Abstract
Background
Relationships among tobacco smoking, tobacco craving, and other drug use and craving may have treatment implications in polydrug-dependent individuals.
Methods
We conducted the first ecological momentary assessment (EMA) study to investigate how smoking is related to other drug use and craving during daily life. For up to 20 weeks, 106 methadone-maintained outpatients carried PalmPilots (PDAs). They reported their craving, mood, behaviors, environment, and cigarette-smoking status in 2 to 5 random-prompt entries per day and initiated PDA entries when they used cocaine or heroin or had a discrete episode of craving for cocaine or heroin.
Results
Smoking frequency increased linearly with random-prompt ratings of tobacco craving, cocaine craving, and craving for both cocaine and heroin. Smoking frequency was greater during discrete episodes of cocaine use and craving than during random-prompt reports of low craving for cocaine. This pattern was also significant for dual cocaine and heroin use and craving. Smoking and tobacco craving were each considerably reduced during periods of urine-verified abstinence from cocaine, and there was a (nonsignificant) tendency for morning smoking to be especially reduced during those periods.
Conclusions
This EMA study confirms that smoking and tobacco craving are strongly associated with the use of and craving for cocaine and heroin. Together with prior findings, our data suggest that tobacco and cocaine may each increase craving for (and likelihood of continued use of) themselves and each other. Treatment for tobacco dependence should probably be offered concurrently with (rather than only after) initiation of treatment for other substance-use disorders.
Keywords: smoking, nicotine, cocaine, heroin, ecological momentary assessment, craving, addiction
1. Introduction
High rates of cigarette smoking are common among polydrug users, especially those with cocaine and opiate dependence. Smoking prevalence is approximately 80–95% among methadone-maintained individuals (Clemmey, Brooner, Chutuape, Kidorf, & Stitzer, 1997), whereas it is 20% among adults in the general population (Centers for Disease Control and Prevention, 2008). Among cocaine-dependent individuals, cigarette smokers report using cocaine at an earlier age (Budney, Higgins, Hughes, & Bickel, 1993), more frequently (Budney et al., 1993; Roll, Higgins, Budney, Bickel, & Badger, 1996), and in greater amounts (Roll et al., 1996) than nonsmokers. Cigarette smoking among substance abusers causes substantial morbidity and mortality (Hurt et al., 1996); it might even be fairly concluded that smoking is “more deadly to substance abuse patients than their primary presenting substance of abuse” (Baca & Yahne, 2009).
Although there is a consensus that tobacco dependence should be addressed in substance-abuse treatment, there is no firm agreement on the best timing. Traditionally, methadone programs have not treated tobacco dependence (Guydish, Passalacqua, Tajima, & Manser, 2007). Some clinicians have argued that a smoking-cessation attempt might interfere with a concurrent attempt to decrease other drug use (Campbell, Wander, Stark, & Holbert, 1995; Weinberger, Reutenauer, Vessicchio, & George, 2008; Weinberger & Sofuoglu, 2009). Most studies, however, have shown that tobacco-dependence treatment offered concurrently with other substance-abuse treatment does not increase use of other drugs and may even improve outcome (Baca & Yahne, 2009; Prochaska, Delucchi, & Hall, 2004). There is also evidence that illicit drug use makes smoking cessation more difficult (Frosch, Nahom, & Shoptaw, 2002; Stapleton, Keaney, & Sutherland, 2009). Quit rates for tobacco smoking among substance abusers are low, even with treatment (Campbell et al., 1995; Kalman et al., 2001). A meta-analysis suggested that tobacco-dependence treatment during other addiction treatment is more effective in the short term than in the long term, but may nonetheless increase long-term abstinence from other substances (Prochaska et al., 2004).
Patients and clinicians might be better equipped to make decisions about the timing of smoking-cessation efforts if they had more systematic information about how smoking interacts with other drug use and craving during daily life. Although a few recent studies have assessed the effects of tobacco-dependence treatment among methadone patients (Frosch, Shoptaw, Nahom, & Jarvik, 2000; Shoptaw, Jarvik, Ling, & Rawson, 1996), no studies have closely examined how ongoing smoking relates to other drug use and craving in the daily lives of methadone patients. In smokers who do not report other substance-use problems, daily patterns of smoking and tobacco craving have been extensively characterized with ecological momentary assessment (EMA) (Shiffman, 2005; Shiffman et al., 2007; Shiffman & Paty, 2006; Shiffman, Stone, & Hufford, 2008). In this study, we used EMA to examine how cigarette smoking and craving are related to cocaine and heroin use and craving in the daily lives of methadone-maintained outpatients.
2. Methods
2.1. Participants and Setting
Participants were methadone-maintained cocaine- and heroin-using outpatients at a treatment-research clinic in Baltimore, MD. The NIDA Institutional Review Board approved the study, and participants gave written informed consent before being enrolled. We reported other results from the same study (Epstein et al., 2009); methodological details are given there and summarized here.
Inclusion criteria were: (1) age between 18 and 65, (2) evidence of physical dependence on opioids (by self-report and physical examination), and (3) evidence of cocaine and opiate use (by self-report and urine screen). Exclusion criteria were: (1) schizophrenia or any other DSM-IV psychotic disorder, history of bipolar disorder, or current major depressive disorder; (2) current dependence on alcohol or any sedative-hypnotic (by DSM-IV criteria); (3) cognitive impairment severe enough to preclude informed consent or valid self-report; and (4) medical illness that would compromise participation in the study.
We planned to enroll at least 100 participants for adequate power to examine differences among episodes of craving, episodes of use, and random prompts. A total of 130 participants enrolled, of whom 114 attended clinic long enough to be issued a PDA. Those 114 carried PDAs for 14,918 person-days (mean 130.9 days per participant, median 162.5, range 6–189). Of those 114, a total of 106 (67 men, 39 women) reported cigarette smoking and were included in the present analyses. Their mean age was 40.9 years (SD 8.2, range 20–58), and they had completed a mean of 11.8 years of education (SD 1.5, range 7–15). Self-reported race/ethnicity was 37% White, 60% Black, and 3% Hispanic. In the 30 days prior to treatment entry, they had used heroin on a mean of 29.2 days (SD 3.3, range 5–30; the participant reporting 5 days of use had transferred from a community methadone program) and cocaine on a mean of 20 days (SD 9, range 4–30). During admission, 102 participants acknowledged a history of smoking (i.e., four of the smokers included in this report had not acknowledged a history of smoking at intake, despite later reporting it via EMA); those 102 reported smoking a mean of 20.2 cigarettes per day (SD 6.8, range 6–40, median 20.0).
2.2. Procedure
The study was designed to assess the natural history of craving and lapse against a background of methadone maintenance, weekly drug counseling, and abstinence reinforcement; all participants received the same treatment. Participants attended clinic 7 days a week for up to 20 weeks; methadone was administered daily (target dose 100 mg/day); urine drug screens were conducted three times per week. Abstinence reinforcement (vouchers given in exchange for urine specimens negative for cocaine, opiates, or both) was in place from weeks 7–18 (12 weeks total; up to $2310 in vouchers were available for participants continuously abstinent from cocaine and opiates); voucher procedures were similar to those used in our prior studies (Epstein, Hawkins, Covi, Umbricht, & Preston, 2003).
A PalmPilot (PDA) was issued to each participant at the end of week 3. The PDA models used were the original Palm Zire and its successor, the Palm Zire 21. Our internally developed Transactional Electronic Diary software (Vahabzadeh, Epstein, Mezghanni, Lin, & Preston, 2004) running on the PDAs triggered 5 random prompts per day for 5 weeks, then 2 random prompts per day for 20 weeks. Random prompts were timed to occur only during each participant's typical waking hours, which were programmed for each day of the week before the PDA was issued, based on the participant's self-reported daily sleep/wake schedule. Participants were also instructed to initiate an event-contingent entry whenever they craved without using or used cocaine or heroin or both drugs; there was no requirement to make an event-contingent entry for every cigarette smoked. At each event-contingent or random-prompt entry, participants reported where they were, whom they were with, how they felt, and what they were doing. For random-prompt entries, this included the question, “When the beep occurred, were you smoking tobacco?”; for event-contingent entries, this included the question, “When the craving/use occurred, were you smoking tobacco?” Each random-prompt entry also included the items, “Right now, do you crave cocaine?,” “Right now, do you crave heroin?,” and “Right now, do you crave tobacco?” These items enabled assessment of drug craving at randomly chosen moments that presumably did not meet the participant's subjective threshold for initiating an event-contingent entry to report craving. The response anchors for the craving questions were “NO!!,” “no??,” “yes??,” and “YES!!”; participants were told that the first and last anchors represented a “strong, definite” feeling. These response anchors have been used in EMA studies by Shiffman et al. (Shiffman et al., 2002; Shiffman & Paty, 2006).
2.3. Data analysis
The responses “NO!!,” “no??,” “yes??,” and “YES!!” were recoded as 0, 1, 2, and 3. To determine relationships between tobacco smoking and tobacco craving (or cocaine or heroin craving) in random-prompt entries, we used repeated-measures logistic regressions (SAS GLIMMIX macro) with smoking as the dichotomous dependent variable. In one analysis, the within-subject independent variable was rating of craving for tobacco (0, 1, 2, or 3). In another analysis, the within-subject independent variables were ratings of craving for cocaine and heroin (0, 1, 2, or 3); the cocaine and heroin ratings were entered into the same analysis so that each would control for the other. Each of these two GLIMMIX analyses also included control terms for sex, race, age, and location. (In two additional GLIMMIX analyses, we controlled for mood rather than location, using 4-point Likert ratings on the adjectives happy, stressed, tired, relaxed, bored, and irritated. The analyses were kept separate to avoid overspecifying the models.) A first-order autoregressive error structure was used. Contrast coefficients were used to test for linear trends.
To determine relationships between tobacco smoking and discrete episodes of other drug craving or other drug use in event-contingent entries, we again used repeated-measures logistic regressions (SAS GLIMMIX macro), with event type (drug craving or drug use) as the within-subject independent variable. A separate analysis was run for each of the three types of craving episodes (cocaine, heroin, or dual) and each of the three types of use episodes (cocaine heroin, or dual). Again, each GLIMMIX included control terms for sex, race, age, and location (mood was not assessed in event-contingent entries), and a first-order autoregressive error structure was used. Additional comparisons between event-contingent and random-prompt entries are described in Results.
To compare smoking-related behaviors (tobacco craving and smoking) during periods of cocaine abstinence versus periods of cocaine use, we used thrice-weekly urine drug screens to identify such periods. Sustained cocaine abstinence was defined as 1 or more weeks of consecutive cocaine-negative specimens; sustained cocaine use was defined as 1 or more weeks of consecutive cocaine-positive specimens. We examined random-prompt entries in each period. For smoking (a dichotomous outcome measure), we again used the SAS GLIMMIX macro; for craving ratings (a continuous outcome measure), we repeated-measures linear regressions (SAS Proc Mixed). Only entries from 6:00 AM to midnight were included due to sparsity of data from postmidnight hours; data were divided into 18 time bins (6:00–7:00 AM and so on). Each of the two models had one dependent variable (tobacco craving or smoking) and three independent variables: Abstinence (a time-varying predictor that could repeatedly alternate between present and absent within each participant), Time of Day (an 18-level categorical variable), and a control variable for the number of data points that each participant contributed to the analysis. The control variable was included to reduce potential bias associated with differences in protocol compliance. A first-order autoregressive error structure provided the best fit to the data. Thirty-four participants contributed data during periods of both use and abstinence; that number is reflected in the denominator degrees of freedom (determined by the between-within method, (SAS Institute, 2008); even though all participants were included in the analyses. The “slice” option was used to generate post hoc F tests between the use and abstinence conditions during each time bin (6:00–7:00 AM, 7:00–8:00 AM, and so on); the 18 resultant p values were Bonferroni-corrected. For the analysis of smoking rates, an overall odds ratio was generated.
The criterion for significance was p ≤ 0.05. The effect size of each linear trend was also expressed as a correlation coefficient (Rosnow & Rosenthal, 1996; Rosnow, Rosenthal, & Rubin, 2000).
3. Results
Included in the data were 25,347 random-prompt entries collected from the 106 participants who smoked; participants reported smoking in 8,173 (32%) of those entries. There were 2,413 event-contingent entries (entries at which participants reported episodes of cocaine or heroin use or craving); participants reported tobacco smoking in 1,260 (52%) of those entries. However, there were far fewer reports of heroin use (n = 60) than of cocaine use (n = 665), cocaine craving (n = 597), heroin craving (n = 257), dual use of heroin and cocaine (n = 229), or dual craving for heroin and cocaine (n = 605). Of the control variables (sex, race, age, location, and mood), only location and mood had significant effects on smoking frequency: smoking was most likely to be reported in a bar/club or at another person's house, and was least likely to be reported in clinic, a store, or a restaurant. Smoking also increased with ratings of being happy, stressed, bored, or irritated and decreased with ratings of being tired (data not shown).
3.1. Smoking and random-prompt ratings of craving
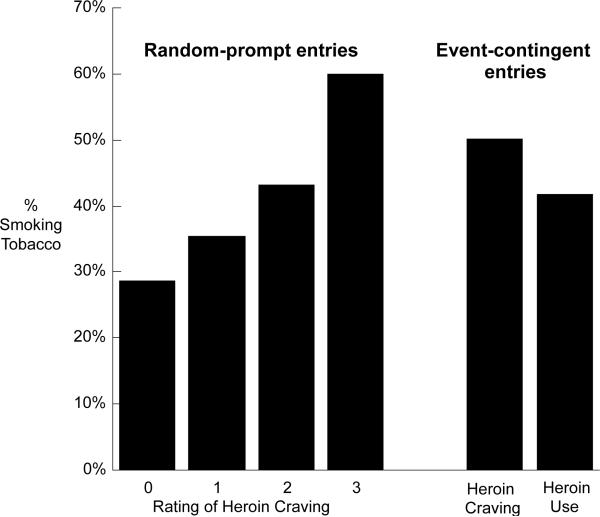
Smoking frequency increased linearly with random-prompt ratings of tobacco craving, F(1,272) = 117.34, p < 0.0001; effect-size r = .55 (Fig. 1). This result is from an analysis controlling for location; results were almost identical in the analysis controlling for mood.
Fig. 1.
Percentage of random prompts at which participants reported smoking tobacco, by degree of reported tobacco craving. The denominators for the four columns are: 8,812 (craving rated 0); 7,245 (craving rated 1); 5,657 (craving rated 2); and 3,110 (craving rated 3). The bars show raw percentages, whereas the statistical results are adjusted for sex, race, age, and location. The pattern of covariate-adjusted percentages (not shown) was similar to the pattern of raw percentages.
Smoking frequency also increased linearly with random-prompt ratings of cocaine craving, F(1,245) = 61.42, p < 0.0001; effect-size r = .45 (Fig. 2, four left columns). Random-prompt ratings of heroin craving were included as a predictor in the same analysis; as heroin craving increased, the linear increase in smoking frequency only approached significance, F(1,216) = 3.10, p = .079, effect-size r = .12 (Fig. 3, four left columns). Finally, smoking frequency increased linearly with random-prompt ratings of dual craving F(1,176) = 33.59, p < 0.0001; effect-size r = .40 (Fig. 3, four left columns). Again, these results are from analyses controlling for location; results were almost identical in analyses controlling for mood.
Fig. 2.
Four left columns: Percentage of random prompts at which participants reported smoking tobacco, by degree of reported cocaine craving. Two right columns: Percentage of event-contingent prompts at which participants reported smoking tobacco. The denominators for the six bars were: 12,569 (cocaine craving rated 0); 8,125 (cocaine craving rated 1); 2,373 (cocaine craving rated 2); 1,792 (cocaine craving rated 3); 597 (event-contingent cocaine-craving entry); 665 (event-contingent cocaine-use entry). The bars show raw percentages, whereas the statistical results are adjusted for sex, race, age, and location. The pattern of covariate-adjusted percentages (not shown) was similar to the pattern of raw percentages.
Fig. 3.
Four left columns: Percentage of random prompts at which participants reported smoking tobacco, by degree of reported heroin craving. Two right columns: Percentage of event-contingent prompts at which participants reported smoking tobacco. The denominators for the six bars were: 15,098 (heroin craving rated 0); 7,647 (heroin craving rated 1); 966 (heroin craving rated 2); 1,103 (heroin craving rated 3); 257 (event-contingent heroin-craving entry); 60 (event-contingent heroin-use entry). The bars show raw percentages, whereas the statistical results are adjusted for sex, race, age, and location. The pattern of covariate-adjusted percentages (not shown) was similar to the pattern of raw percentages.
3.2. Smoking and event-contingent reports of craving and use
Smoking frequency was not significantly different during episodes of cocaine use (53%) compared with episodes of cocaine craving (50%), F(1,55) = 1.04, p = .31 (Fig. 2, two right columns). However, smoking frequency during episodes of cocaine use and craving was significantly greater than during random-prompt reports of low cocaine craving (ratings of 0 or 1 collapsed into a single category; smoking frequency 29%), Tukey-Kramer p < 0.001 for each of two comparisons: “cocaine-craving episode vs. low-craving random prompt” and “cocaine-use episode vs. low-craving random prompt” (Fig. 2).
Similar analyses for heroin use and craving were less well powered due to the few reports of heroin use. Raw percentage data suggested that smoking frequency might be greater during episodes of heroin craving (50.3%) than during episodes of heroin use (41.7%) or random-prompt reports of low heroin craving (31.0%), but none of these differences approached statistical significance (Fig. 3).
Smoking frequency was not significantly different during episodes of dual use (57.2%) compared with episodes of dual craving (52.7%), F(1,41) = 0.25, p = .62. To compare these with random-prompt ratings of dual craving, we used only random-prompt reports in which craving ratings for cocaine and heroin were identical (19,253 of the 27,760 random-prompt reports). Smoking frequency during episodes of dual use or dual craving was significantly greater than during random-prompt reports of low dual craving (ratings of 0 or 1; smoking frequency 29.1%), Tukey-Kramer p < 0.001 for “dual-craving episode vs. low-dual-craving random prompt,” Tukey-Kramer p < .005 for “dual-use episode vs. low-dual-craving random prompt” (Fig. 4).
Fig. 4.
Four left columns: Percentage of random prompts at which participants reported smoking tobacco, by degree of reported dual craving for heroin and cocaine. For dual-craving data, we used only random-prompt reports in which craving ratings for cocaine and heroin were identical (69.4% of the 27,760 random-prompt reports). Two right columns: Percentage of event-contingent prompts at which participants reported smoking tobacco. The denominators for the six bars were: 11,782 (dual craving rated 0); 6,270 (dual craving rated 1); 505 (dual craving rated 2); 696 (dual craving rated 3); 605 (event-contingent dual-craving entry); 229 (event-contingent dual-use entry). The bars show raw percentages, whereas the statistical results are adjusted for sex, race, age, and location. The pattern of covariate-adjusted percentages (not shown) was similar to the pattern of raw percentages.
3.3. Tobacco craving and smoking across the day during urine-verified periods of cocaine use or cocaine abstinence
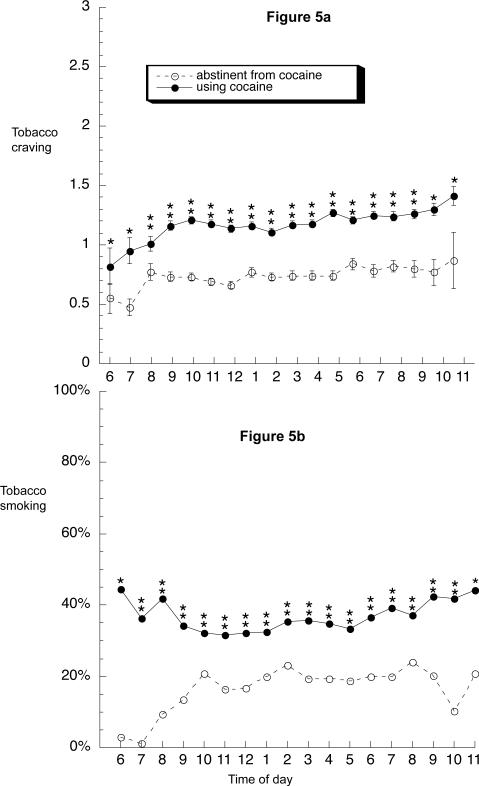
Figure 5a shows that ratings of tobacco craving across the day were significantly higher during periods of cocaine use (least-squares mean = 1.15 ± .02) than during periods of cocaine abstinence (least-squares mean = 0.82 ± .03) [main effect of abstinence: F(1,33) = 112.6, p < .0001]. In both conditions, craving ratings tended to rise slightly in the early hours of the day and then remain relatively constant across the day.
Fig. 5.
Time course of tobacco craving and smoking during periods of 1 or more weeks of cocaine abstinence or cocaine use across the day from 6 AM to 12 PM. (a) Data shown are mean ratings on a four-point (0 to 3) scale. Brackets indicate SEM. (b) Data shown are percentages. Abstinence/use was a time-varying predictor; thus, 34 participants contributed data to both the “abstinence” line and the “use” line. For cocaine abstinence, the median number of datapoints per symbol is 496 (range 24 to 789; all values over 100 from 8:00 am through 9:00 pm); for cocaine use, the median number of datapoints per symbol is 1,058 (range 63 to 1,579; all values over 100 from 7:00 am onward). *Significant difference between use and abstinence at this time point in Bonferroni-corrected post hoc F tests (“slice” option in SAS).
Figure 5b shows that actual rates of smoking across the day were significantly higher during periods of cocaine use than during periods of cocaine abstinence (OR = 2.50, 95% CL 2.13 to 2.86). (Although this GLIMMIX analysis accounted for each participant's pattern of contributions to the overall findings, we performed an additional GLIMMIX using only the subset of 34 participants who contributed data during periods of both cocaine use and cocaine abstinence. In this subset, the cocaine-associated increase in smoking frequency was even more pronounced: OR = 3.02, 95% CL 2.56 to 3.56. Thus, the effect occurred within individuals, not just between individuals.) During periods of cocaine abstinence, smoking seemed especially uncommon in the morning (Figure 5b), although this was not reflected in a significant interaction between group and time, probably because of the relative scarcity of data points from early-morning hours.
4. Discussion
4.1. Summary of Findings
This is the first study to investigate how cigarette smoking relates to other drug use and craving in real time as polydrug-dependent participants engage in their daily activities. As in the studies cited in the Introduction, smoking was very common in our sample. Of the 114 participants who enrolled and provided EMA data, 106 (93%) were smokers. Smoking was reported in approximately one third of all random prompts and on more than 50% of occasions in which cocaine use was reported.
Our study confirms the strong association between smoking and cocaine/heroin use and indicates that smoking frequency and ratings of tobacco craving are lower during periods of cocaine abstinence versus use, even in the absence of treatment for tobacco dependence. Random-prompt data indicated that smoking increased linearly with ratings of tobacco craving, cocaine craving, and dual cocaine/heroin craving. Smoking frequency was greater during discrete episodes of cocaine use and craving than during random-prompt reports of low craving for cocaine. Smoking frequency was also greater during discrete episodes of dual use and craving for cocaine and heroin than during random-prompt reports of low dual craving for cocaine and heroin. Frequency of smoking and craving for tobacco were both significantly lower during periods of extended cocaine abstinence, a striking demonstration of the link between smoking and cocaine use. Our EMA data cannot demonstrate causal relationships between smoking and other drug use and craving, but the fact that the relationships in the data were not affected by participants' locations or mood indicates that our findings were not merely artifacts of these variables.
4.2. Associations between Smoking and Tobacco Craving
The positive relationship between smoking and tobacco craving may strike some readers as counterintuitive: one might expect craving to be satisfied and thus decreased by smoking, whereas we found the highest ratings of “right now” tobacco craving on the random-prompt occasions when participants reported smoking a few seconds earlier (“when the beep occurred”). Shiffman and Paty (2006) found a similar relationship in an EMA study using an extreme-groups case-control design; they compared smoking patterns in a group of longtime tobacco chippers and a matched group of heavy smokers (there was no misuse of other drugs in either group). Both groups reported similarly high tobacco craving during event-contingent reports of smoking compared to random-prompt reports between cigarettes. To some degree, nicotine might share the ability of cocaine to increase appetitive craving for itself, a property we have seen with cocaine both acutely (Preston, Sullivan, Strain, & Bigelow, 1996) and chronically (Preston et al., 2009).
4.3. Associations between Use of and Craving for Tobacco and Other Drugs: Possible Mechanisms
Our data are consistent with a model in which tobacco and cocaine each increase craving for (and likelihood of continued use of) themselves and each other. One possible mechanism is a pharmacological interaction. For example, in rats, pretreatment with nicotine increases cocaine self-administration (Horger, Giles, & Schenk, 1992), and pretreatment with the nicotinic antagonist mecamylamine decreases cocaine self-administration (Levin et al., 2000). In recreational cocaine users, pretreatment with transdermal nicotine increases latency to report cocaine-induced euphoria and attenuates cocaine-induced positive subjective effects (Kouri, Stull, & Lukas, 2001). In heavy cocaine users, however, longer-term pretreatment with transdermal nicotine does not appear to alter cocaine-induced effects (Sobel, Sigmon, & Griffiths, 2004).
Pharmacological interactions could also explain the modest association we saw between smoking and opiate use. In opiate-dependent smokers, cigarette smoking is increased by pretreatment with heroin (Mello, Mendelson, Sellers, & Kuehnle, 1980) or methadone (Chait & Griffiths, 1984), and methadone self-administration is increased by pretreatment with nicotine (Spiga, Schmitz, & Day, 1998).
Another possible mechanism, which could coexist with pharmacological interactions, is an effect of each drug on reactivity to cues associated with the other drugs. In smokers with a history of heavy crack cocaine use, cue-induced cocaine craving is increased by pretreatment with nicotine (Reid, Mickalian, Delucchi, Hall, & Berger, 1998) and attenuated by pretreatment with mecamylamine (Reid, Mickalian, Delucchi, & Berger, 1999). This finding, however, did not translate into a clinically significant effect for mecamylamine in a clinical trial (Reid et al. 2005). In rats, pretreatment with naltrexone attenuates nicotine-cue-maintained responding during extinction and cue-induced reinstatement of nicotine seeking after extinction (Liu et al., 2009) despite not directly altering rates of nicotine self-administration (Corrigall & Coen, 1991; Liu et al., 2009). Among tobacco-dependent humans, cue-induced tobacco craving is attenuated by pretreatment with naltrexone (Hutchison et al., 1999; King & Meyer, 2000). These data suggest that with certain subject populations and dosing procedures, responses to nicotine-associated cues may be affected by cocaine or heroin, and vice versa.
4.4. Study Limitations
One limitation of our study is that we did not require participants to make event-contingent entries for tobacco use or craving; therefore, we do not have a record of every episode of smoking or tobacco craving. Results from thrice-weekly urine tests (data not shown) also indicated that not every episode of cocaine or heroin use was reported. Underreporting of heroin use, possibly because of heroin-related sedation, may explain why cocaine-related findings were more robust than heroin-related findings. Nevertheless, the occurrence of unreported episodes of smoking and other drug use and craving does not change the implications of the results.
4.5. Implications For Treatment
Our participants smoked heavily during periods of cocaine use; they reported smoking tobacco on roughly 40% of random-prompt occasions during such periods (Figure 5b). During periods of cocaine abstinence, however, they may have been functionally similar to tobacco chippers. For example, when Shiffman and Paty (2006) compared EMA data in heavy smokers and chippers, they found that the chippers' smoking frequency was low in the morning and increased throughout the day. This is strikingly similar to the smoking pattern of our participants during periods of cocaine abstinence (Figure 5b). Shiffman and Paty (2006) also found that only heavy smokers, not chippers, reported tobacco craving that persisted between smoking occasions. In our participants, tobacco craving and smoking significantly decreased during periods of cocaine abstinence, suggesting that our participants' smoking and tobacco craving were more situationally driven than might be assumed. Such situational influences could be considered a hallmark of chipping (Shiffman & Paty, 2006). Based on other differences in smoking patterns between chippers and heavy smokers, Shiffman and Paty concluded that chippers smoke primarily to augment the pleasure experienced on “indulgent” leisure occasions such as eating, drinking alcohol, socializing, or relaxing, whereas heavy smokers need to smoke so frequently that no such association is detectable. We speculate that our participants—and, by extension, many cocaine-using methadone-maintained patients—smoke heavily during periods of cocaine use for similarly “indulgent” reasons, but with a frequency that far outpaces that of the classically defined chipper.
Our data suggest that periods of cocaine abstinence are temporal windows of opportunity for treatment of tobacco dependence. As Baca and Yahne (2009) recommend, tobacco treatment could be offered concurrently with other substance-abuse treatment for patients who are willing to address both problems at once (and offered again later for those who prefer to wait). We would add a recommendation that patients should be told that concurrent quitting might be easier than sequential quitting.
Acknowledgements
This research was supported the Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat. 2009;36(2):205–219. doi: 10.1016/j.jsat.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. Journal of Substance Abuse. 1993;5(2):117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Wander N, Stark MJ, Holbert T. Treating cigarette smoking in drug-abusing clients. J Subst Abuse Treat. 1995;12(2):89–94. doi: 10.1016/0740-5472(95)00002-m. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Cigarette smoking among adults—United States, 2007. Morbidity and Mortality Weekly Report. 2008;57(45):1221–1226. [PubMed] [Google Scholar]
- Chait LD, Griffiths RR. Effects of methadone on human cigarette smoking and subjective ratings. J Pharmacol Exp Ther. 1984;229(3):636–640. [PubMed] [Google Scholar]
- Clemmey P, Brooner R, Chutuape MA, Kidorf M, Stitzer M. Smoking habits and attitudes in a methadone maintenance treatment population. Drug Alcohol Depend. 1997;44(2–3):123–132. doi: 10.1016/s0376-8716(96)01331-2. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Opiate antagonists reduce cocaine but not nicotine self-administration. Psychopharmacology. 1991;104(2):167–170. doi: 10.1007/BF02244173. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Hawkins WE, Covi L, Umbricht A, Preston KL. Cognitive-behavioral therapy plus contingency management for cocaine use: Findings during treatment and across 12-month follow-up. Psychology of Addictive Behaviors. 2003;17(1):73–82. doi: 10.1037/0893-164X.17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Archives of General Psychiatry. 2009;66(1):88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch DL, Nahom D, Shoptaw S. Optimizing smoking cessation outcomes among the methadone maintained. J Subst Abuse Treat. 2002;23(4):425–430. doi: 10.1016/s0740-5472(02)00280-5. [DOI] [PubMed] [Google Scholar]
- Frosch DL, Shoptaw S, Nahom D, Jarvik ME. Associations between tobacco smoking and illicit drug use among methadone-maintained opiate-dependent individuals. Experimental and Clinical Psychopharmacology. 2000;8(1):97–103. doi: 10.1037//1064-1297.8.1.97. [DOI] [PubMed] [Google Scholar]
- Guydish J, Passalacqua E, Tajima B, Manser ST. Staff smoking and other barriers to nicotine dependence intervention in addiction treatment settings: a review. J Psychoactive Drugs. 2007;39(4):423–433. doi: 10.1080/02791072.2007.10399881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology. 1992;107(2–3):271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, et al. Mortality following inpatient addictions treatment: role of tobacco use in a community-based cohort. JAMA. 1996;275(14):1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Monti PM, Rohsenow DJ, Swift RM, Colby SM, Gnys M, et al. Effects of naltrexone with nicotine replacement on smoking cue reactivity: preliminary results. Psychopharmacology. 1999;142(2):139–143. doi: 10.1007/s002130050872. [DOI] [PubMed] [Google Scholar]
- Kalman D, Hayes K, Colby SM, Eaton CA, Rohsenow DJ, Monti PM. Concurrent versus delayed smoking cessation treatment for persons in early alcohol recovery: a pilot study. J Subst Abuse Treat. 2001;20(3):233–238. doi: 10.1016/s0740-5472(00)00174-4. [DOI] [PubMed] [Google Scholar]
- King AC, Meyer PJ. Naltrexone alteration of acute smoking response in nicotine-dependent subjects. Pharmacol Biochem Behav. 2000;66(3):563–572. doi: 10.1016/s0091-3057(00)00258-6. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Stull M, Lukas SE. Nicotine alters some of cocaine's subjective effects in the absence of physiological or pharmacokinetic changes. Pharmacology, Biochemistry & Behavior. 2001;69(1–2):209–217. doi: 10.1016/s0091-3057(01)00529-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Mead T, Rezvani AH, Rose JE, Gallivan C, Gross R. The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiology & Behavior. 2000;71(5):565–570. doi: 10.1016/s0031-9384(00)00382-6. [DOI] [PubMed] [Google Scholar]
- Liu X, Palmatier MI, Caggiula AR, Sved AF, Donny EC, Gharib M, et al. Naltrexone attenuation of conditioned but not primary reinforcement of nicotine in rats. Psychopharmacology. 2009;202(4):589–598. doi: 10.1007/s00213-008-1335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Sellers ML, Kuehnle JC. Effects of heroin self-administration on cigarette smoking. Psychopharmacology. 1980;67(1):45–52. doi: 10.1007/BF00427594. [DOI] [PubMed] [Google Scholar]
- Preston KL, Sullivan JT, Strain EC, Bigelow GE. Enhancement of cocaine's abuse liability in methadone maintenance patients. Psychopharmacology. 1996;123(1):15–25. doi: 10.1007/BF02246276. [DOI] [PubMed] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DH. Cocaine craving and use during daily life. Psychopharmacology. 2009 doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol. 2004;72(6):1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Reid MS, Angrist B, Baker SA, O'Leary S, Stone J, Schwartz M, et al. A placebo controlled, double-blind study of mecamylamine treatment for cocaine dependence in patients enrolled in an opiate replacement program. Substance Abuse. 2005;26(2):5–14. doi: 10.1300/j465v26n02_02. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20(3):297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend. 1998;49(2):95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Budney AJ, Bickel WK, Badger GJ. A comparison of cocaine-dependent cigarette smokers and non-smokers on demographic, drug use and other characteristics. Drug and Alcohol Dependence. 1996;40(3):195–201. doi: 10.1016/0376-8716(96)01219-7. [DOI] [PubMed] [Google Scholar]
- Rosnow RL, Rosenthal R. Computing contrasts, effect sizes, and counternulls on other people's published data: General procedures for research consumers. Psychological Methods. 1996;1(4):331–340. [Google Scholar]
- Rosnow RL, Rosenthal R, Rubin DB. Contrasts and correlations in effect-size estimation. Psychological Science. 2000;11(6):446–453. doi: 10.1111/1467-9280.00287. [DOI] [PubMed] [Google Scholar]
- SAS Institute . SAS/STAT 9.2 User's Guide. SAS Institute, Inc.; Cary, NC: 2008. [Google Scholar]
- Shiffman S. Dynamic influences on smoking relapse process. Journal of Personality. 2005;73(6):1715–1748. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, et al. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug and Alcohol Dependence. 2007;91(2–3):159–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, et al. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. Journal of Abnormal Psychology. 2002;111(4):531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty J. Smoking patterns and dependence: contrasting chippers and heavy smokers. Journal of Abnormal Psychology. 2006;115(3):509–523. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Jarvik ME, Ling W, Rawson RA. Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addictive Behaviors. 1996;21(3):409–412. doi: 10.1016/0306-4603(95)00066-6. [DOI] [PubMed] [Google Scholar]
- Sobel BF, Sigmon SC, Griffiths RR. Transdermal nicotine maintenance attenuates the subjective and reinforcing effects of intravenous nicotine, but not cocaine or caffeine, in cigarette-smoking stimulant abusers. Neuropsychopharmacology. 2004;29(5):991–1003. doi: 10.1038/sj.npp.1300415. [DOI] [PubMed] [Google Scholar]
- Spiga R, Schmitz J, Day J., 2nd. Effects of nicotine on methadone self-administration in humans. Drug Alcohol Depend. 1998;50(2):157–165. doi: 10.1016/s0376-8716(98)00020-9. [DOI] [PubMed] [Google Scholar]
- Stapleton JA, Keaney F, Sutherland G. Illicit drug use as a predictor of smoking cessation treatment outcome. Nicotine Tob Res. 2009;11(6):685–689. doi: 10.1093/ntr/ntp050. [DOI] [PubMed] [Google Scholar]
- Vahabzadeh M, Epstein DH, Mezghanni M, Lin J-H, Preston KP. An Electronic Diary Software for Ecological Momentary Assessment (EMA) in Clinical Trials. Proceedings of the 17th IEEE Symposium on Computer-Based Medical Systems (CBMS).2004. pp. 167–172. [Google Scholar]
- Weinberger AH, Reutenauer EL, Vessicchio JC, George TP. Survey of clinician attitudes toward smoking cessation for psychiatric and substance abusing clients. J Addict Dis. 2008;27(1):55–63. doi: 10.1300/J069v27n01_06. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse. 2009;35(1):12–17. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]