Abstract
A total of 7,764 isolates from patients with invasive pneumococcal disease (IPD) were collected from 1992 to June 2006. Data on serotypes were available for 5,022 isolates (64.7% of all invasive isolates). Some 54.0% of the isolates originated from adults ≥16 years of age, and 46.0% were from children <16 years of age. The leading serotypes were 14, 23F, 1, 6B, 7F, 3, and 4. The serotypes significantly more common in children were 14, 6B, 19F, and 18C, while among adults, serotypes 3 and 4 were predominant. Serotype 7F was statistically more prevalent among children <4 months old than among the other age groups. Among children aged ≥4 months and <1 year, serotype 19F occurred statistically more frequently; and among children aged ≥1 year to <5 years, serotypes 14, 6B, and 18C were overrepresented. The serotypes predominantly affecting patients younger than the remaining collective of patients were 14, 6B, 19F, and 18C, while patients with IPD caused by serotypes 3, 4, and 9V were older than the collective, on average.
Streptococcus pneumoniae is among the most important pathogens in bacterial pneumonia, sepsis, and meningitis worldwide (1). The capsular polysaccharide of S. pneumoniae is known to be an important factor in the pathogenicity of the organism (7), and associations of the capsular serotypes with the severity of invasive pneumococcal diseases (IPDs) have been described (2, 14). IPDs are known to be much more frequent among young children and elderly persons than among older children and middle-aged adults (25). The results of several studies of the frequencies of pneumococcal serotypes and pneumococcal vaccine coverage have been published, but only few data on the serotype distribution among children and adults or different age groups are available. Studies from the United States (10, 19, 25), Canada (18), England and Wales (21), and Denmark (9), as well as the available meta-analyses (4, 15), refer to a diversity of age intervals. Although some studies contain statistics on the serotypes covered by the seven-valent pneumococcal conjugate vaccine (PCV7) and those not covered by the vaccine, no information on the association of individual serotypes with age are given. Another meta-analysis reported on the relative risk (odds ratio) of IPD caused by the various serotypes among all age groups subdivided into 10-year bands (26). Furthermore, in a 19-year nationwide surveillance study from Denmark, statistical analyses of the serotype distribution with age have been performed, but that study included only children aged 0 to 6 years (16). Some statistical data about individual serotypes and their association with age can also be found in the report of a study from the United States on the increased prevalence of pediatric pneumococcal serotypes in elderly adults. However, the study contained data only for adults aged ≥35 years (5).
The aim of the study described here was to evaluate the association of serotypes of S. pneumoniae with age in IPD among isolates from all age groups sent to the German National Reference Center for Streptococci (NRCS) between 1992 and 2006.
MATERIALS AND METHODS
Study design.
NRCS has conducted surveillance for invasive pneumococcal disease in Germany since 1992. In the present study, a population- and laboratory-based approach was used to collect data about invasive disease caused by Streptococcus pneumoniae in Germany. Isolates were sent to NRCS by diagnostic microbiological laboratories throughout Germany on a voluntary basis. Cases from 1 January 1992 to 30 June 2006 were included in this study. A case of IPD was defined by the isolation of S. pneumoniae from a normally sterile site. In cases in which the invasiveness was unclear, samples were considered to represent invasive disease only after a review of all available laboratory information, specimen type, the physician's specialty, and comments on request forms. Children <4 months of age were defined as all children who had not completed the 4th month of life.
Microbiological investigations.
The isolates were identified by standard procedures, including tests for bile solubility and optochin sensitivity. Pneumococcal isolates were serotyped by Neufeld's Quellung reaction with type- and factor-specific antisera (Statens Serum Institut, Copenhagen, Denmark). Streptococcus pneumoniae ATCC 49619 was used as a control strain.
Statistical analysis.
All categorical data are expressed as frequencies; continuous variables are expressed as medians and interquartile ranges. Comparisons of serotype frequencies between age groups were done by Fisher's exact test or the chi-square test, as appropriate. Adjusted P values for multiple post-hoc comparisons after classification of the individuals of different ages into six groups were calculated by Fisher's exact test and permutation resampling of 1,000 samples. To assess whether age was generally higher or lower for patients infected with a certain serotype, a two-sided nonparametric unpaired Wilcoxon test was conducted.
As we investigated 12 different serotypes that occurred at high rates within the study population or a subgroup of that population in detail, the overall significance level was adjusted by using the Bonferroni correction method to account for the problem of multiple testing. Thus, test results with P values of ≤0.0042 were considered statistically significantly different.
All statistical analyses were done with the SAS (version 9.1) program (2002-2003; SAS Institute Inc., Cary, NC). Graphics were generated with the SPSS for Windows program (release 17.0.0, 2008; SPSS Inc., Chicago, IL).
RESULTS
A total of 7,764 isolates from patients with invasive pneumococcal disease were collected between 1 January 1992 and 30 June 2006. The numbers of isolates collected per year varied from 297 to 918 (median, 464 isolates). Data on the serotypes were available for 5,022 isolates (64.7% of all invasive isolates). Some 55.5% of these isolates were from male patients, and 42.2% were from female patients; no information on gender was available for 2.3% of the patients. A total of 54.0% of the isolates originated from adults, and 46.0% were from children.
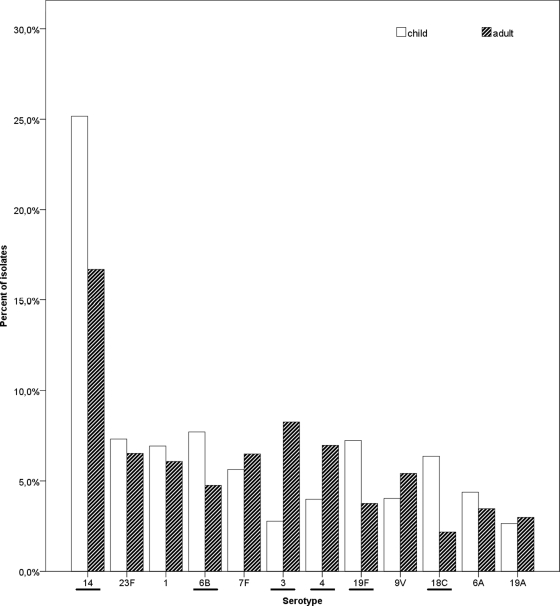
A comparison of the findings for children <16 years of age and adults ≥16 years of age is presented in Table 1. The leading serotypes detected were 14, 23F, 1, 6B, 7F, 3, and 4 (in descending order). The serotypes that were significantly more common in children were 14, 6B, 19F, and 18C, while among adults, serotypes 3 and 4 were predominant (Table 1, columns 3 to 5; Fig. 1). When the median age of patients with IPD caused by particular serotypes was compared with the median age of the remaining patients with IPD caused by other serotypes, which was used as a control group, considerable differences were noticed (Table 1, columns 6 to 8). The serotypes predominantly affecting patients younger than the remaining collective of patients were 14, 6B, 19F, and 18C, while patients with IPD caused by serotypes 3, 4, and 9V were older than the collective, on average.
TABLE 1.
Serotypes tested for significant differences in their distributions among children and adults by age and their distributions among patients with IPD caused by particular serotypes compared to their distributions among patients with IPD not caused by those serotypesa
| Serotype | Overall no. of patients | Distribution by age |
Distribution by serotype |
||||
|---|---|---|---|---|---|---|---|
| % |
P (Fisher's exact test) | Median (IQRb) age (yr) of: |
P (Wilcoxon test) | ||||
| Children (n = 2,309) | Adults (n = 2,713) | Individuals with IPD caused by the indicated serotype | Individuals with IPD not caused by the indicated serotype | ||||
| 14 | 1,034 | 25.16 | 16.70 | <0.0001c | 3 (63) | 38 (65) | <0.0001 |
| 23F | 346 | 7.32 | 6.52 | 0.2882 | 24.5 (66) | 35 (65) | 0.7470 |
| 1 | 325 | 6.93 | 6.08 | 0.2274 | 16 (49) | 36 (66) | 0.9213 |
| 6B | 307 | 7.71 | 4.75 | <0.0001 | 3 (62) | 36 (66) | <0.0001 |
| 7F | 306 | 5.63 | 6.49 | 0.2142 | 38.5 (66) | 34 (65) | 0.8355 |
| 3 | 288 | 2.77 | 8.26 | <0.0001 | 62 (37) | 31 (65) | <0.0001 |
| 4 | 281 | 3.98 | 6.97 | <0.0001 | 47 (66) | 33 (65) | 0.0002 |
| 19F | 269 | 7.23 | 3.76 | <0.0001 | 3 (56) | 36 (65) | <0.0001 |
| 9V | 240 | 4.03 | 5.42 | 0.0239 | 51.5 (65.5) | 33 (65) | 0.0038 |
| 18C | 206 | 6.37 | 2.17 | <0.0001 | 4 (33) | 37 (66) | <0.0001 |
| 6A | 195 | 4.37 | 3.46 | 0.1068 | 11 (69) | 34 (65) | 0.9492 |
| 19A | 142 | 2.64 | 2.99 | 0.4949 | 45 (69) | 34 (65) | 0.7176 |
Only serotypes with at least 100 isolates (overall) were considered.
IQR, interquartile range.
Boldface indicates a statistically significant difference.
FIG. 1.
Comparison of the frequency of serotypes causing IPD among children <16 years of age and adults ≥16 years of age. Differences that reached statistical significance are underlined.
The differences among the age groups from 0 to <4 months, ≥4 months to <1 year, ≥1 to <5 years, ≥5 to <16 years, ≥16 to <65 years, and ≥65 years are shown in Table 2. The chi-square test was used to test whether significant differences existed among the age groups. Verified significant differences were further evaluated by testing each age group against all other age groups combined. For serotypes 1, 14, 3, 18C, and 19F, significant differences were observed for more than one age group compared to the findings for the other age groups, while for serotypes 6B and 7F, significant differences were observed for only one age group. The only positive correlation for children <4 months of age was for serotype 7F, which occurred significantly more frequently in this age group.
TABLE 2.
Serotypes tested for significant differences in serotype distribution among the different age groupsa
| Serotype | Comparison by age group |
Comparison of age groups |
|||||
|---|---|---|---|---|---|---|---|
| Age group | Serotype present | No. of patients | % of patients | P (chi-square test) | Age groups comparedb | Adjusted P value | |
| 14 | <4 mo | 29 | 179 | 16.20 | <0.0001c | <4 mo vs all | 1.0000 |
| ≥4 mo and <1 yr | 129 | 511 | 25.24 | ≥4 mo and <1 yr vs all | 0.3679 | ||
| ≥1 yr and <5 yr | 392 | 1,191 | 32.91 | ≥1 yr and <5 yr vs all (16.76%) | <0.0001 | ||
| ≥5 yr and <16 yr | 31 | 428 | 7.24 | ≥5 yr and <16 yr vs all (21.83%) | <0.0001 | ||
| ≥16 yr and <65 yr | 204 | 1,340 | 15.22 | ≥16 yr and <65 yr vs all (22.54%) | <0.0001 | ||
| ≥65 yr | 249 | 1,373 | 18.14 | ≥65 yr vs all | 0.4010 | ||
| 23F | <4 mo | 10 | 179 | 5.59 | 0.0526 | ||
| ≥4 mo and <1 yr | 37 | 511 | 7.24 | ||||
| ≥1 yr and <5 yr | 100 | 1,191 | 8.40 | ||||
| ≥5 yr and <16 yr | 22 | 428 | 5.14 | ||||
| ≥16 yr and <65 yr | 75 | 1,340 | 5.60 | ||||
| ≥65 yr | 102 | 1,373 | 7.43 | ||||
| 1 | <4 mo | 13 | 179 | 7.26 | <0.0001 | <4 mo vs all | 1.0000 |
| ≥4 mo and <1 yr | 8 | 511 | 1.57 | ≥4 mo and <1 yr vs all (7.03%) | <0.0001 | ||
| ≥1 yr and <5 yr | 42 | 1,191 | 3.53 | ≥1 yr and <5 yr vs all (7.39%) | 0.0001 | ||
| ≥5 yr and <16 yr | 97 | 428 | 22.66 | ≥5 yr and <16 yr vs all (4.96%) | <0.0001 | ||
| ≥16 yr and <65 yr | 114 | 1,340 | 8.51 | ≥16 yr and <65 yr vs all | 0.0317 | ||
| ≥65 yr | 51 | 1,373 | 3.71 | ≥65 yr vs all (7.51%) | 0.0001 | ||
| 6B | <4 mo | 6 | 179 | 3.35 | <0.0001 | <4 mo vs all | 0.9999 |
| ≥4 mo and <1 yr | 45 | 511 | 8.81 | ≥4 mo and <1 yr vs all | 0.4828 | ||
| ≥1 yr and <5 yr | 117 | 1,191 | 9.82 | ≥1 yr and <5 yr vs all (4.96%) | <0.0001 | ||
| ≥5 yr and <16 yr | 10 | 428 | 2.34 | ≥5 yr and <16 yr vs all | 0.0116 | ||
| ≥16 yr and <65 yr | 57 | 1,340 | 4.25 | ≥16 yr and <65 yr vs all | 0.0377 | ||
| ≥65 yr | 72 | 1,373 | 5.24 | ≥65 yr vs all | 0.9998 | ||
| 7F | <4 mo | 32 | 179 | 17.88 | <0.0001 | <4 mo vs all (5.66%) | <0.0001 |
| ≥4 mo and <1 yr | 31 | 511 | 6.07 | ≥4 mo and <1 yr vs all | 1.0000 | ||
| ≥1 yr and <5 yr | 46 | 1,191 | 3.86 | ≥1 yr and <5 yr vs all | 0.0096 | ||
| ≥5 yr and <16 yr | 21 | 428 | 4.91 | ≥5 yr and <16 yr vs all | 1.0000 | ||
| ≥16 yr and <65 yr | 89 | 1,340 | 6.64 | ≥16 yr and <65 yr vs all | 1.0000 | ||
| ≥65 yr | 87 | 1,373 | 6.34 | ≥65 yr vs all | 1.0000 | ||
| 3 | <4 mo | 13 | 179 | 7.26 | <0.0001 | <4 mo vs all | 1.0000 |
| ≥4 mo and <1 yr | 16 | 511 | 3.13 | ≥4 mo and <1 yr vs all | 0.3103 | ||
| ≥1 yr and <5 yr | 11 | 1,191 | 0.92 | ≥1 yr and <5 yr vs all (7.23%) | <0.0001 | ||
| ≥5 yr and <16 yr | 24 | 428 | 5.61 | ≥5 yr and <16 yr vs all | 1.0000 | ||
| ≥16 yr and <65 yr | 100 | 1,340 | 7.46 | ≥16 yr and <65 yr vs all | 0.1113 | ||
| ≥65 yr | 124 | 1,373 | 9.03 | ≥65 yr vs all (4.49%) | <0.0001 | ||
| 4 | <4 mo | 5 | 179 | 2.79 | 0.0001 | <4 mo vs all | 0.9998 |
| ≥4 mo and <1 yr | 13 | 511 | 2.54 | ≥4 mo and <1 yr vs all | 0.0412 | ||
| ≥1 yr and <5 yr | 56 | 1,191 | 4.70 | ≥1 yr and <5 yr vs all | 0.9998 | ||
| ≥5 yr and <16 yr | 18 | 428 | 4.21 | ≥5 yr and <16 yr vs all | 1.0000 | ||
| ≥16 yr and <65 yr | 97 | 1,340 | 7.24 | ≥16 yr and <65 yr vs all | 0.1493 | ||
| ≥65 yr | 92 | 1,373 | 6.70 | ≥65 yr vs all | 0.9030 | ||
| 19F | <4 mo | 12 | 179 | 6.70 | <0.0001 | <4 mo vs all | 1.0000 |
| ≥4 mo and <1 yr | 53 | 511 | 10.37 | ≥4 mo and <1 yr vs all (4.79%) | 0.0002 | ||
| ≥1 yr and <5 yr | 86 | 1191 | 7.22 | ≥1 yr and <5 yr vs all | 0.0842 | ||
| ≥5 yr and <16 yr | 16 | 428 | 3.74 | ≥5 yr and <16 yr vs all | 0.9999 | ||
| ≥16 yr and <65 yr | 58 | 1,340 | 4.33 | ≥16 yr and <65 yr vs all | 0.9647 | ||
| ≥65 yr | 44 | 1,373 | 3.20 | ≥65 yr vs all (6.17%) | 0.0012 | ||
| 9V | <4 mo | 11 | 179 | 6.15 | 0.0319 | ||
| ≥4 mo and <1 yr | 19 | 511 | 3.72 | ||||
| ≥1 yr and <5 yr | 41 | 1,191 | 3.44 | ||||
| ≥5 yr and <16 yr | 22 | 428 | 5.14 | ||||
| ≥16 yr and <65 yr | 63 | 1340 | 4.70 | ||||
| ≥65 yr | 84 | 1,373 | 6.12 | ||||
| 18C | <4 mo | 2 | 179 | 1.12 | <0.0001 | <4 mo vs all | 0.8692 |
| ≥4 mo and <1 yr | 32 | 511 | 6.26 | ≥4 mo and <1 yr vs all | 0.5462 | ||
| ≥1 yr and <5 yr | 79 | 1,191 | 6.63 | ≥1 yr and <5 yr vs all (3.32%) | 0.0003 | ||
| ≥5 yr and <16 yr | 34 | 428 | 7.94 | ≥5 yr and <16 yr vs all | 0.0096 | ||
| ≥16 yr and <65 yr | 39 | 1,340 | 2.91 | ≥16 yr and <65 yr vs all | 0.4488 | ||
| ≥65 yr | 20 | 1,373 | 1.46 | ≥65 yr vs all (5.1%) | <0.0001 | ||
| 6A | <4 mo | 1 | 179 | 0.56 | 0.0003 | <4 mo vs all | 0.5978 |
| ≥4 mo and <1 yr | 27 | 511 | 5.28 | ≥4 mo and <1 yr vs all | 0.9957 | ||
| ≥1 yr and <5 yr | 48 | 1,191 | 4.03 | ≥1 yr and <5 yr vs all | 1.0000 | ||
| ≥5 yr and <16 yr | 25 | 428 | 5.84 | ≥5 yr and <16 yr vs all | 0.8816 | ||
| ≥16 yr and <65 yr | 31 | 1,340 | 2.31 | ≥16 yr and <65 yr vs all | 0.0198 | ||
| ≥65 yr | 63 | 1,373 | 4.59 | ≥65 yr vs all | 0.9994 | ||
| 19A | <4 mo | 7 | 179 | 3.91 | 0.1496 | ||
| ≥4 mo and <1 yr | 18 | 511 | 3.52 | ||||
| ≥1 yr and <5 yr | 30 | 1,191 | 2.52 | ||||
| ≥5 yr and <16 yr | 6 | 428 | 1.40 | ||||
| ≥16 yr and <65 yr | 33 | 1,340 | 2.46 | ||||
| ≥65 yr | 48 | 1,373 | 3.50 | ||||
Only serotypes with at least 100 isolates (overall) were considered.
All, the remaining collective of patients, i.e., all patients except the group tested. Percent values for all are given when the adjusted P value was statistically significant.
Boldface indicates a statistically significant difference.
DISCUSSION
In the study described in this paper, we analyzed the association of serotypes of S. pneumoniae with age in invasive pneumococcal disease, on the basis of data collected over 14.5 years of surveillance of invasive pneumococcal disease in Germany.
Overall, the most frequent serotypes were 14, 23F, 1, 6B, and 7F (in descending order), which is similar to the most frequent serotypes published in reports of other studies conducted over the same period in Germany (11, 12). They deviate in part from older German data (17, 20), but they are generally in line with the results reported from other countries (3, 5, 6, 8, 22, 27).
In the present study, serotype 7F was statistically more prevalent among children <4 months of age than among the individuals in the other age groups. Among children aged ≥4 months and <1 year, serotype 19F was statistically significantly more frequent; and among children from ≥1 year to <5 years of age, serotypes 14, 6B, and 18C were overrepresented. The serotypes commonly defined as “pediatric serotypes” are 6B, 9V, 14, 19F, and 23F (5). Besides these, serotypes 18C, 7F, 1, 6A, and 4 are reported to be among the 10 serotypes responsible for about 80% of cases of IPD in children younger than age 5 years (8). Among neonates, the infecting pneumococcal serogroups were predominantly 19, 9, 3, 18, 1, 6, 14, 5, and 12 (10). In a study from Denmark, the proportion of serotype 7F isolates has been reported to be higher among invasive isolates from children aged <6 months than among children from 6 months to <2 years of age (16), which is comparable to our results. Data on serotype 3 are heterogeneous in the literature. Serotype 3 has been described to be frequent across all age groups except children younger than 5 years of age (8), but it has also been described to be among the most frequent pneumococcal serogroups infecting neonates (10). In our study, serotype 3 showed a disproportionately low prevalence among children ≥1 and <5 years of age compared to its prevalence among the other age groups. When our results for serotypes 19A and 19F of serogroup 19 are summarized, serogroup 19 was found to be the third most common cause of pneumococcal infection among children <4 months of age. While serotype 19A was not statistically significantly associated with IPD in any particular age group, serotype 19F was statistically significantly more frequent among children ≥4 months and <1 year than among the other age groups. Among neonates in the United States, serogroup 19 has been described to be the leading pneumococcal serogroup (10).
All serotypes that were significantly more common in children <16 years of age in this study (serotypes 14, 6B, 19F, and 18C) than among adults ≥16 years of age are among the serotypes defined as pediatric serotypes (serotypes 14, 6B, and 19F) (5) or are shown to have similar epidemiologic characteristics (serotype 18C) (5, 26).
Among the adults in our study, serotypes 3 and 4 were statistically significantly predominant. The relative risk of disease with serotypes 3 has been described in a meta-analysis to be increased among middle-aged people, whereas the risk increases progressively up to a peak in the seventh decade of life (26). Serotype 4 is known to cause a larger percentage of cases of IPD in adults than in children, even though the prevalence of serotype 4 decreased significantly with age during adulthood (5). Another study described serotypes 1, 4, 14, 7F, 3, 9V, 12F, 8, and 23F to be the most frequent among patients aged ≥5 years. Since approximately 90% of the patients were in this age group, the results might be at least partly related (8).
However, the differences in the ages of individuals infected with particular IPD serotypes and the ages of the rest of the population not infected with these serotypes were the most prominent for serotypes 14 (median age, 3 years versus 38 years), 6B and 19F (3 years versus 36 years each), and 18C (4 years versus 37 years).
Nevertheless, some factors and limitations must be considered when the results of this study are interpreted. The isolates were sent by the participating laboratories on a voluntary basis, since participation in surveillance is not mandatory in Germany. Moreover, the systematic sampling of invasive isolates from adults (1992) and children (1997) was taken up at different times and at least partly included isolates from population-based studies conducted in three German federal states starting in 2001 (North Rhine-Westphalia) and 2006 (Bavaria and Saxony). In a previous study conducted by NRCS (24), a laboratory underreporting factor of 1.86, which indicates that 46.2% of the IPD isolates had not been sent to NRCS, was calculated for IPD among adults in North-Rhine Westphalia. If it is assumed that this factor is transferable to all German federal states, and if a similar underreporting is assumed for childhood isolates, the number of potentially available IPD isolates would accumulate to 14,441 cases. Assuming the same serotype rates, this would result in 9,341 serotyped isolates. However, since we suppose that the rate of underreporting was lower among children, the number of cases is likely to be the hypothetical maximum value and the true numbers of IPD cases might be in between. Furthermore, the structure of the surveillance has continuously been improved, and over the years, the percentage of isolates serotyped has continuously increased. During the first years of the study, serotyping of all isolates was not conducted due to the excessive costs, and high levels of resistance were the main trigger for the initiation of serotyping. However, a similar bias might be present among the laboratories/hospitals sending the isolates, and isolates from severe clinical cases or resistant isolates might preferably be sent to NRCS. Therefore, the isolates may not be fully representative of all isolates causing IPD in Germany. In addition, the possibility that temporal (4, 13) and regional (5, 12, 23) changes in serotype distribution have occurred must be kept in mind when the results of this study are interpreted.
Acknowledgments
We thank the microbiological laboratories in Germany for their cooperation and for providing the isolates.
This study was supported, in part, by Wyeth Pharma GmbH, Germany.
Ralf René Reinert has been an employee of Wyeth Vaccines Research, Paris La Défense, Paris, France, since 2007.
Footnotes
Published ahead of print on 27 January 2010.
REFERENCES
- 1.Austrian, R. 1981. Pneumococcus: the first one hundred years. Rev. Infect. Dis. 3:183-189. [DOI] [PubMed] [Google Scholar]
- 2.Brueggemann, A. B., T. E. Peto, D. W. Crook, J. C. Butler, K. G. Kristinsson, and B. G. Spratt. 2004. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J. Infect. Dis. 190:1203-1211. [DOI] [PubMed] [Google Scholar]
- 3.de Cunto Brandileone, M. C., D. V. V. Simonsen, S. Tadeu Casagrande, R. Cobo Zanella, M. L. Leopoldo Silva Guerra, A. Pires Brandao, C. E. de Andrade Melles, A. C. C. Pignatari, J. L. Di Fabio, and R. Austrian. 1998. Characteristics of isolates of Streptococcus pneumoniae from middle-aged and elderly adults in Brazil: capsular serotypes and antimicrobial sensitivity to invasive infections. Braz. J. Infect. Dis. 2:90-96. [PubMed] [Google Scholar]
- 4.Feikin, D. R., and K. P. Klugman. 2002. Historical changes in pneumococcal serogroup distribution: implications for the era of pneumococcal conjugate vaccines. Clin. Infect. Dis. 35:547-555. [DOI] [PubMed] [Google Scholar]
- 5.Feikin, D. R., K. P. Klugman, R. R. Facklam, E. R. Zell, A. Schuchat, and C. G. Whitney. 2005. Increased prevalence of pediatric pneumococcal serotypes in elderly adults. Clin. Infect. Dis. 41:481-487. [DOI] [PubMed] [Google Scholar]
- 6.Flamaing, J., J. Verhaegen, J. Vandeven, N. Verbiest, and W. E. Peetermans. 2008. Pneumococcal bacteraemia in Belgium (1994 2004): the pre-conjugate vaccine era. J. Antimicrob. Chemother. 61:143-149. [DOI] [PubMed] [Google Scholar]
- 7.Hammerschmidt, S., S. Wolff, A. Hocke, S. Rosseau, E. Muller, and M. Rohde. 2005. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect. Immun. 73:4653-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harboe, Z. B., R. W. Thomsen, A. Riis, P. Valentiner-Branth, J. J. Christensen, L. Lambertsen, K. A. Krogfelt, H. B. Konradsen, and T. L. Benfield. 2009. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 6:e1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harboe, Z. B., P. Valentiner-Branth, T. L. Benfield, J. J. Christensen, T. Hjuler, M. Kaltoft, K. A. Krogfelt, L. Lambertsen, and H. B. Konradsen. 2008. Estimated effect of pneumococcal conjugate vaccination on invasive pneumococcal disease and associated mortality, Denmark 2000-2005. Vaccine 26:3765-3771. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman, J. A., E. O. Mason, G. E. Schutze, T. Q. Tan, W. J. Barson, L. B. Givner, E. R. Wald, J. S. Bradley, R. Yogev, and S. L. Kaplan. 2003. Streptococcus pneumoniae infections in the neonate. Pediatrics 112:1095-1102. [DOI] [PubMed] [Google Scholar]
- 11.Imöhl, M., R. R. Reinert, and M. van der Linden. 2009. Adult invasive pneumococcal disease between 2003 and 2006 in North-Rhine Westphalia, Germany: serotype distribution before recommendation for general pneumococcal conjugate vaccination for children <2 years of age. Clin. Microbiol. Infect. 15:1008-1012. [DOI] [PubMed] [Google Scholar]
- 12.Imöhl, M., R. R. Reinert, and M. van der Linden. 13 July 2009, posting date. Regional differences in serotype distribution, pneumococcal vaccine coverage, and antimicrobial resistance of invasive pneumococcal disease among German federal states, Int. J. Med. Microbiol. [Epub ahead of print.] [DOI] [PubMed]
- 13.Jacobs, M. R., C. E. Good, B. Beall, S. Bajaksouzian, A. R. Windau, and C. G. Whitney. 2008. Changes in serotypes and antimicrobial susceptibility of invasive Streptococcus pneumoniae strains in Cleveland: a quarter century of experience. J. Clin. Microbiol. 46:982-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansen, A. G., G. D. Rodenburg, A. van der Ende, L. van Alphen, R. H. Veenhoven, L. Spanjaard, E. A. Sanders, and E. Hak. 2009. Invasive pneumococcal disease among adults: associations among serotypes, disease characteristics, and outcome. Clin. Infect. Dis. 49:e23-e29. [DOI] [PubMed] [Google Scholar]
- 15.Jefferson, T., E. Ferroni, F. Curtale, P. Giorgi Rossi, and P. Borgia. 2006. Streptococcus pneumoniae in western Europe: serotype distribution and incidence in children less than 2 years old. Lancet Infect. Dis. 6:405-410. [DOI] [PubMed] [Google Scholar]
- 16.Kaltoft, M. S., N. Zeuthen, and H. B. Konradsen. 2000. Epidemiology of invasive pneumococcal infections in children aged 0-6 years in Denmark: a 19-year nationwide surveillance study. Acta Paediatr. Suppl. 89:3-10. [DOI] [PubMed] [Google Scholar]
- 17.Kaufhold, A., R. Lütticken, and J. Henrichsen. 1987. Capsular types and antibiotic susceptibility of Streptococcus pneumoniae isolated from patients with systemic infections in West Germany. Eur. J. Clin. Microbiol. 6:696-697. [DOI] [PubMed] [Google Scholar]
- 18.Kellner, J. D., O. G. Vanderkooi, J. MacDonald, D. L. Church, G. J. Tyrrell, and D. W. Scheifele. 2009. Changing epidemiology of invasive pneumococcal disease in Canada, 1998-2007: update from the Calgary-area Streptococcus pneumoniae research (CASPER) study. Clin. Infect. Dis. 49:205-212. [DOI] [PubMed] [Google Scholar]
- 19.Lacapa, R., S. J. Bliss, F. Larzelere-Hinton, K. J. Eagle, D. J. McGinty, A. J. Parkinson, M. Santosham, M. J. Craig, and K. L. O'Brien. 2008. Changing epidemiology of invasive pneumococcal disease among White Mountain Apache persons in the era of the pneumococcal conjugate vaccine. Clin. Infect. Dis. 47:476-484. [DOI] [PubMed] [Google Scholar]
- 20.Lütticken, R., and A. Kaufhold. 1985. Serotypen und Antibiotikaempfindlichkeit von Streptococcus pneumoniae (Pneumokokken) im Raum Köln. Immun. Infekt. 13:99-107. [PubMed] [Google Scholar]
- 21.Miller, E., P. Waight, A. Efstratiou, M. Brisson, A. Johnson, and R. George. 2000. Epidemiology of invasive and other pneumococcal disease in children in England and Wales 1996-1998. Acta Paediatr. Suppl. 89:11-16. [DOI] [PubMed] [Google Scholar]
- 22.Plouffe, J. F., S. K. Moore, R. Davis, and R. R. Facklam. 1994. Serotypes of Streptococcus pneumoniae blood culture isolates from adults in Franklin County, Ohio. J. Clin. Microbiol. 32:1606-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinert, R. R. 2004. Pneumococcal conjugate vaccines—a European perspective. Int. J. Med. Microbiol. 294:277-294. [DOI] [PubMed] [Google Scholar]
- 24.Reinert, R. R., S. Haupts, M. van der Linden, C. Heeg, M. Y. Cil, A. Al-Lahham, and D. S. Fedson. 2005. Invasive pneumococcal disease in adults in North-Rhine Westphalia, Germany, 2001-2003. Clin. Microbiol. Infect. 11:985-991. [DOI] [PubMed] [Google Scholar]
- 25.Robinson, K. A., W. Baughman, G. Rothrock, N. L. Barrett, M. Pass, C. Lexau, B. Damaske, K. Stefonek, B. Barnes, J. Patterson, E. R. Zell, A. Schuchat, and C. G. Whitney. 2001. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995-1998: opportunities for prevention in the conjugate vaccine era. JAMA 285:1729-1735. [DOI] [PubMed] [Google Scholar]
- 26.Scott, J. A., A. J. Hall, R. Dagan, J. M. Dixon, S. J. Eykyn, A. Fenoll, M. Hortal, L. P. Jette, J. H. Jorgensen, F. Lamothe, C. Latorre, J. T. Macfarlane, D. M. Shlaes, L. E. Smart, and A. Taunay. 1996. Serogroup-specific epidemiology of Streptococcus pneumoniae: associations with age, sex, and geography in 7,000 episodes of invasive disease. Clin. Infect. Dis. 22:973-981. [DOI] [PubMed] [Google Scholar]
- 27.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, C. Lexau, A. Reingold, L. Lefkowitz, P. R. Cieslak, M. Cetron, E. R. Zell, J. H. Jorgensen, and A. Schuchat. 2000. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343:1917-1924. [DOI] [PubMed] [Google Scholar]