Abstract
We report the use of PCR techniques on a formalin-fixed and paraffin-embedded tissue specimen for direct detection of one dominant azole resistance mechanism in a case of disseminated invasive aspergillosis. Rapid detection of mutations associated with azole resistance directly in tissue significantly reduces diagnostic delay.
Invasive infections due to Aspergillus fumigatus are associated with significant morbidity and mortality, although the prognosis of patients with invasive aspergillosis has improved with the clinical use of mold-active antifungal azoles, most notably voriconazole (9, 11). However, the survival of patients may be threatened by the emergence of azole resistance of aspergilli (1, 7, 13). Resistance is commonly due to point mutations in the cyp51A gene, which is the target for antifungal azoles (1, 4, 8, 13, 14). The isolates commonly exhibit a cross-resistant phenotype (4), and patients with azole-resistant disease may fail azole therapy (1, 7, 10, 12). One problem in the management of azole-resistant aspergillosis is the early detection of resistance as cultures are negative in up to 50% of patients with focal pulmonary lesions (2), and in vitro susceptibility testing takes at least 5 to 7 days to complete. In this report, molecular tools were utilized to rapidly confirm the diagnosis of disseminated azole-resistant aspergillosis.
Case report.
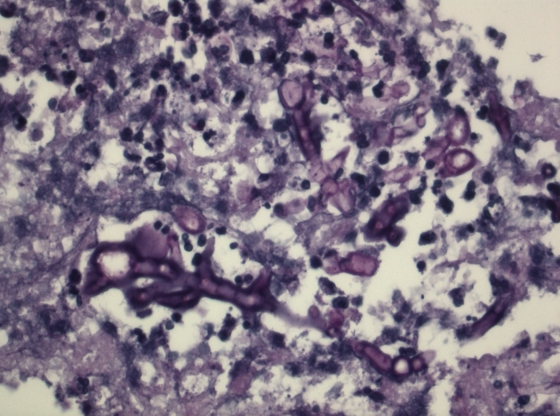
A 60-year-old man was diagnosed with acute myeloid leukemia and underwent an allogeneic hematopoietic stem cell transplantation. Six months later, he developed grade 2 to 3 graft-versus-host disease (GvHD) of the gastrointestinal tract and skin. He was treated with 1 mg prednisone/kg/day plus 3 mg b.i.d. Entocort. His neutrophil count was 0.77 × 109/liter. Diarrhea responded quickly. However, 10 days later, he developed fever, and high-resolution computed tomography (CT) of the thorax showed the presence of multiple nodular pulmonary infiltrates. Aspergillus fumigatus was cultured from the sputum, and circulating galactomannan was detected in serum. Under the suspicion of invasive pulmonary aspergillosis, treatment with voriconazole was started immediately, and the prednisone dose was halved and, within a few weeks, further reduced to a maintenance dose of 5 mg daily. The GvHD did not relapse, but as the clinical condition of the patient did not improve and sputum cultures remained positive with A. fumigatus, caspofungin was added. One week after the start of the antifungal therapy, paresis of the abducens nerve developed, and brain magnetic resonance imaging (MRI) showed multiple lesions. A brain biopsy was performed, and histological examination showed septate hyphae (Fig. 1); however, cultures remained negative. The sputum isolate was resistant to itraconazole and voriconazole and intermediate susceptible to posaconazole (Table 1) (13). As the cerebral lesion might be due to azole-resistant A. fumigatus, it was decided to replace voriconazole by liposomal amphotericin B. The patient again developed fever, and posaconazole was added to the treatment regimen.
FIG. 1.
Histology of the brain biopsy specimen showing septate hyphae (PAS staining, ×400 magnification).
TABLE 1.
Results of the diagnostic assays for the clinical specimens obtained from the patient and for three controls
| Date | Sample | Microscopy | Culture | Molecular identificationa | Detected cyp51A substitutionb | MIC (mg/liter)c |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMB | ITZ | VCZ | POS | CAS | ||||||
| 26 September 2008 | Sputum | Not done | A. fumigatus | A. fumigatus | TR+L98H | 0.5 | >16 | 4 | 0.5 | 0.25 |
| 24 October | Brain biopsy sample | Septate hyphae | Negative | A. fumigatus | TR+L98H | |||||
| 2008 | Lung biopsy sample (control) | Septate hyphae | A. fumigatus | A. fumigatus | TR+L98H | 0.25 | >16 | 4 | 0.5 | 0.5 |
| Lung biopsy sample (control) | Negative | A. fumigatus | A. fumigatus | Negative | 0.5 | 1 | 0.5 | 0.063 | 0.125 | |
| Brain biopsy sample (control) | Negative | Negative | Negative | Negative | ||||||
Molecular identification by sequencing the 28S ribosomal DNA directly on isolates if cultures were positive or directly on the tissue when cultures were negative.
For the sputum isolate, the full sequence of the cyp51A gene was determined. For the biopsy specimens, two PCR assays were used to detect the tandem repeat and L98H substitution directly on formalin-fixed, paraffin-embedded biopsy samples. TR, 34-bp tandem repeat; L98H, substitution of leucine for histidine at codon 98.
MIC determined using the CLSI M38-A2 protocol. AMB, amphotericin B; ITZ, itraconazole; VCZ, voriconazole; POS, posaconazole; CAS, caspofungin. For caspofungin, the minimum effective concentration was determined.
We utilized the sputum isolate and three formalin-fixed and paraffin-embedded tissue sections from the brain biopsy for identification of the resistance mechanism. Amplification of the 28S ribosomal DNA confirmed the presence of Aspergillus in the biopsy samples. Sequence-based analysis of the cyp51A gene (using reference sequence of strain AF338659 from GenBank) of the sputum isolate showed a substitution of leucine for histidine at codon 98 and a 34-bp tandem repeat in the gene promoter region (TR+L98H). The morphological species identification of A. fumigatus was confirmed by sequencing of the highly conserved β-tubulin gene (7). Two PCR assays (Table 2) targeted at the tandem repeat and the mutation at codon 98 were performed directly on the brain biopsy specimen and were both positive (Table 1). DNA was extracted using proteinase K and the Qiagen EZ1 robot (Qiagen, The Netherlands). For the real-time detection of L98H, a 122-bp region was amplified, bridging codon 98 of the cyp51A gene by using a forward primer and a reverse primer (Table 2). Using hybridization probes, the wild-type L98 codon could be differentiated from the mutated L98H codon. A sensor probe was designed to span the mutation and to be separated from an anchor probe by 1 nucleotide. The melting temperature (Tm) of the sensor probe was approximately 6.6°C lower than that of the anchor probe; a wild-type DNA template will result in a mismatch giving a lower Tm than that observed for the mutated DNA template. The LightCycler FastStart DNA Master HybProbe reaction mix was used.
TABLE 2.
PCR assays targeted at the tandem repeat and the mutation at codon 98
| Targeta | Primer | Sequenceb | Binding positions (bp)c |
|---|---|---|---|
| TR | Forward primer | 5′-CAGCACCACTCCAGAGTTGTCT-3′ | 8-29 |
| Reverse primer | 5′-GTGTATGGTATGCTGGAACTACACCT-3′ | 109-84 | |
| TaqMan probe (TR) | 3′-FAM-TGCTGAGCCGAATGAATCACGC-TMR-5′ | 26-47 | |
| TaqMan probe (wild-type sequence) | 3′-FAM-ATTAGGCAACTTTCATTCGGCTCAGC-BBQ-5′ | 78-53 | |
| L98H | Forward primer | 5′-CAAAAAACCACAGTCTACCTG-3′ | 658-678 |
| Reverse primer | 5′-GAATTGGGACAATCATACACC-3′ | 800-780 | |
| Sensor probe | 5′-ACGGCAAGCACAAGGATG-3′, fluorescein-labeled | 707-724 | |
| Anchor probe | 5′-CAATGCGGAAGAGGTCTATAGTCCATTGA-3′-LC-red 640-labeled | 723-754 |
TR, 34-bp tandem repeat; L98H, substitution of leucine for histidine at codon 98.
FAM, 6-carboxyfluorescein; TMR, 6-TAMRA (6-carboxytetramethylrhodamine); BBQ, 4.4-bis-(2-butyloctyloxy)-p-quaterphenyl; LC-red 640, LightCycler Red 640-aminohexyl-spacer-5′-OH.
Binding positions of oligonucleotides on the basis of reference strain AF 338659.
To detect the 34-bp repetitive insertion, a TaqMan probe (TR) (Table 2) was designed to bind to the last 13 bp and the first 9 bp of the repetitive insertion, thereby binding specifically to a mutated DNA template. To control for a successful amplification of the 110-bp fragment of the promoter region by a forward primer and a reverse primer of the cyp51A gene, an additional TaqMan probe (wild-type sequence) (Table 2) was designed to detect the gene in general, independent from the presence of the repetition. Besides that, sequence analysis of a 160-bp fragment of the cyp51A gene from the biopsy sample confirmed the presence of the TR in the A. fumigatus in the tissue sample.
The clinical condition of the patient gradually improved, and imaging showed regression of the pulmonary and brain lesions. Liposomal amphotericin B and caspofungin therapy was discontinued after 12 weeks. Posaconazole was continued, and a plasma level of 1.7 mg/liter was achieved. Repeat CT of the brain showed further improvement of the lesions 6 months after initiation of antifungal therapy.
Azole resistance has emerged in The Netherlands, and the prevalence among clinical A. fumigatus isolates was 6% in 2007 (7). Resistance significantly complicates the treatment of invasive aspergillosis, as alternative drugs with efficacy similar to that of voriconazole in central nervous system aspergillosis are not available (5, 6, 10). The TR+L98H resistance mechanism was found to be present in more than 90% of Dutch azole-resistant A. fumigatus isolates and was always associated with a multi-azole-resistant phenotype (7, 10, 12). In an experimental model of disseminated aspergillosis, the efficacy of posaconazole against TR+L98H isolates was reduced by 50% compared to that of wild-type isolates (3). Furthermore, it remains unclear if adequate posaconazole levels can be achieved at the site of infection in the brain. Nevertheless, as there were no oral alternatives, we used posaconazole for step-down therapy in our patient and were able to obtain high plasma levels. The lesions continued to improve during posaconazole monotherapy.
The detection of resistance in invasive aspergillosis may be problematic, as positive cultures are required to test for a resistant phenotype, while the infecting isolate is obtained in only a minority of cases. However, even if Aspergillus is cultured, adequate treatment may be delayed for 5 to 7 days due to the time needed for MIC results to become available. Given a median survival of central nervous system aspergillosis of only 10 days (6), this is an unacceptable delay. Although in our patient resistance was expected early due to MIC testing of the sputum isolate, we were able to diagnose azole-resistant disseminated aspergillosis directly in the brain biopsy by using specifically designed real-time PCR assays. This appears to be a feasible approach, as mutations in the cyp51A gene are the primary mechanism of azole resistance in A. fumigatus, and the TR+L98H is the dominant change in Dutch azole-resistant A. fumigatus isolates (7). Provided that the resistance mechanism is known, molecular techniques are suitable to detect multiple resistance mechanisms simultaneously.
Acknowledgments
P.E.V. has received research grants from Bio-Rad, Pfizer, Gilead, Schering-Plough, Merck, and Basilea. Other authors have no conflicts of interest.
Footnotes
Published ahead of print on 27 January 2010.
REFERENCES
- 1.Howard, S. J., D. Cerar, M. J. Anderson, A. Albarrag, M. C. Fisher, A. C. Pasqualotto, M. Laverdiere, M. C. Arendrup, D. S. Perlin, and D. W. Denning. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 15:1068-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine, S. J. 1992. An approach to the diagnosis of pulmonary infections in immunosuppressed patients. Semin. Respir. Infect. 7:81-95. [PubMed] [Google Scholar]
- 3.Mavridou, E., R. J. Brüggemann, W. J. G. Melchers, J. W. Mouton, and P. E. Verweij. 2010. Efficacy of posaconazole against three clinical Aspergillus fumigatus isolates with mutations in the cyp51A gene. Antimicrob. Agents Chemother. 54:860-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellado, E., G. Garcia-Effron, L. Alcazar-Fuoli, W. J. G. Melchers, P. E. Verweij, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2007. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob. Agents Chemother. 51:1897-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz, S., M. Ruhnke, P. Ribaud, L. Corey, T. Driscoll, O. A. Cornely, U. Schuler, I. Lutsar, P. Troke, and E. Thiel. 2005. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood 106:2641-2645. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz, S., M. Ruhnke, P. Ribaud, E. Reed, P. Troke, and E. Thiel. 2007. Poor efficacy of amphotericin B-based therapy in CNS aspergillosis. Mycoses 50:196-200. [DOI] [PubMed] [Google Scholar]
- 7.Snelders, E., H. A. L. van der Lee, J. Kuijpers, A. J. M. M. Rijs, J. Varga, R. A. Samson, E. Mellado, W. J. G. Melchers, and P. E. Verweij. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 5:e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snelders, E., R. A. G. Huis in ′t Veld, A. J. M. M. Rijs, G. H. J. Kema, W. J. G. Melchers, and P. E. Verweij. 2009. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl. Environ. Microbiol. 75:4053-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upton, A., K. A. Kirby, P. Carpenter, M. Boeckh, and K. A. Marr. 2007. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin. Infect. Dis. 44:531-540. [DOI] [PubMed] [Google Scholar]
- 10.van der Linden, J. W. M., R. R. Jansen, D. Bresters, C. Visser, S. Geerlins, E. J. Kuijper, W. J. G. Melchers, and P. E. Verweij. 2009. Azole resistant central nervous system aspergillosis. Clin. Infect. Dis. 48:1111-1113. [DOI] [PubMed] [Google Scholar]
- 11.van ′t Hek, L. G., P. E. Verweij, C. M. Weemaes, R. Van Dalen, J. B. Yntema, and J. F. Meis. 1998. Successful treatment with voriconazole of invasive aspergillosis in chronic granulomatous disease. Am. J. Respir. Crit. Care Med. 157:1694-1696. [DOI] [PubMed] [Google Scholar]
- 12.Verweij, P. E., E. Mellado, and W. J. Melchers. 2007. Multiple-triazole-resistant aspergillosis. N. Engl. J. Med. 356:1481-1483. [DOI] [PubMed] [Google Scholar]
- 13.Verweij, P. E., S. J. Howard, W. J. G. Melchers, and D. W. Denning. 2009. Azole resistance in Aspergillus: proposed nomenclature and breakpoints. Drug Resist. Updat. 12:141-147. [DOI] [PubMed] [Google Scholar]
- 14.Verweij, P. E., E. Snelders, G. H. J. Kema, E. Mellado, and W. J. G. Melchers. 2009. Azole-resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect. Dis. 9:789-795. [DOI] [PubMed] [Google Scholar]