Abstract
Salt taste in mammals can trigger two divergent behavioural responses. In general, concentrated saline solutions elicit robust behavioural aversion, while low concentrations of NaCl are typically attractive, particularly after sodium depletion1-5. Notably, the attractive salt pathway is selectively responsive to sodium and inhibited by amiloride, while the aversive one functions as a non-selective detector for a wide range of salts1-3, 6-9. Since amiloride is a potent inhibitor of the epithelial sodium channel (ENaC), ENaC has been proposed to function as a component of the salt taste receptor system1, 3, 6-14. Here, we examine the basis of sodium sensing in the mammalian taste system. Previously, we showed that four of the five basic taste qualities, sweet, sour, bitter and umami are mediated by separate taste receptor cells (TRC) each tuned to a single taste modality, and wired to elicit stereotypical behavioural responses5, 15-18. We now demonstrate that sodium sensing is also mediated by a dedicated population of TRCs. These taste cells express the epithelial sodium channel ENaC19, 20, and mediate behavioural attraction to NaCl. We genetically engineered mice lacking ENaCα in TRCs, and produced animals exhibiting a complete loss of salt attraction and sodium taste responses. Together, these studies substantiate independent cellular substrates for all five basic taste qualities, and validate the essential role of ENaC for sodium taste in mice.
Sodium is the major cation of extracellular fluids and an essential component of every fluid compartment in the body. It is therefore not surprising that animals have evolved dedicated salt-sensing systems, including prominent detectors in the taste system1-3. These salt sensitive receptors are crucial for the acceptance of low concentrations of sodium (e.g. to satisfy the “salt appetite”)1, 3 while simultaneously serving as a warning mechanism against hyper-salinity2, 3, thus helping maintain ion and water homeostasis. For humans, the “taste for salt” also has direct bearing on excessive Na+-consumption, which is believed to be a significant dietary risk factor in hypertension, particularly in the developed world21. In mice, the low-concentration, and behaviourally “attractive” salt taste pathway has three salient properties: it is activated at NaCl concentrations as low as 10 mM, it is highly selective for sodium versus other cations, and it is blocked by lingual application of the ion-channel inhibitor amiloride8, 9, 14, 22. The high-concentration (aversive) pathway, on the other hand, begins to be significant only at concentrations greater than 150 mM NaCl, it is non-selective for sodium (i.e. other salts are equally effective), and it is amiloride insensitive9, 14, 22.
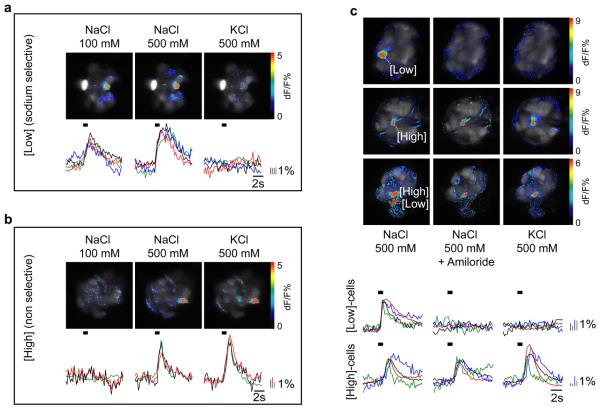
In order to explore the cellular basis for the taste of NaCl (i.e. are the distinct physiological and behavioural responses mediated by the same or by separate TRCs?), we developed a novel preparation that allows functional imaging of TRCs in response to salt stimulation. In essence, TRCs in fungiform papillae were loaded with the calcium-sensitive dye calcium green23 in vivo, and then were stimulated and imaged, ex-vivo, with a regime that either preferentially activated the low-concentration pathway (100 mM NaCl), or activated both the high- and low-concentration pathways (500 mM NaCl). To separate the contribution of each of the two salt-sensing systems at high-stimulus concentrations, we examined the salt responses in the presence and absence of 10 μM amiloride (Fig. 1). Receptor cells that are only activated by high concentrations of salt also respond to a wide range of non-sodium salts (e.g. from KCl to NMDG.Cl, Fig. 1 and Supplementary Fig. S1), and their activity is unaffected by the presence of amiloride (Fig. 1b and c). In contrast, low-concentrations of NaCl activates a completely separate population of TRCs; these cells do not respond to non-sodium salts (Fig. 1a and c, see also Supplementary Fig. S1), and their responses are blocked by amiloride (Fig. 1c). These results demonstrate the presence of two anatomically distinct salt sensing systems, and accordingly suggest that the appetitive and aversive behaviors are likely to be mediated by non-overlapping populations of TRCs. As the TRCs activated by low concentrations of NaCl are highly selective for sodium salts, we consider them to be the dedicated sodium sensing system and thus are the subject of this study.
Figure 1. Two classes of TRCs mediate distinct salt taste responses.
Fungiform taste buds loaded with the activity sensor calcium green respond with high selectivity and specificity to different concentrations of salt. (a) A unique subset of TRCs (labeled as [Low]) respond to low concentrations of sodium chloride (100 mM) as well as higher concentrations (500 mM NaCl) but not other salts (KCl). Shown below the imaging data are individual traces from 4 different TRCs depicting the kinetic and amplitude changes in intracellular calcium levels following salt stimulation; calcium changes were pseudo-coloured as depicted (b) A different population of TRCs (labeled as [High]) are activated only at elevated concentrations of NaCl (500 mM) and are also stimulated by KCl; shown are individual traces for 3 different TRCs. (c) Amiloride selectively blocks [Low] responses but has no effect on [High] responses; shown are individual traces for 4 different TRCs; the duration of tastant application is denoted by black bars. See supplementary Fig. S1 for a diagram of the preparation, quantitations and responses to additional salts.
Because amiloride is an inhibitor of the epithelial sodium channel (ENaC), ENaC has been hypothesized as a potential component of the salt taste receptor system1, 6, 8, 10-12, 14. The ENaC channel is made up of 3 subunits (α, β, and γ), and plays a key role in regulating trans-epithelial transport of Na+ in a wide range of tissues, including kidneys, airway cells of the lung, epithelial skin cells, and the ducts of salivary and sweat glands19, 20. While a knockout of any ENaC subunit is sufficient to completely abolish ENaC function20, conventional ENaC knockouts die within a few days of birth20 precluding their use in physiological and behavioural studies of taste.
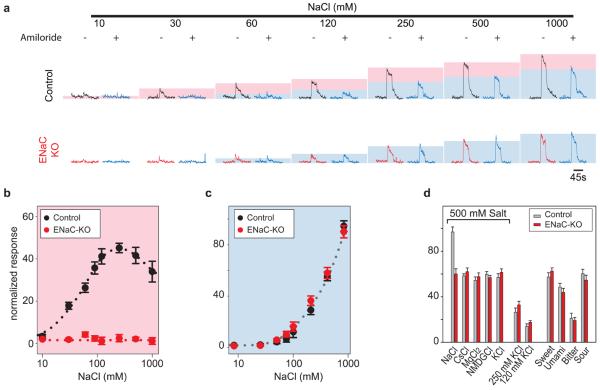
To examine the role of ENaCs in the taste system, we utilized a floxed ENaCα conditional knockout strategy (Scnn1aflox)24. In essence, we generated animals in which ENaC function was selectively eliminated in all differentiated TRCs by using the cytokeratin19 gene, a marker for all mature taste cells (see Supplementary Fig. S2) to drive the expression of Cre-recombinase in the taste system. To investigate the taste responses of the conditional ENaCα knockout mice, we recorded tastant-induced action potentials from nerves innervating the taste cells of the tongue; this physiological assay monitors the activity of the taste system at the periphery and provides a reliable measure of TRC function9, 18, 25. In wild-type mice, NaCl elicits a dose-dependent increase in action potentials in the chorda tympani nerve, with a physiological response threshold of approximately 10 mM (Fig. 2). Loss of ENaCα in the taste system does not affect responses to four of the five basic taste qualities: sweet, bitter, umami and sour stimuli (Fig 2d and Supplementary Fig. S3). In contrast, ENaCα knockouts display a complete loss of the responses to low-concentrations of NaCl (Fig. 2). As would be expected if ENaC was the sodium sensor, these animals are also missing all amiloride sensitivity in their NaCl responses (Fig. 2). Importantly, the knockout mice retain all responses to non-sodium salts (Fig. 2d and Supplementary Fig. S3). These results demonstrate that taste responses to salts are mediated by genetically separable components.
Figure 2. ENaC is necessary for high sensitivity taste responses to sodium salts.
Conditional knockout of ENaCα in TRCs (ENaC-KO) abolishes responses to low concentrations of NaCl, and eliminates amiloride-sensitivity. (a) Integrated neural recordings from the chorda tympani nerve of normal (Control) and ENaC-KO mice in the presence (blue traces) or absence of 10 μM amiloride. Shaded boxes illustrate the amiloride-sensitive (pink) and insensitive (blue) components. (b-c) Quantitations of integrated neural responses of control and KO animals; the coloured boxes are as in panel a (n=4, mean ± s.e.m, P < 0.001 for amiloride sensititive responses of control and mutant groups at 30 - 1000 mM NaCl). (d) ENaC-KO mice retain normal responses to other salts or other taste qualities (n=4, mean ± s.e.m); see Methods for details of calculations, tastants used, concentrations, genotype of strains and abbreviations.
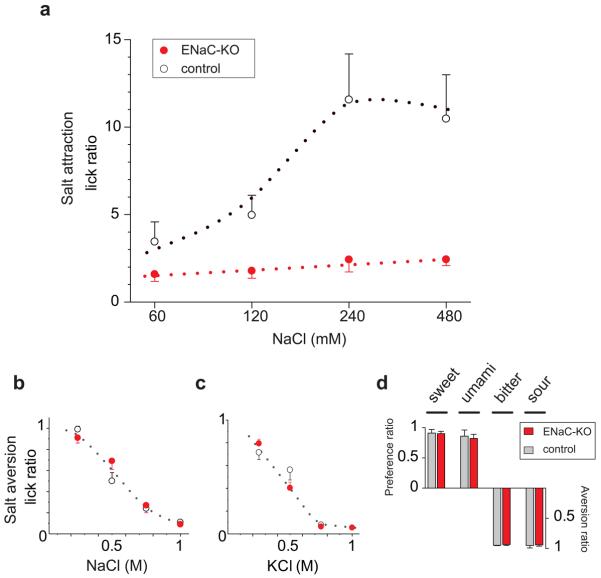
Animals ranging from simple invertebrates to mammals readily consume low to moderate concentrations of Na+, and actively seek it under conditions of salt deprivation1, 2, 4, 26. Therefore, we carried out behavioural tests of salt consumption to examine the taste behavior of the ENaCα conditional knockout animals both under conditions of salt depletion (to test attraction) and under water deprivation (to test aversion)5. We reasoned that if ENaC encodes the principal sodium taste sensor, it should mediate all attraction to salt, and consequently, the knockout mice should have a total loss of behavioural attraction to NaCl. Indeed, ENaCα-KO mice show no significant attraction to salt, even under conditions where control animals exhibit extraordinary appetite for sodium (Fig. 3a). In contrast, the aversive responses to high concentrations of NaCl (and KCl) are unaltered in the same knockout animals (Fig. 3b and c). Importantly, behavioural responses to sweet, sour, umami and bitter tastants are indistinguishable from control animals (Fig. 3d). These results validate ENaC as the mammalian taste receptor responsible for behavioural acceptance of (and attraction to) NaCl.
Figure 3. ENaC function in TRCs is required for behavioural attraction to salt.
Conditional knockout of ENaCα in TRCs (ENaC-KO) selectively abolishes the attractive taste of NaCl. (a) After diuretic induced Na+-depletion, ENaC-KO mice show little or no preference for NaCl solutions relative to water whereas littermate controls exhibit very robust attractive responses (P < 0.02 between control and mutant groups at 120 - 480 mM NaCl). In contrast, in salt aversion assays, water deprived ENaC-KO and controls are indistinguishable in their responses to (b) NaCl or (c) KCl. (d) Behavioural responses to other taste qualities are unaffected in the ENaC-KO animals; shown are means ± s.e.m. (n≥7); see Methods for additional details.
Our previous studies have shown that sweet, bitter, umami and sour tastes are mediated by independent populations of TRCs, each tuned to a single taste modality5, 15, 17, 18. If this labeled-line logic of taste coding at the periphery extends to all five basic taste modalities, then sodium taste should also be mediated by a unique population of TRCs. Thus, we examined whether the amiloride-sensitive salt sensing cells indeed define a sub-population of taste receptor cells separate from sweet, bitter, sour and umami TRCs. We engineered mice expressing Cre-recombinase under the control of the ENaCα gene, and then crossed them to a floxed GFP reporter line (Z/EG). To validate the fidelity of Cre expression in ENaCα-expressing cells we analyzed progeny from four independent Cre-driver founders and confirmed proper GFP reporter expression in the airway cells of the lung as well as in the kidney cortical collecting duct cells and distal convoluted tubules, well-characterized sites of ENaCα expression19 (see Supplementary Figs. S4).
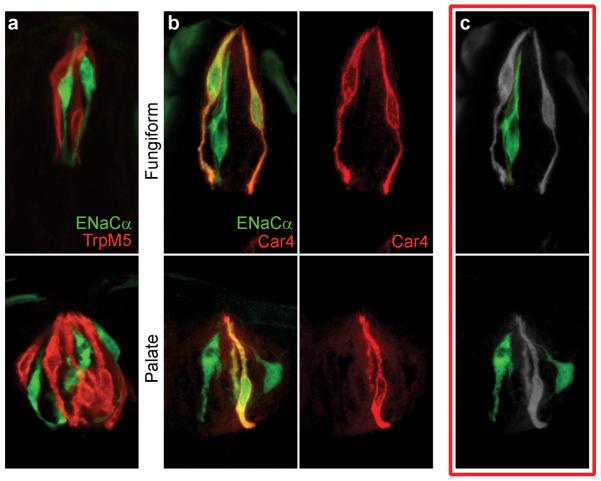
Co-labeling with the sweet/umami/bitter TRC marker, TRPM517, demonstrated that ENaCα expressing cells are distinct from sweet-, bitter- or umami-TRCs (Fig. 4a and Supplementary Fig. S6). In fungiform and palate taste buds co-localization with a sour cell marker, Car427, revealed the presence of two populations of TRCs: one exhibiting co-expression of ENaCα and Car4 (Fig. 4b), and importantly, a second one expressing ENaCα, but not sour, sweet, bitter or umami markers (referred to as “ENaC-alone” cells, Fig 4b and c). We hypothesized that the ENaC-alone cells are the bona-fide sodium taste sensors, and that the expression of ENaCα in sour cells may just be a consequence (i.e. non-functional) of a common lineage between the cells mediating ionic tastes. Thus, we carried out additional studies. First, we generated animals lacking ENaCα solely in Car4-expressing cells by using a sour-cell Cre driver to excise the conditional ENaCα knockout allele. As expected, these mice display wild-type responses to sweet, bitter, umami and sour stimuli. Importantly, they exhibit normal salt responses that are indistinguishable from wild-type controls (Supplementary Fig. S5) thus demonstrating that the ENaCα expression in the sour cells is in fact not required for salt taste. In a complementary study, we also generated mice entirely lacking sour-sensing cells15; these animals display a total loss of sour sensing, yet they maintain normal salt responses (Supplementary Fig. S5). Most critically, we directly imaged salt and sour responses using our novel peeled epithelium preparation. Indeed, there is total segregation of the cells responding to salt (low and high concentrations) versus those responding to acid stimulation (i.e. sour cells never respond to salt stimuli13; see Supplementary Fig. S1). Taken together, these results substantiate the functional and anatomical segregation of sodium-sensing TRCs, and prove that all 5 basic taste modalities are mediated by separate and dedicated receptor cells at the periphery.
Figure 4. ENaC defines a novel population of TRCs.
Transgenic mice expressing GFP under the control of ENaCα (see Methods for details) were immunostained for markers of known classes of TRC. (a) No overlap in the expression of ENaCα (green) and TrpM5 (red; a marker of sweet, bitter and umami TRCs) was observed in fungiform or palate taste buds. In contrast, (b) Car4-expressing sour cells (red label) co-express ENaCα (green label; compare red and yellow-labeled cells). However, ENaCα is also expressed in a unique subset of "ENaC-alone" TRCs (green-only cells in panels b and c); see Methods for details of mice and the illustration in panel c. Note that since Car4-expressing sour cells do not express the essential ENaCβ subunit they do not respond to salt (see Supplementary Fig. S6).
An unusual feature of the physiology of sodium taste in mice has been the observation that the back of the tongue (circumvallate papillae) harbors no sodium-selective (amiloride-sensitive) responses10, 22, 28, highlighting a strong topographic segregation (front to back) in salt taste (see below). With the identity of the amiloride-sensitive salt taste receptor at hand, we reasoned it should now be possible to explore the molecular basis of the absence of sodium sensing at the back of the tongue. ENaC channels are composed of 3 essential subunits (α, β and γ), thus we hypothesized this Na+ and amiloride insensitivity could be easily explained if the functional ENaC heterotrimeric channel were not found in the circumvallate papillae. Indeed, our results show that at the back of the tongue ENaCα and ENaCβ subunits are found in completely non-overlapping populations of TRCs (Supplementary Fig. S6). Therefore, amiloride-insensitivity at the back of the tongue is due to the lack of functional ENaC channels.
In this study we have shown that ENaC, first proposed to play a role in salt taste over 25 years ago8, 12, functions as the sodium taste receptor. We also demonstrated that sodium taste is mediated by a dedicated population of TRCs separate from those mediating sweet, umami, bitter and sour taste. Notably, the taste of sodium and non-sodium salts are detected by genetically, pharmacologically and physiologically distinguishable TRCs. The availabity of two channels (and cellular pathways) for salt-sensing endows animals with the ability to distinguish sodium-containing salts from other salts; this affords mammals with a powerful mechanism to select food sources containing adequate sodium but at the same time to avoid ingesting excessive amounts of salt.
The presence of salt shakers on dinner tables around the world attests to the appetitive role of salt taste in the human diet. Indeed, salt has been a food additive shared by humans for thousands of years, with empires from the Roman (for salary) to the British (for taxes) valuing it as a precious commodity. Does ENaC function in human salt taste? Physiological recordings in non-human primates have clearly demonstrated an amiloride-sensitive component in taste responses to salt stimuli29, 30. However, psychophysical experiments in humans remain inconclusive7, with some reports of amiloride altering salt taste7, 12, and several failing to substantiate a significant effect for amiloride in the perception of saltiness (reviewed, ref 7). Given the molecular similarities between mice and humans in all other other taste modalities16, a “human-specific” molecular mechanism for salt taste would be surprising. Perhaps more likely, the contribution of ENaC to human salt taste may be masked as a result of experience, exposure to salt, and diet. Future experiments studying people subjected to controlled salt intake may help clarify the role, if any, of ENaC in human taste.
Methods Summary
Transgenic animals and mouse strains
ENaCα-IRES-Cre, ENaCβ-IRES-tTA, cytokeratin19-IRES-Cre are BAC-transgenics engineered to express Cre recombinase or the tetracycline dependent transactivator (tTA) by inserting an IRES-Cre or IRES-tTA transgene 3′ to the Scnn1a, Scnn1b or Krt19 stop codon. Other strains have been described previously15, 24.
Calcium imaging
Fungiform TRCs were pre-loaded in vivo with Calcium Green-1 dextran 3kD (Invitrogen) by electroporating single taste buds. After 24-36 h taste epithelium was enzymatically peeled11 18and placed on a recording chamber with the apical side of TRCs facing up (Supplementary Fig. S1). Taste stimuli were delivered in artificial saliva by focal application. Changes in [Ca2+]i were imaged using a 5-Live confocal microscope (Zeiss) and dF/F from individual TRCs analyzed and pseudo-coloured as described previously23.
Nerve recording, behavioural and localization studies
All procedures were as described previously 5, 17, 18, 25, 27.
Methods
Transgenic animals and mouse strains
The PKD2L1-IRES-Cre, ROSA-DTA, Z/EG and ENaCα conditional knockouts (Scnn1aflox/flox) strains have been described previously15, 24. ENaCα-IRES-Cre, ENaCβ-IRES-tTA, cytokeratin19-IRES-Cre are BAC-transgenics engineered to express Cre recombinase or the tetracycline dependent transactivator (tTA) by inserting an IRES-Cre or IRES-tTA transgene 3′ to the Scnn1a, Scnn1b or Krt19 stop codon. The ENaCβ-IRES-tTA transgenic mice also carried a TetO-sapphire (modified GFP) reporter in the BAC-transgene. Four independent lines of ENaCα-IRES-Cre expressed the Cre transgene appropriately in kidney and lung and labeled equivalent populations of taste cells (Supplementary Fig. S2). Similarly, three founder lines expressing ENaCβ-IRES-tTA showed equivalent tTA-expression in lung, kidney and taste tissue. Mice were inter-crossed as described in the text to generate appropriate genotypes for physiological, behavioural and anatomical experiments. Control groups were littermates either not carrying the IRES-Cre transgene and/or animals with at least one copy of the wildtype Scnn1a allele. Wild type, heterozygous and the conditional ENaCα taste knockouts had no obvious differences in size, weight, fertility, life expectancy, or food consumption.
Calcium imaging
Fungiform TRCs were pre-loaded in vivo with calcium green-1 dextran 3kD (Invitrogen) by electroporating single taste buds in the tongue of anesthesized mice using a 3.5 μA, 50 pulses / s × 16 cycles regime. On average, 8-9 taste buds were loaded per animal. After 24-36 h of recovery, tongues were removed and the taste epithelium enzymatically peeled as described previously11. The epithelium was then placed on a recording chamber with the apical side of TRCs facing up (Supplementary Fig. S1), ensuring that the integrity and polarity of taste buds is maintained. The apical surface of the preparation was bathed in artificial saliva at a constant flow rate of 4.5 ml / min, and taste stimuli were delivered by focal application using a custom made dispensing pipette (800 μm diameter). Tastant application was for 1 s, with a minimum of 10 s of artificial saliva between stimuli. Changes in [Ca2+]i were imaged using a 5-Live confocal microscope (Zeiss) using a 40x C-Apochromat 1.20W objective; images were captured at 4 Hz, and dF/F from individual TRCs analyzed and pseudo-coloured as described previously23.
Nerve recordings
Lingual stimulation and recording procedures were performed as previously described18, 25. All data analyses used the integrated response over a 25 s period immediately after the application of the stimulus. Tastants used for nerve recordings were: 3 - 30 mM acesulfameK (sweet); 1 - 100 mM monopotassium glutamate + 1.0 mM inosine monophosphate (umami); 1 - 10 mM quinine hydrochloride (bitter); 1 - 50 mM citric acid (sour). The responses to 50 mM citric acid were used to normalize responses to each experimental series in control and ENaCα-KO (Fig. 2). To compute the amiloride-sensitive salt component (Fig. 2b) the stimulation regime involved sequential applications of NaCl solutions first without and then with amiloride (in the same experimental series). The amiloride insensitive component was defined as the response in the presence of amiloride (Fig 2c). The fraction of the response inhibited by amiloride was defined as the amiloride sensitive component (amiloride sensitive component= Response without amiloride - Response with amiloride; Fig. 2b). Responses in experiments involving PKD2L1-IRES-Cre / Rosa-DTA (PKD2L1-DTA) and PKD2L1-IRES-Cre / ENaCα flox/flox (PKD2L1-ENaC-KO) were normalized to responses obtained with 30 mM acesulfameK (Supplementary Fig. S5). NaCl solutions used in dose-response studies for measuring the amiloride-insensitve sodium responses (Fig. 2c) included 10 μM amiloride. Differences between knockout and control responses were analyzed for statistical significance using an unpaired, two-tailed Student's t-test and 95 % confidence limits.
Behavioural assays
Behavioural assays used a custom made gustometer to measure immediate lick responses as described previously17, 18. For salt attraction assays, mice were injected with furosemide (50 mg/kg) and were placed on a low sodium diet with unrestricted water 16-20 h to deplete sodium before testing5. For salt aversion assays, mice were water deprived for 24 h before testing5, 17, 18. Control tastants were 32 mM acesulfameK (sweet), 100 mM monosodium glutamate + 1 mM inosine monophosphate + 0.1 mM amiloride (umami), 1 mM quinine sulfate (bitter) and 150 mM citric acid (sour). Differences between knockout and control responses were analyzed for statistical significance using an unpaired, two-tailed Student's t-test and 95 % confidence limits; for Supplementary Fig. S5b a one way ANOVA with Newman-Keuls posterior test was used to compare datasets.
Immunohistochemistry and cell labelling
Immunostaining, whole-mount imaging (GFP) and in situ hybridization were performed as described previously17, 18; animals were perfused with 4% paraformaldehyde and tissue post-fixed for 6-48 h to allow localization of GFP. Images were obtained using a Leica SP2 TSC or a Zeiss 510 LSM meta confocal microscope. Anti-TRPM5 and anti-Car4 antibodies were as described previously17, 27. The illustration in Fig. 4c, was composed by converting the red and green channels in Fig. 4b to grayscale and overlaying with the ENaC-alone (green-only) cells using Adobe Photoshop.
Supplementary Material
Acknowledgements
we thank Wei Guo and Ann Becker for generation and maintenance of mouse lines. We thank Kristin Scott and members of our labs for valuable comments. This research was supported in part by the intramural research program of the NIH, NIDCR (N.J.P.R.). C.S.Z. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Supplementary Information accompanies the paper.
Competing interest statement, C.S.Z. is a scientific founder and scientific advisory board member of Senomyx.
References
- 1.Contreras R,J. Gustatory mechanisms of a specific appetite. In: Cagan RH, editor. Neural Mechanisms in Taste. CRC; Boca Raton, FL: 1989. pp. 119–145. [Google Scholar]
- 2.Duncan CJ. Salt Preferences of Birds and Mammals. Physiological Zoology. 1962;35:120–132. [Google Scholar]
- 3.Lindemann B. Receptors and transduction in taste. Nature. 2001;413:219–225. doi: 10.1038/35093032. [DOI] [PubMed] [Google Scholar]
- 4.Beauchamp GK, Bertino M, Burke D, Engelman K. Experimental sodium depletion and salt taste in normal human volunteers. Am J Clin Nutr. 1990;51:881–889. doi: 10.1093/ajcn/51.5.881. [DOI] [PubMed] [Google Scholar]
- 5.Mueller KL, et al. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- 6.Eylam S, Spector AC. Taste discrimination between NaCl and KCl is disrupted by amiloride in inbred mice with amiloride-insensitive chorda tympani nerves. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1361–1368. doi: 10.1152/ajpregu.00796.2004. [DOI] [PubMed] [Google Scholar]
- 7.Halpern BP. Amiloride and vertebrate gustatory responses to NaCl. Neuroscience and biobehavioral reviews. 1998;23:5–47. doi: 10.1016/s0149-7634(97)00063-8. [DOI] [PubMed] [Google Scholar]
- 8.Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 9.Hettinger TP, Frank ME. Specificity of amiloride inhibition of hamster taste responses. Brain research. 1990;513:24–34. doi: 10.1016/0006-8993(90)91085-u. [DOI] [PubMed] [Google Scholar]
- 10.Doolin RE, Gilbertson TA. Distribution and characterization of functional amiloride-sensitive sodium channels in rat tongue. J Gen Physiol. 1996;107:545–554. doi: 10.1085/jgp.107.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kretz O, Barbry P, Bock R, Lindemann B. Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J Histochem Cytochem. 1999;47:51–64. doi: 10.1177/002215549904700106. [DOI] [PubMed] [Google Scholar]
- 12.Schiffman SS, Lockhead E, Maes FW. Amiloride reduces the taste intensity of Na+ and Li+ salts and sweeteners. Proc Natl Acad Sci U S A. 1983;80:6136–6140. doi: 10.1073/pnas.80.19.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC neuroscience. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida R, et al. NaCl responsive taste cells in the mouse fungiform taste buds. Neuroscience. 2009;159:795–803. doi: 10.1016/j.neuroscience.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 15.Huang AL, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 18.Zhao GQ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 19.Canessa CM, et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 20.Hummler E, Beermann F. Scnn1 sodium channel gene family in genetically engineered mice. J Am Soc Nephrol. 2000;11(Suppl 16):S129–134. [PubMed] [Google Scholar]
- 21.Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. In: Clinical research, editor. BMJ. Vol. 339. 2009. p. b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ninomiya Y. Reinnervation of cross-regenerated gustatory nerve fibers into amiloride-sensitive and amiloride-insensitive taste receptor cells. Proc Natl Acad Sci U S A. 1998;95:5347–5350. doi: 10.1073/pnas.95.9.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oka Y, et al. Odorant receptor map in the mouse olfactory bulb: in vivo sensitivity and specificity of receptor-defined glomeruli. Neuron. 2006;52:857–869. doi: 10.1016/j.neuron.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Hummler E, Merillat AM, Rubera I, Rossier BC, Beermann F. Conditional gene targeting of the Scnn1a (alphaENaC) gene locus. Genesis. 2002;32:169–172. doi: 10.1002/gene.10041. [DOI] [PubMed] [Google Scholar]
- 25.Nelson G, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 26.Dethier VG. The taste of salt. Am Sci. 1977;65:744–751. [PubMed] [Google Scholar]
- 27.Chandrashekar J, et al. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank ME. Taste-responsive neurons of the glossopharyngeal nerve of the rat. Journal of neurophysiology. 1991;65:1452–1463. doi: 10.1152/jn.1991.65.6.1452. [DOI] [PubMed] [Google Scholar]
- 29.Hellekant G, Danilova V, Ninomiya Y. Primate sense of taste: behavioral and single chorda tympani and glossopharyngeal nerve fiber recordings in the rhesus monkey, Macaca mulatta. Journal of neurophysiology. 1997;77:978–993. doi: 10.1152/jn.1997.77.2.978. [DOI] [PubMed] [Google Scholar]
- 30.Hellekant G, Ninomiya Y. Bitter taste in single chorda tympani taste fibers from chimpanzee. Physiology & behavior. 1994;56:1185–1188. doi: 10.1016/0031-9384(94)90364-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.