Abstract
Scrub typhus, caused by Orientia tsutsugamushi, has emerged recently in areas of northern China where the disease had not been known to exist. We analyzed epidemiological, clinical, and laboratory data for 104 patients who were admitted to a hospital in Fuyang City between 26 September and 1 November 2008. We showed that the major clinical manifestations of the patients were fever (100%), headache (82%), myalgias (77%), eschar (67%), rash (52%), and unusual facial flushing (62%). Among the 104 patients, the sera of 98% contained IgM antibodies to O. tsutsugamushi detected by indirect immunofluorescence assays (IFA), and DNA of the O. tsutsugamushi 56-kDa gene was amplified by PCR from the blood of 36 patients. We conclude that 104 patients were infected with scrub typhus in Fuyang City, Anhui Province. Our study indicates that physicians need to consider the diagnosis of scrub typhus for febrile patients living in northern China, where scrub typhus had not been considered to exist in the past.
Scrub typhus, also known as tsutsugamushi disease, is an acute, febrile infectious illness. It is a zoonosis that is widespread in southern Asia, in a triangle from northern Japan and far-eastern Russia in the north to northern Australia in the south and to Pakistan and Afghanistan in the west, as well as in the islands of the western Pacific and Indian Oceans. More than half (55%) of the world's population lives in areas where scrub typhus is endemic. The causative agent, Orientia tsutsugamushi (formerly Rickettsia tsutsugamushi), is an obligately intracellular bacterium and is transmitted to humans by the bite of a larval trombiculid mite, popularly known as a chigger. Scrub typhus has been known in southern China for thousands of years (5). However, the disease has emerged in northern China only in the last 2 decades (3). Here we report an outbreak of scrub typhus cases in the fall of 2008 in northwestern Anhui Province in central China, where the disease had not been known to occur previously.
MATERIALS AND METHODS
Study site.
Fuyang City, with a total population of more than 800,000, is located in northwestern Anhui Province, China (longitude, 114°9′ to 116°5′E; latitude, 32°4′ to 33°6′N) (Fig. 1). The city covers 1,830 km2 of alluvial plains with an average altitude of about 32 m. The area has temperate seasonal weather (average annual temperature, 14.5 to 15.0°C; frost-free period, 213 to 223 days), adequate rainfall (average annual precipitation, 821 to 938 mm), and adequate sunlight (the total solar radiation from north to south ranges from 5,000 to 5,150 MJ/mi2).
FIG. 1.
Scrub typhus study site: Fuyang City, Anhui Province, China. (Modified from a map in Wikimedia Commons.)
Informed consent and permission to perform the study.
The study was approved by the ethics committee of the Chinese National CDC, according to the medical research regulations of the Ministry of Health of China. Informed oral consent was obtained from all study participants.
Case definition.
The criteria for the clinical diagnosis of scrub typhus comprise fever plus one of the following symptoms: eschar, lymphadenopathy, and rash. Patients with confirmed scrub typhus cases were those who fulfilled these clinical criteria and had serum antibodies to O. tsutsugamushi (an IgM titer of ≥1:32 or an IgG titer of ≥1:64) and/or PCR detection of the O. tsutsugamushi 56-kDa gene. A total of 129 patients were diagnosed with scrub typhus in a hospital in Fuyang City according to the criteria described above. However, only 105 patients had complete clinical data and blood samples. For 1 of these 105 patients, no antibodies were detected by an indirect immunofluorescence assay (IFA) and no O. tsutsugamushi DNA was detected by PCR; this patient was therefore excluded from analysis. Thus, we report on 104 patients in this study who had clinical data and blood samples and who were confirmed as having scrub typhus by serology and/or PCR.
IFA.
We obtained a single acute-phase coagulated blood sample from each of 105 patients. Sera from 50 normal healthy persons (21 males and 29 females) ranging in age from 20 to 68 years were used as controls. The serum was examined for titers of antibody to O. tsutsugamushi by an IFA using antigen slides from Panbio (Queensland, Australia), which mixed Gilliam, Kato, and Karp antigens together. Serum was diluted from 1:32 in twofold increments; dilutions from 1:32 to 1:512 were evaluated for IgM, and dilutions from 1:64 to 1:2,048 were evaluated for IgG. The cutoff of a positive result was 1:32 for IgM and 1:64 for IgG.
PCR.
DNA was extracted from blood clots by using a blood DNA extraction kit (Qiagen, Valencia, CA). The O. tsutsugamushi 56-kDa protein gene was amplified by nested PCR as described previously (11). The outside primer pair comprised TACATTAGCTGCGGGTATGACA and CCAGCATAATTCTTCAACCAAG. The nested primer pair comprised GAGCAGAGCTAGGTGTTATGTA and TAGGCATTATAGTAGGCTGAGG. PCR products were 306 to 339 bp for the outside primer pair and 150 to 168 bp for the nested primer pair. PCR conditions were the same for both primer pairs, with initial denaturation for 5 min at 94°C, followed by 35 cycles of 1 min at 94°C, 1 min at 50°C, and 1 min at 72°C, and a final extension of 5 min at 72°C. PCR products were separated by electrophoresis, stained with ethidium bromide, and recorded by using the Bio-Rad gel imaging system.
Nucleotide sequence accession number.
The sequence of the O. tsutsugamushi 56-kDa gene sequenced in this study has been deposited in GenBank under accession number GQ468796.
RESULTS
Epidemiological data.
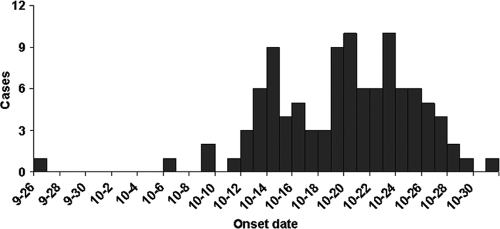
Analysis of clinical data from the 104 patients revealed that the proportions of patients with the disease did not differ between the genders; 51% of the patients were male, and 49.0% were female. Patient ages ranged from 7 to 83 years, with a median age of 52 years. The age distribution was as follows: 4% were less than 19 years old; 40% were 20 to 49 years old; 44% were 50 to 69 years old; and 12% were older than 70 years. Patients were predominantly farmers, who accounted for 80.8% of all cases. The first case was reported on 26 September; the last was reported on 1 November; and the majority of cases occurred from mid- to late October (Fig. 2).
FIG. 2.
Temporal distribution of onsets of Fuyang scrub typhus cases.
Clinical manifestations.
All patients (n = 104) had high fevers, and the average highest body temperature was 39.3°C. The duration of fever was 2 to 20 days (average, 10 days). Other major clinical manifestations included headache (82.7%), fatigue (81.7%), myalgias (77.9%), chills (75%), eschar (67.3%), facial flushing (62.5%), and rash (52.9%) (Table 1).
TABLE 1.
Clinical and laboratory findings for Fuyang scrub typhus patients
| Finding | % of patients |
|---|---|
| Clinical | |
| Fever | 100 |
| Headache | 82.7 |
| Fatigue | 81.7 |
| Myalgias | 77.9 |
| Chill | 75 |
| Eschar or skin ulcer | 67.3 |
| Facial flushing | 62.5 |
| Rash | 52.9 |
| Lymphadenopathy | 17.3 |
| Hepatosplenomegaly | 6.7 |
| Bronchopneumonia | 3.9 |
| Laboratory | |
| Increased WBC counta (10.0 × 109/liter to 18.6 × 109/liter) | 20.2 |
| Decreased platelet countb (30 × 109 to 100 × 109/liter) | 24 |
| Transaminase level increase | |
| ALT (>40 U/liter) | 79 |
| AST (>40 U/liter) | 81 |
| C-reactive protein increase | 28 |
Normal WBC counts are 4.3 × 109 to 10 × 109/liter.
Normal platelet counts are 150 × 109 to 400 × 109/liter.
Clinical laboratory results.
Analysis of 57 cases of scrub typhus revealed that the majority of patients had normal white blood cell (WBC) counts, while 20% had elevated WBC counts (>10.0 × 109/liter to 18.6 × 109/liter). Seventy-nine percent of patients had increased serum alanine aminotransferase (ALT) concentrations, and 81% had increased serum aspartate aminotransferase (AST) levels (Table 1).
PCR.
PCR using DNA extracted from 104 patients' blood clots revealed, by amplification of the 56-kDa protein gene, that 36 patients' samples contained O. tsutsugamushi DNA. DNA sequencing revealed that the sequences from all patients were identical. This sequence was 99% similar to the Shandong and Kawasaki genotypes on the nucleotide level and was 100% identical to the Shandong genotype and 99% identical to the Kawasaki genotype on the amino acid level.
IFA.
We tested the blood samples of all 104 patients and of 50 healthy persons for IgM and IgG antibodies. None of the healthy persons had serum antibodies to O. tsutsugamushi (IgM titer, <1:32; IgG titer, <1:64). IFA showed that 98% of patients had IgM antibodies (≥1:32) and 85.6% (89/104) had IgG antibodies (≥1:64) (Table 2). Two patients had no IgM antibodies by IFA, but both were positive by PCR and one had IgG antibodies at a titer of 1:64. The sensitivity of the IFA, as determined with PCR-positive patients, was 94% for IgM at a 1:32 dilution and 88% for IgG at 1:64 (Table 3). When IgM and IgG were combined, the sensitivity of the IFA increased to 97%.
TABLE 2.
IFA titers and numbers of patients positive for IgM or IgG antibodies
| Patient group | No. of patients with the following IFA titer: |
IFA-positive rate (%)a | ||||||
|---|---|---|---|---|---|---|---|---|
| 32 | 64 | 128 | 256 | 512 | 1,024 | 2,048 | ||
| IgM positive | 27 | 30 | 33 | 9 | 3 | 98 | ||
| IgG positive | 10 | 7 | 7 | 16 | 19 | 30 | 85 | |
Calculated as (number of IgM- or IgG-positive patients)/(total number of patients) × 100. The total number of patients was 104.
TABLE 3.
IFA sensitivity determined on the basis of 36 PCR-positive patients
| Patient group | No. of patients with the following IFA titer: |
Sensitivity (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 32 | 64 | 128 | 256 | 512 | 1,024 | 2,048 | ||
| PCR positive for IgM | 6 | 12 | 10 | 4 | 2 | 95 | ||
| PCR positive for IgG | 4 | 3 | 2 | 6 | 10 | 7 | 88 | |
Treatment.
We compared the periods from the initiation of antimicrobial treatment until defervescence for doxycycline-treated (n = 26) and azithromycin-treated (n = 16) patients. Both doxycycline and azithromycin were effective in the treatment of these patients with scrub typhus. Only 12.5% of patients remained febrile at 24 h after treatment with azithromycin, whereas at the same time 23% of patients treated with doxycycline remained febrile. No patients remained febrile after 48 h of treatment with either azithromycin or doxycycline. The median time to defervescence was 24 h for both the doxycycline- and azithromycin-treated groups. There were no serious adverse events among the study patients.
DISCUSSION
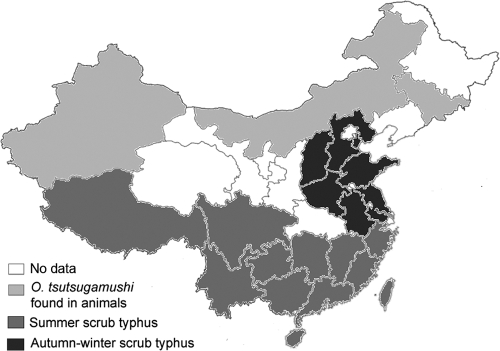
Before 1985, scrub typhus was known to be endemic in areas south of the Yangtze River (below latitude 31°N) in China. It has emerged in northern China since the disease was first reported in Shandong Province in 1986. Outbreaks of scrub typhus have now been reported in several northern provinces, including Tianjin (16), Hebei (1), Shanxi (2), Henan (15), Jiangsu (7), and Anhui (this study). The areas of scrub typhus epidemicity in China include three types: areas of summer scrub typhus endemicity, south of the Yangtze River; areas of autumn-winter scrub typhus endemicity, north of the Yangtze River; and areas in northern China where there is evidence of O. tsutsugamushi in animals but no patients with the disease have been reported (Fig. 3) (9, 14, 17). Autumn-winter scrub typhus occurs between September and December, with a peak in October, whereas summer scrub typhus occurs with a peak between June and July (9, 14). The serotype of O. tsutsugamushi in northern China is predominantly the Gilliam type, and the genotype is predominantly that of the Kawasaki strain (6, 10, 14). The discrepancy between the serotype and the genotype might be caused by the facts that the Gilliam and Kawasaki strains are closely related antigenically and that Gilliam strain antigens instead of Kawasaki antigens were used for IFA testing in China. There is an incomplete correlation between the serotype designation and the genotype. More serotypes of O. tsutsugamushi have been found in southern China, including Gilliam, Kato, and Karp strains. In northern China the Leptotrombidium scutellare chigger is the predominant vector of O. tsutsugamushi, whereas in southern China L. deliense is the predominant vector. Several other vectors have also been found in southern China, including L. rubellum in the coastal area of Fujian Province, L. gaohuense and L. insulare in Zhejiang Province, and L. jishoum in Hunan Province (9, 12, 14). The animals found to be infected with O. tsutsugamushi in southern China include Rattus (Rattus losea, R. flavipectus, R. norvegicus, R. confucianus), Apodemus agrarius, and Suncus murinus, whereas Apodemus agrarius is the dominant vertebrate host in Shandong Province in northern China (9, 12, 14). The fall outbreaks of scrub typhus in northern China appear to be associated with adequate temperature and rainfall in these areas during this season, which are suitable for the reproduction of L. scutellare.
FIG. 3.
Areas of scrub typhus endemicity in China. (Modified from a map in Wikimedia Commons.)
Fuyang, in northwest Anhui Province, had never reported scrub typhus before. The disease most likely had existed in the area previously but was not recognized until the fall 2008 outbreak. The clinical diagnostic criterion for scrub typhus used in the hospital in Fuyang City was quite a narrow definition, considering that a substantial proportion of patients with this disease do not have an eschar, lymphadenopathy, or a rash, which is confirmed in our study (Table 1). Thus, our cases likely represent an underestimate of the true incidence. Scrub typhus may be quite common in Anhui Province, as well as in other areas in northern China.
In China, hospitals usually do not perform specific laboratory diagnostic tests for scrub typhus using an IFA and/or PCR. The only laboratory diagnostic method generally available is the Weil-Felix Proteus OXK agglutination reaction, which was not used in this outbreak, because initial testing in a hospital in Fuyang showed that there is no difference in Weil-Felix reactivity between scrub typhus patients and normal healthy persons in the local population. Our study showed that the sensitivity of the IFA was 94% for IgM at 1:32 but only 88% for IgG at 1:64. The specificity of the IFA was 100% for both IgM and IgG, since none of the healthy persons had IgM or IgG antibodies. These observations suggest that an IFA with a 1:32 cutoff for IgM is a good criterion for initial screening for scrub typhus when a patient is first admitted to the hospital. However, treatment should not be withheld if the clinical features suggest scrub typhus in a patient who has not developed antibodies.
The epidemiological data showed that the majority of patients were farmers 20 to 69 years old, and the disease occurred during the fall harvest season. Two weeks before the outbreak, there had been moderate rainfall in the area. That may have promoted an increase in the chigger population, which increased the risk of transmission of scrub typhus when farmers harvested crops. The natural host and vector of O. tsutsugamushi in the area have not yet been investigated. Because Anhui Province is adjacent to Jiangsu Province and is not far from Shandong Province, where L. scutellare chiggers are the host and vector of O. tsutsugamushi (10, 13), it is most likely that L. scutellare is also the major host and vector of O. tsutsugamushi in Anhui Province.
Previous studies have demonstrated that azithromycin is effective in the treatment of scrub typhus (4, 8). Our study further confirmed that azithromycin is as effective as doxycycline for the treatment of scrub typhus and that it is a valid choice for such treatment. This is especially important for physicians in China, where the use of chloramphenicol and doxycycline has been restricted. Outbreaks of scrub typhus in northern China have challenged both physicians and policy makers in China. In China scrub typhus has not been a reportable infectious disease since 1989, and little attention has been paid to the disease, as reflected by the lack of diagnostic methods and epidemiological data. Most scrub typhus outbreaks in China in recent years were not recognized by physicians initially. Even in southern China, where scrub typhus is well known, the disease has a 77 to 79% misdiagnosis rate (18, 19). Thus, physicians need to be trained in the diagnosis of scrub typhus; specific laboratory diagnostic methods need to be established; and the prevalences of the vector and etiological agent of scrub typhus in northern China need to be investigated.
Footnotes
Published ahead of print on 3 February 2010.
REFERENCES
- 1.Chen, S., C. Li, L. Kon, Q. Shi, L. Zhou, X. Ji, Z. Li, and J. Xu. 2001. Observation on clinical characteristics of tsutsugamushi disease in the epidemic focus, Hebei Province. Chin. J. Vector Biol. Control 12:65-66. [Google Scholar]
- 2.Chen, X., X. Zheng, Q. Yu, Y. Zhang, L. Duan, Q. Huo, and X. Zhang. 1996. Outbreak of scrub typhus in Shanxi Province. Chin. J. Zoonoses 12:2. [Google Scholar]
- 3.Chen, X. R., Y. G. Zhang, and J. J. Wei. 1990. The biology and immunology properties of Orientia tsutsugamushi. Chin. J. Zoonoses 6:12-14. [Google Scholar]
- 4.Choi, E. K., and H. Pai. 1998. Azithromycin therapy for scrub typhus during pregnancy. Clin. Infect. Dis. 27:1538-1539. [DOI] [PubMed] [Google Scholar]
- 5.Fan, M. Y., D. H. Walker, S. R. Yu, and Q. H. Liu. 1987. Epidemiology and ecology of rickettsial diseases in the People's Republic of China. Rev. Infect. Dis. 9:823-840. [PubMed] [Google Scholar]
- 6.Guo, H., G. Wu, J. Tang, X. Li, M. Yu, Y. Zhang, S. Pan, and Y. Li. 1995. Finding out Kawasaki serotype Rickettsia tsutsugamushi by nested polymerase chain reaction in China. Chin. J. Zoonoses 11:22-24. [Google Scholar]
- 7.Guo, H. B., G. H. Wu, and M. H. Xu. 1994. Study on natural foci of tsutsugamushi disease of the autumn-winter type. Chin. J. Epidemiol. 15:27-30. [PubMed] [Google Scholar]
- 8.Kim, Y. S., H. J. Yun, S. K. Shim, S. H. Koo, S. Y. Kim, and S. Kim. 2004. A comparative trial of a single dose of azithromycin versus doxycycline for the treatment of mild scrub typhus. Clin. Infect. Dis. 39:1329-1335. [DOI] [PubMed] [Google Scholar]
- 9.Li, Z., X. Li, and Y. Liu. 2005. Epidemiology and reservoirs of scrub typhus in China. Practical Prev. Med. 12:1251-1253. [Google Scholar]
- 10.Liu, Y. X., Y. Gao, Z. T. Zhao, J. L. Zhang, Z. Q. Yang, X. P. Bu, and J. J. Su. 2004. Amplification and typing of sta56 gene of Orientia tsutsugamushi from Shangdong province. Chin. J. Epidemiol. 25:698-701. [PubMed] [Google Scholar]
- 11.Ohashi, N., H. Nashimoto, H. Ikeda, and A. Tamura. 1992. Diversity of immunodominant 56-kDa type-specific antigen (TSA) of Rickettsia tsutsugamushi. Sequence and comparative analyses of the genes encoding TSA homologues from four antigenic variants. J. Biol. Chem. 267:12728-12735. [PubMed] [Google Scholar]
- 12.Wang, Q. Z., H. Cui, Z. Li, S. He, and S. Q. Wang. 1995. The etiologic agent of scrub typhus in Shandong province. Chin. J. Zoonoses 11:47-48. [Google Scholar]
- 13.Wu, G., H. B. Guo, and M. Yu. 2000. Studies on three types of natural foci of tsutsugamushi disease in eastern part of China. Chin. J. Epidemiol. 21:34-36. [PubMed] [Google Scholar]
- 14.Wu, G. H. 2000. The epidemiology of scrub typhus in China. Chin. J. Infect. Dis. 18:142-144. [Google Scholar]
- 15.Xu, B. L., H. M. Chen, J. I. Zhang, S. L. Xia, M. L. Li, X. G. Li, L. Chen, X. W. Huang, and Z. C. Zhang. 2006. The epidemiological investigation on the first outbreak of tsutsugamushi disease in Henan Province. Henan J. Prev. Med. 17:129-131. [Google Scholar]
- 16.Yu, C. S., M. H. Xiao, and Z. L. Zhang. 1992. The first report of scrub typhus in countryside of Tianjin. Chin. J. Epidemiol. 13:212. [Google Scholar]
- 17.Zhang, Q., Y. X. Liu, X. M. Wu, Q. M. Zhao, P. H. Zhang, H. Yang, and W. C. Cao. 2006. Investigation on rodents natural infection of Orientia tsutsugamushi in some areas of Inner Mongolia and Xinjinag. Chin. J. Epidemiol. 27:475-478. [PubMed] [Google Scholar]
- 18.Zhao, L. Y. 2008. Clinical analysis of 48 cases of children tsutsugamushi. Occup. Health 24:799-800. [Google Scholar]
- 19.Zhao, Z. M. 2008. Analysis of 134 misdiagnosed scrub typhus cases. Chin. J. Rural Med. Pharm. 15:45-46. [Google Scholar]