Abstract
Objectives: To assess whether antibiotic treatment for acute cough is effective and to measure the side effects of such treatment.
Design: Quantitative systematic review of randomised placebo controlled trials.
Data sources: Nine trials (8 published, 1 unpublished) retrieved from a systematic search (electronic databases, contact with authors, contact with drug manufacturers, reference lists); no restriction on language.
Main outcome measures: Proportion of subjects with productive cough at follow up (7-11 days after consultation with general practitioner); proportion of subjects who had not improved clinically at follow up; proportion of subjects who reported side effects from taking antibiotic or placebo.
Results: Eight trials contributed to the meta-analysis. Resolution of cough was not affected by antibiotic treatment (relative risk 0.85 (95% confidence interval 0.73 to 1.00)), neither was clinical improvement at re-examination (relative risk 0.62 (0.36 to 1.09)). The side effects of antibiotic were more common in the antibiotic group when compared to placebo (relative risk 1.51 (0.86 to 2.64)).
Conclusions: Treatment with antibiotic does not affect the resolution of cough or alter the course of illness. The benefits of antibiotic treatment are marginal for most patients with acute cough and may be outweighed by the side effects of treatment.
Key messages
Acute cough, with or without sputum, is a common reason for consulting a general practitioner
Although antibiotic treatment is common for this condition, its likely benefits and side effects have not been measured
This systematic review reports the outcome of nine randomised controlled trials that compared antibiotic with placebo in patients with acute cough
Resolution of cough and clinical improvement at follow up was no different in the two groups
The benefits of antibiotic treatment seem to be marginal for most patients with acute cough and may be outweighed by the side effects of treatment
Introduction
Acute cough and respiratory tract infection are terms used to describe a wide variety of clinical syndromes. Symptoms range from cough without sputum to an illness characterised by expectoration of mucopurulent sputum, fever, general malaise, and dyspnoea,1 but coughing is nearly always present.1–4 Therefore, although the terms acute bronchitis, upper respiratory tract infection, common cold, and chest infection are used in a clinical context to define separate disease entities, they represent a range of respiratory tract infection whose symptoms, causative agents, and resolution vary.1,2
Acute cough is a common reason for consulting a general practitioner. The fourth national morbidity survey in the United Kingdom found that the overall consultation rate for acute upper respiratory infections (code 465 of the international classification of diseases, ninth revision (ICD-9)) and acute bronchitis and bronchiolitis (ICD-9 code 466) was 772 and 719 per 10 000 person years at risk.5
The clinical syndrome of cough is nearly always preceded and associated with a viral nasopharyngitis.1,2 The causes of such infection are usually influenza virus, para-influenza virus, respiratory syncytial virus, rhinovirus, coronavirus, and adenovirus.2,6,7 Infection with non-viral organisms such as Bordetella pertussis, Mycoplasma pneumoniae, and Chlamydia pneumonia may also occur, some studies reporting a high prevalence of infection with Mycoplasma spp, particularly in young adults.7,8 Secondary bacterial infection occurs in a certain proportion of cases, usually with Haemophilus influenzae and Streptococcus pneumoniae.1,2,7 Because bacteria are carried as normal resident flora in the upper respiratory tract, the aetiological role of bacteria cultured from sputum samples is unclear.2 In a study based in the United Kingdom 25% of sputum culture samples from people being treated for acute bronchitis grew recognised or potential respiratory bacterial pathogens.9 A community based longitudinal study in the United Kingdom showed that a potential pathogen was cultured in only 29% of cases, with viruses being identified more frequently than Mycoplasma spp and bacteria being identified least of all.4 In a community based study in the United Kingdom of 206 patients with more severe respiratory tract infection (inclusion criteria were productive cough, focal signs on chest examination, and prescription of antibiotic) an aetiological diagnosis was established in 91 (44%) patients.10 The most commonly identified pathogens were S pneumoniae (36%), H influenzae (10%), and influenza viruses (13%).10 An accompanying editorial highlighted the difficulty in clinically differentiating between the more severe forms of bronchitis and pneumonia in the community.11
Microbiological investigation of acute bronchitis is rare in general practice.1,9,12 Differentiation between viral and bacterial infection is difficult on the basis of symptoms alone,1 and therefore general practitioners have substantially different diagnostic and treatment thresholds for respiratory tract infection in the community.1,12,13
Concern about the treatment of acute cough with antibiotics is not new.14,15 Review articles have questioned the value of antibiotic treatment for acute bronchitis and related conditions.1,16–19 To our knowledge, the absolute risk of illness without antibiotic treatment, the likely benefits and risks of treatment, and the balance of risk and benefit for individual patients have not been measured. We therefore carried out a systematic review of randomised controlled trials to establish whether antibiotics are effective in the treatment of acute cough in the community.
Methods
Inclusion and exclusion criteria
We included studies of patients aged greater than 12 years who were attending a family practice clinic, community based outpatient department, or an outpatient department attached to a hospital. We included patients who complained of acute cough with or without purulent sputum that had not been treated in the preceding week with antibiotic. Patients with chronic obstructive airways disease were excluded. The included studies were prospective trials in which antibiotic was allocated by formal randomisation or by quasi-randomisation, such as alternate allocation to treatment and placebo groups. Only placebo controlled trials were included; comparative studies between different classes of antibiotics were excluded. Categorical and continuous outcomes were reported in the randomised controlled trials identified at the start of the review.20–28 Many different outcomes were reported in individual randomised controlled trials; we concentrated on the three most commonly reported outcomes: the proportion of subjects reporting productive cough, the proportion of subjects who had not improved clinically at re-examination, and the proportion of subjects who reported side effects from taking antibiotic or placebo.
Systematic search
We searched Medline and EMBASE databases from 1966 and 1982 respectively using the recommended Cochrane Collaboration search strategy29 and the medical subject heading (MeSH) terms “cough,” “bronchitis,” “sputum,” and “respiratory tract infections.” The search was not restricted to the English language. We also searched for references from published research by using the Science Citation Index and searching references in published studies and abstracts, particularly for those published before 1966. We conducted a search on the Controlled Trials Register from the Cochrane Library30 with the search terms “bronchitis,” “chest infection,” and “common cold.” We contacted authors of published trials requesting knowledge of any unpublished studies. We also wrote to drug companies in the United Kingdom that manufacture antibiotics (as given in the British National Formulary) requesting unpublished trials.
Assessment of quality and extraction of data
Each trial was read independently by TF and NS, who then assessed the quality of each study according to the four criteria outlined in the Cochrane Collaboration Handbook.31 Each criterion—selection bias, performance bias, attrition bias, and detection bias—was scored from 1 to 3, so the highest score for an individual trial was 12. Measurement of agreement between reviewers was calculated by means of the kappa statistic and disagreement resolved by consensus. Data were extracted independently; when data were missing or incomplete we contacted the authors of the trial for clarification.
Analysis
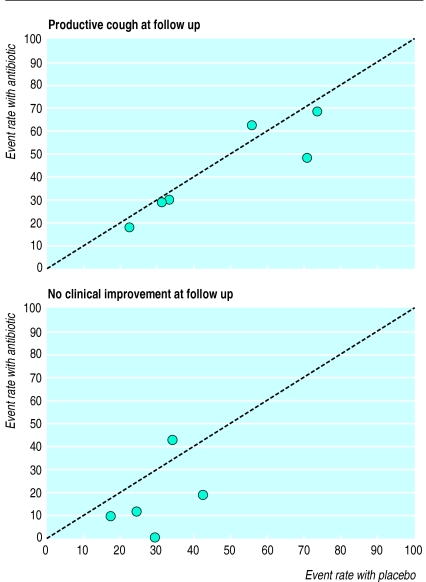
Because the events in the treatment and control arms occurred frequently, significance and clinical importance were evaluated by estimating relative risk.32 We explored differences in baseline risk and heterogeneity between studies by using L’Abbe plots (see fig 1).33 As the inclusion criteria and event rates reported in the control arms varied, the pooled relative risks were estimated with 95% confidence intervals by means of both random effects and fixed effects models.34 Antibiotic is significantly better than placebo in improving a condition when the upper limit of the 95% confidence interval is <1. Conversely, side effects of antibiotic treatment are significant when the lower 95% confidence limit of the relative risk is >1.
Figure 1.
L’Abbe plots of proportion of subjects with productive cough at follow up at 7-11 days (six trials) and of proportion of subjects who had not improved clinically at 7-11 days (five trials)
Relative risks were calculated with revman 3.0 (Update Software 1996). We calculated the numbers needed to treat with a spreadsheet (Microsoft excel 5.0).35
Results
Trials found
Our search uncovered nine trials that met the inclusion criteria for this review (M Stephenson, unpublished data).21–28 The losses to follow up, antibiotic regimen, outcome measured, recommendation for antibiotic treatment, and characteristics of patients for these nine trials are available as two tables on the BMJ’s website (www.bmj.com).
We excluded Howie and Clark’s trial from the 1970s in 829 patients.20 Although the unit of randomisation was patients who were instructed to take either antibiotic or placebo at the start of a respiratory illness, the unit of analysis was episodes of illness. Some patients did not contribute any episodes of illness to the analysis (198/829 participants, or 24% of those randomised) while others reported more than one episode of illness (1.52 and 1.55 courses in the antibiotic and placebo arms respectively).20 This trial reported no difference between antibiotic and placebo in all outcomes reported at the end of the trial. One other unpublished trial that had reported no difference in outcome between antibiotic and placebo in 33 patients,36 had no original data remaining (S Thomas, personal communication). Franks and Gleiner reported the average percentage of days with cough over a period of seven days (all subjects in placebo arm, 92% of subjects in antibiotic arm) but not the number of patients with cough at a specified end point.22 No further data were available (P Franks, personal communication), so this trial contributed data to the part of the meta-analysis which examined the side effects of treatment only. King et al included patients aged 8 years and over, but the average age of participants was 37 years and so we included this trial.27 Finally, one trial by Scherl et al did not contribute data to the meta-analysis because it reported on a continuous variable, the mean number of days with cough.28 No additional information could be obtained because the author of the report had died.
This left us with a total of eight trials reporting on the three specified outcome measures. We excluded several trials we judged to be inadequately randomised, case series of early antibiotic use, three trials of the common cold, and eleven trials in children (references available on website (www.bmj.com)). A subgroup of 75 patients with tracheobronchitis from a trial with the diagnostic label of the common cold (L Kaiser, personal communication) was included in a sensitivity analysis on the outcome of resolution of illness.37
Assessment of quality
The kappa scores for agreement between reviewers for each of the four variables measuring the quality of trials were 0.5 (moderate agreement) for selection, 0.57 (moderate agreement) for performance, 0.85 (substantial agreement) for attrition, and 1 (almost perfect agreement) for blinding. The overall kappa for trial quality was 0.54 (moderate agreement).
Baseline risk and diagnosis
The six trials that reported resolution of productive cough as an outcome measure had varied considerably in this measure (fig 1) (M Stephenson, unpublished data).21,23,25–27 Such differences highlight the range of illness and the differences between trials in diagnosis of acute cough. However, the five trials that had outcome data on the course of clinical improvement were similar in the reported resolution of illness (fig 1).21,23–26
Efficacy of antibiotic
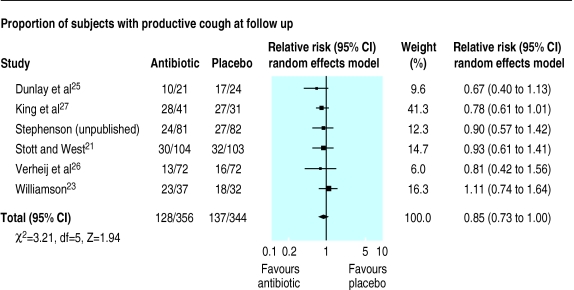
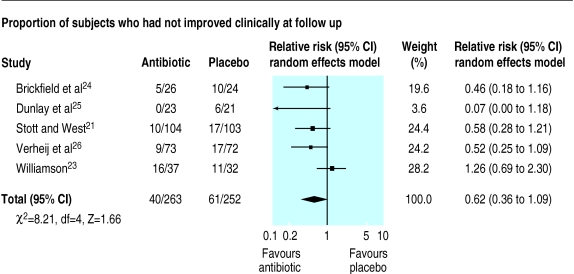
Antibiotic treatment was no better than placebo when the resolution of cough at days 7-11 was assessed (relative risk 0.85 (95% confidence interval 0.73 to 1.00)) (fig 2). Similarly, when the proportion of subjects who had not improved clinically was assessed at days 7-11 in five trials antibiotic treatment did not significantly improve the resolution of illness (relative risk 0.62 (0.36 to 1.09)) (fig 3).21,23–26 Inclusion of a subgroup of 75 patients with tracheobronchitis in a trial of the common cold who were randomly allocated to co-amoxiclav or placebo37 did not alter the pooled results for resolution of illness (relative risk 0.71 (0.43 to 1.18), χ2 test for heterogeneity=16.87, df=5, P<0.5).
Figure 2.
Comparison of antibiotic and placebo treatment on resolution of productive cough at days 7-11
Figure 3.
Comparison of antibiotic and placebo treatment on clinical improvement at days 7-11
Side effects of treatment
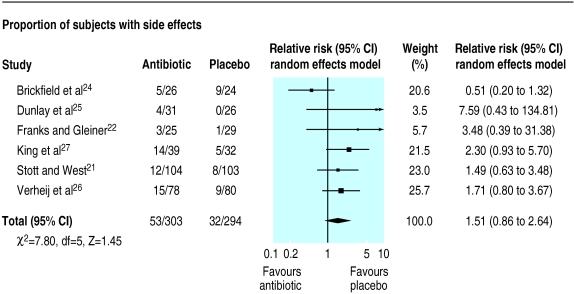
The mean percentage of subjects reporting side effects from antibiotic treatment in seven trials was 19% (range 12% to 36%). In all but one trial24 the percentage of subjects reporting side effects was higher in the antibiotic arm; subsequent pooling of data showed that a course of antibiotic was associated with a non-significant increase in the risk of side effects from antibiotic (relative risk 1.51 (0.86 to 2.64)) (fig 4). When the one trial which reported an increase in side effects from placebo was excluded,24 the heterogeneity between trials was reduced and side effects were significantly associated with antibiotic use (relative risk 1.9 (1.19 to 3.02), χ2 test for heterogeneity=1.73, df=4, P>0.5).
Figure 4.
Comparison of antibiotic and placebo treatment on rate of reporting of side effects
Discussion
This systematic review shows that antibiotic treatment has no effect on the resolution of acute cough. For both measures of efficacy—the proportion of subjects coughing and the proportion whose symptoms had not improved at days 7-11—antibiotic was no different from placebo. Furthermore, treatment with antibiotic may incur side effects in a few patients.
Shortcomings
This review has several shortcomings. Firstly, the outcomes chosen and assessed in each of the randomised trials were varied and different. Consequently, when the results were pooled several important outcomes were reported only in some of the trials and were measured in different ways. For example, time off work was measured as a continuous outcome in two trials,23,28 as a categorical outcome in three others,21,22,25 as a categorical and continuous outcome in one trial,27 and not at all in the remaining trials (M Stephenson, unpublished data).24,26
Secondly, more recent generic scores for measuring the quality of life were not used in any of the trials, once again limiting the propensity to combine the results. Therefore important information for patients such as the effect of antibiotic on quality of life and on return to work is not reported.
Finally, the timing of assessment differed between trials. Such differences make it difficult to measure the clinical course of acute cough. These shortcomings reflect the difficulty in combining results from pragmatic randomised trials that examine outcomes based on illness in general practice. Nevertheless, substantially important differences between antibiotic and placebo are unlikely to be present in these other outcomes: individual trials did not report any substantial benefit of antibiotic in the outcomes that we did not consider in this systematic review.
Diagnosis and prognosis
The clinical course of resolution of acute cough was different between trials (fig 1). Such differences reflect the fact that acute cough is primarily diagnosed on history and examination alone. Additional diagnostic tests such as sputum culture and chest radiography are seldom used in general practice,1,9,12 so diagnostic classification is imprecise. The diagnostic nomenclature has also changed over time. For example, an early non-randomised study of acute respiratory infection38 found that the signs reported by the enrolled cohort were no different from those in subjects classed as having acute bronchitis in the 1980s.22–25,27 In addition, the relation between diagnostic category and likelihood of bacterial infection is poorly defined and uncertain in clinical practice.
Inevitably, the diagnostic heterogeneity in each of the randomised controlled trials has been reflected in differences in the reported resolution of cough or illness (fig 1). Results from cohort studies suggest that it may take up to three or four weeks before cough has resolved and general wellbeing returned in patients with acute bronchitis.39
Implications
In the clinical context of everyday management of acute cough in general practice, treatment with antibiotics is common. The variation in rate of prescribing antibiotics varies substantially between countries. One fifth of consultations in the Netherlands end with an antibiotic being prescribed, up to 80% in the United Kingdom, and an even higher proportion in the United States.40–42 Results from our systematic review suggest that most patients receive no benefit from antibiotic treatment.
We calculated the number needed to treat (11) and the number needed to harm (15) if we accepted that the outcome of proportion of subjects who reported clinical improvement was balanced with the proportion likely to have side effects from taking antibiotic. For every 100 people treated with antibiotic, nine would report an improvement after 7-11 days if they revisited their general practitioner but at the expense of seven others who would have side effects from the antibiotic. The resolution of illness in the remaining 84 people would not be affected by treatment with antibiotic.
One of the higher quality trials reported that the prognostic factors of frequent cough combined with feeling ill at entry were associated with beneficial effects from antibiotic treatment.26 The same trial also reported that people aged over 55 derived benefit from such treatment.26 These findings are consistent with the greater prevalence of bacterial infection and subsequent infection of the lower respiratory tract in people aged over 55 and increased rates of hospital admission of elderly people.7,10,43 However, until prospective randomised controlled trials test whether age, feeling unwell, and frequent cough predict a poor clinical outcome or bacterial infection and also influence clinical and quality of life outcomes associated with antibiotic treatment, treatment based on these prognostic variables will remain speculative.
Apart from the side effects of treatment, three other factors need to be considered by a clinician before prescribing antibiotics. Firstly, the cost of prescribing antibiotic is important. A recent decision analysis suggests that the most cost effective strategy is to withold antibiotic and treat only patients with persistent cough.44 Secondly, treatment with antibiotic may change patients’ expectations about future episodes of acute cough. Observational research suggests that patients’ expectations may increase and influence subsequent workload among general practitioners.41,45 A recently published open randomised trial of antibiotic for sore throat showed that patients prescribed antibiotic were more likely to consult in the future than were those whose symptoms were treated.46 Qualitative research suggests that patients’ satisfaction is likely to be higher when their expectations are addressed than when antibiotic is prescribed.47 Finally, bacterial resistance to antibiotics is becoming increasingly common.48 A liberal prescribing policy by general practitioners managing acute bronchitis is likely to make this situation worse.
Conclusions
This systematic review shows that antibiotic is unlikely to alter the course of illness in most adult patients presenting with acute cough. A minority may have side effects from treatment. When managing individual patients the potential risks from treatment—including side effects, costs of antibiotic, alteration in consulting behaviour, and increased bacterial resistance—should all be considered before initiating treatment.
Figure.

Data from the nine included trials are available on our website at www.bmj.com, as well as citation details of excluded trials
Acknowledgments
We thank the following for providing extra data, clarifying data from published studies, or providing information about unpublished studies: Chris van Weel, Peter Franks, John Howie, Laurent Kaiser, Dana King, Amy Schende, Mike Stephenson, Siewert Thomas, Nigel Stott, Murray Tilyard, and Theo Verheij. We also thank the following for comments on the manuscript: Mike Crilly, Jon Deeks, Tim Lancaster, Debbie Sharp, and Michael Whitfield. Finally, we would particularly like to thank Debbie Jones for help with the searching and Matthias Egger for helping us with methodological dilemmas.
Footnotes
Funding: The Royal College of General Practitioners Scientific Foundation Board.
Conflict of interest: None.
References
- 1.Verheij T, Kaptein A, Mulder J. Acute bronchitis: aetiology, symptoms and treatment. Fam Pract. 1989;6:66–69. doi: 10.1093/fampra/6.1.66. [DOI] [PubMed] [Google Scholar]
- 2.Gwaltney JM. Acute bronchitis. In: Mandell GL, Bennet JE, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York: Churchill Livingstone; 1995. pp. 606–608. [Google Scholar]
- 3.World Organisation of National Colleges and Academies of General Practice. An international classification of health problems of primary health care (ICHPPC-2). 3rd ed. Oxford: Oxford University Press; 1983. [Google Scholar]
- 4.Boldy DA, Skidmore SJ, Ayres JG. Acute bronchitis in the community: clinical features, infective factors, changes in pulmonary function and bronchial reactivity to histamine. Respir Med. 1990;84:377–385. doi: 10.1016/S0954-6111(08)80072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Royal College of General Practitioners; Office of Population Censuses and Surveys; Department of Health. Morbidity statistics from general practice. Fourth national study, 1991-1992. London: HMSO; 1995. [Google Scholar]
- 6.MRC Working Party on Acute Respiratory Virus Infections. A collaborative study of the aetiology of acute respiratory infections in Britain, 1961-4. BMJ 1965;ii:319-26. [DOI] [PMC free article] [PubMed]
- 7.Garibaldi RA. Epidemiology of community-aquired respiratory tract infections in adults. Am J Med. 1985;78:32–37. doi: 10.1016/0002-9343(85)90361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King DE, Muncie HL. High prevalence of Mycoplasma pneumoniae in patients with respiratory tract symptoms: a rapid detection method. J Fam Pract. 1991;32:529–531. [PubMed] [Google Scholar]
- 9.Johnson PH, MacFarlane JT, Humphreys H. How is sputum microbiology used in general practice? Respir Med. 1996;90:87–88. doi: 10.1016/s0954-6111(96)90203-6. [DOI] [PubMed] [Google Scholar]
- 10.Macfarlane J, Colville A, Guion A, Macfarlane R, Rose D. Prospective study of aetiology and outcome of adult lower-respiratory-tract infections in the community. Lancet. 1993;341:511–514. doi: 10.1016/0140-6736(93)90275-l. [DOI] [PubMed] [Google Scholar]
- 11.Örtqvist A. Respiratory infection: community acquired respiratory infection. Lancet 1993;341:529-30. [DOI] [PubMed]
- 12.Verheij T, Hermans J, Kaptein A, Wijkel D, Mulder J. Acute bronchitis: general practitioners’ views regarding diagnosis and treatment. Fam Pract. 1990;7:175–180. doi: 10.1093/fampra/7.3.175. [DOI] [PubMed] [Google Scholar]
- 13.Howie JR, Richardson IM, Gill G, Durno D. Respiratory illness and antibiotic use in general practice. J R Coll Gen Pract. 1971;21:657–663. [PMC free article] [PubMed] [Google Scholar]
- 14.Antibiotics and respiratory illness [editorial]. BMJ 1974;ii:1. [DOI] [PMC free article] [PubMed]
- 15.Wyatt T, Passmore C, Morrow N, Reilly P. Antibiotic prescribing: the need for a policy in general practice. BMJ. 1990;300:441–444. doi: 10.1136/bmj.300.6722.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orr P, Scherer K, Macdonald A, Moffatt M. Randomised placebo-controlled trials of antibiotics for acute bronchitis: a critical review of the literature. J Fam Pract. 1993;36:507–512. [PubMed] [Google Scholar]
- 17.MacKay DN. Treatment of acute bronchitis in adults without underlying lung disease. J Gen Intern Med. 1996;11:557–562. doi: 10.1007/BF02599608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzales R, Sande M. What will it take to stop physicians from prescribing antibiotics in acute bronchitis? Lancet. 1995;345:665–666. doi: 10.1016/s0140-6736(95)90861-7. [DOI] [PubMed] [Google Scholar]
- 19.Wise R. Antibiotics for the uncommon cold. Lancet. 1996;347:1499. doi: 10.1016/s0140-6736(96)90664-9. [DOI] [PubMed] [Google Scholar]
- 20.Howie J, Clark G. Double-blind trial of early demethylchlortetracycline in minor respiratory illness in general practice. Lancet. 1970;ii:1099–1102. doi: 10.1016/s0140-6736(70)92294-4. [DOI] [PubMed] [Google Scholar]
- 21.Stott N, West R. Randomised controlled trial of antibiotics in patients with cough and purulent sputum. BMJ. 1976;ii:556–559. doi: 10.1136/bmj.2.6035.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franks P, Gleiner J. The treatment of acute bronchitis with trimethoprim and sulfamethoxazole. J Fam Pract. 1984;19:185–190. [PubMed] [Google Scholar]
- 23.Williamson H. A randomised, controlled trial of doxycycline in the treatment of acute bronchitis. J Fam Pract. 1984;19:481–486. [PubMed] [Google Scholar]
- 24.Brickfield F, Carter W, Johnson R. Erythromycin in the treatment of acute bronchitis in a community practice. J Fam Pract. 1986;23:119–122. [PubMed] [Google Scholar]
- 25.Dunlay J, Reinhardt R, Donn L. A placebo-controlled, double blind trial of erythromycin in adults with acute bronchitis. J Fam Pract. 1987;25:137–141. [PubMed] [Google Scholar]
- 26.Verheij T, Hermans J, Mulder J. Effects of doxycycline in patients with acute cough and purulent sputum: a double blind placebo controlled trial. Br J Gen Pract. 1994;44:400–404. [PMC free article] [PubMed] [Google Scholar]
- 27.King D, Williams CW, Bishop L, Shechter A. Effectiveness of erythromycin in the treatment of acute bronchitis. J Fam Pract. 1996;42:601–605. [PubMed] [Google Scholar]
- 28.Scherl E, Riegler S, Cooper J. Doxycycline in acute bronchitis: a randomized double-blind trial. Journal of the Kentucky Medical Association. 1987;85:539–541. [PubMed] [Google Scholar]
- 29.Dickerson K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. In: Chalmers I, Altman D, editors. Systematic reviews. London: BMJ Publishing Group; 1995. pp. 17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cochrane Collaboration. Oxford: Update Software; 1997. The Cochrane Library. Issue 2 ed. [Google Scholar]
- 31.Mulrow CD, Oxman AD. Cochrane Collaboration Handbook (updated 9 December 1996). In: The Cochrane Collaboration, ed. The Cochrane Library (database on disc and CDROM). Issue 2 ed. Oxford: Update Software, 1996.
- 32.Hennekens C, Buring J. Epidemiology in medicine. Boston: Little, Brown; 1987. [Google Scholar]
- 33.L’Abbe KA, Detsky AS, O’Rourke K. Meta-analysis in clinical research. Ann Intern Med 1987;107:224-33. [DOI] [PubMed]
- 34.Petitti DB. Meta-analysis, decision analysis and cost-effectiveness analysis. Methods for quantitative synthesis in medicine. New York: Oxford University Press; 1994. [Google Scholar]
- 35.Sackett D, Richardson WS, Rosenberg W, Haynes RB. Evidence-based medicine: how to practise and teach EBM. London: Churchill Livingstone; 1996. [Google Scholar]
- 36.Thomas S. Antibiotics for cough and purulent sputum. BMJ. 1978;i:1374. doi: 10.1136/bmj.2.6148.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaiser L, Lew D, Hirschel B, Auckenthaler R, Morabia A, Heald A, et al. Effects of antibiotic treatment in the subset of common-cold patients who have bacteria in nasopharyngeal secretions. Lancet. 1996;347:1507–1510. doi: 10.1016/s0140-6736(96)90670-4. [DOI] [PubMed] [Google Scholar]
- 38.Jones PH, Bigham R, Manning PR. Use of antibiotics in nonbacterial respiratory infections. JAMA. 1953;99:262–264. doi: 10.1001/jama.1953.02940210004002. [DOI] [PubMed] [Google Scholar]
- 39.Verheij T, Hermans J, Kaptein A, Mulder J. Acute bronchitis: course of symptoms and restrictions in patients daily activities. Scand J Primary Health Care. 1995;13:8–12. doi: 10.3109/02813439508996728. [DOI] [PubMed] [Google Scholar]
- 40.Kuyvenhoven M, de Melker R, Van der Velden K. Prescription of antibiotics and prescribers’ characteristics. A study into prescription of antibiotics in upper respiratory tract infections in general practice. Fam Pract. 1993;10:366–370. doi: 10.1093/fampra/10.4.366. [DOI] [PubMed] [Google Scholar]
- 41.Davey P, Rutherford D, Graham B, Lynch B, Malek M. Repeat consultations after antibiotic prescribing for respiratory infections: a study in one general practice. Br J Gen Pract. 1994;44:509–513. [PMC free article] [PubMed] [Google Scholar]
- 42.Mainous AG, Hueston W, Clark JR. Antibiotics and upper respiratory infection. J Fam Pract. 1996;42:357–361. [PubMed] [Google Scholar]
- 43.Verheij T. Antibiotics in acute bronchitis. Lancet. 1995;345:1244. [PubMed] [Google Scholar]
- 44.Hueston WJ. Antibiotics: neither cost effective nor “cough” effective. J Fam Pract. 1997;44:261–265. [PubMed] [Google Scholar]
- 45.Howie JGR, Hutchison KR. Antibiotics and respiratory illness in general practice: prescribing policy and work load. BMJ. 1978;ii:1342. doi: 10.1136/bmj.2.6148.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ. 1997;315:350–352. doi: 10.1136/bmj.315.7104.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamm RM, Hicks RJ, Bemben DA. Antibiotics and respiratory infections: are patients more satisfied when expectations are met? J Fam Pract. 1996;43:56–62. [PubMed] [Google Scholar]
- 48.Verkatesum P, Innes JA. Antibiotic resistance in common acute respiratory pathogens. Thorax. 1995;50:481–483. doi: 10.1136/thx.50.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]