Abstract
Oligodendrocytes are sensitive to ischemic damage. The Sonic hedgehog (Shh) pathway is critical in oligodendrogenesis; Gli1 is the principal effector of Shh signaling. We investigated oligodendrogenesis and Shh/Gli1 pathway activation after bone marrow stromal cell (BMSC) treatment of stroke in rats. Rats were subjected to the middle cerebral artery occlusion (MCAo). BMSCs have been shown to promote functional recovery post stroke. A therapeutic dose of BMSC (3×106 cells) treatment was initiated 1 day after MCAo. Immunohistochemistry was carried out to measure the oligodendrocyte progenitor cells, oligodendrocytes, myelin, and expressions of Shh and Gli1 at 14 days after MCAo. Gene expression of Shh and Gli1 was tested at 2 days after MCAo. An in vitro study was used to investigate the effects of BMSC on a premature oligodendrocyte cell line (N20.1 cells). BMSC treatment significantly increased O4+ oligodendrocytes, MBP+ area, and bromodeoxyuridine (BrdU)+, NG2+, BrdU+–NG2+ cells, and mRNA and protein expressions of Shh and Gli1 in the ipsilateral brain of the MCAo rats than that in phosphate buffered saline (PBS)-treated rats. BMSCs promoted N20.1 cell proliferation and Gli1 mRNA expression, and these effects were abolished by the Shh pathway inhibitor cyclopamine. These data indicate that the BMSC treatment stimulates oligodendrogenesis by activation of the Shh/Gli1 pathway post stroke.
Keywords: bone marrow stromal cells, Gli1, MCAo, oligodendrocyte, oligodendrogenesis, sonic hedgehog
Introduction
Oligodendrocytes generate the myelin sheaths that enwrap axons, thereby facilitating saltatory conduction (McTigue and Tripathi, 2008); they are vulnerable to damage in a variety of neurologic diseases, such as ischemic cerebrovascular diseases, and oligodendrocyte damage leads to demyelination, which contributes to neurologic functional deficits (Dewar et al, 2003; McTigue and Tripathi, 2008; Micu et al, 2006). Recruited oligodendrocytes can remyelinate axons, raising the possibility of therapeutic intervention (Chang et al, 2000). Mature oligodendrocytes in the adult mammalian central nervous system (CNS) were considered to be postmitotic and are unable to proliferate in response to injury (McTigue and Tripathi, 2008). However, abundant oligodendrocyte progenitor cells exist in the white and gray matter of normal CNS, and are present in the lesions (Komitova et al, 2006; McTigue and Tripathi, 2008; Tanaka et al, 2003), and proliferating oligodendrocyte progenitor cells contribute to remyelination (Carroll and Jennings, 1994; Gensert and Goldman, 1997; Jiang et al, 2008; Keirstead et al, 1998; Prineas et al, 1989).
Our previous in vivo studies found that bone marrow stromal cells (BMSCs) stimulate the production of restorative factors by parenchymal cells and evoke white matter remodeling in the injured brain (Chopp and Li, 2002; Chopp et al, 2009), which lead to improvement in neurologic function post stroke (Chen et al, 2000; Chen et al, 2001a; Shen et al, 2007; Zhang et al, 2004), and our in vitro results suggest that BMSCs protect oligodendrocytes subjected to oxygen–glucose deprivation (Zhang et al, 2008). These studies provide insight into white matter damage and the therapeutic benefits of BMSC cell based-remyelinating therapy after stroke. In this study, we investigated the effects of BMSC treatment of stroke on oligodendrogenesis.
To elucidate the underlying mechanisms of oligodendrocyte progenitor cell proliferation after treatment, we used N20.1 cells, which are premature oligodendrocytes (Paez et al, 2004). These cells are widely used to study the cellular and molecular mechanisms involved in the development, maturation, and formation of myelin by oligodendrocytes in the CNS (Garcia et al, 2007; Paez et al, 2004; Zhang et al, 2008).
A signaling pathway by which BMSCs induce oligodendrogenesis was investigated. Sonic hedgehog (Shh) is a member of the family of the hedgehog proteins; it plays a critical role in the induction, survival, proliferation, and migration of oligodendrocytes (Marti and Bovolenta, 2002; Seifert et al, 2005; Sussman et al, 2002). The Shh signaling pathway is well conserved (Marti and Bovolenta, 2002), Shh binds to the transmembrane receptor protein, patched, to activate another transmembrane receptor, smoothened (Ingham and McMahon, 2001), and induces intracellular reactions that target the Gli family of transcription factors (Ruiz i Altaba et al, 2002). Gli1 is the principal effector of Shh signaling in neural progenitor cells (Ahn and Joyner, 2005; Wang et al, 2007). In this article, we show that BMSC treatment, as an effective restorative treatment for stroke, enhances white matter remodeling and stimulates the Shh pathway.
Materials and methods
All experimental procedures have been approved by the Institutional Animal Care and Use Committee of Henry Ford Health System.
Bone Marrow Stromal Cell Preparation
Rat (r) and mouse (m) BMSCs were generously provided by Cognate Therapeutics Inc. (Baltimore, MD, USA). BMSCs were isolated, grown, and tested, as described previously (Zhang et al, 2004). Briefly, the bone marrow was obtained from adult donors and placed into media with Dulbecco’s modified Eagle’s medium-low glucose and 10% selected fetal bovine serum (FBS). The adherent cells were fed and generally lasted for about 2 weeks. When dense colonies of spindle-shaped cells covered greater than 80% of the dish, the cells were passaged into the secondary culture. When dose and purity levels were achieved, cells were harvested and cryopreserved in appropriate dose-related aliquots in Plasma-Lyte (Baxter Healthcare Corporation, Deerfield, IL, USA) containing serum albumin and dimethyl sulfoxide.
Animal Model
Adult male Wistar rats (270 to 300 g, n = 27; Charles River Laboratories, Wilmington, MA, USA) were used in our experiments. Middle cerebral artery occlusion (MCAo) was induced by a method of intraluminal vascular occlusion modified in our laboratory (Zhang et al, 2004). Briefly, a length of 4-0 monofilament nylon suture (18.5 to 19.5 mm) was advanced from the external carotid artery into the lumen of the internal carotid artery until it blocked the origin of the MCA. Rats were randomly divided into the following:
Normal control group
This control group consisted of naive rats (n = 9) without surgery.
MCAo control group
Rats (n = 9) were subjected to permanent MCAo and 1 mL phosphate buffered saline (PBS) was injected into a tail vein at 1 day after MCAo.
BMSC treatment group
After washing with PBS and subsequent centrifugation, rBMSCs (rat BMSC) (3 × 106) in 1 mL of PBS were injected into a tail vein at 1 day after MCAo (n = 9). This dose of cells was shown earlier to be highly effective in improving functional recovery after stroke (Chen et al, 2001a, 2003; Zhang et al, 2004). Bromodeoxyuridine (BrdU, Sigma Chemical) was used for mitotic labeling. It (100 mg/kg) was intraperitoneally injected twice a day for 7 consecutive days into normal or ischemic rats starting 1 day after MCAo.
Tissue Preparation
To measure the expressions of Shh and Gli1 mRNA, normal rats (n = 3) and ischemic rats with BMSC or PBS treatment were killed at 2 days after MCAo (n=3 per treatment group). The ischemic boundary zone (IBZ) was extracted from the brains of the rats subjected to stroke (Zhang et al, 2004). For immunohistochemical evaluation, normal rats (n = 6) were killed at 7 days after BrdU injection. Rats treated with BMSCs or PBS were killed at 14 days (n = 6 per treatment group) after MCAo. Brains were fixed in 4% of paraformaldehyde and embedded in paraffin. A coronal slide (6 μm thick) from the center of the ischemic core (bregma 0.2 mm) (Zhang et al, 2004) was used for immunohistochemical staining.
Real-Time Reverse Transcriptase-PCR Analysis
Quantitative PCR was carried out using the SYBR Green real-time PCR method. Total RNA was isolated from the IBZ using the RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA, USA). Quantitative reverse transcriptase-PCR (RT-PCR) was carried out on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA, USA) using three-stage program parameters provided by the manufacturer as follows: 2 mins at 50°C, 10 mins at 95°C, and then 40 cycles of 15 secs at 95°C and 1 min at 60°C. Specificity of the produced amplification product was confirmed by the examination of dissociation reaction plots. A distinct single peak indicated that a single DNA sequence was amplified during PCR. Polymerase chain reaction products were run on 2% agarose gels to confirm that correct molecular sizes were present. Each sample was tested in triplicate, and samples obtained from three independent experiments were used for analysis of relative gene expression using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Primer sets used to detect Shh and Gli1 mRNAs were listed as follows: Shh (Fwd, ATAACCTTGCCTGCTGTTGC; Rev, GAGACCCAACTCCGATGTGT), Gli1 (Fwd, CAGCTCAAAGCTCAGCTCCT; Rev, CTTGGGGCTCTGATATGGAA), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Fwd, AGAACATCATCCCTGCATCC; Rev, CACATTGGGGGTAGGAACAC). One-way ANOVA (analysis of variance) followed by the Student–Newman–Keuls test was carried out. The data are presented as means ± s.d. A value of P < 0.05 is considered significant.
Immunohistochemistry and Quantification
Oligodendrocytes were identified by antibodies O4 (1:100, Chemicon, Billerica, MA, USA) or myelin basic protein (MBP) (1:50, Abcam, Cambridge, MA, USA). The NG2 antibody (1:100, Chemicon) was used to mark oligodendrocyte progenitor cells. To test the protein expressions of Shh and Gli1 in the MCAo brains with or without BMSC treatment, immunohistochemistry stainings were carried out using a goat polyclonal antibody (pAb) against Shh (1:50, Santa Cruz Biotechnology) and a rabbit pAb against Gli1 (1:200, Santa Cruz Biotechnology).
Double immunostaining for NG2 with BrdU (a mouse monoclonal antibody, 1:100, Dako, Carpinteria, CA, USA) was used to identify oligodendrocyte progenitor cell proliferation. To clarify the relationship of the expressions of Shh and Gli1 in neural cells, double immunostaining for Shh with NeuN (marker of neurons, Chemicon), glial fibrillary acidic protein (GFAP, marker for astrocytes, Dako), and O4, as well as Gli1 with NG2 were used.
Immunostaining was carried out following standard protocols. Slides were treated first with the primary antibody and then with the antibody conjugated to fluorescein isothiocyanate (FITC, Jackson ImmunoResearch, West Grove, PA, USA). These slides were then treated with a second primary antibody and then incubated with an antibody conjugated to Cy3 (Jackson ImmunoResearch). Negative control slides for each animal received identical preparations for immunostaining, except that primary antibodies were omitted.
To measure immunoreactive cells, numbers of O4+, Shh+, Gli1+, NG2+, BrdU+, and NG2+–BrdU+ cells were counted in eight fields of view from the IBZ of a standard section from the center of the ischemia core (bregma 0.2 mm) and in all the fields from the ipsilateral subventricular zone (SVZ) in the same slide. The fields were digitized under the light microscope (Olympus BX40, Tokyo, Japan) using a 3-CCD color video camera (Sony DXC-970 MD, Tokyo, Japan) interfaced with Micro Computer Imaging Device (MCID) analysis system (Imaging Research, Saint Catharines, ON, Canada). The immunopositive cells were calculated and divided by the measured areas, and presented as numbers per mm2. The density of the MBP+ area was measured in eight fields of view in the IBZ and presented as a proportional area by dividing the measured MBP+ area by the total scanned area. Double immunostaining images were taken using Zeiss (Oberkochen, Germany) laser-scanning confocal microscopy (LSM 510 META). 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei were visualized by two-photon microscopy using a Chameleon pulsed laser (Coherent Inc., Santa Clara, CA, USA) set at 720 nm excitation. These regions were scanned in 512 × 512 pixel (279 × 279 μm2) format in the x–y direction using a × 5 frame-scan average, and five optical sections along the z-axis with a 1-μm step size were obtained under a × 63 objective.
Data are presented as mean ± s.d. Significance between the two groups was examined using ANOVA analysis. Avalue of P < 0.05 was considered significant.
In Vitro Oligodendrocyte Proliferation
We used an immortalized mouse premature oligodendrocyte cell line (N20.1, generously provided by Dr Anthony Campagnoni, University of California at Los Angeles) to measure oligodendrocyte proliferation and differentiation. N20.1 cells were obtained from mouse primary cultures of oligodendrocytes conditionally immortalized by transformation with a temperature-sensitive large T-antigen (Paez et al, 2004). N20.1 cells were cultured in Dulbecco’s modified Eagle’s medium/F12 (Invitrogen, Carlsbad, CA, USA) with 3.6 g/L of dextrose anhydrous, 3.38 g/L of HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 2.16 g/L of sodium bicarbonate, 90 mg/L of gentamicin, 1% of FBS, and G418 (100 μg/mL) at 39°C (Paez et al, 2004).
N20.1 cells were incubated in the following groups (n = 3 per group, three wells were used in each group): (1) regular culture medium for control; (2) mouse BMSCs (mBMSCs) cocultured with normal N20.1 cells (oligodendrocytes: mBMSCs = 10:1), an insert (0.4 μm, BD Biosciences, Franklin Lakes, NJ, USA) was used to contain mBMSCs. Oligodendrocytes were plated on the base of the culture wells and the upper transwell compartments were seeded with mBMSCs; (3) mBMSCs cocultured with 5 μmol/L cyclopamine (Calbiochem, Gibbstown, NJ, USA)-treated N20.1 cells. Cyclopamine is a specific inhibitor of smoothened (Wang et al, 2007).
For investigation of oligodendrocyte proliferation, N20.1 cells were treated for 12 h and 50 μg/mL BrdU (Sigma) was added to the cell cultures for 1 h. BrdU immunostaining of the N20.1 cells was measured. Numbers of BrdU+ cells were calculated by counting 10 random fields in each well with six wells per group. The results are presented as a percentage (positive cells divided by total cells).
In Vitro Activation of the Shh/Gli Pathway in Oligodendrocytes
An additional set of in vitro experimental groups was used for mRNA analyses (n = 3 per group). Primer sets used to detect mouse NG2 and Gli1 mRNAs were listed as follows: Gli1 (Fwd, TGTGTGAGCAAGAAGGTTGC; Rev, TTGCACACGTATGGCTTCTC), NG2 (Fwd, CGGCCAACAGTGGTTTCAAGT; Rev, CTTCTGTGAAGGCTGTCGATG), GAPDH (Fwd, AG AACATCATCCCTGCATCC; Rev, CACATTGGGGGTAGGAACAC). One-way ANOVA followed by the Student–Newman–Keuls test was carried out. The data are presented as means ± s.d. A value of P < 0.05 is considered significant.
Statistical Analyses
Data were evaluated by an investigator blinded to the treatment status of each animal. Data were evaluated for normality, and data transformation or nonparametric analysis was considered if data were not normal. Repeated analysis of covariance was used to test the BMSC effects on measurements. The analysis began testing for BMSC by time interaction, followed by testing the main effect of BMSCs if no interaction was observed at the 0.05 level.
Results
BMSC Treatment Increases the Area of Myelin in the Brain of Rats Subjected to MCAo
Given the significant improvement in neurologic outcome when rodents subjected to MCAo were treated with BMSCs (Chen et al, 2001a; Chopp et al, 2009; Shen et al, 2007; Zhang et al, 2004), we sought to address whether BMSC treatment affects myelin. As indicated by MBP immunostaining, myelin damage was obvious in the IBZ of MCAo rats at 14 days (Figure 1A). After BMSC treatment, the proportional area of myelin (MBP+) density was significantly increased in the IBZ of BMSC-treated rats than that of PBS-treated rats (73.7% ± 4.8% versus 64.9% ± 2.1%, P < 0.05, Figures 1B and 1C). These data indicate that BMSC treatment protects myelin and/or induces oligodendrogenesis and the remyelination in the white matter of MCAo rats.
Figure 1.
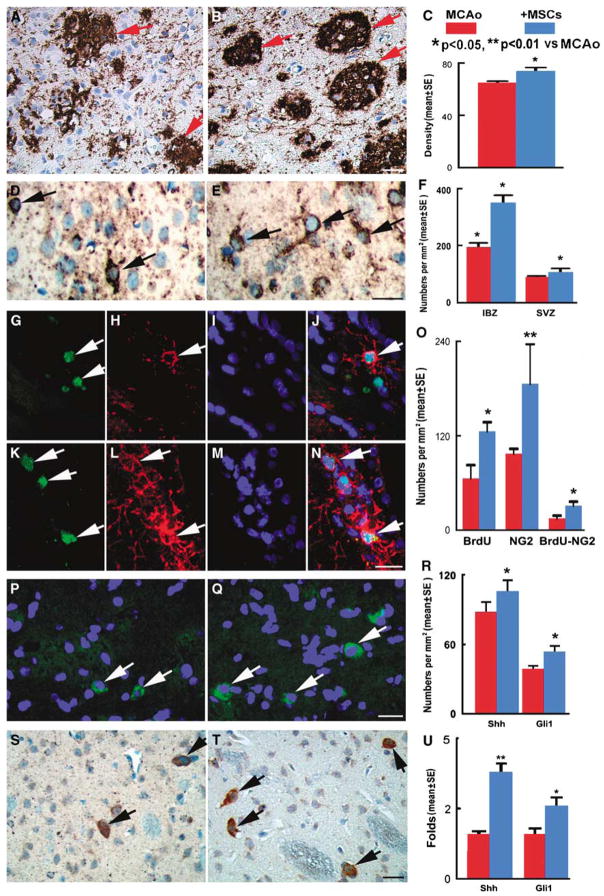
(A to C) The staining by MBP shows the myelin (red arrows) in the IBZ of MCAo rats treated by PBS (A) and BMSCs (B). Quantitative data show that MBP+ areas (C) were significantly increased at 14 days after MCAo in the BMSC treatment group compared with those in the PBS group. (D to F) The staining by O4 shows the oligodendrocytes in the white matter of MCAo rats treated by PBS (D) and BMSCs (E). Quantitative data (F) show that O4+ cells were significantly increased at 14 days after MCAo in the BMSC treatment group compared with those in the PBS group. (G to O) Double immunofluorescence staining (nuclei, DAPI, blue, I, M) indicated that BrdU+ cells (FITC, green, G, K) were colocalized with NG2+ oligodendrocyte progenitor cells (CY3, red, H, L). Quantitative data (O) show that BrdU+, NG2+, and BrdU+–NG2+ cells were significantly increased at 14 days after MCAo in the BMSC treatment group (K to N) compared with those in the PBS group (G to J). (P to T) The immunostaining data show that the Shh+ (merged, green; nuclei, blue) in the white matter of MCAo rats treated by PBS (P) and BMSCs (Q), and Gli1+ cells in the PBS group (S) and BMSC group (T). Quantitative data (R) show that Shh+ and Gli1+ cells were increased significantly at 14 days after MCAo in the BMSC treatment group compared with those in the PBS group. RT-PCR (U) analysis shows the mRNA expressions of Shh and Gli1 were significantly increased at 2 days in the MCAo rats treated by BMSCs compared with those in the PBS-treated rats. Bars=25 μm (A, B, D, E, G to N, P, Q, S, and T). *P < 0.05, **P < 0.01 versus MCAo.
BMSC Treatment Increases Oligodendrogenesis in the Brain of MCAo Rats
Given the significant increase of the myelin area, we sought to address whether BMSC treatment affects oligodendrocyte proliferation and remyelination. After ischemic injury, O4+ oligodendrocytes were significantly increased in the IBZ and the SVZ of BMSC-treated rats than those of the PBS treatment group (350.7 ± 44.9 per mm2 versus 195 ± 25 per mm2 in IBZ; 107 ± 23.1 per mm2 versus 90 ± 7.5 per mm2 in SVZ, P < 0.05, Figures 1D to 1F).
Using double BrdU and NG2 immunostaining, we found that BrdU+ proliferating cells, NG2+ oligodendrocyte progenitor cells, and BrdU+–NG2+ proliferating oligodendrocyte progenitor cells were significantly increased in the ipsilateral brain of BMSC-treated MCAo rats than that of PBS-treated rats (125.9 ± 11.5 per mm2 versus 65.8 ± 17 per mm2; 186.1 ± 20.7 per mm2 versus 97.2 ± 6.3 per mm2; 31.3 ± 5.1 per mm2 versus 15.2 ± 3.3 per mm2, respectively, P < 0.05, Figures 1G to 1O). These data indicate that BMSC treatment stimulates oligodendrogenesis and evokes white matter remyelination in the injured brain.
BMSC Treatment Activates the Shh/Gli1 Signaling Pathway
The Shh/Gli1 signaling pathway regulates oligodendrogenesis in the adult rodent CNS (Wang et al, 2007). To determine whether BMSCs induce Shh/Gli1 signaling activation, we examined the expression of Shh and its effector Gli1. Immunostaining showed that Shh+ and Gli1+ cells are present in the ipsilateral brain at 14 days after MCAo, and significantly increased after the BMSC treatment compared with the PBS treatment (Figures 1P to 1T). Real-time RT-PCR analysis showed that Shh and Gli1 mRNA expressions are present in the IBZ at 2 days after MCAo. After BMSC treatment, gene expressions of Shh and Gli1 were significantly increased in the IBZ of BMSC-treated rats than that of the PBS-treated controls (Figure 1U).
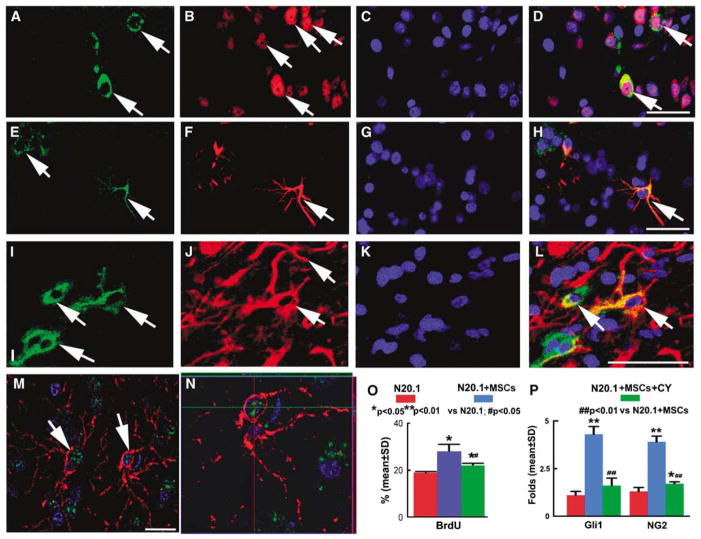
Using double immunostaining of Shh with NeuN, GFAP, and O4, respectively, we found that Shh+ cells colocalized with NeuN+ neurons (Figures 2A to 2D), GFAP+ astrocytes (Figures 2E to 2H), and O4+ oligodendrocytes (Figures 2I to 2L). Thus, Shh protein may be released from neurons, astrocytes, and oligodendrocytes (Jiao and Chen, 2008; Paintlia et al, 2005; Seifert et al, 2005; Wallace, 1999; Wallace and Raff, 1999; Wechsler-Reya and Scott, 1999). Double immunostaining data showed that Gli1+ cells colocalized with NG2+ oligodendrocyte progenitor cells (Figures 2M and 2N), implying that Gli1 activity was involved in oligodendrogenesis after BMSC treatment. These data suggest that BMSCs stimulate the production of Shh by parenchymal cells, evoke oligodendrogenesis, and lead to white matter remodeling in the injured brain.
Figure 2.
Double immunofluorescence staining (nuclei, DAPI, blue) showed that Shh+ cells (FITC, green) were reactive to NeuN+ (CY3, red) (A to D), GFAP+ (CY3, red) (E to H), and O4+ cells (CY3, red) (I to L). Confocal images show that the Gli1+ cells (merged, green) were NG2+ (merged, red) (M). A × 3 zoom (with orthogonal views shown above and to the right in highlighted boxes) of panel M reveals colocalization of NG2, Gli1, and DAPI (N). Bars=50 μm (A to D, E to H, and I to L); Bar=25 μm (M). Quantitative data (O) of BrdU immunostaining in N20.1 cells show that the proliferation rate of N20.1 cells cocultured with BMSCs significantly increased compared with normal cells (P < 0.05). The N20.1 cell proliferation significantly decreased after BMSC coculture plus cyclopamine treatment compared with BMSC coculture. RT-PCR analysis (P) shows the mRNA expression in N20.1 cells. mRNA expressions of NG2 and Gli1 were significantly increased after BMSC coculture compared with the normal N20.1 cells (P < 0.01), and cyclopamine treatment significantly reduces these increases induced by BMSC treatment. *P < 0.05, **P < 0.01 versus N20.1 cells; #P < 0.05, ##P < 0.01 versus N20.1 + MSCs.
BMSC Treatment Promotes Oligodendrocyte Proliferation in Cell Culture
As in vivo data suggest that activation of the Shh/Gli1 pathway induces oligodendrogenesis, and BMSC treatment after MCAo promotes Shh/Gli1 gene and protein expression leading to remyelination, we carried out an in vitro study to clarify the relationships among proliferation of N20.1 cells, the Shh/Gli1 pathway, and BMSC treatment. N20.1 cells are premature oligodendrocytes (Paez et al, 2004). Under 1% FBS and 39°C conditions, most of N20.1 cells exhibited a significantly decreased growth rate. BMSC treatment significantly increased the N20.1 cell proliferation, as indicated by BrdU immunostaining, compared with the normal medium group (P < 0.01, Figure 2O). BMSC-induced N20.1 cell proliferation was significantly reduced by cyclopamine, a pharmacological inhibitor of the Shh signaling pathway (P < 0.05). These data indicate that BMSCs induce N20.1 cell proliferation through the Shh pathway.
BMSC Treatment Activates the Shh/Gli1 Signaling Pathway in Oligodendrocyte Cell Culture
To test the hypothesis that the effect of BMSCs on oligodendrogenesis is mediated through the Shh/Gli pathways, we used RT-PCR to measure NG2 and Gli1 gene expressions. RT-PCR data showed that the NG2 mRNA level was increased significantly after 12 h of BMSC coculture, implying an increase in the proliferation of N20.1 cell. Gli1 mRNA expression was increased significantly in N20.1 cells after coculture with BMSCs. All these BMSC effects were blocked by the Shh/Gli1 pathway inhibitor, cyclopamine (Figure 2P). These data suggest that activation of the Shh/Gli1 pathway is involved in oligodendrogenesis, and BMSC treatment promotes this activation.
Discussion
BMSCs, including multipotential mesenchymal stem and precursor cells, have broad therapeutic applications to the treatment of neurologic diseases (Chopp and Li, 2002; Chopp et al, 2009). Functional recovery is evident after BMSC treatment of rodents with stroke from 14 days (Chen et al, 2001a, 2003; Shen et al, 2007; Zhang et al, 2004) and up to 1 year (Shen et al, 2007) after ischemic injury. BMSCs stimulate the production of restorative factors by parenchymal cells, evoke white matter remodeling in the injured brain, and lead to improvement in neurologic function post stroke (Chopp and Li, 2002; Chopp et al, 2009). Similar restorative benefits were also obtained using other cell sources for transplantation in stroke (Borlongan, 2009; Chen et al, 2001b). In this study, we show that BMSC treatment of rats subjected to MCAo induces oligodendrogenesis and activates the Shh/Gli1 pathway, which may contribute to functional recovery. We note, however, that our data do not show a causal relationship between oligodendrocyte proliferation and functional benefit. There are likely many factors that contribute to the BMSC-mediated functional improvement post stroke (Chen et al, 2002; Chopp and Li, 2002; Chopp et al, 2009; Qu et al, 2007), among which are angiogenesis, neurogenesis, and synaptogenesis (Chopp and Li, 2002; Chopp et al, 2009). As axonal integrity and myelination are necessary for brain function, and stroke often disrupts and damages white matter, it is reasonable to consider the BMSC-mediated induction of oligodendrogenesis as a potential contributing factor in promoting recovery post stroke. To our knowledge, this is the first study to show that a cell-based therapy stimulates oligodendrogenesis and that the Shh pathway may mediate this restorative process.
Oligodendrocytes are the only myelin-producing cells in the CNS that enable rapid electrical conduction of impulses, and they are vulnerable to the ischemic injury (Dewar et al, 2003; Domercq and Matute, 2004). The lack of oxygen and glucose, as well as free radicals, contributes to oligodendrocyte damage after stroke (Shibata et al, 2000). The death of oligodendrocytes after MCAo leads to demyelination, and axonal conduction will be subsequently impaired or lost (Dewar et al, 2003; McTigue and Tripathi, 2008; Micu et al, 2006).
In this study, we focus on the effects of BMSCs on oligodendrogenesis and myelin remyelination. Abundant oligodendrocyte progenitor cells (NG2+) are present in the adult CNS (McTigue and Tripathi, 2008). BrdU and NG2 double immunostaining showed oligodendrocyte progenitor cell proliferation in the CNS after MCAo, and BMSC treatment significantly enhanced this proliferation. This proliferation may contribute to the remyelination, which is suggested by the significantly increased O4+ cells and MBP+ area. These data suggests that BMSC treatment promotes oligodendrocyte progenitor cell proliferation and differentiation into myelinated oligodendrocytes. Remyelination is likely a requisite for functional recovery, and recruited oligodendrocytes can replace the damaged oligodendrocytes, remyelinate axons, and reduce axonal loss.
Shh plays a critical regulatory role in oligodendrogenesis and facilitates remyelination (Marti and Bovolenta, 2002; Murray et al, 2002; Nery et al, 2001; Orentas et al, 1999; Seifert et al, 2005; Sussman et al, 2002), and Gli1 is the principal effector of Shh (Ahn and Joyner, 2005; Wang et al, 2007). Our data show Shh and Gli1 mRNAs are present in the IBZ at 2 days, and protein expressions of Shh and Gli1 were present in the ipsilateral brain at 14 days after MCAo, and BMSC treatment significantly enhanced these expressions. Double immunostaining data suggest that the Shh protein expressed in neurons, astrocytes, and oligodendrocytes and in the Gli1 activity are involved in oligodendrogenesis. These data suggest that BMSC treatment-mediated enhancement of oligodendrogenesis may occur by promoting the production of Shh by parenchymal cells and activation of the Shh/Gli1 pathway in the ischemic brain. Also, our in vitro study shows that BMSCs stimulate N20.1 cell proliferation and Gli1 mRNA expression. Blockage of the Shh pathway with the Shh antagonist, cyclopamine, abolished the BMSC-induced proliferation of N20.1 cells, and also decreased Gli1 mRNA expression in N20.1 cells, implying that BMSCs promote oligodendrocyte proliferation by activation of the Shh/Gli1 pathway. These data suggest that activation of the Shh/Gli1 pathway underlies BMSC-induced oligodendrogenesis.
In summary, our data indicate that BMSC treatment promotes oligodendrogenesis and remyelination. Activation of the Shh/Gli1 pathway may underlie the restorative effects of BMSCs after MCAo in rats.
Acknowledgments
The authors thank Mr. Siamak Pourabdollah, Dr Lei Wang, Cynthia Roberts, Supata Santra and Qinge Lu for their technical assistance.
This work was supported by the NIH Grants P01 NS42345 and P01 NS23393 and by the Benson Ford Foundation.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–7. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Borlongan CV. Cell therapy for stroke: remaining issues to address before embarking on clinical trials. Stroke. 2009;40:S146–8. doi: 10.1161/STROKEAHA.108.533091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll WM, Jennings AR. Early recruitment of oligodendrocyte precursors in CNS demyelination. Brain. 1994;117(Part 3):563–78. doi: 10.1093/brain/117.3.563. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–12. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Chopp M. Intracerebral transplantation of bone marrow with BDNF after MCAo in rat. Neuropharmacology. 2000;39:711–6. doi: 10.1016/s0028-3908(00)00006-x. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–86. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001a;32:1005–11. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001b;32:2682–8. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–9. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y, Zhang ZG. Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke. 2009;40:S143–5. doi: 10.1161/STROKEAHA.108.533141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23:263–74. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- Domercq M, Matute C. Brain damage and repair. Netherlands: Springer; 2004. [Google Scholar]
- Garcia CI, Paez PM, Soto EF, Pasquini JM. Differential gene expression during development in two oligodendroglial cell lines overexpressing transferrin: a cDNA array analysis. Dev Neurosci. 2007;29:413–26. doi: 10.1159/000097317. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Jiang S, Ballerini P, Buccella S, Giuliani P, Jiang C, Huang X, Rathbone MP. Remyelination after chronic spinal cord injury is associated with proliferation of endogenous adult progenitor cells after systemic administration of guanosine. Purinergic Signal. 2008;4:61–71. doi: 10.1007/s11302-007-9093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Chen DF. Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells. 2008;26:1221–30. doi: 10.1634/stemcells.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–70. [PubMed] [Google Scholar]
- Komitova M, Perfilieva E, Mattsson B, Eriksson PS, Johansson BB. Enriched environment after focal cortical ischemia enhances the generation of astroglia and NG2 positive polydendrocytes in adult rat neocortex. Exp Neurol. 2006;199:113–21. doi: 10.1016/j.expneurol.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marti E, Bovolenta P. Sonic hedgehog in CNS development: one signal, multiple outputs. Trends Neurosci. 2002;25:89–96. doi: 10.1016/s0166-2236(02)02062-3. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–92. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Murray K, Calaora V, Rottkamp C, Guicherit O, Dubois-Dalcq M. Sonic hedgehog is a potent inducer of rat oligodendrocyte development from cortical precursors in vitro. Mol Cell Neurosci. 2002;19:320–32. doi: 10.1006/mcne.2001.1079. [DOI] [PubMed] [Google Scholar]
- Nery S, Wichterle H, Fishell G. Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development. 2001;128:527–40. doi: 10.1242/dev.128.4.527. [DOI] [PubMed] [Google Scholar]
- Orentas DM, Hayes JE, Dyer KL, Miller RH. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development. 1999;126:2419–29. doi: 10.1242/dev.126.11.2419. [DOI] [PubMed] [Google Scholar]
- Paez PM, Garcia CI, Davio C, Campagnoni AT, Soto EF, Pasquini JM. Apotransferrin promotes the differentiation of two oligodendroglial cell lines. Glia. 2004;46:207–17. doi: 10.1002/glia.20001. [DOI] [PubMed] [Google Scholar]
- Paintlia AS, Paintlia MK, Khan M, Vollmer T, Singh AK, Singh I. HMG-CoA reductase inhibitor augments survival and differentiation of oligodendrocyte progenitors in animal model of multiple sclerosis. FASEB J. 2005;19:1407–21. doi: 10.1096/fj.05-3861com. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Kwon EE, Goldenberg PZ, Ilyas AA, Quarles RH, Benjamins JA, Sprinkle TJ. Multiple sclerosis. Oligodendrocyte proliferation and differentiation in fresh lesions. Lab Invest. 1989;61:489–503. [PubMed] [Google Scholar]
- Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z, Chen X, Chopp M. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007;27:355–63. doi: 10.1111/j.1440-1789.2007.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361–72. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- Seifert T, Bauer J, Weissert R, Fazekas F, Storch MK. Differential expression of sonic hedgehog immunoreactivity during lesion evolution in autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 2005;64:404–11. doi: 10.1093/jnen/64.5.404. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A, Lu M, Savant-Bhonsale S, Chopp M. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke. 2007;38:2150–6. doi: 10.1161/STROKEAHA.106.481218. [DOI] [PubMed] [Google Scholar]
- Shibata M, Hisahara S, Hara H, Yamawaki T, Fukuuchi Y, Yuan J, Okano H, Miura M. Caspases determine the vulnerability of oligodendrocytes in the ischemic brain. J Clin Invest. 2000;106:643–53. doi: 10.1172/JCI10203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman CR, Davies JE, Miller RH. Extracellular and intracellular regulation of oligodendrocyte development: roles of Sonic hedgehog and expression of E proteins. Glia. 2002;40:55–64. doi: 10.1002/glia.10114. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Suzuki S, Dembo T, Kosakai A. Upregulation of oligodendrocyte progenitor cells associated with restoration of mature oligodendrocytes and myelination in peri-infarct area in the rat brain. Brain Res. 2003;989:172–9. doi: 10.1016/s0006-8993(03)03317-1. [DOI] [PubMed] [Google Scholar]
- Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–8. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- Wallace VA, Raff MC. A role for Sonic hedgehog in axon-to-astrocyte signalling in the rodent optic nerve. Development. 1999;126:2901–9. doi: 10.1242/dev.126.13.2901. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Gregg SR, Zhang RL, Jiao Z, LeTourneau Y, Liu X, Feng Y, Gerwien J, Torup L, Leist M, Noguchi CT, Chen ZY, Chopp M. The Sonic hedgehog pathway mediates carbamylated erythropoietin-enhanced proliferation and differentiation of adult neural progenitor cells. J Biol Chem. 2007;282:32462–70. doi: 10.1074/jbc.M706880200. [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic hedgehog. Neuron. 1999;22:103–14. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Chen J, Yang M, Katakowski M, Lu M, Chopp M. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004;1030:19–27. doi: 10.1016/j.brainres.2004.09.061. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Zheng X, Gao Q, Liu Z, Qu R, Borneman J, Elias SB, Chopp M. Bone marrow stromal cells protect oligodendrocytes from oxygen–glucose deprivation injury. J Neurosci Res. 2008;86:1501–10. doi: 10.1002/jnr.21617. [DOI] [PMC free article] [PubMed] [Google Scholar]