Abstract
Prognostic features of serum galactomannan (GM) remain poorly defined in patients with GM-positive invasive aspergillosis (GPA). We identified 93 patients with proven or probable invasive aspergillosis (IA) and GM values of ≥0.50 from January 2005 to March 2009. We used Cox modeling of time to 6- and 12-week mortality for the GM level at the time of diagnosis (GM0), GM decay in the week following diagnosis in 72 patients with ≥2 GM values, other predictors of mortality, and antifungal use during the week following diagnosis. Six-week mortality was 55% in the whole cohort and 43% in patients with ≥2 GM determinations. The hazard ratio (HR) of GM0 per unit increase and 1-week GM decay per unit decline per week were 1.25 (95% confidence interval [CI], 1.01 to 1.54; P = 0.04) and 0.78 (95% CI, 0.63 to 0.96; P = 0.02), respectively, adjusting for other predictors of IA mortality; these values remained stable after adjusting for antifungal use and were predictive of all-cause mortality at 12 weeks with similar adjusted HR values. We conclude that the combination of GM0 and 1-week GM decay is predictive of all-cause mortality in patients with GPA, independent of other traditional risk factors for mortality and antifungal exposure, supporting GM decay as a potential surrogate endpoint for future antifungal therapeutic trials.
Galactomannan (GM) is a cell wall polysaccharide released by growing Aspergillus hyphae (14, 20). In experimental animal models of pulmonary invasive aspergillosis (IA), serum GM antigenemia correlates with tissue fungal burden, increasing with progressive disease and declining with effective antifungal therapy (1, 2, 6, 12, 16, 17, 24). In animal models, rising GM antigenemia has been associated with mortality, while clearance of antigenemia has been associated with survival (2, 12, 16, 17).
A similar relationship has been observed empirically in humans with IA. Soon after the development of serum GM testing, patients who died of IA were often noted to have progressively rising GM levels, while patients who survived IA gradually cleared their antigenemia (3, 4, 10, 11, 21, 23). It has also been observed that the use of mold-active antifungal therapy blunts GM diagnostic sensitivity in this setting, as antigenemia declines below the diagnostic threshold with effective therapy (12, 13).
Recently, two studies proposed a binarized GM outcome as a possible surrogate outcome measure for IA, based on a strong κ correlation between GM outcome and poor clinical outcomes in hematologic malignancy and hematopoietic stem cell transplantation (HSCT) patients (9, 27). Success was defined in these studies as a repeatedly negative serum GM in the absence of new extrapulmonary lesions and failure as a persistently positive GM level or death within 2 weeks of GM normalization unless autopsy failed to show evidence of IA. A review of 27 studies of serial GM screening for the diagnosis of IA in hematologic malignancy and HSCT patients also found a correlation between GM levels in the week preceding IA outcome and clinical outcome (15). A simple correlation, however, is insufficient for establishing surrogacy; measurements that correlate with the outcome of interest are not useful surrogates unless they also capture the net effects of treatment on outcome (19, 25). These studies also stratified patients by GM features available late in the course of IA, just proximal to the clinical outcome of interest, rather than by baseline and early GM features.
We hypothesized that early GM features, namely, the height of the initial GM at the time of diagnosis and the subsequent rate of GM decay within the initial week following diagnosis, were important factors in predicting clinical outcome, and we sought to further refine the relationship between GM kinetics and mortality, including the effect of antifungal therapy on this relationship.
MATERIALS AND METHODS
Patient selection and data collection.
We reviewed the results of all GM values from 1 January 2005 to 31 March 2009 at Brigham & Women's Hospital/Dana-Farber Cancer Institute. All patients with proven or probable IA by 2008 European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) classification criteria (5) and at least one serum GM value of ≥0.50 were included in this analysis. Demographic data, baseline diagnoses, reasons for GM testing, details of immunosuppressive and systemic antifungal therapy, and results of relevant laboratory, microbiology, radiology, and pathology studies were recorded. We recorded the results of all GM values following the first value of ≥0.50. Mortality and cause of death were recorded at 6 weeks, after which mortality attributable to IA wanes (22, 26), and at 12 weeks, recommended as a secondary time point for IA outcome assessment by the recent EORTC/MSG therapeutic response and outcome consensus statement (22).
GM testing.
All serum GM assessments were performed with the Platelia Aspergillus enzyme immunoassay (EIA) (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's standard protocol. GM testing was performed at the discretion of clinical care teams.
Definitions.
The GM level at the time of diagnosis (GM0) was defined as the first GM value of ≥0.5 in the setting of appropriate EORTC/MSG host factors, clinical criteria, and mycologic criteria (5). Patients who did not otherwise meet criteria for proven or probable IA were not included. No patients received piperacillin-tazobactam or any other parenteral β-lactam/β-lactamase combinations.
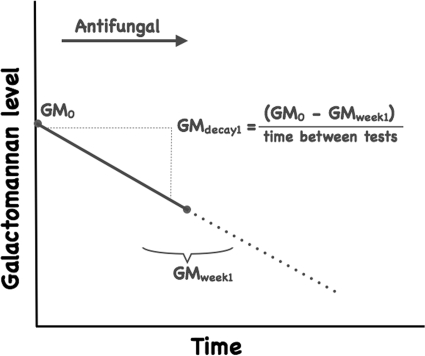
For patients with ≥2 serum GM values, a daily GM decay value was calculated by dividing the difference between GM0 and a GM measurement obtained around 1 week after GM0 by the number of days between the tests (Fig. 1). If a day 7 GM was unavailable, the GM value soonest after day 7 was preferentially used for the calculation, dividing by the actual number of days between tests. Daily GM decay values were multiplied by 7 to yield a 1-week GM decay value in EIA units per week.
FIG. 1.
Schematic diagram of GM0 and determination of 1-week GM decay for a patient with GM-positive IA (GMdecay1). GMweek1, GM measurement obtained around 1 week after GM0.
Statistical methods.
Wilcoxon rank-sum tests were used to compare medians. We used the method of Kaplan and Meier to estimate all-cause survival at 6 and 12 weeks for the whole cohort and for the subset of patients with ≥2 GM values.
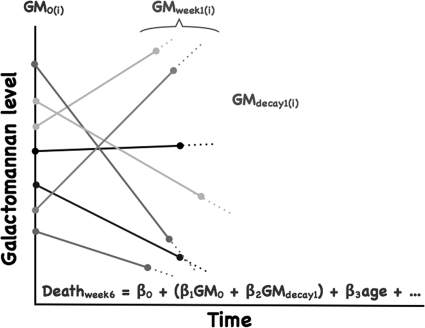
Cox regression modeling was used to generate unadjusted hazard ratios (HRs) for GM0, 1-week GM decay, and other potential predictors of mortality, including age, gender, and EORTC/MSG-defined host risk factors (Fig. 2). GM0, 1-week GM decay, and age were modeled as continuous covariates. Univariate HRs were also generated for voriconazole, liposomal amphotericin B, and echinocandin use during the 1 week following GM0 as daily time-dependent covariates.
FIG. 2.
Schema of the derivation of the Cox proportional hazards model for GM0, 1-week GM decay, and other predictors on the probability of death at 6 weeks following the diagnosis of GM-positive IA. Each line represents a patient (i) with a specific GM0 and 1-week GM decay slope.
Cox proportional hazards modeling was used to generate adjusted HRs of time to all-cause mortality at 6 and 12 weeks. Possible predictors of mortality were included in the multivariable Cox regression model if they were associated with this outcome in the univariable analysis or previously identified in the literature as significant risk factors. We assessed the impact of the inclusion of an interaction term between GM0 and 1-week GM decay. We also assessed the impact of adjusting for time-dependent daily voriconazole, liposomal amphotericin B, and echinocandin (caspofungin or micafungin) use on our multivariable regression model.
All analyses were performed using STATA version 10 (STATA Corporation, College Station, TX). The hospital's Human Research Committee approved this study.
RESULTS
Patient characteristics.
We identified 93 patients with GM-positive IA (GPA) during the study period; 23 patients had proven IA, and 70 had probable IA. Baseline characteristics are presented in Table 1. While most patients had at least one traditional EORTC/MSG host factor, there were cases of proven IA in patients who were exposed to corticosteroids and other immunosuppressants who did not meet the dosing or duration definitions of the current classification criteria (5).
TABLE 1.
Baseline characteristics
| Characteristic | Value (%) for: |
|
|---|---|---|
| All patients | Patients with ≥2 GM values | |
| Total no. | 93 | 72 |
| Age (yr) | 55 (42-63, 17-93)a | 53 (41-62, 17-78)a |
| Female | 42 (45.2) | 37 (51.4) |
| Solid organ transplant | 11 (11.8) | 9 (12.5) |
| Malignancy | 66 (71.0) | 53 (73.6) |
| Hematologic malignancy | 58 (62.4) | 49 (68.1) |
| Allogeneic HSCTb | 34 (36.6) | 33 (45.8) |
| Acute grade III to IV GVHD | 5 (5.4) | 5 (6.9) |
| High-risk neutropeniac | 35 (37.6) | 30 (41.7) |
| Duration of high-risk neutropenia in 60-day period prior to GM0 (days)c | 35 (23-60, 13-60)a | 37 (27-60, 13-60)a |
| Prolonged corticosteroid use within 90 days of GM0d | 37 (39.8) | 29 (40.3) |
| T-cell immunosuppressants within 90 days of GM0e | 45 (48.4) | 39 (54.2) |
Median, IQR, and range.
There were no autologous HSCT patients in this cohort.
High-risk neutropenia was defined according to the 2008 EORTC/MSG criteria for IFD classification as a recent history of neutropenia (<0.5 × 109 neutrophils/liter for >10 days) temporally related to the onset of fungal disease.
Prolonged corticosteroid use was defined according to 2008 EORTC/MSG IFD classification criteria as a mean minimum dose of 0.3 mg/kg/day of prednisone equivalent for >3 weeks.
T-cell immunosuppressants were defined according to 2008 EORTC/MSG IFD classification criteria as recognized T-cell immunosuppressants, such as cyclosporine, tumor necrosis factor alpha blockers, specific monoclonal antibodies such as alemtuzumab, or nucleoside analogues.
GM testing was triggered by a pneumonic syndrome in 66 patients (71.0%), febrile neutropenia in 17 patients (18.3%), sepsis in four patients (4.3%), skin nodules in two patients (2.2%), sinus or ear symptoms in two patients (2.2%), and microbiologic findings in two patients (2.2%).
Only one patient with GPA had isolated sinus disease. The remaining 92 patients had pulmonary IA at minimum, and eight of these patients had multifocal disseminated IA.
Forty-three patients (46.2%) had definitive microbiologic identification of their causative Aspergillus species; 33 grew A. fumigatus, six A. flavus, two A. niger, and two A. terreus.
Antifungal therapy.
Ninety patients (96.8%) received systemic antifungal therapy. Forty-three patients (46.2%) were receiving antifungal therapy at the time of GM0 for a median of 7 days (interquartile range [IQR], 2 to 17; range, 1 to 60) prior to GM0; 24 (55.8%) were receiving echinocandins, 11 (25.6%) voriconazole, seven (16.3%) liposomal amphotericin B, and one (2.3%) itraconazole. These antifungals were prescribed for empirical treatment of febrile neutropenia or pulmonary syndromes. Of the 24 patients receiving echinocandins on the day of GM0, 21 (87.5%) were switched to voriconazole during the week following GM0, while the other three patients remained on echinocandin therapy.
Forty-three additional patients (46.2%) started antifungal therapy during the week following GM0, 18 (41.9%) on the day of GM0 and 11 (25.6%) the day following GM0. Of these patients, 18 (41.9%) received voriconazole, 17 (39.5%) echinocandins, and eight (18.6%) liposomal amphotericin B as initial antifungal therapy. There were numerous antifungal therapeutic changes in the days following GM0.
Three patients started voriconazole therapy 8, 9, and 10 days following GM0, and one patient with slowly eroding pulmonary mycetomas started voriconazole therapy 33 days after GM0. The three patients who did not receive systemic antifungal therapy died 1, 4, and 7 days after GM0.
GM test use.
Of the 93 GPA patients, 72 had ≥2 GM values during the 6 weeks following GM0. Patients in this subset had a median of four GM values during this period (IQR, 2 to 7; range, 2 to 17). HSCT recipients had significantly more GM values than did non-HSCT patients, with a median of six (IQR, 3 to 9; range, 2 to 17) compared to four (IQR, 2 to 5; range, 2 to 11) values.
Of the 72 GPA patients with ≥2 GM values, 22 (30.6%) had 1-week GM drawn on day 7, and 11 (15.3%) had GM drawn on day 8. The median number of days between GM0 and 1-week GM was 7 (IQR, 7 to 9; range, 1 to 40), and the distribution was positively skewed, with a skewness of +3.2.
GM parameters.
The median GM0 for all patients was 1.02 (IQR, 0.72 to 2.04; range, 0.50 to 8.45). The median GM0 for the 72 patients with ≥2 GM values was 1.01 (IQR, 0.72 to 1.95; range, 0.50 to 8.45).
Median GM decay was 0.32 EIA units per week (IQR, −0.12 to 0.95; range, −9.73 to 6.94). A negative number indicates an increase in GM in the week following GM0.
Outcomes.
Actuarial all-cause mortality was 0.55 (95% confidence interval [CI], 0.45 to 0.65) at 6 weeks and 0.62 (95% CI, 0.52 to 0.72) at 12 weeks in the whole cohort, and 0.44 (95% CI, 0.34 to 0.57) at 6 weeks and 0.54 (95% CI, 0.43 to 0.66) at 12 weeks in the 72 patients with ≥2 GM values.
Of the 51 patients who died during the 6 weeks following GM0, 9 (17.6%) underwent autopsy, with confirmation of IA in all cases. There were no autopsies performed in the additional seven patients who died 6 to 12 weeks after GM0.
Cox proportional hazards models at 6 weeks.
Among all 93 patients with GPA, the crude HR for GM0 for time to mortality at 6 weeks was 1.23 (95% CI, 1.07 to 1.40; P = 0.003) per unit increase in EIA. Adjusting for age, allogeneic HSCT, prolonged corticosteroid use, and high-risk neutropenia, the adjusted HR for GM0 for the full cohort of 93 GPA patients was 1.13 (95% CI, 0.97 to 1.32; P = 0.11) per unit increase in EIA.
Unadjusted and adjusted HR values for GM0, 1-week GM decay, and other potential predictors of time to 6-week mortality are presented in Table 2 for the 72 GPA patients with ≥2 GM values. The adjusted HR for GM0 for time to mortality at 6 weeks was 1.25 (95% CI, 1.01 to 1.54; P = 0.04) per unit increase in EIA. The adjusted HR for 1-week GM decay was 0.78 (95% CI, 0.63 to 0.96; P = 0.02) per EIA unit decline over the week following GM0. There was no evidence of confounding of the combination of GM0 and 1-week GM decay in the adjusted model. Grade III to IV acute graft-versus-host disease (GVHD) was excluded from the adjusted proportional hazards model because of the small number of observations in our cohort. T-cell immunosuppressants were also excluded from the adjusted proportional hazards model because the model became overspecified with the addition of this covariate, and there was colinearity between T-cell immunosuppressants and HSCT. There were no significant changes in the adjusted HR estimates of the covariates that remained in the final model with the addition of T-cell immunosuppressants as an additional variable. Baseline renal dysfunction (modeled as continuous glomerular filtration rate at IA diagnosis) was not a significant predictor of 6-week mortality alone or when added to the multivariable model. There were no significant changes in the adjusted HR estimates with the inclusion of a GM0 × 1-week GM decay interaction term.
TABLE 2.
Cox proportional hazards model for GM0, 1-week GM decay, and other potential predictors of 6-week mortality
| Covariate | Univariate HR (95% CI) | P value | Adjusted HR (95% CI) | P value |
|---|---|---|---|---|
| GM0 (per EIA unit increase) | 1.27 (1.08-1.49) | 0.005 | 1.25 (1.01-1.54) | 0.039 |
| 1-wk GM decay (per EIA unit/week decline)a | 0.82 (0.66-1.02) | 0.075 | 0.78 (0.63-0.96) | 0.020 |
| Age, per decade | 1.16 (0.91-1.48) | 0.230 | 1.06 (0.83-1.35) | 0.625 |
| Allogeneic HSCTb | 0.64 (0.31-1.31) | 0.225 | 0.59 (0.26-1.33) | 0.200 |
| Acute grade III to IV GVHD | 1.95 (0.59-6.41) | 0.274 | ||
| High-risk neutropeniac | 0.79 (0.39-1.62) | 0.521 | 1.84 (0.71-4.75) | 0.211 |
| Prolonged corticosteroids within 90 days of GM0d | 3.27 (1.61-6.65) | 0.001 | 3.53 (1.40-8.94) | 0.008 |
| T-cell immunosuppressants within 90 days of GM0e | 0.45 (0.22-0.91) | 0.026 |
Negative GM decay values reflect an increase in GM EIA a week from GM0; the inverse of the HR (1/0.78 = 1.28) should be used to calculate the HR in those cases.
There were no autologous HSCT patients in this cohort.
High-risk neutropenia was defined according to the 2008 EORTC/MSG criteria for IFD classification as a recent history of neutropenia (<0.5 × 109 neutrophils/liter for >10 days) temporally related to the onset of fungal disease.
Prolonged corticosteroid use was defined according to 2008 EORTC/MSG IFD classification criteria as a mean minimum dose of 0.3 mg/kg/day of prednisone equivalent for >3 weeks.
T-cell immunosuppressants were defined according to 2008 EORTC/MSG IFD classification criteria as recognized T-cell immunosuppressants, such as cyclosporine, tumor necrosis factor alpha blockers, specific monoclonal antibodies such as alemtuzumab, or nucleoside analogues.
Assessment of effect of antifungal therapy.
Unadjusted HRs for antifungal therapy with azoles, liposomal amphotericin B, and echinocandins in the week following GM0 for 6-week all-cause mortality are presented in Table 3. The impact of adjusting for daily administration of each systemic antifungal agent on the multivariable Cox proportional hazards model developed above is summarized in Table 3. Adjusting for systemic voriconazole, liposomal amphotericin B, or echinocandin use during the week following GM0, controlling for age, allogeneic HSCT, high-risk neutropenia, and prolonged corticosteroid use had minimal effect on HR estimates for GM0 or 1-week GM decay. Voriconazole use was protective against mortality at 6 weeks in the adjusted model, and while liposomal amphotericin B and echinocandins were associated with elevated HRs for mortality at 6 weeks, they did not reach statistical significance.
TABLE 3.
Cox proportional hazards model for GM0 and 1-week GM decay, adjusting for antifungal exposure in the week following GM0 as time-dependent covariates
| Characteristic | HR (95% CI) | P value |
|---|---|---|
| Unadjusted antifungal drug exposure of: | ||
| Voriconazolea | 0.40 (0.22-0.70) | 0.002 |
| Liposomal amphotericin B | 1.59 (0.58-4.35) | 0.364 |
| Echinocandinsb | 1.45 (0.75-2.83) | 0.273 |
| Effect of adjusting multivariable model for voriconazole use onc: | ||
| GM0 (EIA, per unit increase) | 1.30 (1.06-1.59) | 0.011 |
| 1-wk GM decay (EIA units/week, per EIA unit/week decline) | 0.76 (0.60-0.96) | 0.019 |
| Voriconazolea | 0.42 (0.20-0.87) | 0.020 |
| Effect of adjusting multivariable model for liposomal amphotericin B use onb: | ||
| GM0 (EIA, per unit increase) | 1.27 (1.03-1.58) | 0.027 |
| 1-wk GM decay (EIA units/week, per EIA unit/week decline) | 0.76 (0.61-0.94) | 0.012 |
| Liposomal amphotericin B | 2.49 (0.49-12.79) | 0.274 |
| Effect of adjusting multivariable model for echinocandin use onb: | ||
| GM0 (EIA, per unit increase) | 1.23 (0.99-1.53) | 0.062 |
| 1-wk GM decay (EIA units/week, per EIA unit/week decline) | 0.80 (0.64-1.01) | 0.057 |
| Echinocandins | 1.57 (0.59-4.17) | 0.367 |
One patient in the voriconazole analysis received itraconazole rather than voriconazole.
Caspofungin was used until November 2007; micafungin was used thereafter.
Also adjusting for age, allogeneic HSCT, high-risk neutropenia, and prolonged corticosteroid use (individual adjusted HR not shown; see Table 2).
There was no evidence of confounding or effect modification by the receipt of empirical antifungal therapy prior to GM0 when this covariate was included in the model.
Secondary analysis of 12-week outcome.
GM0 and 1-week GM decay were also predictive of outcome at 12 weeks. In patients with ≥2 GM values, the crude HR was 1.19 (95% CI, 1.04 to 1.37; P = 0.011) for GM0 and 0.79 (95% CI, 0.64 to 0.96; P = 0.020) for 1-week GM decay for 12-week all-cause mortality. In the multivariable model, the adjusted HR was 1.27 (95% CI, 1.03 to 1.55; P = 0.024) per rise in EIA unit for GM0 and 0.75 (95% CI, 0.61 to 0.92; P = 0.006) per EIA unit decline per week for 1-week GM decay.
DISCUSSION
We analyzed early GM prognostic features in all patients with GPA at our institution and found both GM0 and 1-week GM decay to be predictive of time to all-cause mortality at 6 and 12 weeks, after adjusting for other traditional risk factors for mortality. Each EIA unit increase in GM0 increased the hazard of time to all-cause mortality at 6 weeks by 25%, while each GM EIA unit decline in the week following GM0 decreased the risk of time to all-cause mortality at 6 weeks by 22%.
In an unadjusted analysis, the colinear covariates HSCT and T-cell immunosuppressant use decreased the hazard of time to mortality, possibly because of a higher clinical index of suspicion and more-intensive serum GM surveillance in this subgroup, with a lower threshold for initiation of systemic antifungal therapy, but these factors were not predictive of time to mortality in either the 6- or 12-week multivariable model. In our adjusted Cox proportional hazards model, only GM0, 1-week GM decay, and prolonged exposure to corticosteroids within the preceding 60 days predicted time to mortality at 6 and 12 weeks. These hazard ratios remained stable even after adjusting for receipt of systemic antifungal therapy with voriconazole, liposomal amphotericin B, and echinocandins in the week following GM0.
Strengths of this study include its relatively large and unselected sample of all patients with GPA at our institution over 4 years and its nuanced assessment of early GM kinetic features in predicting time to mortality. A significant limitation of our study was the irregularity of GM monitoring after GM0, given its retrospective nature and GM testing at the discretion of clinical care teams. For 1-week GM, we preferentially considered the GM value soonest after day 7 if a day 7 GM was unavailable, evident in the positively skewed distribution of the number of days between GM0 and 1-week GM, and likely underestimated true 1-week GM decay in patients who survived past 1 week, thus underestimating the protective effect of 1-week GM decay on all-cause mortality. Our autopsy rates were also relatively low, which limited our ability to analyze GM prognostic features for IA-specific mortality.
An ideal surrogate outcome marker meets the following criteria: (i) replaces a comparatively remote but most clinically meaningful “true” endpoint with one more proximate in time to the study intervention; (ii) is convincingly related to the hazard rate for the true endpoint; and (iii) mediates at least a substantial portion of treatment effect on the true endpoint (18, 19, 25). Proving surrogacy is a challenging endeavor, due to the tremendous biological complexity of the relationship between the surrogate candidate, the true outcome, and the effects of various therapeutic measures on both of these parameters. In the subset of IA patients with GPA, for example, serum GM is a product of numerous factors, including the virulence of the Aspergillus species, infecting inoculum, location of the infection, timeliness of diagnosis, immune status of the host, the effectiveness of host hepatic and renal metabolism in clearing fungal mannans from the bloodstream, and the effectiveness of various antifungal therapy regimens, among many others (14). In addition, patients with IA are usually at risk of death due to their underlying conditions, aggressive treatments and their toxicities, and other potentially fatal nonfungal infections.
Many empirical experimental and clinical observations indirectly support serum GM as a surrogate outcome marker in GPA. Serum GM is standardized, reproducible, continuous, and highly specific for IA in the absence of certain parenteral β-lactam antibiotics and other products industrially produced in Aspergillus species (8). GM correlates closely with fungal burden in in vivo models and appears to be inextricably linked to Aspergillus pathogenesis; in an elegant alveolar epithelial-endothelial cell bilayer model, the first appearance of GM in the endothelial compartment occurred as hyphae invaded the endothelial compartment (7). Prior studies have described a correlative relationship between failure to clear GM and poor clinical outcomes (9, 27) and a correlative relationship between elevated GM values the week prior to IA outcome and poor clinical outcomes (15). One study from the prevoriconazole era found that a rise in GM of 1.0 EIA units above baseline during the first week of each treatment episode was a marker of therapeutic failure, with a sensitivity of 44%, specificity of 87%, and positive predictive value of 94% (3).
We propose that the simple, early parameter of 1-week GM decay, accounting for GM0, has value as a surrogate endpoint candidate; it has prognostic value for the true endpoint of mortality at 6 and 12 weeks and appears to be stably predictive of the true endpoint even after adjusting for treatment with distinct antifungal therapies, including voriconazole, liposomal amphotericin B, or echinocandins, during the initial week. Our findings will need validation in other data sets of patients who underwent more systematic GM testing.
IA has a wide spectrum of disease manifestations, and the subset of IA patients with elevated GM values at diagnosis may represent a biologically distinct stratum of patients that can be studied separately, analogous to the stratification of acute coronary syndrome patients by troponin and creatine phosphokinase biomarkers to unstable angina and non-heat-stable enterotoxin (non-ST) elevation myocardial infarction cohorts for the purposes of clinical trials. If the relationship between GM decay and IA outcome is confirmed in other data sets, using a surrogate endpoint of a difference in 1-week GM decay, accounting for GM0, has the potential to accelerate development of future antifungal therapies by sharply reducing trial duration and sample size in GPA patients.
Acknowledgments
We report no potential conflicts of interest or financial support relevant to this study.
We are grateful to Susan Hadley and David Andes for their invitation to discuss “The Use of Surrogate Markers to Assess Fungal Disease Burden, Outcomes and Antifungal Responses” for the Mycoses Study Group Annual Meeting Symposium entitled Clinical Mycology Studies: What We Don't Know, Why, and Potential Solutions, held in Philadelphia, PA, on 2 April 2009. The study of the topic and the discussion during the symposium inspired the conception and pursuit of this project.
Footnotes
Published ahead of print on 10 February 2010.
REFERENCES
- 1.Becker, M. J., S. de Marie, M. H. Fens, H. A. Verbrugh, and I. A. Bakker-Woudenberg. 2003. Effect of amphotericin B treatment on kinetics of cytokines and parameters of fungal load in neutropenic rats with invasive pulmonary aspergillosis. J. Antimicrob. Chemother. 52:428-434. [DOI] [PubMed] [Google Scholar]
- 2.Becker, M. J., S. de Marie, D. Willemse, H. A. Verbrugh, and I. A. Bakker-Woudenberg. 2000. Quantitative galactomannan detection is superior to PCR in diagnosing and monitoring invasive pulmonary aspergillosis in an experimental rat model. J. Clin. Microbiol. 38:1434-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutboul, F., C. Alberti, T. Leblanc, A. Sulahian, E. Gluckman, F. Derouin, and P. Ribaud. 2002. Invasive aspergillosis in allogeneic stem cell transplant recipients: increasing antigenemia is associated with progressive disease. Clin. Infect. Dis. 34:939-943. [DOI] [PubMed] [Google Scholar]
- 4.Bretagne, S., A. Marmorat-Khuong, M. Kuentz, J. P. Latge, E. Bart-Delabesse, and C. Cordonnier. 1997. Serum Aspergillus galactomannan antigen testing by sandwich ELISA: practical use in neutropenic patients. J. Infect. 35:7-15. [DOI] [PubMed] [Google Scholar]
- 5.De Pauw, B., T. J. Walsh, J. P. Donnelly, D. A. Stevens, J. E. Edwards, T. Calandra, P. G. Pappas, J. Maertens, O. Lortholary, C. A. Kauffman, D. W. Denning, T. F. Patterson, G. Maschmeyer, J. Bille, W. E. Dismukes, R. Herbrecht, W. W. Hope, C. C. Kibbler, B. J. Kullberg, K. A. Marr, P. Munoz, F. C. Odds, J. R. Perfect, A. Restrepo, M. Ruhnke, B. H. Segal, J. D. Sobel, T. C. Sorrell, C. Viscoli, J. R. Wingard, T. Zaoutis, and J. E. Bennett. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis, P., J. W. Lee, A. Hoffman, J. Peter, A. Francesconi, J. Bacher, J. Shelhamer, P. A. Pizzo, and T. J. Walsh. 1994. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar d-mannitol and serum galactomannan as markers of infection. J. Infect. Dis. 169:356-368. [DOI] [PubMed] [Google Scholar]
- 7.Hope, W. W., M. J. Kruhlak, C. A. Lyman, R. Petraitiene, V. Petraitis, A. Francesconi, M. Kasai, D. Mickiene, T. Sein, J. Peter, A. M. Kelaher, J. E. Hughes, M. P. Cotton, C. J. Cotten, J. Bacher, S. Tripathi, L. Bermudez, T. K. Maugel, P. M. Zerfas, J. R. Wingard, G. L. Drusano, and T. J. Walsh. 2007. Pathogenesis of Aspergillus fumigatus and the kinetics of galactomannan in an in vitro model of early invasive pulmonary aspergillosis: implications for antifungal therapy. J. Infect. Dis. 195:455-466. [DOI] [PubMed] [Google Scholar]
- 8.Hope, W. W., T. J. Walsh, and D. W. Denning. 2005. Laboratory diagnosis of invasive aspergillosis. Lancet Infect. Dis. 5:609-622. [DOI] [PubMed] [Google Scholar]
- 9.Maertens, J., K. Buve, K. Theunissen, W. Meersseman, E. Verbeken, G. Verhoef, J. Van Eldere, and K. Lagrou. 2009. Galactomannan serves as a surrogate endpoint for outcome of pulmonary invasive aspergillosis in neutropenic hematology patients. Cancer 115:355-362. [DOI] [PubMed] [Google Scholar]
- 10.Maertens, J., K. Theunissen, E. Verbeken, K. Lagrou, J. Verhaegen, M. Boogaerts, and J. V. Eldere. 2004. Prospective clinical evaluation of lower cut-offs for galactomannan detection in adult neutropenic cancer patients and haematological stem cell transplant recipients. Br. J. Haematol. 126:852-860. [DOI] [PubMed] [Google Scholar]
- 11.Maertens, J., J. Verhaegen, K. Lagrou, J. Van Eldere, and M. Boogaerts. 2001. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood 97:1604-1610. [DOI] [PubMed] [Google Scholar]
- 12.Marr, K. A., S. A. Balajee, L. McLaughlin, M. Tabouret, C. Bentsen, and T. J. Walsh. 2004. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. J. Infect. Dis. 190:641-649. [DOI] [PubMed] [Google Scholar]
- 13.Marr, K. A., M. Laverdiere, A. Gugel, and W. Leisenring. 2005. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin. Infect. Dis. 40:1762-1769. [DOI] [PubMed] [Google Scholar]
- 14.Mennink-Kersten, M. A., J. P. Donnelly, and P. E. Verweij. 2004. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect. Dis. 4:349-357. [DOI] [PubMed] [Google Scholar]
- 15.Miceli, M. H., M. L. Grazziutti, G. Woods, W. Zhao, M. H. Kocoglu, B. Barlogie, and E. Anaissie. 2008. Strong correlation between serum aspergillus galactomannan index and outcome of aspergillosis in patients with hematological cancer: clinical and research implications. Clin. Infect. Dis. 46:1412-1422. [DOI] [PubMed] [Google Scholar]
- 16.Petraitiene, R., V. Petraitis, A. H. Groll, T. Sein, S. Piscitelli, M. Candelario, A. Field-Ridley, N. Avila, J. Bacher, and T. J. Walsh. 2001. Antifungal activity and pharmacokinetics of posaconazole (SCH 56592) in treatment and prevention of experimental invasive pulmonary aspergillosis: correlation with galactomannan antigenemia. Antimicrob. Agents Chemother. 45:857-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petraitis, V., R. Petraitiene, A. H. Groll, K. Roussillon, M. Hemmings, C. A. Lyman, T. Sein, J. Bacher, I. Bekersky, and T. J. Walsh. 2002. Comparative antifungal activities and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 46:1857-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prentice, R. L. 2009. Surrogate and mediating endpoints: current status and future directions. J. Natl. Cancer Inst. 101:216-217. [DOI] [PubMed] [Google Scholar]
- 19.Prentice, R. L. 1989. Surrogate endpoints in clinical trials: definition and operational criteria. Stat. Med. 8:431-440. [DOI] [PubMed] [Google Scholar]
- 20.Reiss, E., and P. F. Lehmann. 1979. Galactomannan antigenemia in invasive aspergillosis. Infect. Immun. 25:357-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohrlich, P., J. Sarfati, P. Mariani, M. Duval, A. Carol, C. Saint-Martin, E. Bingen, J. P. Latge, and E. Vilmer. 1996. Prospective sandwich enzyme-linked immunosorbent assay for serum galactomannan: early predictive value and clinical use in invasive aspergillosis. Pediatr. Infect. Dis. J. 15:232-237. [DOI] [PubMed] [Google Scholar]
- 22.Segal, B. H., R. Herbrecht, D. A. Stevens, L. Ostrosky-Zeichner, J. Sobel, C. Viscoli, T. J. Walsh, J. Maertens, T. F. Patterson, J. R. Perfect, B. Dupont, J. R. Wingard, T. Calandra, C. A. Kauffman, J. R. Graybill, L. R. Baden, P. G. Pappas, J. E. Bennett, D. P. Kontoyiannis, C. Cordonnier, M. A. Viviani, J. Bille, N. G. Almyroudis, L. J. Wheat, W. Graninger, E. J. Bow, S. M. Holland, B. J. Kullberg, W. E. Dismukes, and B. E. De Pauw. 2008. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin. Infect. Dis. 47:674-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stynen, D., A. Goris, J. Sarfati, and J. P. Latge. 1995. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J. Clin. Microbiol. 33:497-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallor, A. C., W. R. Kirkpatrick, L. K. Najvar, R. Bocanegra, M. C. Kinney, A. W. Fothergill, M. L. Herrera, B. L. Wickes, J. R. Graybill, and T. F. Patterson. 2008. Assessment of Aspergillus fumigatus burden in pulmonary tissue of guinea pigs by quantitative PCR, galactomannan enzyme immunoassay, and quantitative culture. Antimicrob. Agents Chemother. 52:2593-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weir, C. J., and R. J. Walley. 2006. Statistical evaluation of biomarkers as surrogate endpoints: a literature review. Stat. Med. 25:183-203. [DOI] [PubMed] [Google Scholar]
- 26.Wingard, J. R., P. Ribaud, H. T. Schlamm, and R. Herbrecht. 2008. Changes in causes of death over time after treatment for invasive aspergillosis. Cancer 112:2309-2312. [DOI] [PubMed] [Google Scholar]
- 27.Woods, G., M. H. Miceli, M. L. Grazziutti, W. Zhao, B. Barlogie, and E. Anaissie. 2007. Serum Aspergillus galactomannan antigen values strongly correlate with outcome of invasive aspergillosis: a study of 56 patients with hematologic cancer. Cancer 110:830-834. [DOI] [PubMed] [Google Scholar]