2008, nasal and pharyngeal swab specimens from 10 dogs were immediately collected (Table 1) and submitted for examination for viruses and bacteria. Serum samples were taken from five dogs (dogs 71995, 71802, 71927, 71982, and 71579) in the middle of the epidemic (11 and 13 June). An external commercial laboratory conducted bacterial examination of the swabs: no significant respiratory pathogens such as Mycoplasma spp. or Bordetella spp. were detected. A further three dogs (Table 1, dogs 71917, 71947, and 71927) died on 13, 18, and 23 June, respectively. Necropsy of the dogs was not performed.
TABLE 1.
CHV detection and clinical specimens from inpatients at an animal referral center
| Dog identifier | Breeda | Sexb | Age (yr) | Vaccination recordc or date (yr.mo.day) | Clinical signd and outcome | Treatment |
Specimen (originf) | CHV detection |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroidse | Surgery | Chemotherapy | Radiation | VIg | PCR | |||||||
| 71923 | MDH | FS | 11 | 2007.11.10 | Coughing, pyrexia, recovery | Yes | Yes | 08/005 (N) | + | + | ||
| 08/006 (P) | − | + | ||||||||||
| 71995 | Shih tzu | F | 8 | Vaccinated | Pyrexia, sneezing, coughing, recovery | Yes | 08/009 (N) | − | − | |||
| 08/010 (P) | − | + | ||||||||||
| 71802 | Pomeranian | FS | 1 | 2007.9.1 | Pyrexia, sneezing, recovery | Yes | Yes | 08/011 (N) | + | + | ||
| 08/012 (P) | − | + | ||||||||||
| 71927 | Shih tzu | M | 12 | 2007.5.22 | Coughing, sneezing, nasal discharge, death | Yes | 08/013 (N) | − | − | |||
| 08/014 (P) | − | + | ||||||||||
| 71982 | MDH | M | 7 | 2007.9.21 | Sneezing, nasal discharge, recovery | Yes | 08/015 (N) | + | + | |||
| 08/016 (P) | − | + | ||||||||||
| 71579 | Mongrel | M | 10 | Unknown | Pyrexia, sneezing, recovery | Yes | 08/017 (N) | − | + | |||
| 08/019 (P) | − | + | ||||||||||
| 71917 | Shelty | M | 8 | Vaccinated | Vomiting, pyrexia, coughing, death | Yes | Yes | 08/021 (N) | + | + | ||
| 71993 | MDH | M | 7 | 2007.5.30 | Pyrexia, sneezing, nasal discharge, recovery | Yes | Yes | 08/022 (N) | − | + | ||
| 71947 | SSD | F | 9 | Vaccinated | Interstitial pneumonia, death | Yes | 08/026 (N) | − | + | |||
| 71705 | Yorky | M | 11 | 2007.5.23 | Diarrhea, nasal discharge, recovery | Yes | Yes | 08/027 (N) | − | + | ||
| 08/028 (P) | − | + | ||||||||||
MDH, miniature dachshund; Yorky, Yorkshire terrier; SSD, Shetland sheepdog.
F, female; M, male; FS, female spayed.
The last inoculation date with canine combined vaccines; vaccinated, vaccinated but the date of inoculation is unknown.
Clinical signs at the time of sampling.
Prednisolone at 0.25 to 5 mg/kg/day for 5 to 29 consecutive days.
N, nasal; P, pharyngeal.
VI, virus isolation with MDCK cell culture.
The other samples were swab specimens cryopreserved after primary examination in our laboratory and comprised a total of 176 respiratory clinical specimens (81 oropharyngeal, 63 nasal cavity, 28 conjunctival, and 4 other specimens) from 137 dogs brought to animal hospitals in various areas of Japan between 1998 and 2008. These samples had been tested for CAV, CPIV, canine coronavirus (CCoV), canine respiratory coronavirus (CRCoV), canine distemper virus (CDV), minute virus of canines (MVC), and influenza A virus (IAV). Some of the results of that examination were published previously (21, 23, 38). However, detection of CHV in these samples was not attempted, mainly because of the classical impression that CHV was not important in canine IBT (11).
Reference CHV strains and cells.
Canine herpesvirus strains GCH-1 (12) and D004 (ATCC VR-552) (3) were used as reference CHV strains in the PCR, restriction length polymorphism (RFLP), and serum neutralization assays. Madin-Darby canine kidney (MDCK) (20), A-72 (ATCC CRL-1542), primary dog kidney (DK), and Felis catus whole fetus 4 (fcwf-4) (ATCC CRL-2787) (26) cells were used either for culturing for CHV or for the detection of virus from clinical specimens. The cells were grown with Dulbecco's modified Eagle's medium (MEM) supplemented with 10% fetal calf serum (FCS) and antibiotics.
Detection of viral agent in clinical specimens.
Swabs were placed in 2 ml of MEM and the extracts were clarified by centrifugation at 15,000 rpm for 20 min. The resulting supernatant was examined for viruses. For general virus isolation, the supernatant was inoculated into both MDCK and fcwf-4 cell cultures at 37°C. The cultures were examined for cytopathic effects (CPEs) and were blind passaged a further two times when no CPE was apparent. The samples were especially examined for the following viruses by previously described molecular methods (21, 23, 38): CDV, CPIV, CCoV, CRCoV, MVC, and IAV.
CHV detection was specifically performed by both virus isolation with either MDCK or primary DK cells and two kinds of PCRs: one was a PCR designed for the detection of CHV (CHV PCR) (19), and the other was a PCR designed for the detection of diverse herpesvirus species by the use of consensus primers (consensus primer PCR) (33). The PCRs were performed precisely by the methods originally described; CHV strain D004 was used as a positive reference.
Molecular characterization of CHV isolates.
For viral DNA extraction, A-72 cells were infected with CHV at a low multiplicity of infection. When the CPE was prominent, the cells were collected and treated with a 0.1 M Tris-HCl (pH 9.0) solution containing 0.1 M NaCl2, 5 mM EDTA, 1% sodium dodecyl sulfate, and 0.1 mg/ml of proteinase K at 37°C overnight. DNA was extracted with a mixture of phenol and chloroform-isoamyl alcohol (25:24:1), precipitated with ethanol, and then dissolved in water.
To examine whether the CHV isolates originated from either single or multiple sources, viral DNA was digested with restriction endonucleases HindIII and XbaI for RFLP analysis (37). The digested fragments were separated by electrophoresis on a 0.7% agarose gel for 18.5 h at 20 V. The gels were stained with ethidium bromide.
For nucleotide sequencing, viral DNA (a portion of the DNA polymerase gene) was amplified from isolate 08/005 by the PCR with herpesviral consensus primers TGV (5′-TGTAACTCGGTGTAYGGNTTYACNGGNGT-3′) and IYG (5′-CACAGAGTCCGTRTCNCCRTADAT-3′), as described previously (33). The PCR product was purified with a QIAquick PCR purification kit (Qiagen, Germany) and sequenced with primers TGV and IYG by using a BigDye Terminator (version 3.1) cycle sequencing kit (Applied Biosystems Inc.).
Serology.
Blood samples collected from both the inpatient dogs and experimentally infected dogs were titrated for the detection of CHV neutralization (NT) antibodies. Neutralization was performed with complement supplementation, and the titer was determined by a plaque reduction method. Twofold serial dilutions of heat-inactivated sera were made in MEM and transferred to 96-well plates at 60 μl per well. Approximately 100 PFU of CHV strain D004 in 30 μl and complement (a 15% concentration of fresh guinea pig serum in MEM) in 30 μl were added to each well, and the plates were incubated at 37°C for 1 h in 5% CO2. One hundred microliters of the mixture was then inoculated onto a MDCK cell sheet formed in 24-well plates and left for adsorption at 37°C for 1 h in a chamber containing 5% CO2. After the unabsorbed viruses were washed out, all wells received 1 ml of MEM containing 2% FCS and 1% agar and the plates were incubated at 37°C for 4 to 5 days. The plaques were visualized by staining with 0.005% neutral red. The antibody titer was defined as the reciprocal of the highest dilution of serum that reduced the number of plaques to less than half of that for the virus control without serum.
Field serum samples.
The serum samples that had been cryopreserved in our laboratory were screened for NT antibodies against CHV by complement supplementation. The samples had been collected from dogs presenting at veterinary teaching hospitals located in Yamaguchi (western Japan; 48 dogs), Tokyo (eastern Japan; 60 dogs), and Sapporo (northern Japan; 30 dogs) in 2000 and 2001 for a canine calicivirus study (22). One hundred thirty-eight samples were available for the present examination. The reasons that the dogs had visited the hospitals were not limited to respiratory disease.
Experimental infection of SPF dogs with the CHV isolate.
Four 26-week-old specific-pathogen-free (SPF) beagle dogs (dogs 2, 8, 12, and 16) were used for experimental infection. The dogs did not possess anti-CHV NT antibody before challenge. Isolate 08/005, passaged twice in MDCK cells and once in A-72 cell cultures, was used as the inoculum. Each dog received 3 ml of culture fluid containing 105 50% tissue culture infective doses of infective virus as 1 ml orally and 2 ml intranasally. The clinical condition of each dog was observed for 2 weeks after inoculation. Oral swab samples were taken daily to detect virus excretion by the CHV PCR, and blood samples were obtained every other day for the first week and additionally on the 10th and 14th days.
The virulence of CHV strain GCH-1 was also examined for comparison. The strain was isolated in 1975 from newborn pups with typical fatal hemorrhagic syndrome (12) and had been passaged 31 times in DK cell culture. Two 28-week-old seronegative SPF beagle dogs (dogs 13 and 14) were challenged and observed under the same conditions used for isolate 08/005.
RESULTS
Viruses detected from hospitalized dogs with ITB.
CAV, CPIV, CCoV, CRCoV, CDV, MVC, and IAV were not detected in any of the respiratory specimens except specimen 08/026, in which a CPIV RNA fragment was detected by reverse transcription-PCR. In contrast, as shown in Table 1, CHV was isolated from four nasal swab specimens (from dogs 08/005, 08/011, 08/015, and 08/021) in MDCK cell culture. The viruses showed a typical herpesvirus-like CPE in the primary culture at 37°C, as described previously (12). Since this result was unexpected, all specimens were then screened by the CHV PCR. Either nasal or oropharyngeal specimens, or both, for 10 dogs, including the 4 dogs that harbored infectious CHV in the nasal cavity, were found to be positive for the CHV DNA fragment (data not shown).
Antibodies in the serum samples taken from inpatient dogs in the middle of the epidemic.
Serum samples from five dogs were examined for NT antibodies against CHV strain D004 (Table 2). No NT activity was detected in three samples (from dogs 71995, 71927, and 71982); however, NT titers of 64 and 2,048 were found in the two other dogs (dogs 71802 and 71579, respectively). Although all dogs possessed antibody titers sufficient to prevent CPIV, CAV-2, and canine parvovirus type 2 (CPV-2) infections, the anti-CDV titers for some dogs were comparatively low (Table 2).
TABLE 2.
Antibody titers of sera taken from inpatient dogs in the middle of the epidemica
| Dog identifier | Hospitalization record of date that the dog: |
Antibody titerb against: |
|||||
|---|---|---|---|---|---|---|---|
| Entered | Left | CHV | CPIV | CAV-2 | CDV | CPV-2 | |
| 71927 | 22 May | 23 Junec | <2 | 64 | 64 | 25 | 256 |
| 71802 | 9 June | 15 June | 64 | 128 | 512 | 73 | 16,000 |
| 71982 | 31 May | 14 June | <2 | 8 | 256 | 9 | 2,048 |
| 71995 | 2 June | 19 June | <2 | 256 | 32 | 33 | 2,048 |
| 71579 | 16 May | 14 June | 2,048 | 32 | 2,048 | 56 | 64 |
Serum was taken on 11 or 13 June 2008.
Neutralization titer for CHV, CPIV, CAV-2, and CDV and hemagglutination inhibition titer for CPV-2.
The animal left the medical center because it had died.
Cross-neutralization test between reference strain CHV D004 and CHV isolate 08/005.
In the cross-neutralization test, anti-CHV D004 rabbit hyperimmunized serum and CHV 08/005-infected dog serum taken at 4 weeks postchallenge were used. Both isolate 08/005 and strain D004 were neutralized by the homologous and heterologous serum samples, with the difference in the NT titers not being significantly (data not shown), indicating that there was no antigenic difference between the viruses.
Sequencing and RFLP analysis of CHV isolates.
The nucleotide sequence of the PCR product obtained from isolate 08/005 was compared with the nucleotide sequences of herpesviruses deposited in databases. The product was 234 bp and was a fragment of the DNA polymerase gene. Perfect identity of the sequence over 185 bp, except at the two ends of the primer sequences, to the sequence of CHV reported previously (GenBank accession no. EU531507) was obtained (data not shown), indicating that the PCR product was of CHV origin.
The viral DNA obtained from the isolates was compared with those of reference CHV strains D004 and GCH-1 by RFLP analysis (Fig. 1). The migration patterns of the digested fragments were almost identical among all four isolates (from dogs 08/005, 08/011, 08/015, and 08/021), although some subtle differences were observed between the present isolates and the reference strains. Several differences were detected in the HindIII digestion pattern: the gain of a 4.4-kbp fragment and the loss of a 3.1-kbp fragment were clear for the present four isolates. In the XbaI digestion pattern, it was clear that the four isolates showed a gain of a 10-kbp fragment instead of the loss of an 11.4-kbp fragment.
FIG. 1.
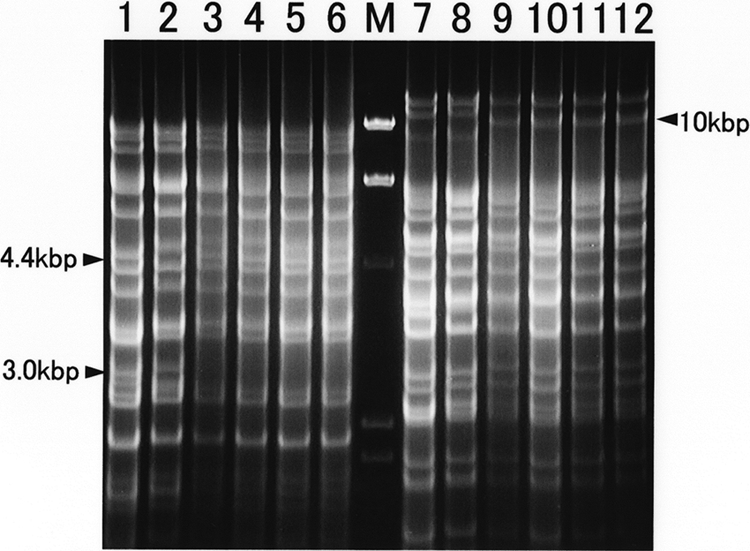
RFLP of viral DNA of the present CHV isolates and reference CHV strains. DNA extracted from virus-infected cells was digested with the endonucleases HindIII (lanes 1 to 6) and XbaI (lanes 7 to 12). Lanes: 1 and 7, reference strain D004; 2 and 8, reference strain GCH-1; 3 and 9, isolate 08/005; 4 and 10, isolate 08/011; 5 and 11, isolate 08/015; 6 and 12, isolate 08/021; M, bacteriophage lambda DNA digested with HindIII as a size marker. Arrowheads indicate the different fragments clearly detected between the isolates tested in the present study and the reference CHV strains.
Virulence of CHV isolate to SPF dogs.
In the experimental dogs infected with isolate 08/005, the prominent clinical signs were pyrexia and serous nasal discharge. Dog 12 had a high fever (temperature, 39.9°C to 40.1°C) from the second day after challenge which continued for 5 days and a serous nasal discharge for 5 days from the fifth day. The other three dogs, dogs 2, 8, and 16, also showed similar clinical signs, although these were less serious and of shorter duration than those for dog 12. A slight lacrimation was observed in dog 16. Virus excretion was detected in the nasal cavities of all dogs for 5 days between the 3rd and 7th days after challenge. The serum NT titers of the samples taken 2 weeks after challenge were elevated to 32 to 1,024.
Of the dogs infected with strain GCH-1, dog 13 had a temperature of 39.4°C on the 4th day and had a serous nasal discharge for 2 days from the 5th day. Virus excretion was detected in the nasal cavity on the 5th day. Dog 14 had a temperature of 39.5°C on the 3rd day and a serous nasal discharge for 5 days from the 3rd day. The feces of this dog changed from loose to diarrheic during the same period. Virus excretion was detected in the nasal cavity for 3 days between the 3rd and 5th days. The serum NT titers of the samples taken 2 week after challenge were elevated to 1,024 to 2,048 in both dogs.
CHV detection in cryopreserved clinical specimens.
Herpesvirus DNA was detected in the oropharyngeal swabs of two dogs by CHV PCR, with the rate of positivity being 1.46%. However, the specimens were negative by the consensus primer PCR. CAV, CPIV, CDV, and CRCoV were not detected in the same specimens. The samples were from 2- to 3-month-old pups that had not received canine combined vaccine; and their principal clinical signs were coughing, nasal discharge, and dyspnea. Slight conjunctivitis was observed in one pup. An attempt to isolate CHV from the CHV DNA-positive specimens by primary DK cell culture at 34°C did not succeed.
Survey of field serum samples for CHV NT antibodies.
In all, 21.7% of the dogs had NT antibodies to CHV, with the mean positive antibody titer being 19.1. However, a regional variation was noted. There was higher seroprevalence among dogs from the Tokyo area: the rate of antibody positivity was 40%, and the mean positive antibody titer was 29.6. In contrast, the seroprevalence rates among dogs from the Yamaguchi and Sapporo areas were 10.4% and 3.3%, respectively, and the antibody titer for the seropositive dogs in these areas was 10 for all dogs.
DISCUSSION
When dogs are housed in a group into which newcomers are constantly introduced, canine ITB is typically caused by a complex of pathogens (9). CHV has been regarded as a secondary agent in ITB because of its infrequent association with the syndrome. Actually, in the present study, the rate of detection of CHV from the dogs with respiratory disease in the field was low (1.46%) compared with the rates of detection of CPIV (7.4%) and B. bronchiseptica (10.3%), as revealed by our recent study in which the same clinical specimens were examined (23). The rate of detection of CHV was similar to the rates for CRCoV and CDV (1.5%), suggesting that CHV is of secondary significance as a pathogen, as previously regarded. However, from a recent epidemiological study in a rehoming center in England, Erles et al. (8) proposed a different scenario for the pathogenic role of CHV in canine ITB. They proposed that (i) the CHV latently harbored in some dogs can become reactivated during the course of ITB due to the stress caused by other viral and bacterial infections; hence, (ii) CHV infection is associated with ITB in which more severe respiratory signs are present, and therefore, (iii) CHV infection may have an exacerbating effect on other viral or bacterial infections. There are both common and different epidemiological features of the ITB outbreaks involving CHV between the animal medical center evaluated in the present study and the rehoming center described previously (8). The present study introduces a new concept about the role of CHV in canine ITB and serves as a warning to facilities where dogs are kept in a group and are subject to stress.
Most of the dogs that were inpatients at the animal medical center were aged and had been vaccinated with combined core vaccines against primary agents of ITB, such as CPIV and CAV-2 (Table 1). The dogs were very likely to have been immune to infections with such canine pathogens since they had high titers of antibodies against each virus (Table 2). Indeed, except for one dog (Table 1, dog 71947) in which CPIV was detected, neither primary viral nor bacterial ITB pathogens were associated with the clinical syndrome. In the end, it was unexpectedly found that almost all cases were solely attributable to CHV. Unfortunately some dogs died during the epidemic, but no conclusive pathological reasons for that outcome were obtained, since no postmortem examination was performed, in accordance with the dog owners' wishes. Although most inpatient dogs had received some medication before as well as during the CHV epidemic, it was considered very unlikely that such medical treatments were responsible for the fatalities. To elucidate the reasons for the ITB outbreak in the animal medical center, the following points should be considered: first, the origin and virulence of the causative CHV isolate and, second, the factor(s) which made the situation very serious.
The findings of previous studies showing very low levels of antigenic and genomic divergence of global CHV isolates indicate that CHV is a monotypic virus (8, 29). In the present study, no significant antigenic difference between the reference CHV strain and the present CHV isolate was observed by the cross-neutralization test. Sequence analysis of the DNA polymerase gene of isolate 08/005 indicated 100% identity of the sequenced region with the sequence of the reference CHV strain. Although these results were indeed useful for antigenic and molecular identification of the isolate as CHV, they were not helpful for discriminating between isolates. Xuan et al. (37) described the value of RFLP analysis of genomic DNA for the differentiation of CHV isolates by using the endonucleases HindIII, XbaI, and PvuII. They differentiated unrelated individual CHV isolates from each other. In contrast, CHV isolates from the same origin showed almost identical restriction cleavage patterns. Indeed, the results of RFLP analysis obtained in the present study (Fig. 1) were sufficiently clear to show that the four CHV isolates tested in the present study were of the same origin because they showed the same migration patterns. Therefore, it was hypothesized that the ITB outbreak in the animal medical center was caused by a CHV strain of a single origin.
Although the analysis of anti-CHV NT antibodies was performed for only a limited number of the inpatient dogs (Table 2), the findings provide a hint about the suspect dog that introduced CHV into the center. The characteristics needed to identify the dog are that it had been hospitalized before the epidemic and that it had anti-CHV antibodies and therefore had been infected with CHV. For example, dog 71579 met this requirement. However the evidence may not be enough to definitely identify the suspect dog since we could not examine all dogs hospitalized before the outbreak.
The virulence of the CHV isolates from the present study remains unresolved. Some previous animal experiments showed that CHV induces a mild upper respiratory disease in pups aged less than 12 weeks (1, 15, 33), while other CHV isolates were avirulent in young pups (24, 35). However, it was confirmed experimentally that isolate 08/005 from the present study possesses some pathogenic potential for the respiratory tracts of adolescent dogs. In addition, reference CHV strain GCH-1, which was isolated 30 years ago in Japan from pups with fatal hemorrhagic syndrome, also showed virulence similar to but rather less than that of isolate 08/005 for the respiratory tracts as well as the alimentary tracts of the dogs. These results lend support to the recent proposal of Erles et al. (8) that CHV is a more significant pathogen than was previously believed, especially when canine ITB occurring among stressed dogs in a group is evaluated. Further experiments will be required to clarify the pathogenic diversities of different CHV strains, especially for immunosuppressed dogs.
A possible source of infection for the present ITB outbreak is a latently infected dog that was introduced into the animal hospital some time before the outbreak, as discussed above. The serological survey conducted in the present study indicated that the dogs in the area of Tokyo adjoining the hospital possess a higher prevalence of anti-CHV antibodies than the dogs raised in the suburban areas of Yamaguchi or Sapporo. Almost half of the dogs had NT antibodies against CHV, indicating that they harbored latent CHV (4, 19). Following treatment with agents that induce stress, the recrudescence of latent CHV infection occurs within a week (11). According to a recent study of adult dogs latently infected with CHV (18), the virus was activated and detected on the 10th day after the systemic administration of prednisolone (3.0 mg/kg of body weight/day for 7 consecutive days from the first day). This dose and duration of treatment are within the standard ranges used in small-animal clinics, and most dogs in the animal medical center described here also received a similar dose of prednisolone (0.25 to 5 mg/kg/day for 5 to 29 consecutive days; Table 1). Even without prednisolone treatment, the dogs might have become immunosuppressed, owing to the stress caused by the hospitalization itself, surgery, chemotherapy, or radiation, as listed in Table 1.
The present study demonstrated that CHV was the causative agent of a serious outbreak of canine ITB that occurred in a large animal medical center. Although the virulence of the CHV isolated from cases of disease seemed to be slightly higher than that of previously tested CHV strains, the most significant reason for the spread of infection in the center may be the immunosuppressed status of the susceptible inpatient dogs. It is logical to assume that the number of immunosuppressed dogs was high enough to perpetuate transmission after the initial introduction of CHV. This situation might develop anywhere that canine ITB occurs. Although herpesvirus vaccines are generally not sufficient to prevent infection, vaccination might be one of the choices applied to animals under such special circumstances. An inactivated vaccine is available in Europe to protect newborn pups from systemic infection. Vaccination of aged dogs may have some effect in reducing virus excretion from infected individuals, consequently controlling the spread of infection in a group.
Acknowledgments
We are much obliged to Oswald Jarrett of the University of Glasgow for valuable discussions in the preparation of the manuscript. Akiko Yachi of Kyoritsu Seiyaku Corporation provided expert technical assistance.
Footnotes
Published ahead of print on 27 January 2010.
REFERENCES
- 1.Appel, M. J., M. Menegus, I. M. Parsonson, and L. E. Carmichael. 1969. Pathogenesis of canine herpesvirus in specific-pathogen-free dogs: 5- to 12-week-old pups. Am. J. Vet. Res. 30:2067-2073. [PubMed] [Google Scholar]
- 2.Bartsch, R. C., O. J. B. Hübschle, and H. J. Els. 1974. Canine herpesvirus infection: literature review and case report. J. S. Afr. Vet. Med. Assoc. 45:81-85. [PubMed] [Google Scholar]
- 3.Binn, L. N., G. A. Eddy, E. E. Lazar, J. Helm, and T. Murnane. 1967. Virus recovery from laboratory dogs with respiratory disease. Proc. Soc. Exp. Biol. Med. 126:140-145. [DOI] [PubMed] [Google Scholar]
- 4.Burr, P. D., M. E. Campbell, L. Nicolson, and D. E. Onions. 1996. Detection of canine herpesvirus 1 in a wide range of tissues using the polymerase chain reaction. Vet. Microbiol. 53:227-237. [DOI] [PubMed] [Google Scholar]
- 5.Carmichael, L. E., R. A. Squire, and L. Krook. 1965. Clinical and pathologic features of a fatal viral disease of newborn pups. Am. J. Vet. Res. 26:803-814. [PubMed] [Google Scholar]
- 6.Dahlbom, M., M. Johnsson, V. Myllys, J. Taponen, and M. Andersson. 2009. Seroprevalence of canine herpesvirus-1 and Brucella canis in Finnish breeding kennels with and without reproductive problems. Reprod. Domest. Anim. 44:128-131. [DOI] [PubMed] [Google Scholar]
- 7.de Ratuld, Y., and G. H. Werner. 1965. Le virus herpétique canin. Ann. Inst. Pasteur 112:802-807. [PubMed] [Google Scholar]
- 8.Erles, K., E. J. Dubovi, H. W. Brooks, and J. Brownlie. 2004. Longitudinal study of viruses associated with canine infectious respiratory disease. J. Clin. Microbiol. 42:4524-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford, R. B. 2006. Canine infectious tracheobronchitis, p. 54-61. In C. E. Greene (ed.), Infectious diseases of the dog and cat, 3rd ed. Elsevier Inc., St. Louis, MO.
- 10.Geldard, H., W. A. Geering, and T. J. Bagust. 1971. Isolation of a herpesvirus from neonatal dogs in Australia. Aust. Vet. J. 47:286. [DOI] [PubMed] [Google Scholar]
- 11.Greene, C. E., and L. E. Carmichael. 2006. Canine herpesvirus infection, p. 47-53. In C. E. Greene (ed.), Infectious diseases of the dog and cat, 3rd ed. Elsevier Inc., St. Louis, MO.
- 12.Hashimoto, A., K. Hirai, A. Miyoshi, S. Shimakura, K. Yagami, N. Kato, K. Kunihiro, A. Fujiura, K. Kitazawa, K. Okada, and Y. Fujimoto. 1978. Naturally occurring canine herpesvirus infection in Japan. Jpn. J. Vet. Sci. 40:157-169. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto, A., K. Hirai, Y. Suzuki, and Y. Fujimoto. 1983. Experimental transplacental transmission of canine herpesvirus in pregnant bitches during the second trimester of gestation. Am. J. Vet. Res. 44:610-614. [PubMed] [Google Scholar]
- 14.Hashimoto, A., K. Hirai, T. Yamaguchi, and Y. Fujimoto. 1982. Experimental transplacental infection of pregnant dogs with canine herpesvirus. Am. J. Vet. Res. 43:844-850. [PubMed] [Google Scholar]
- 15.Karpas, A., F. G. Garcia, F. Calvo, and R. E. Cross. 1968. Experimental production of canine tracheobronchitis (kennel cough) with canine herpesvirus isolated from naturally infected dogs. Am. J. Vet. Res. 29:1251-1257. [PubMed] [Google Scholar]
- 16.Karpas, A., N. W. King, and F. G. Garcia. 1967. Isolation of herpes-like virus from dogs with tracheobronchitis (kennel cough). Bacteriol. Proc. 67:157. [Google Scholar]
- 17.Ledbetter, E. C., S. G. Kim, and E. J. Dubovi. 2009. Outbreak of ocular disease associated with naturally-acquired canine herpesvirus-1 infection in a closed domestic dog colony. Vet. Ophthalmol. 12:242-247. [DOI] [PubMed] [Google Scholar]
- 18.Ledbetter, E. C., S. G. Kim, E. J. Dubovi, and R. C. Bicalho. 2009. Experimental reactivation of latent canine herpesvirus-1 and induction of recurrent ocular disease in adult dogs. Vet. Microbiol. 138:98-105. [DOI] [PubMed] [Google Scholar]
- 19.Miyoshi, M., Y. Ishii, M. Takiguchi, A. Takada, J. Yasuda, A. Hashimoto, K. Okazaki, and H. Kida. 1999. Detection of canine herpesvirus DNA in the ganglionic neurons and the lymph node lymphocytes of latently infected dogs. J. Vet. Med. Sci. 61:375-379. [DOI] [PubMed] [Google Scholar]
- 20.Mochizuki, M. 2006. Growth characteristics of canine pathogenic viruses in MDCK cells cultured in RPMI 1640 medium without animal protein. Vaccine 24:1744-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mochizuki, M., M. Hashimoto, T. Hajima, M. Takiguchi, A. Hashimoto, Y. Une, F. Roerink, T. Ohshima, C. R. Parrish, and L. E. Carmichael. 2002. Virologic and serologic identification of minute virus of canines (canine parvovirus type 1) from dogs in Japan. J. Clin. Microbiol. 40:3993-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mochizuki, M., M. Hashimoto, F. Roerink, Y. Tohya, Y. Matsuura, and N. Sasaki. 2002. Molecular and seroepidemiological evidence of canine calicivirus infections in Japan. J. Clin. Microbiol. 40:2629-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mochizuki, M., A. Yachi, T. Ohshima, A. Ohuchi, and T. Ishida. 2008. Etiologic study of upper respiratory infections of household dogs. J. Vet. Med. Sci. 70:563-569. [DOI] [PubMed] [Google Scholar]
- 24.Motohashi, T., and M. Tajima. 1966. Isolation of a herpes virus from a diseased adult dog in Japan. Jpn. J. Vet. Sci. 28:307-314. [DOI] [PubMed] [Google Scholar]
- 25.Nöthling, J. O., D. Hussy, D. Steckler, and M. Ackermann. 2008. Seroprevalence of canine herpesvirus in breeding kennels in the Gauteng province of South Africa. Theriogenology 69:276-282. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen, N. C., J. F. Boyle, and K. Floyd. 1981. Infection studies in kittens, using feline infectious peritonitis virus propagated in cell culture. Am. J. Vet. Res. 42:363-367. [PubMed] [Google Scholar]
- 27.Prydie, J., M. J. Harrison, and J. Graham. 1966. Isolation of a canine herpes virus. Vet. Rec. 79:660-661. [DOI] [PubMed] [Google Scholar]
- 28.Reading, M. J., and H. J. Field. 1998. A serological study on canine herpesvirus-1 infection in the English dog population. Arch. Virol. 143:1477-1488. [DOI] [PubMed] [Google Scholar]
- 29.Reubel, G. H., J. Pekin, K. Webb-Wagg, and C. M. Hardy. 2002. Nucleotide sequence of glycoprotein genes B, C, D, G, H and I, the thymidine kinase and protein kinase genes and gene homologue UL24 of an Australian isolate of canine herpesvirus. Virus Genes 25:195-200. [DOI] [PubMed] [Google Scholar]
- 30.Rijsewijk, F. A., E. J. Luiten, F. J. Daus, R. W. van der Heijden, and J. T. van Oirschot. 1999. Prevalence of antibodies against canine herpesvirus 1 in dogs in The Netherlands in 1997-1998. Vet. Microbiol. 65:1-7. [DOI] [PubMed] [Google Scholar]
- 31.Ronsse, V., J. Verstegen, K. Onclin, A. L. Guiot, C. Aeberlé, H. J. Nauwynck, and H. Poulet. 2002. Seroprevalence of canine herpesvirus-1 in the Belgian dog population in 2000. Reprod. Domest. Anim. 37:299-304. [DOI] [PubMed] [Google Scholar]
- 32.Thompson, H., N. G. Wright, and H. J. Cornwell. 1972. Canine herpesvirus respiratory infection. Res. Vet. Sci. 13:123-126. [PubMed] [Google Scholar]
- 33.VanDevanter, D. R., P. Warrener, L. Bennett, E. R. Schultz, S. Coulter, R. L. Garber, and T. M. Rose. 1996. Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 34:1666-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von König, M., J. Neiseke, and H. J. Thiel. 2004. Prevalence of canine herpesvirus 1 (CHV-1) in German kennels. Tieraerztl. Umschau. 59:559-565 (In German.) [Google Scholar]
- 35.Wright, N. G., and H. J. C. Cornwell. 1968. Experimental herpes virus infection in young puppies. Res. Vet. Sci. 9:295-299. [PubMed] [Google Scholar]
- 36.Wright, N. G., H. J. C. Cornwell, H. Thompson, and M. Stewart. 1970. Canine herpesvirus respiratory infection. Vet. Rec. 87:108-109. [DOI] [PubMed] [Google Scholar]
- 37.Xuan, X., T. Horimoto, M. Ono, J. A. Limcumpao, Y. Tohya, M. Azetaka, E. Takahashi, and T. Mikami. 1990. Restriction endonuclease analysis of canine herpesviruses isolated in Japan. Jpn. J. Vet. Sci. 52:1181-1188. [DOI] [PubMed] [Google Scholar]
- 38.Yachi, A., and M. Mochizuki. 2006. Survey of dogs in Japan for group 2 canine coronavirus infection. J. Clin. Microbiol. 44:2615-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]


