One or more members of the family of epidermal growth factor receptor (EGFR) genes are overexpressed or otherwise deregulated in virtually all epithelial tumors, including non–small cell lung cancers (NSCLC). This and related observations on the importance of protein phosphorylation and the discovery that the first identified oncogene, v-Src, is a protein kinase led John Mendelsohn and Gordon Sato to select EGFR as the first target of molecular targeted therapy more than 20 years ago (1, 2). EGFR family members are deregulated in cancers by the following three fundamental mechanisms: activating gene mutations, increased gene copy number (via amplification or polysomy), and altered ligand expression (with possible formation of autocrine loops; ref. 3). Two reports in this issue of the journal advance our understanding of the role of all three mechanisms in the pathogenesis and progression of NSCLC (4, 5). Before discussing these reports, however, we will present background information on EGFR signaling and its deregulation in cancers.
Reversible protein phosphorylation as a crucial regulator of many essential cell functions has been elucidated over the past 50 years. A superfamily of more than 500 highly conserved protein kinase genes contains about 2% of the genome (6). Specific kinases phosphorylate serine/threonine or tyrosine residues or have dual specificity. The tyrosine kinases, which catalyze the transfer of γ phosphate of ATP to tyrosine residues on protein substrates, fall into two classes: transmembrane receptors (receptor tyrosine kinase) and nonreceptors. Subclass I of the receptor tyrosine kinases is the EGFR family, which consists of four members: EGFR (or EGFR1, ERBB2, HER1), EGFR2 (or ERBB2, HER2), EGFR3 (or ERBB3, HER3), and EGFR4 (or ERBB4, HER4; ref. 3). Receptor-ligand interaction results in formation of homodimers or heterodimers (between family members), activation of the intrinsic kinase domain, and phosphorylation of specific tyrosine residues in the cytoplasmic tail of the receptor. The phosphorylated residues become docking sites for multiple proteins, which in turn activate downstream signaling pathways including the PI3K/AKT prosurvival, STAT transcription, and RAS/RAF/MEK proliferation pathways.
Eleven members of the EGF family have been identified as ligands for the EGFR family. HER2 is not ligand activated because of its unique extracellular spatial structure but is the preferred dimerization partner for other family members; its heterodimers preferentially enhance ligand binding (7). EGFR3 is “kinase dead” (i.e., it lacks intrinsic kinase activity) and, as with HER2, functions via heterodimerization. The EGF ligands show specificity for multiple homodimers or heterodimers (7). Epiregulin is a pan-EGFR family ligand that preferentially activates heterodimeric receptor complexes (8). The EGF ligands are produced as transmembrane precursors that are cleaved into their soluble forms by proteases (“sheddases”) of the ADAM family (especially ADAM10 and ADAM17) or by matrix metalloproteinases, a process known as ectodomain shedding (9). Other receptor pathways also may activate EGFR signaling by activating the EGFR pathway via “cross talk” and/or “transactivation.” An important new example of this with relevance to EGFR is the inflammatory cytokine interleukin-6, which activates the Janus-activated kinase/signal transducer and activator of transcription system, which in turn activates EGFR pathway signaling. High levels of interleukin-6 have been described in many cancers, including EGFR-mutant lung cancers, providing an additional method for EGFR activation and a new therapeutic target.
NSCLC cells can produce and release several of the EGF ligands (10–12). Under certain circumstances, the membrane-anchored isoforms and soluble growth factors also may act as biologically active ligands. Therefore, depending on the circumstances, these ligands may induce juxtacrine, autocrine, paracrine, and/or endocrine signaling (13). Establishing EGFR autocrine loops renders the cells sensitive to inhibition by tyrosine kinase inhibitors (10, 12). Zhou et al. (14) described the presence of an autocrine heregulin-EGFR3 loop associated with up-regulation of the sheddase ADAM10. Inhibiting ADAM10 with a specific inhibitor prevented the processing and activation of multiple EGF ligands. Recent reports indicate that breast and NSCLC cells (especially those with EGFR mutations) may produce large amounts of interleukin-6, activating another autocrine loop that drives tumorigenesis (15, 16).
Mutations of EGFR may target many regions of the gene, especially the extracellular domain in glioblastomas (17) and the kinase domain in lung cancers (18, 19). EGFR mutations may play a major role in lung tumorigenesis but also leave lung tumor cells dependent on EGFR signaling pathway activation for growth and survival (“oncogene addiction”; refs. 19, 20). Therefore, inhibition of EGFR signaling by tyrosine kinase inhibitors rapidly leads to apoptosis and growth cessation. In the 4 years since the discovery of the mutations, however, it was realized that primary tumor response and resistance to tyrosine kinase inhibitors are influenced by many factors, including mutations, mutation type, and copy numbers of EGFR; EGFR3 activation; KRAS mutations; MET amplification, and others (21–23). Therefore, although some studies (usually from single institutions analyzing highly selected patient populations) have shown very high response rates of EGFR-mutant tumors to tyrosine kinase inhibitors, large multi-institutional clinical trials have often failed to show a survival benefit of this approach, although increased copy number of EGFR (and HER2 in some series) was associated with a good treatment outcome (24, 25). Although EGFR mutations and copy number gains may occur independently, they occur together more frequently than alone (26, 27). In addition, as with glioblastomas (17), the mutant allele is preferentially amplified in such cases (26). Therefore, “triple whammy” tumors (i.e., those with mutations, copy number gains, and mutant allele-specific amplifications) are in all probability highly oncogene addicted and likely to show dramatic and sustained responses to appropriate targeted therapies. Autocrine loops and other derangements of EGFR signaling are frequent in all forms of NSCLC, which therefore may involve tumors with more than three EGFR aberrations, or “multiple whammy” tumors.
The finding that all of these different mechanisms activate EGFR signaling in lung cancers signifies the presence and great importance of strong selective pressures on the EGFR signaling pathway in these cancers. This selectivity was dramatically highlighted by the finding of EGFR tyrosine kinase domain mutations, but lung cancer use of all these alternative mechanisms is equally important in underscoring the key role of the EGFR pathway in driving lung cancer pathogenesis. Of course, these findings also highlight how versatile tumor cells are in finding ways to activate the pathway. On a related note, the relapse and subsequent drug resistance of lung cancers that had responded to EGFR-targeting drugs (such as EGFR tyrosine kinase inhibitors) show the resourcefulness of these cancers in finding other ways to use the EGFR or other pathways (e.g., KRAS, c-MET) to ward off extinction. Relapse and resistance also highlight the need for tools that can determine whether the pathway is active in and identify “sensitive” therapeutic target(s) for individual lung cancers. It is also important to realize that the target is constantly changing, and thus different therapeutic options are needed at different disease stages.
We now evaluate the contributions of the articles by Zhang et al. (4) and Tang et al. (5) in the context of the EGFR signaling background detailed above. Lung cancer has a high mortality that usually is due to the development of metastatic lesions. Although relatively few studies have directly compared the molecular changes in primary tumors with those in corresponding metastatic tumors, the metastatic phenotype is characterized by changes in multiple cellular pathways (28). The study by Zhang et al. (4) was stimulated by previous work from their laboratory showing that epiregulin is one of the several highly expressed EGF ligands in EGFR-mutant NSCLC cells (10). This group tested the hypothesis that epiregulin is involved in the development of the metastatic phenotype. Immunostaining studies confirmed their previous observation that primary NSCLC tumors with localized disease stages frequently (in 65% of cases) expressed the ligand. They reported a significant correlation between ligand expression and advanced nodal stage (stage II) and a trend toward shorter survival. In vitro studies confirmed the role of epiregulin in promoting tumor growth and invasion. These analyses show a clear role for epiregulin in tumor cell survival, invasion, and metastasis. Because epiregulin can stimulate multiple members of the EGFR receptor family, activation of both EGFR and EGFR3 signaling may contribute to carcinogenesis. Because ligand expression is much more frequent than are EGFR mutations or copy number gains, these findings provide further evidence that autocrine loops may be the major mechanism by which EGRF signaling is deregulated in all histologic forms of NSCLC. Future studies should comprehensively analyze all 11 EGF ligands found in lung cancers because other members of this ligand group may have similar tumor-promoting actions.
As mentioned earlier, EGFR mutations and copy gains occur frequently in the same tumors. Previous studies have shown widespread field effects throughout the respiratory epithelium of smokers (29, 30), suggesting that tobacco exposure damages the entire respiratory epithelium. Most EGFR mutations occur in lung cancers of lifetime never smokers, which have a largely unknown etiology (31). In their earlier work, the authors carefully microdissected histologically normal respiratory epithelium from small airways surrounding mutation-containing tumors (32); often present in airways within or near the tumor but seldom in distant sites, the mutations reflected a limited field effect. Therefore, exposure and damage seem to be much more limited in never smokers than in current or former smokers. In their present study, Tang et al. conducted a more extensive field study, assessing the presence of mutations and copy number gains (by fluorescence in situ hybridization technique) in primary NSCLC, corresponding metastases, and histologically normal respiratory epithelium. As in their previous study, mutations and EGFR protein overexpression were a localized field effect. The key present findings are that copy number gains were absent in normal epithelium and were distributed heterogeneously in primary tumors and more evenly in metastases. Tang et al. (5) have answered the question, “Which came first, the chicken (copy number gains) or the egg (mutations)?” The finding of mutant allele-specific gains gives the nod to the egg.
The prototype EGFR gene is not the only EGFR pathway gene amplified in NSCLC. A recent report describes amplification of other pathway members including HER2, SHC1, and AKT (33). Our unpublished work indicates that other pathway genes including KRAS and BRAF may also be amplified in NSCLC. Although mutations of pathway genes are usually mutually exclusive, single tumors may contain copy number gains for multiple genes or a single pathway mutation and one or more pathway gene copy number gains. 1
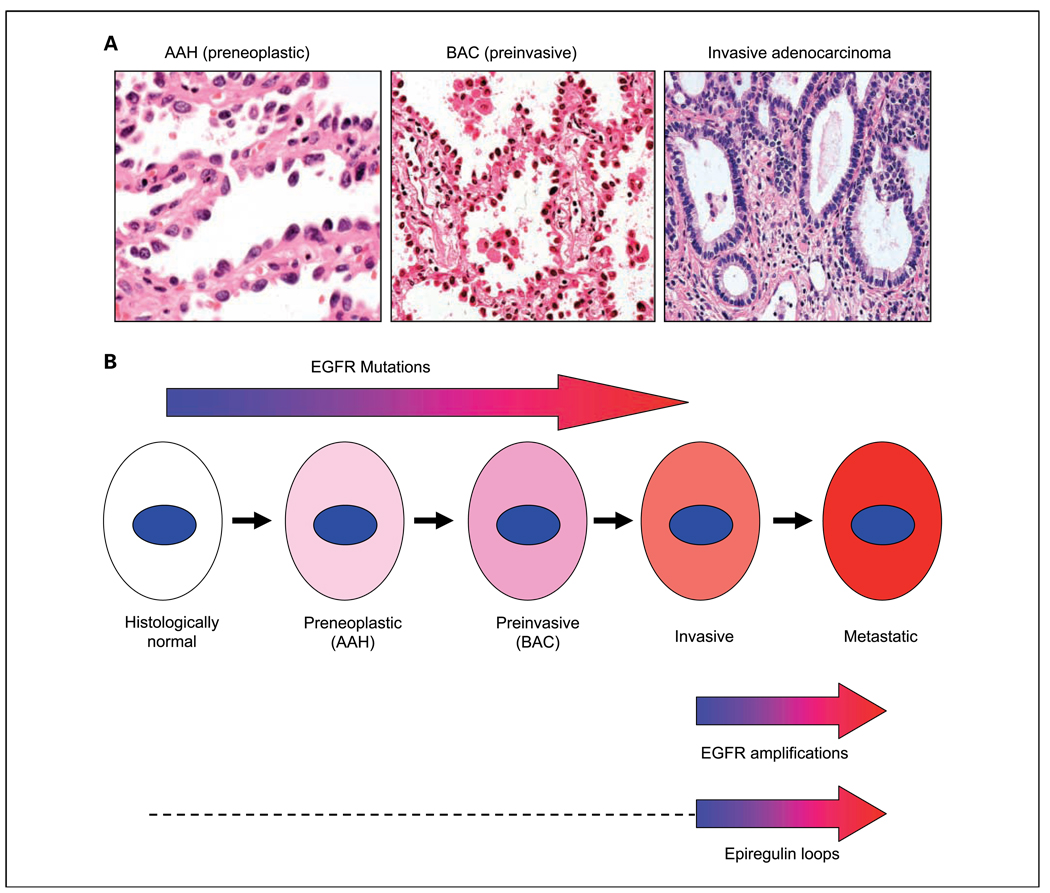
Two other recently published studies (34, 35) are consistent with the findings of Tang et al. (5). Cancers arise as a result of multistage processes, and a lesion known as atypical adenomatous hyperplasia is recognized as a precursor or premalignant lesion for peripheral lung adenocarcinomas. Atypical adenomatous hyperplasia lesions progress to noninvasive cancers known as bronchioloalveolar carcinomas as defined by the strict criteria of WHO classification (36). Bronchioloalveolar carcinoma tumors may become invasive and eventually metastatic. Early invasive cancers may contain invasive and noninvasive components that can be microdissected and examined separately. By examining the various stages of lung pathogenesis for EGFR mutations and copy gains, both reports (34, 35) conclude that mutations are early, preinvasive changes, whereas copy number gains are later events associated with the invasive phenotype (Fig. 1).
Fig. 1.
Deregulation of the EGFR gene during the multistage pathogenesis of peripheral lung adenocarcinomas. A, peripheral adenocarcinomas are believed to arise from preneoplastic lesions known as atypical adenomatous hyperplasias (AAH), which first progress to a preinvasive neoplastic stage called bronchioloalveolar carcinoma (BAC). Foci of invasion may develop in the fibrotic centers of bronchioloalveolar carcinomas, which then are called invasive adenocarcinomas, although noninvasive elements may persist at the edges of the tumors. Metastases ultimately develop (not shown). B, from the article by Tang et al. (5) and from the literature cited in the text, EGFR mutations commence early during pathogenesis and can be detected in histologically normal respiratory epithelium near tumors (localized field effect). Mutations are more frequent in preneoplastic (atypical adenomatous hyperplasia) and preinvasive (bronchioloalveolar carcinoma) stages than in normal epithelium. Therefore, there is relatively little heterogeneity of mutations in invasive carcinomas, and the mutations contribute to tumor pathogenesis. In contrast, gene copy number gains, often in the form of amplifications, commence relatively late in pathogenesis, usually at the tumor stage. They are more frequent in metastatic lesions, suggesting that they may be progression events involved in the metastatic phenotype. Much less is known about the timing of epiregulin loops (either autocrine, paracrine, or juxtacrine). From the data of Zhang et al. (4), however, it would seem that epiregulin loops can be detected in primary invasive tumors but are more frequent or active during the metastatic stage. The dashed line indicates that the timing of the appearance of these loops during earlier preinvasive stages is unknown.
All of these findings are consistent with the hypothesis that mutations precede copy number gains, which may be associated with the metastatic phenotype. Therefore, mutations are likely to show little or no heterogeneity in primary or metastatic tumors, and copy number gains may be absent or heterogeneously distributed in primary tumors and relatively evenly distributed within metastatic sites. Further work will be needed to confirm that copy number gains are part of the metastatic phenotype.
What are the clinical implications of these findings? The data of Zhang et al. (4) suggest that about two thirds of all NSCLCs express at least one of the EGF ligands. Testing the expression of the other 10 known ligands in this cohort presumably would have shown an even higher percentage. The expression of EGFR protein in most NSCLCs, including squamous cell carcinomas, raises the question of what mechanism causes deregulation. Mutations and copy number gains explain only a minority of these cases and probably are not important mechanisms in squamous cell carcinomas. As suggested by the data of Zhang et al. (4), activation of autocrine (or paracrine or juxtacrine) loops is an attractive alternative mechanism. If this loop is dependent on continued EGFR signaling and is inhibited by tyrosine kinase inhibitor therapy, as suggested by the data, this would be a plausible explanation for why some nonmutant tumors of all histologic types with nearly diploid copy number respond to tyrosine kinase inhibitor therapy (24, 37). Future retrospective and prospective studies are needed to determine whether EGF ligand expression is an additional predictive factor for tyrosine kinase inhibitor response. The concept that the driving force behind many or most NSCLC tumors is EGF ligand receptor loops offers the clinician the following additional avenues for potential targeted therapies: preventing sheddase up-regulation or activity, preventing ligand production directly or by inhibition of the loop at a more upstream stage, targeting the soluble form of the ligand, and preventing ligand-receptor interaction.
With the identification of deregulated expression of EGF family ligands in lung cancer pathogenesis, we can now consider using the relevant ligands for early cancer diagnosis, identifying key therapeutic targets, and as biomarkers to monitor response to chemoprevention or very early treatment. Because the ligands are soluble, they potentially could be detected in blood or bronchial lavage specimens in addition to biopsy and brushing specimens. Furthermore, while exploring their diagnostic and therapeutic targeting roles, we need to understand the molecular mechanisms leading to the deregulated expression of these ligands. Copy number changes, mutations, promoter alterations (including epigenetic changes), the role of specific transcription factors (such as the lineage-specific oncogene TITF1), and altered miRNA expression are all potential mechanisms that need to be explored, as does ligand expression in cancer stem cells.
Another major clinical interest is to understand the sequential appearance of molecular changes during multistage pathogenesis. The appearance of EGFR mutations at a preinvasive and even at a premalignant phase creates opportunities to use EGFR mutation markers for risk identification, early detection, and prevention, particularly for never smokers, who are at most risk for EGFR-mutant tumors and for whom no such markers currently exist (31). Early EGFR mutations also have important implications for the study of EGFR tyrosine kinase inhibitors in the adjuvant/second primary tumor prevention setting. Whereas mutations seem to be initiating events, copy number gains are related to progression and metastatic events. Therefore, heterogeneity may occur both within the primary tumor and between the primary tumor and metastatic sites. These considerations are important if copy number gains are used as a marker for selecting targeted therapies, and they indicate the importance of testing for this marker in tumor samples obtained immediately before therapy versus relying on marker data from earlier samples.
The reports of Zhang et al. and Tang et al. in this issue of the journal shed new light on the highly complex, multifaceted, and as yet incompletely understood nature of the EGFR signaling pathway. This pathway in NSCLCs and in the bronchial epithelium of patients at a high lung-cancer risk will be a critical focus of diagnostic, preventive, and therapeutic efforts for the foreseeable future.
Acknowledgments
Grant support: NCI Lung Cancer Specialized Program of Research Excellence grant P50CA70907, Early Detection Research Network, National Cancer Institute, and Department of Defense VITAL and PROSPECT grants.
Footnotes
Unpublished data.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Cohen P. Protein kinases-the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1:309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 2.Mendelsohn J. Antibody-mediated EGF receptor blockade as an anticancer therapy: from the laboratory to the clinic. Cancer Immunol Immunother. 2003;52:342–346. doi: 10.1007/s00262-002-0354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nature reviews. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Iwanaga K, Choi CC, et al. Intratumoral epirgulin is a marker of advanced disease in non-small cell lung cancer patients and confers invasive properties on EGFR-mutant cells. Cancer Prev Res. 2008 doi: 10.1158/1940-6207.CAPR-08-0014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang X, Varella-Garcia M, Xavier AC, et al. EGFR abnormalities in the pathogenesis and progression of lung adenocarcinoma. Cancer Prev Res. 2008 doi: 10.1158/1940-6207.CAPR-08-0032. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manning G, Plowman GD, Hunter T, Sudarsanam S. Evolution of protein kinase signaling from yeast to man. Trends Biochem Sci. 2002;27:514–520. doi: 10.1016/s0968-0004(02)02179-5. [DOI] [PubMed] [Google Scholar]
- 7.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 8.Shelly M, Pinkas-Kramarski R, Guarino BC, et al. Epiregulin is a potent pan-ErbB ligand that preferentially activates heterodimeric receptor complexes. J Biol Chem. 1998;273:10496–10505. doi: 10.1074/jbc.273.17.10496. [DOI] [PubMed] [Google Scholar]
- 9.Hynes NE, Schlange T. Targeting ADAMS and ERBBs in lung cancer. Cancer cell. 2006;10:7–11. doi: 10.1016/j.ccr.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto N, Wislez M, Zhang J, et al. High expression of ErbB family members and their ligands in lung adenocarcinomas that are sensitive to inhibition of epidermal growth factor receptor. Cancer Res. 2005;65:11478–11485. doi: 10.1158/0008-5472.CAN-05-1977. [DOI] [PubMed] [Google Scholar]
- 11.Volante M, Saviozzi S, Rapa I, et al. Epidermal growth factor ligand/receptor loop and down-stream signaling activation pattern in completely resected nonsmall cell lung cancer. Cancer. 2007;110:1321–1328. doi: 10.1002/cncr.22903. [DOI] [PubMed] [Google Scholar]
- 12.Wu W, O'Reilly MS, Langley RR, et al. Expression of epidermal growth factor (EGF)/transforming growth factor-α by human lung cancer cells determines their response to EGF receptor tyrosine kinase-inhibition in the lungs of mice. Mol Cancer Ther. 2007;6:2652–2663. doi: 10.1158/1535-7163.MCT-06-0759. [DOI] [PubMed] [Google Scholar]
- 13.Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal. 2005;17:1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Zhou BB, Peyton M, He B, et al. Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in non-small cell lung cancer. Cancer Cell. 2006;10:39–50. doi: 10.1016/j.ccr.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schafer ZT, Brugge JS. IL-6 involvement in epithelial cancers. J Clin Invest. 2007;117:3660–3663. doi: 10.1172/JCI34237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao SP, Mark KG, Leslie K, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frederick L, Eley G, Wang XY, James CD. Analysis of genomic rearrangements associated with EGRFvIII expression suggests involvement of Alu repeat elements. Neuro-oncol. 2000;2:159–163. doi: 10.1093/neuonc/2.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006;118:257–262. doi: 10.1002/ijc.21496. [DOI] [PubMed] [Google Scholar]
- 19.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nature reviews. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 20.Gazdar AF, Shigematsu H, Herz J, Minna JD. Mutations and addiction to EGFR: the Achilles 'heal' of lung cancers? Trends Mol Med. 2004;10:481–486. doi: 10.1016/j.molmed.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Engelman JA, Cantley LC. The role of the ErbB family members in non-small cell lung cancers sensitive to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2006;12:4372s–4376s. doi: 10.1158/1078-0432.CCR-06-0795. [DOI] [PubMed] [Google Scholar]
- 22.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–7241. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 24.Shepherd FA, Rosell R. Weighing tumor biology in treatment decisions for patients with non-small cell lung cancer. J Thorac Oncol. 2007;2 Suppl 2:S68–S76. doi: 10.1097/01.JTO.0000269737.05962.a0. [DOI] [PubMed] [Google Scholar]
- 25.Cappuzzo F, Varella-Garcia M, Shigematsu H, et al. Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive non-small-cell lung cancer patients. J Clin Oncol. 2005;23:5007–5018. doi: 10.1200/JCO.2005.09.111. [DOI] [PubMed] [Google Scholar]
- 26.Nomura M, Shigematsu H, Li L, et al. Polymorphisms, mutations, and amplification of the EGFR gene in non-small cell lung cancers. PLoS Med. 2007;4:e125. doi: 10.1371/journal.pmed.0040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okabe T, Okamoto I, Tamura K, et al. Differential constitutive activation of the epidermal growth factor receptor in non-small cell lung cancer cells bearing EGFR gene mutation and amplification. Cancer Res. 2007;67:2046–2053. doi: 10.1158/0008-5472.CAN-06-3339. [DOI] [PubMed] [Google Scholar]
- 28.Minn AJ, Gupta GP, Siegel PM, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao L, Lee JS, Kurie JM, et al. Clonal genetic alterations in the lungs of current and former smokers [see comments] J Natl Cancer Inst. 1997;89:857–862. doi: 10.1093/jnci/89.12.857. [DOI] [PubMed] [Google Scholar]
- 30.Wistuba II, Lam S, Behrens C, et al. Molecular damage in the bronchial epithelium of current and former smokers. J Natl Cancer Inst. 1997;89:1366–1373. doi: 10.1093/jnci/89.18.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers—a different disease. Nat. Rev. 2007;7:778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 32.Tang X, Shigematsu H, Bekele BN, et al. EGFR tyrosine kinase domain mutations are detected in histologically normal respiratory epithelium in lung cancer patients. Cancer Res. 2005;65:7568–7572. doi: 10.1158/0008-5472.CAN-05-1705. [DOI] [PubMed] [Google Scholar]
- 33.Lockwood WW, Chari R, Coe BP, et al. DNA amplification is a ubiquitous mechanism of oncogene activation in lung and other cancers. Oncogene. 2008 doi: 10.1038/onc.2008.98. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soh J, Toyooka S, Ichihara S, et al. Sequential molecular changes during multistage pathogenesis of small peripheral adenocarcinomas of the lung. J Thorac Oncol. 2008;3:340–347. doi: 10.1097/JTO.0b013e318168d20a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yatabe Y, Takahashi T, Mitsudomi T. Epidermal growth factor receptor gene amplification is acquired in association with tumor progression of EGFR-mutated lung cancer. Cancer Res. 2008;68:2106–2111. doi: 10.1158/0008-5472.CAN-07-5211. [DOI] [PubMed] [Google Scholar]
- 36.Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18:1059–1068. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- 37.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]