Abstract
Purpose
To study the histopathologic features of CT screen-detected Stage IA adenocarcinomas to determine whether survival differed by the proportion of bronchioloalveolar component (BAC) or by the presence of multiple lesions in node-negative patients.
Methods
Five pathologists with expertise in pulmonary pathology examined 279 resected cases of adenocarcinomas, 30 mm or less in length diagnosed by CT screening for lung cancer. The panel determined the consensus diagnosis for each case, identified additional cancers, and classified each case as solitary or non-solitary. The presence and proportion of BAC was also documented.
Results
Of the cases of adenocarcinoma, 20 (7%) were BAC subtype, 246 (88%) mixed subtype and 13 (5%) adenocarcinoma-OTHER. BAC cases manifested as non-solid and part solid nodules, mixed as solid and part-solid, and other as solid only. Kaplan–Meier 10-year survival rates were 100% for BAC and adeno-MIXED with 90–99% BAC cases, 95% for mixed with 1–90% BAC, 90% for those without a BAC component, and 75% for other cases. Fifty (18%) cases were non-solitary carcinomas and 44 of these were node negative; the non-solitary node-negative cases had the same excellent prognosis as solitary node-negative cases.
Conclusions
The proportion of BAC component was a positive prognostic factor and correlated with CT consistency. Contrary to staging predictions, cases of non-solitary node-negative adenocarcinoma had the same excellent prognosis as solitary node-negative cases, suggesting that most of the small, node-negative multiple carcinomas probably represent multiple primaries rather than intrapulmonary metastasis.
Keywords: Adenocarcinoma, Bronchioloalveolar carcinoma, Lung cancer, Satellite nodules, Screening, Stage, WHO classification
1. Introduction
Prior to 1970, the most frequent type of lung cancer in the United States was squamous cell carcinoma, and extensive study of those cases, especially the very early cases, led to identification of its precursor lesions, progression and the pathologic features of significance in treatment planning and prognosis [5–7,9,10,23,41]. Since then, the relative frequency of adenocarcinoma has increased 8-fold [3,11,17,18,39] to make it the predominant type of lung cancer and therefore it is important to determine its precursors, progression and prognostically relevant pathologic features. As with squamous cell carcinoma, study of the early cases is valuable for determining critical features of adenocarcinoma. In 1995, Noguchi et al. [27] described a series of 236 patients with small, resected Stage I adenocarcinomas and identified the pathologic features of prognostic significance. Since then, others have used the Noguchi histologic classification and found similar results [1,16].
In 1997, the current Staging TNM criteria for lung cancer were changed by the American Joint Commission on Cancer (AJCC) to stage cases with satellite nodules as T4—Stage IIIB or M1—Stage IV depending on their location in the same or a different lobe from the index carcinoma [24]. Criteria for distinguishing intrapulmonary metastasis from a second primary of the same cell type were not given, and, in fact, remain elusive to this day. Detection of a second adenocarcinoma, preoperatively or intra-operatively, may therefore forestall surgical resection and hence loss of an opportunity for cure. In 1999 and again in 2004, the World Health Organization histologic classification of lung cancer [35] incorporated the data from Noguchi et al. [27] and defined bronchioloalveolar carcinoma (BAC) as a carcinoma with entirely lepidic spread without stromal invasion. Most adenocarcinomas, however, were classified in an Adenocarcinoma-MIXED category in which 2 or more patterns (BAC, invasive acinar, solid or papillary) were present. We previously described the difficulties pathologists had with this new formulation and how histologic distinctions were made in a group of baseline CT-detected adenocarcinomas reviewed by a pathology panel [8]. In this study, we describe the prognostic implications of currently acknowledged histologic features of 279 small adenocarcinomas, detected in baseline or annual rescreen, in the International Early Lung Cancer Action Program (I-ELCAP) [12–15].
2. Methods
There were 338 patients diagnosed with adenocarcinoma resulting from CT screening between 1993 and 2007; 299 as a result of baseline screening, 39 as a result of annual repeat screening [9,10,12]. Informed consent at each participating institution was obtained from all those enrolled in the screening program. Once diagnosed with lung cancer, patient was classified as a baseline diagnosis when the nodule was first identified on the baseline CT regardless of when the diagnosis was achieved. When the nodule was first identified on an annual repeat CT, it was a annual repeat case. There were no interim-diagnosed cases prompted by symptoms among these 338 patients.
Of these 338 patients diagnosed with adenocarcinoma, 312 underwent resection and 299 were less than 30 mm in length. The surgical specimens were examined by the pathologist at the hospital where the resection was performed according to the I-ELCAP pathology protocol [36–38] which specified specimen preparation and findings to be documented: presence of lymph node metastases, additional cancers, invasion of the basement membrane, and invasion of pleural, angiolymphatic or bronchial structural. Pathology slides of 279 (93%) of the 299 patients were received at the I-ELCAP Coordinating Center and they are the focus of this paper.
A 5-member pathology review panel (including MN) determined the consensus diagnosis according to the I-ELCAP protocol using the 2004 World Health Organization criteria [35]. The panel also identified additional cancers in the specimen; each patient was classified as having a solitary or non-solitary cancer, the dominant cancer being the largest one. Based on the consensus diagnosis (dominant one if non-solitary), each patient was classified into one of the three categories: adenocarcinoma, bronchioloalveolar subtype (adeno-BAC); adenocarcinoma, mixed subtype (adeno-MIXED); and adenocarcinoma, other (adeno-OTHER). The latter included all other subtypes of adenocarcinoma (colloid, clear cell, fetal, or signet ring, etc.).
One panel member (D.C.) reviewed each case to determine whether there was invasion of stroma, pleura, lymphatics, blood vessels, and bronchi. Pleural invasion was assessed according to the Shimuzi et al. criteria [34]. For the cases of adeno-MIXED, the proportion of the tumor that was composed of the BAC component was estimated and each case of adeno-MIXED was classified into one of the four categories based on the proportion of BAC: 0%, 1–50%, 50–90%, and 90–99% (Fig. 1). By definition, adeno-BAC had 100% BAC component and adeno-OTHER had 0% BAC component (Fig. 2). Proportions of acinar, solid and papillary components were also recorded. Criteria for distinction between BAC and invasive carcinoma were those of Noguchi et al. [27] and the WHO [3]; briefly, a desmoplastic reaction in the stroma was evidence of invasion. Bronchial invasion was invasion of the wall of a cartilage-bearing bronchus.
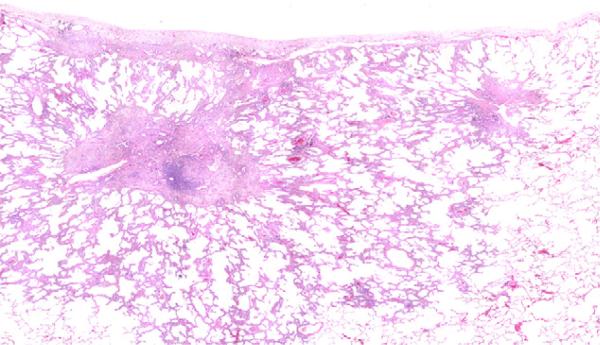
Fig. 1.
Adeno-MIXED. Over 90% of the carcinoma is BAC, but the central nodule of invasive acinar carcinoma comprises 7% of the surface area of the tumor.
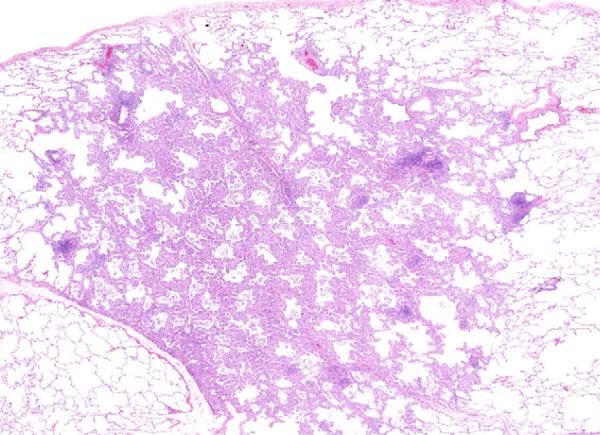
Fig. 2.
Adeno-BAC (100% BAC). The figure shows the BAC which has several lymphoid nodules, but no invasion.
2.1. CT findings
Two chest radiologists (YH, CH) reviewed the CT of each patient closest to the resection and documented the consistency (solid, part-solid, non-solid) of the nodule (dominant one if non-solitary) by consensus. Consistency was classified as solid if the nodule completely obscured the lung parenchyma within the nodule, part-solid if it obscured part of the lung parenchyma within it, and non-solid if there was no solid component [14].
2.2. Data analysis
All frequency tabulations and Kaplan–Meier analyses were carried out using the Statistical Analysis System, SAS Institute, Cary, NC. The Chi-square test was used to test for significant differences in the proportions. The 95% confidence interval (CI) for the survival rate was based on the log-rank test.
3. Results
Among the 279 patients with a diagnosis of adenocarcinoma who underwent resection and in whom the length in the pathology specimen was less than 30 mm, adeno-BAC occurred in 20 cases (7%), adeno-MIXED in 246 (88%), and adeno-OTHER in 13 (5%) (Table 1). Median age in years at time of diagnosis was 66, 66 and 64 years, respectively. The ratio of men to women was 9:11, 104:142, and 8:5, respectively. Thus, women had slightly higher proportion of adeno-BAC (55%) and adeno-MIXED (58%) than men, but a lower proportion (38%) of adeno-OTHER. The median tumor size was 10 mm for adeno-BAC, 13 mm for adeno-MIXED, and 15 mm for adeno-OTHER.
Table 1.
279 patients with adenocarcinoma by subtype, tumor diameter, proportion without pleural, angiolymphatic or bronchial invasion, Stage I (proportion without lymph node invasion) and CT consistency.
| Subtypes | Total | Tumor diameter (mm) |
Without invasion, N (%) | Stage I, N (%) | CT consistency |
||||
|---|---|---|---|---|---|---|---|---|---|
| <10 | 11–20 | 21–30 | NS | PS | Solid | ||||
| Adeno-BAC | 20 | 11 | 8 | 1 | 20 (100) | 20 (100) | 11 | 9 | 0 |
| Adeno-MIXED with BAC | 207 | 58 | 121 | 28 | 148 (72) | 194 (94) | 41 | 69 | 97 |
| Adeno-MIXED w/o BAC | 39 | 13 | 21 | 5 | 24 (62) | 33 (85) | 2 | 2 | 35 |
| Adeno-OTHER | 13 | 4 | 5 | 4 | 10 (77) | 10 (77) | 0 | 0 | 13 |
| Total | 279 | 86 | 155 | 38 | 202 (72) | 257 (92) | 54 | 80 | 145 |
| Percentage | 100 | 31 | 55 | 14 | 72 | 92 | 19 | 29 | 52 |
Of the 279 resected cases, 244 (87%) resulted from baseline and 35 (13%) from annual repeat screening. Although adeno-BAC was about equally frequent at baseline than at annual screening (7% vs. 6%), adeno-MIXED was slightly more frequent at baseline (89% vs. 86%), and adeno-OTHER was less frequent at baseline (4% vs. 9%).
Frequency of manifestation as a solid nodule increased from 49% on baseline to 71% on annual repeat screening. Adeno-BAC never manifested as a solid nodule, while adeno-MIXED with a BAC component manifested as a solid nodule in 97 (47%) of the 207 cases, adeno-MIXED without a BAC component in 35 (90%) of the 39 cases, and adeno-OTHER always manifested as a solid nodule (Table 1).
The overall proportion of pathologic Stage IA disease was 92% (257/279). The proportion of Stage IA decreased from 100% among the adeno-BAC cases, to 94% among the adeno-MIXED with any BAC-component, to 85% among the adeno-MIXED without any BAC component, and to 77% among the adeno-OTHER (Table 1). Of the adeno-BAC and adeno-MIXED, the proportion of cases without any pleural, angiolymphatic, or bronchial invasion decreased with decreasing proportion of BAC (100% vs. 72% vs. 62%).
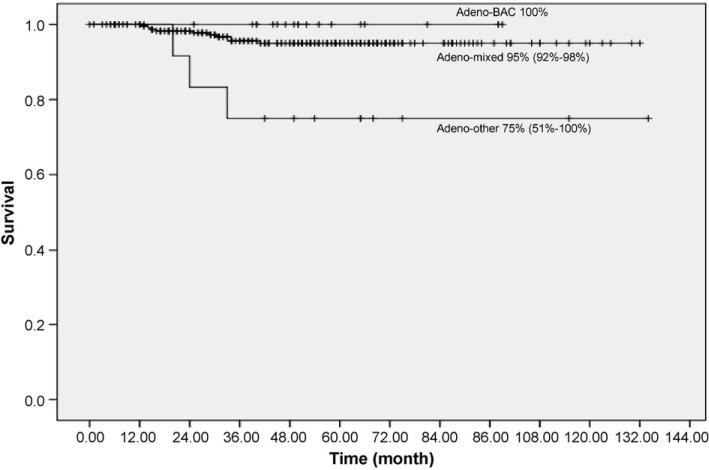
The 10-year lung-cancer specific Kaplan–Meier survival rate for 20 patients with adeno-BAC was 100% (Fig. 3). For the 246 patients with adeno-MIXED, it was 95% (95% CI: 92–98%) and for 13 patients with adeno-OTHER it was 75% (95% CI: 51–100%).
Fig. 3.
Kaplan–Meier survival rates in patients with resected adenocarcinoma (n = 279) by bronchioloalveolar, mixed, or other subtype, regardless of stage and treatment.
Of the 246 adeno-MIXED, 117 had no papillary component, 102 had less than 50% and 27 had 50% or more. The 39 cases of adeno-MIXED without any BAC component all had an acinar component with the added component being papillary (n = 29) and/or solid (n = 20). One adeno-OTHER had more than 50% papillary component; the other 12 had none. The 10-year Kaplan–Meier survival rate for those 102 patients with less than 50% papillary component as compared to the 27 patients with 50% or more was not significantly different (P = 0.87).
3.1. Adenocarcinoma classified by the bronchioloalveolar component
Adeno-BAC (20 patients) is totally (100%) comprised of BAC component by definition while for adeno-MIXED (246 patients) the BAC component can vary between 0% and 99%. Among these combined 266 patients, those having less than 50% BAC were most frequent (61% = 161/266), followed by those with 50–90% BAC (22% = 59/266), those with 90–99% BAC (10% = 26/266), and finally those with 100% BAC (8% = 20/266) (Table 2). As the percentage of BAC decreased, the proportion of cases with pleural, angiolymphatic, and bronchial invasion increased, the nodule consistency changed from non-solid to solid (Table 2). Among the 19 patients whose stage was greater than IA, none had 100% or 90–99% BAC, 2 had 50–89%, 11 had 1-50% and 6 cases had no BAC component.
Table 2.
266 patients with adenocarcinoma, bronchioloalveolar or mixed subtype by the proportion of the bronchioloalveolar (BAC) component, proportion without pleural, angiolymphatic or bronchial invasion, Stage I (proportion without lymph node invasion), proportion of non-solitary cases, and CT consistency.
| Proportion of BAC | Total N | Without invasion, N (%) | Stage I, N (%) | Non-solitary, N (%) | CT consistency, N (%) |
||
|---|---|---|---|---|---|---|---|
| Non-solid | Part-solid | Solid | |||||
| 100% | 20 | 20 (100) | 20 (100) | 3 (15) | 11 (55) | 9 (45) | 0 (0) |
| 90–99% | 26 | 25 (96) | 26 (100) | 3 (12) | 3 (12) | 16 (61) | 7 (27) |
| 50–90% | 59 | 45 (76) | 57 (97) | 13 (22) | 21 (36) | 23 (39) | 15 (25) |
| 1–50% | 122 | 78 (64) | 111 (91) | 24 (20) | 17 (14) | 30 (25) | 75 (61) |
| 0% | 39 | 24 (62) | 33 (85) | 6 (15) | 2 (5) | 2 (5) | 35 (90) |
| Total | 266 | 192 (72) | 247 (93) | 49 (18) | 54 (20) | 80 (30) | 132 (50) |
| Non-solitary | 49 | - | - | - | 11 (22) | 17 (35) | 21 (43) |
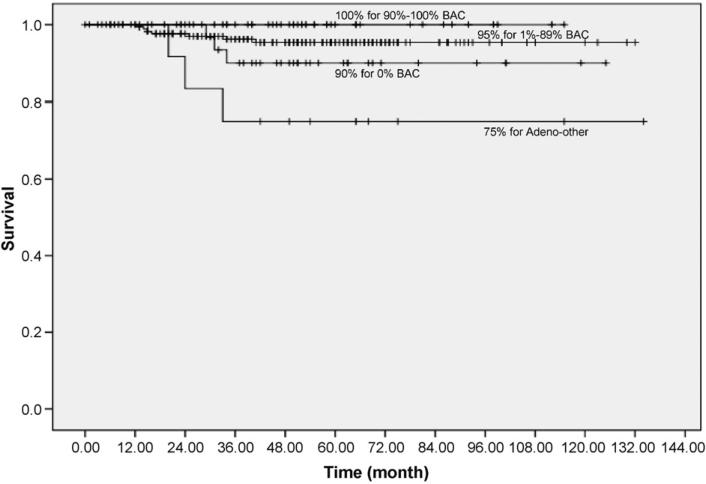
The 10-year Kaplan–Meier lung cancer-specific survival rate for patients was 100% for those with 100% BAC and also for those with 90–99% BAC (Fig. 4). For those with 1–90% BAC, it was 95% (95% Confidence Interval (CI): 92–99%) and for those with 0% BAC, it was 90% (95% CI: 81–100%).
Fig. 4.
Kaplan–Meier survival rates for patients with adenocarcinoma (n = 279) by percentage of bronchioloalveolar component.
3.2. Solitary and non-solitary adenocarcinoma
Non-solitary cancers were seen in 49 (18%) of the 279 resected cases. Non-solitary cancers were most frequent (46/246 = 19%) in patients with adenocarcinoma-MIXED, slightly less frequent in patients with adeno-BAC (3/20 = 15%) and least frequent in patient of adeno-OTHER (1/13 = 8%) (Table 2). As to the nodule consistency, the frequency of a dominant non-solitary cancer manifesting as part-solid nodule was 17/80 = 21%, as non-solid, it was 11/54 = 20%, and as solid, it was 21/145 = 15%.
Of the 257 patients without lymph node metastases, 44 (17%) were non-solitary. Of these 44, another lung cancer was diagnosed in the same lobe in 21, in another lobe in 14, and in the same and another lobe in 9. The cell subtype was the same in 16 and different in 19; in another 9, more than 2 cancers were found. In 34 of the 44 cases, the additional lung cancer(s) could be seen on the CT prior to resection, but in the remaining 10 cases, the additional cancer(s) could not be identified even in retrospective re-review. The median diameter of the dominant cancer of the non-solitary ones was 12.5 mm, the same as for the solitary cancer.
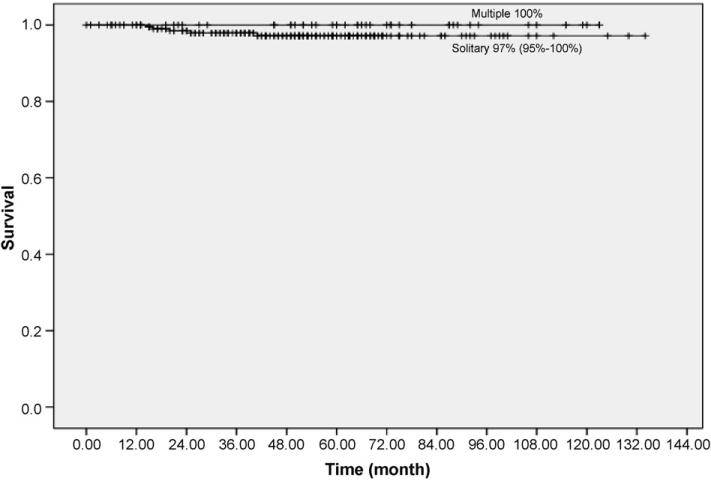
Fig. 5 shows the 10-year Kaplan–Meier survival rate of the 257 patients with pathologic node negative solitary lung cancer was 97% (95% CI: 95–100%) and it was 100% for patients with non-solitary malignancy, there being no statistically significant difference between these two rates (P = 0.29).
Fig. 5.
Kaplan–Meier survival rates for patients diagnosed with adenocarcinoma, solitary (n = 213) or non-solitary (n = 44) with length 30 mm or less and without pathologic evidence of lymph node or distant metastases.
Among the 247 patients with pathologic stage I, the percentage of BAC in the dominant carcinoma did not correlate with presence of multiple lesions (P = 0.79). The presence of multiple lesions in these resected cases did not impact prognosis. In fact, there were no deaths among the multiple-lesion cases. Similarly, the CT appearance and histology of the second lesions did not influence survival.
4. Discussion
The World Health Organization criteria for Adeno-BAC and adeno-MIXED [35] most closely corresponds to Noguchi et al. [27] Type A or B and C, respectively, and adeno-OTHER to their Types D, E and F. Our results show that prognosis was related to the proportion of BAC. Adeno-BAC is, by definition, 100% BAC and had a 100% 10 year survival, whereas carcinomas with less than 90% BAC had a lessened survival, which was still better than for those without a BAC component. Travis et al. [36] indicated that a definition of minimally invasive adenocarcinoma of the lung had not yet been identified. Our data suggest that carcinomas with greater than 90% BAC might be considered minimally invasive. The data are also consistent with the notion that there may be three prognostically different classes of pulmonary adenocarcinomas—pure BAC, BAC with invasive components and adenocarcinomas which are entirely invasive. The fact that adeno-OTHER occurred more frequently in annual repeat screenings also suggests that it typically has a more aggressive course than adeno-MIXED and its lower survival rate is consistent with the findings of Noguchi et al. [27] and others including Rena et al. [32], Okada et al. [28], Lin et al. [19] and Volpino et al. [40]. Yim et al. [42] divided adenocarcinomas into 4 categories and reported little or no difference between BAC and those with less than 5 mm invasive carcinoma and a lesser, but not different survival between those with greater than 5 mm invasion plus BAC and those without BAC. Yokose et al. [43] found no deaths in adenocarcinomas of the lung with greater than 75% lepidic growth pattern, but decreased survival in patients with less than 25% lepidic growth and also those with a papillary component. Sakao et al. [33] divided 82 adenocarcinomas 2 cm or smaller into mixed adenocarcinomas with and without BAC component and found this to be prognostically significant. Overall size was not a prognostic factor, but size excluding BAC component was. Our long-term survival rates for adenocarcinoma, mixed subtype are better than those reported by the others, likely because our patients were all detected as a result of CT screening [9,10,12].
The CT nodule consistency appears to closely reflect the proportion of BAC [1,28]. Although histology was the best indicator of prognosis, it correlated closely with CT appearance, which was also a good indicator of prognosis. Adenocarcinoma with a high proportion of BAC typically manifested as non-solid nodules on CT, and these patients have a very high 10-year Kaplan–Meier survival rate. This suggests that when a non-solid nodule is seen on CT it is reasonable to wait until growth is identified or a solid component becomes evident as this reflects invasion by the tumor. It is also important to understand that when a part-solid nodule is identified in the baseline screening, the solid component may be due to scar formation rather than due to stromal invasion and thus growth assessment is still important prior to biopsy.
As previously demonstrated by the long-term follow-up of diagnosed cases of lung cancer resulting from CT screening, the proportion of Stage I cases closely correlates with the overall long-term survival rate [15]. This was also true in this study as both adeno-BAC and adeno-MIXED subtypes had a higher proportion of Stage I than adeno-OTHER and also had a better prognosis. Among resected adenocarcinomas, the frequency of pure adeno-BAC was low (7%) and this is similar to our prior study, which reported that 4.0% had this subtype among the 250 patients with any CT screen-detected lung cancer [8] (Figs. 1 and 2). In contrast, the study of 59 patients diagnosed with lung cancer as a result of CT screening by Lindell et al. [20], 9 (15%) of the 59 patients diagnosed with lung cancer had adeno-BAC subtype. Parenchymal invasion was found in 96% of cases in the series by Lin et al. [19].
Makimoto et al. [22] reported a survival difference of 10% between cases of adenocarcinoma 2 cm or smaller when a papillary carcinoma component was present. Miyoshi et al. [25] reported a 14% decrease in survival in Stage I adenocarcinomas with a micropapillary component. Our data did not show a statistically significant prognostic difference relative to papillary configuration or solid pattern.
Perhaps the most significant finding in the study is the outcome of non-solitary cancers in patients without evidence of lymph node or distant metastases. Based on the current staging criteria [24], a patient with 2 or more carcinomas might not be offered surgical resection, unless it can be shown that the cell-types are different. Since adenocarcinoma has become the most frequent cell-type, there is a high likelihood that any additional carcinoma will be of the same cell-type, even the same subtype, and Staged IIIB or IV according to location of the satellite nodule. We found that 18% of our cases diagnosed adeno-MIXED or adeno-BAC were non-solitary (Table 2). This was higher than the 2 (3%) of 59 patients reported with non-solitary carcinomas in the series reported by Lindell et al. [20]. Contrary to the current staging scheme in which T4 cases had a cumulative 5-year survival rate of 7%, diagnosis of non-solitary adenocarcinoma of the lung resulting from CT screening for lung cancer, whether in the same or in different lobes in the absence of lymph node metastases, did not convey a poor prognosis in these resected cases. In fact, the estimated 10-year Kaplan–Meier survival rate of patients with non-solitary lung cancer was excellent. Battafarano et al. [2] reported 3 year survival of 80% for T1 resected cases and 67% for T4 multifocal carcinomas which was not statistically significant. Bryant et al. [4] reported 57% survival for T4 N0M0 resected due to the presence of intralobar satellites. Lucchi et al. [21] reported no difference of survival in 24 resected cases with intralobar satellites. Nakata et al. [26] found 93% 3-year survival in 26 synchronously multiple adenocarcinomas. Osaki et al. [29] reported 27% survival among 36 resected T4 multifocal adenocarcinomas. Port et al. [30] found 48% survival in 53 resected adenocarcinomas with intralobar satellites, only 8 of which were evident preoperatively. Rao et al. [31] reported 57% 5 year survival in 35 resected adenocarcinoma with T4 satellites. Suzuki et al. [44] studied 1360 resected cases and found no impact on survival in the 137 cases in whom AAH was present. However, in the 46 cases of intrapulmonary metastases, the prognosis was significantly worse. It appears that experience indicates that T4 and even M1 based solely on satellites, especially when nodes are negative, does not convey the Stage IIIB or IV mortality previously indicated. Our data show that, in cases with satellite lesions, survival figures are higher than previously reported, but all of our cases were asymptomatic, CT screen detected, and mostly found in Stage I.
At the present time, it is extremely difficult to distinguish independent primaries from intrapulmonary metastases by any means. Sozzi et al. [45] studies p53 mutations, 3p chromosome deletions and K-ras mutations in 11 synchronous cases and found different genetic lesions in each patient. Huang et al. [46] compared patterns of loss of heterozygosity in multiple genes and chromosomal loce between paired tumors and found the lesions to differ genetically in most. Our follow-up data is consistent with the interpretation that, at least in the setting of small, resected, node negative carcinomas detected by CT scan screening, histologically identical satellite carcinomas behaved as though they were independent primaries and were surgically cured.
Prior to our long-term follow-up of resected cases, consensus within I-ELCAP had already developed that all cases of non-solitary adenocarcinoma without evidence of lymph node or distant metastases, even if both were of the same histologic subtype, should be classified as T and M status indeterminate (Stage I*) and treated according to the recommendations for Stage I lung cancer. The outcome data presented here support that view and we hope that this report will stimulate reassessment of the current staging criteria.
Acknowledgements
Partially funded by the American Cancer Society and the National Cancer Institute grant (R01-CA-78905).
Footnotes
Conflicts of interest
The authors other than Claudia I. Henschke and David F. Yankelevitz indicated no potential conflicts of interest. The potential conflicts of Claudia I. Henschke and David F. Yankelevitz are not related to pathology, but could be interpreted as being related to screening.
References
- 1.Aoki T, Nakata H, Watanabe H, et al. Evolution of peripheral lung adenocarcinomas: CT findings correlated with histology and tumor doubling time. Am J Roentgoenol. 2000;174:763–8. doi: 10.2214/ajr.174.3.1740763. [DOI] [PubMed] [Google Scholar]
- 2.Battafarano RJ, Meyers BF, Guthrie TJ, et al. Surgical resection of multifocal non-small cell lung cancer is associated with prolonged survival. An Thorac Surg. 2002;74:988–93. doi: 10.1016/s0003-4975(02)03878-x. [DOI] [PubMed] [Google Scholar]
- 3.Beard CM, Jedd MB, Woolner LB, et al. Fifty-year trend in incidence rates of bronchogenic carcinoma by cell type in Olmsted County, Minnesota. J Natl Cancer Inst. 1988;80:1404–7. doi: 10.1093/jnci/80.17.1404. [DOI] [PubMed] [Google Scholar]
- 4.Bryant AS, Pereira SJ, Miller DL, et al. Satellite pulmonary nodule in the same lobe (T4N0) should not be staged IIIB non-small cell lung cancer. Ann Thorac Surg. 2006;82:1808–13. doi: 10.1016/j.athoracsur.2006.03.123. [DOI] [PubMed] [Google Scholar]
- 5.Carter D, Marsh BR, Baker RR, et al. Relationship of morphology to clinical presentation in 10 cases of early squamous cell carcinoma of the lung. Cancer. 1976;37:1389–96. doi: 10.1002/1097-0142(197603)37:3<1389::aid-cncr2820370320>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 6.Carter D, Vazquez M, Flieder DB, et al. Comparison of pathologic findings of baseline and annual repeat cancers diagnosed on CT screening. Lung Cancer. 2007;56:193–9. doi: 10.1016/j.lungcan.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Flehinger BJ, Melamed MR, Zaman MB, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Memorial-Sloan Kettering study. Am Rev Respir Dis. 1984;130:555–60. doi: 10.1164/arrd.1984.130.4.555. [DOI] [PubMed] [Google Scholar]
- 8.Flieder DB, Vazquez M, Carter D, et al. Pathologic findings in lung tumors diagnosed on baseline CT screening. Am J Surg Pathol. 2006;30:606–13. doi: 10.1097/01.pas.0000202040.51967.d0. [DOI] [PubMed] [Google Scholar]
- 9.Fontana RS, Sanderson DR, Taylor WF, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Mayo Clinic study. Am Rev Respir Dis. 1984;130:561–5. doi: 10.1164/arrd.1984.130.4.561. [DOI] [PubMed] [Google Scholar]
- 10.Frost JK, Ball Jr WC, Levin ML, et al. Early Lung Cancer Detection: Results of the initial (prevalence) radiologic and cytologic screening in the Johns Hopkins study. Am Rev Respir Dis. 1984;130:549–54. doi: 10.1164/arrd.1984.130.4.549. [DOI] [PubMed] [Google Scholar]
- 11.Fu JB, Kau Y, Severson RK, et al. Lung cancer in women: analysis of the national Surveillance, Epidemiology and End Results database. Chest. 2005;127:768–77. doi: 10.1378/chest.127.3.768. [DOI] [PubMed] [Google Scholar]
- 12.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: Overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 13.Henschke CI, Naidich DP, Yankelevitz DF, et al. Early Lung Cancer Action Project: Initial results of annual repeat screening. Cancer. 2001;92:153–9. doi: 10.1002/1097-0142(20010701)92:1<153::aid-cncr1303>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR. 2002;178:1053–7. doi: 10.2214/ajr.178.5.1781053. [DOI] [PubMed] [Google Scholar]
- 15.The International Early Lung Cancer Action Program Investigators Survival of Patients with Stage I lung cancer detected on CT screening. N Engl J Med. 2006;355:1763–71. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 16.Kishi K, Homma S, Kurosaki A, et al. Small lung tumors with the size of 1 cm or less in diameter: clinical radiological and histopathologic characteristics. Lung Cancer. 2004;44:43–51. doi: 10.1016/j.lungcan.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Koyt H, Hillerdahl G, Branden E. A prospective study of the total material of lung cancer from a county in Sweden 1997-1999: gender, symptoms, type, stage and smoking habits. Lung Cancer. 2002;36:9–14. doi: 10.1016/s0169-5002(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 18.Levi F, Francheschi S, LaVecchia C, et al. Lung carcinoma trends by histologic type in Vaud and Neuchatel, Switzerland, 1974–1994. Cancer. 1997;79:906–14. doi: 10.1002/(sici)1097-0142(19970301)79:5<906::aid-cncr6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Lin DM, Ma Y, Zheng S, et al. Prognostic value of bronchioloalveolar carcinoma component in lung adenocarcinoma. Histol Histopathol. 2006;21:627–32. doi: 10.14670/HH-21.627. [DOI] [PubMed] [Google Scholar]
- 20.Lindell RM, Hartman TE, Swensen SJ, et al. Five year lung cancer screening experience: CT appearance, growth rate, Location and histologic features of 61 lung cancers. Radiology. 2007;242:555–62. doi: 10.1148/radiol.2422052090. [DOI] [PubMed] [Google Scholar]
- 21.Lucchi M, Viti A, Meifi F, et al. IIIB-T4 non-small cell lung cancer: indications and results of surgical treatment. J Cardiovasc Surg (Torino) 2007;48:369–74. [PubMed] [Google Scholar]
- 22.Makimoto Y, Nabeshima K, Iwasaki H, et al. Micropapillary pattern: a distinct pathological marker to subclassify tumours with a significantly poor prognosis within small peripheral adenocarcinoma (<+20 mm) with mixed bronchioloalveolar and invasive subtypes (Noguchi's type C tumours). Histopathology. 2005;46:677–84. doi: 10.1111/j.1365-2559.2005.02126.x. [DOI] [PubMed] [Google Scholar]
- 23.Melamed MR, Flehinger BJ, Zaman MB, et al. Detection of true pathologic Stage I lung cancer in a screening program and its effect on survival. Cancer. 1981;47:1182–7. doi: 10.1002/1097-0142(19810301)47:5+<1182::aid-cncr2820471322>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–7. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 25.Miyoshi T, Satoh Y, Okumura S, et al. Earlystage adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol. 2003;27:101–9. doi: 10.1097/00000478-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Nakata M, Sawada S, Yamashita M, et al. Surgical treatment for multiple primary adenocarcinomas of the lung. Ann Thorac Surg. 2004;78:1194–9. doi: 10.1016/j.athoracsur.2004.03.102. [DOI] [PubMed] [Google Scholar]
- 27.Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung: histologic characteristics and prognosis. Cancer. 1995:2844–52. doi: 10.1002/1097-0142(19950615)75:12<2844::aid-cncr2820751209>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Okada M, Nishio W, Sakamoto T, et al. Correlation between computed tomographic findings, bronchioloalveolar carcinoma component and biologic behaviour of small-sized lung adenocarcinomas. J Thorac Cardiovasc Surg. 2004;127:857–61. doi: 10.1016/j.jtcvs.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 29.Osaki T, Sugio K, Hanagiri T, et al. Survival and prognostic factors of surgically resected T4 non-small cell lung cancer. Ann Thorac Surg. 2003;75:1745–51. doi: 10.1016/s0003-4975(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 30.Port JL, Korst RJ, Lee PC, et al. Surgical resection for multifocal (T4) non-small cell lung cancer: is the T4 designation valid? Ann Thorac Surg. 2007;83:397–400. doi: 10.1016/j.athoracsur.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Rao J, Sayeed RA, Tomaszek S, et al. Prognostic factors in resected satellite nodule T4 non-small cell lung cancer. Ann Thorac Surg. 2007;84:934–8. doi: 10.1016/j.athoracsur.2007.04.097. [DOI] [PubMed] [Google Scholar]
- 32.Rena O, Papalia E, Ruffini E, et al. Stage I pure bronchioloalveolar carcinomas: recurrences, survival and comparison with adenocarcinoma of the Lung. Eur J Cardio-thoracic Surg. 2003;23:409–14. doi: 10.1016/s1010-7940(02)00830-8. [DOI] [PubMed] [Google Scholar]
- 33.Sakao Y, Miyamoto H, Sakuraba M, et al. Prognostic significance of a histologic subtype in small adenocarcinoma of the lung: the impact of bronchioloalveolar carcinoma components. Ann Thorac Surg. 2007;83:209–14. doi: 10.1016/j.athoracsur.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 34.Shimuzi K, Yoshida J, Nagai K, et al. Visceral pleural invasion classification in non-small cell lung cancer: a proposal on the basis of outcome assessment. J Thorac Cardiovasc Surg. 2004;127:1574–8. doi: 10.1016/j.jtcvs.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Travis WD, Brambilla E, Muller-Hermelink HK, et al. Pathology and genetics of tumours of the lung, pleura, thymus and heart. IARC Press; Lyon: 2004. World Health Organization classification of tumours. [Google Scholar]
- 36.Travis WD, Garg K, Franklin WA, et al. Bronchioloalveolar carcinoma and lung adenocarcinoma: the clinical importance and research relevance of the 2004 World Health Organization pathologic criteria. J Thorac Oncol. 2006;1:S13–9. [PubMed] [Google Scholar]
- 37.Vazquez M, Flieder D, Travis W, et al. Early Lung Cancer Action Project pathology protocol. Lung Cancer. 2003;39:231–2. doi: 10.1016/s0169-5002(02)00452-x. [DOI] [PubMed] [Google Scholar]
- 38.Vazquez M, Flieder D, Travis W, et al. Early Lung Cancer Action Project pathology protocol. doi: 10.1016/s0169-5002(02)00452-x. http://www.ielcap.org. [DOI] [PubMed]
- 39.Vincent RG, Pickren JW, Lane WN, et al. The changing histopathology of lung cancer: a review of 1682 cases. Cancer. 1977;39:1647–55. doi: 10.1002/1097-0142(197704)39:4<1647::aid-cncr2820390439>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 40.Volpino P, Cavallaro A, Cangemi R, et al. Comparative analysis of clinical features and prognostic factors in resected bronchioloalveolar carcinoma and adenocarcinoma of the lung. Anticancer Res. 2003;23:4959–65. [PubMed] [Google Scholar]
- 41.Woolner LB, David E, Fontana RS, et al. In situ and early invasive bronchogenic carcinoma: Report of 28 cases with postoperative survival data. J Thorac Cardiovasc Surg. 1970;60:275–90. [PubMed] [Google Scholar]
- 42.Yim J, Zhu LC, Chiriboga L, et al. Histologic features are important prognostic indicators in early stage lung cancer. Mod Pathol. 2007;20:233–41. doi: 10.1038/modpathol.3800734. [DOI] [PubMed] [Google Scholar]
- 43.Yokose T, Suzuki K, Nagai K, et al. Favorable and unfavorable morphological prognostic factors in peripheral adenocarcinoma of the lung 3 cm or less in diameter. Lung Cancer. 2000;29:179–88. doi: 10.1016/s0169-5002(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki K, Nagai K, Yoshida J, et al. The prognosis of resected lung carcinoma associated with atypical adenomatous hyperplasia: a comparison of the prognosis of well-differentiated adenocarcinoma associated with atypical adenomatous hyperplasia and intrapulmonary metastases. Cancer. 1997;79:1521–6. doi: 10.1002/(sici)1097-0142(19970415)79:8<1521::aid-cncr12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 45.Sozzi G, Miozzo M, Pastorino U, et al. Genetic evidence for independent origin of multiple preneoplastic and neoplastic lung lesions. Cancer Res. 1995;55:135–40. [PubMed] [Google Scholar]
- 46.Huang J, Behrens C, Wistuba I, Gazdar AF, Jagirdar J. Molecular analysis of synchronous and metachronous tumors of the lung: Impact on management and prognosis. Ann Diagn Pathol. 2001;5:321–9. doi: 10.1053/adpa.2001.29338. [DOI] [PubMed] [Google Scholar]