Abstract
In this work, we describe a novel polyamidoamine (PAMAM) dendrimer hydrogel (DH) platform with potential for tissue engineering and drug delivery. With PAMAM dendrimer G3.0 being the underlying carrier, polyethylene glycol (PEG) chains of various lengths (MW=1500, 6000, or 12000 gmol−1) were coupled to the dendrimer to different extents, and the resulting PEGylated PAMAM dendrimers were further coupled with acrylate groups to yield photoreactive dendrimer macromonomers for gel formation. It was found that gelation based on photoreactive PAMAM G3.0 macromonomers was restricted by the degree of PEGylation, PEG chain length, and the distribution of acrylate groups on the dendrimer surface. Further, the architecture of the photoreactive macromonomers affects the structural stability and swelling of the resultant networks. A completely crosslinked network (DH-G3.0–12000H) with a high water swelling ratio was created by UV-curing of PAMAM dendrimer G3.0 coupled with 28 PEG 12000 chains in the presence of the eosin Y-based photoinitiating system. The disintegration of DH-G3.0–12000H was pH-insensitive. DH-G3.0–12000H was found to have similar cytocompatibility to uncrosslinked G3.0–12000H but have a significantly lower cellular uptake by macrophages. With PAMAM dendrimer G3.5 being the underlying carrier, the dendrimer modified with 43 PEG 1500 chains was able to form a completely crosslinked network (DH-G3.50–1500H) by UV-curing in the presence of the eosin Y-based photoinitiating system. DH-G3.50–1500H exhibited pH-dependent disintegration. Its disintegration ratio increased with pH. PAMAM dendrimer hydrogels uniquely express the structural characteristics of both PEG hydrogel and PAMAM dendrimer and have potential for various applications in tissue engineering and drug delivery.
Keywords: amphiphilic hydrogel network, dendrimer, dendritic PEG acrylate, PEGylation, tissue engineering
Introduction
Dendrimers possess a well-defined highly branched nanoscale architecture with many reactive surface groups. Numerous biomedical applications such as drug delivery have been explored based on dendrimers because the size, geometry, and building blocks of dendritic structures, as well as the functionality and quantity of their surface groups, can be finely modulated.1–6 Drug molecules can be either physically entrapped inside the dendritic structure or covalently attached onto the surface. Their highly clustered surface groups allow targeted drug delivery and high drug payloads that would enhance therapeutic effectiveness. Further, dendrimers are an ideal type of platform for combined delivery of diagnostic and therapeutic agents, namely, theranostics. Nonetheless, dendrimer-based biomedical applications rely heavily on the construction of nanostructured bioactive dendritic delivery systems. Recently, it has drawn attention to employ dendritic polymers to synthesize hydrogels.7–10 Hydrogels are crosslinked insoluble networks of polymer chains that swell in aqueous solutions. Hydrogels are useful in drug delivery and tissue engineering because of their biocompatibility, high water content, low surface tension, hydrodynamic properties similar to those of natural biological gels and tissues, and their minimal mechanical irritation due to their soft and rubbery state.
The multifunctionality of dendrimers makes themselves a new class of crosslinking agent for producing crosslinked networks, which have been demonstrated in previous studies.11, 12 Photocrosslinkable dendrimers have also been explored to generate tissue adhesive scaffolds for tissue engineering applications. Grinstaff and coworkers synthesized aliphatic polyester-ether hybrid dendritic-linear polymers and reported that these new dendritic structures could be further coupled with photoreactive groups to generate tissue adhesive dendritic gels with potential application in corneal laceration repair.8–10 The emergence of dendrimer-based scaffolds suggested a new direction for the design of tissue engineering scaffolds and drug delivery systems.13 It has been demonstrated that the inclusion of dendritic polymers affords hydrogels with better control over chemical, physical, and biological properties on the basis of their versatile structures. Due to many potential applications, there is an ongoing need to develop dendritic hydrogels with increasingly flexible architectures, which are capable of being adapted to a variety of uses and conditions.
Being the most investigated family of dendrimers, polyamidoamine (PAMAM) dendrimers have been exhaustively evaluated and utilized for targeted drug and gene delivery, diagnosis, and imaging.13–16 Beyond the role of crosslinking agent previously explored for PAMAM dendrimers,12 we designed a new enabling PAMAM dendrimer hydrogel platform with combined structural characteristics and properties of dendritic nanoparticle and hydrogel, which can be tailored for broad applications. In this work, we explored the synthesis and characterization of PAMAM dendrimer hydrogels based on amine-terminated PAMAM dendrimer G3.0 and carboxyl-terminated PAMAM dendrimer G3.5. PEG was used as spacer to facilitate with crosslinking of individual dendrimer molecules. Covalent attachment of PEG to the dendrimer was on the basis of its many useful properties such as biocompatibility, stealth properties, prevention of protein absorption, etc. Photopolymerizable acrylate end groups were introduced to the PEGylated dendrimers for crosslinking dendrimers. One of the envisioned compelling characteristics of the PAMAM dendrimer hydrogel networks is that they allow simultaneous delivery of both hydrophobic and hydrophilic drugs (Figure 1). In particular, the interior hydrophobic core of the dendrimer would encapsulate hydrophobic compounds, thus increasing their water solubility and loading amounts, while the crosslinked PEG network enables the encapsulation of hydrophilic drugs. In addition, the surface charges conferred by terminal groups on the dendrimer surface can be modulated to make the hydrogel polyionic with controllable charge density. Additionally, their unique spatial structure and configuration with high adaptability may present themselves a suitable platform for fine tuning of cell-scaffold interactions in tissue engineering. The focus of this work was to identify optimal conditions for gel formation, elucidate the impact of dendritic structure on gelation, and study the properties of the formed hydrogels.
Figure 1.
Schematic of a crosslinked PAMAM dendrimer network.
Experimental Section
Materials
Polyethylene glycol diol (OH-PEG-OH) (MW=1500, 6000, and 12000 g/mol), triethylamine (TEA), 1-vinyl-2 pyrrolidinone (NVP), 4-nitrophenyl chloroformate (NPC), ethyl ether(anhydrous), N,N-dimethylformamide (DMF), ethanol, fluorescein isothiocynate (FITC), ninhydrin, eosin Y, triethanolamine (TEOA), dimethoxyphenyl acetophone (DMPA), tetrahydrofuran (THF), hydrochloric acid (HCl), N,N'-dicyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine (DMAP), phosphate buffer solution (PBS), and sodium hydroxide were purchased from Sigma-Aldrich (St. Louis, MO). PAMAM dendrimers G3.0 and G3.5 were purchased from Dendritech (Midland, MI). Irgacure 2959 was given by Ciba Corporation (Newport, DE).
Synthesis of Photoreactive Dendrimer Macromonomers
G3.0-PEG-acrylate
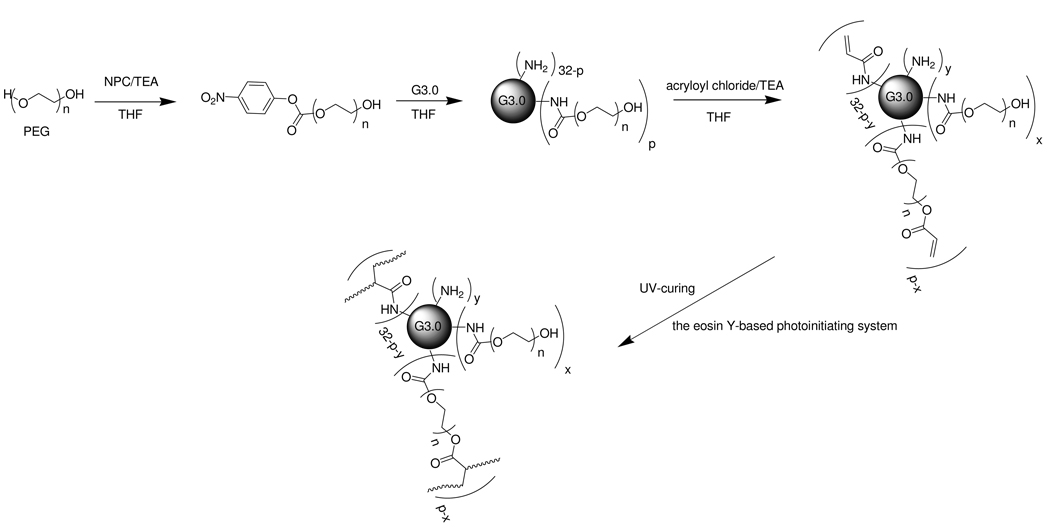
As illustrated in Scheme 1, one hydroxyl end group of PEG diol (MW=1500, 6000, or 12000 g·mol−1) was activated with NPC and TEA to form OH-PEG-NPC conjugates. Briefly, PEG diol was dissolved in a large volume of THF. To this solution were added equimolar amounts of NPC and TEA. The mixture was stirred for 24 hours, and then centrifuged to remove salt. The resultant OH-PEG-NPC conjugates were precipitated in cold ether, collected, and freeze dried. OH-PEG-NPC was then reacted with PAMAM dendrimer G3.0 in DMF for 72 hours where the molar feed ratio of OH-PEG-NPC/dendrimer was 32:1. The resulting G3.0-PEG-OH was precipitated in cold ethyl ether and then dialyzed for purification. To introduce photoreactive acrylate groups to PEGylated PAMAM dendrimer G3.0, G3.0-PEG-OH was dissolved in THF. Acryloyl chloride and TEA were slowly added to the solution. The solution was stirred for 4 hours to generate G3.0-PEG-acrylate. The molar feed ratio of PEG coupled to the dendrimer: acryloyl chloride: TEA was 1:4:6. Following the removal of the salt through centrifugation, G3.0-PEG-acrylate was precipitated in cold ethyl ether, dialyzed against deionized water, and freeze dried. Linear PEG acrylate (MW=1500 g·mol−1) was also synthesized by heterobifunctionalizing PEG diol with acryloyl chloride and used as a control for hydrogel formation.
Scheme 1.
Synthesis of photoreactive G3.0-PEG-acrylate macromonomer and its photoinitiated crosslinking reaction.
G3.5-PEG-acrylate
As shown in Scheme 2, PEG diol (MW=1500 g/mol) was directly conjugated to half generation PAMAM dendrimer G3.5 following the DCC/DMAP coupling chemistry detailed our previous work 17. The molar feed ratio of PEG diol: DCC: DMAP: G3.5 was 64:64:64:1. Photoreactive acrylate groups were introduced to G3.5-PEG-OH following the procedure described above. Upon the removal of the salt produced in the reaction, the resultant G3.5-PEG 1500-acrylate was precipitated in cold ethyl ether, dialyzed against deionized water, and freeze dried. G%-#p denotes photoreactive dendrimer macromonomer, where % is the generation of PAMAM dendrimer (3.0 or 3.5), # is the molecular weight of PEG (gmol−1), and subscript p indicates the level of the degree of PEGylation (H (high) or L (low)).
Scheme 2.
Synthesis of photoreactive G3.5-PEG-acrylate macromonomer and its photoinitiated crosslinking reaction.
Preparation of Dendrimer Hydrogels
Three types of photoinitiators (i.e., DMPA, Irgacure 2959, and eosin Y-based photoinitiating system) were attempted for forming dendrimer hydrogels. For each trial, 100 µL of dendrimer-PEG acrylate solution (7.5–40 wt.%) was mixed with either 2 mg of DMPA, 2 mg of Irgacure 2959, or 5 µL solution of an eosin Y photoinitiator solution containing eosin Y (0.1 wt.%), TEOA (40 wt.%), and NVP (4 wt.%),18 and then subjected to UV radiation at 325 nm for up to 30 minutes. Hydrogel formation (i.e., sol-gel phase transition) was estimated using the inverted test tube method.19 DH-G%-#p denotes each UV-cured sample where % is the generation of PAMAM dendrimer (3.0 or 3.5), # is the molecular weight of PEG (gmol−1), and subscript p indicates the level of the degree of PEGylation (H (high) or L (low)).
Characterization
1H NMR Spectroscopy
The 1H NMR spectra of dendrimer derivatives were recorded on a 300 MHz NMR spectrometer (Mercury-300). The solvent used was deuterium water (D2O), which has a chemical shift of 4.8 ppm.
1H-NMR for G3.0-PEG-OH (300 MHz, D2O): δ 3.6 ppm, methylene of PEG; 2.4–3.4 ppm, methylene of G3.0. According to the integration of corresponding proton peaks, the degrees of PEGylation (p) were determined and listed in Table 1.
Table 1.
Dendrimer hydrogels and qualitative description on their flow property.
| Dendrimer hydrogel (DH-G%-#p)a |
Generation of dendrimer (%) |
PEG length (#, gmol−1) |
Degree of PEGylation b |
Flow property c |
|---|---|---|---|---|
| DH-G3.0–1500L | 3.0 | 1500 | 4 | − |
| DH-G3.0–1500H | 3.0 | 1500 | 29 | + |
| DH-G3.0–6000L | 3.0 | 6000 | 3 | − |
| DH-G3.0–6000H | 3.0 | 6000 | 23 | + |
| DH-G3.0–12000L | 3.0 | 12000 | 3 | − |
| DH-G3.0–12000H | 3.0 | 12000 | 28 | ++ |
| DH-G3.5–1500H | 3.5 | 1500 | 43 | ++ |
The macromonomer solutions (7.5 wt%) were mixed with the eosin Y-based photoinitiating system and exposed to UV light (325 nm) for 30 minutes.
determined by 1H-NMR.
−, neither increase in the viscosity of the solution nor gel formation was observed; +, viscosity of the solution increased; however, no gel formed; ++, gel formed determined by the inverted test tube method.
1H-NMR for G3.5-PEG-OH (300 MHz, D2O): δ 3.6 ppm, methylene of PEG; 2.4–3.4 ppm, methylene of G3.5. The degree of PEGylation for G3.5-PEG1500-OH was 43 per dendrimer.
Scanning Electron Microscopy (SEM)
SEM images were taken under a scanning electron microscope ZESIS EVO50. Each freeze-dried sample was mounted on the stub over a double sided sticky carbon sheet, and then the stub with the mounted sample was coated with gold for 2 minutes and 40 seconds at 30 milliamps. The gold plated stub was placed inside the SEM chamber under high vacuum for image taking.
Water Adsorption Kinetics
Water adsorption of dendrimer hydrogels as a function of time was determined. Each weighed dry sample was immersed into PBS (pH 7.4). The sample was taken out from the swelling medium, blot dried, and weighed at different time intervals until a constant weight was reached. Alternatively, when the swollen sample became viscous or disintegrated, the sample was subjected to centrifugal ultrafiltration (MWCO 300) at a low speed to remove unbound water prior to weighing. Each water adsorption measurement was carried out over a period of 24 hours. The water adsorption ratio of the sample was calculated using the following equation: Water adsorption ratio , where Ws is the weight of swollen sample and Wd is the weight of dry sample. The reported values of water uptakes were averaged over at least three measurements.
pH-Dependent Hydrogel Disintegration Study
Freeze-dried hydrogel samples were weighed and incubated at room temperature in 2 mL-centrifuge tubes containing media of different pHs (i.e., pH 2 (prepared with HCl), pH 4.4 (prepared with HCl), pH 7.4 (prepared with PBS), and pH 10 (prepared with NaOH)). At 24 hours, centrifugation was carried out. The sample residue from the bottom portion of the centrifuge tube was freeze dried and weighed to determine the disintegration degree. The disintegration extent was calculated using the following equation: Disintegration , where Wd0 is the weight of initial dry sample, Wd24h is the weight of freeze dried sample after 24 hours of incubation in the medium.
Cytotoxicity and Cellular Uptake Studies
The cytotoxicity and cellular uptake of uncrosslinked or crosslinked G3.0-PEG-acrylate were examined in vitro using RAW264.7 mouse macrophages. Prior to the tests, G3.0-PEG-acrylate was labeled with florescent dye, i.e. FITC. Briefly, G3.0-PEG-acrylate was dissolved in 2 mL of PBS buffer, and FITC was dissolved in 1 mL of methanol. The FITC solution was added dropwise to the PBS solution of G3.0-PEG acrylate, in which the molar ratio of amine groups of dendrimer to FITC was 1:1.25. This mixture solution was then stirred in dark for 24 hours. Dialysis was then performed to remove the excess amount of FITC. The solution was freeze dried to obtain FITC labeled G3.0-PEG-acrylate.
The macrophages were seeded in a 24-well cell culture plate (1×103 cells/well) at 37 °C in 1 mL of growth medium (DMEM medium supplemented with 10% fetal calf serum, 100 UI/ml penicillin-streptomycin) in an atmosphere of 10% CO2. After 24 hours, the culture medium was replaced with fresh growth medium containing different concentrations of uncrosslinked or crosslinked G3.0-PEG-acrylate (i.e., 0.2, 2, 20, 50, or 100 µM). Fluorescence images of FITC-labeled dendrimers taken up by the cells were obtained from a Zeiss Axiovert 200 inverted fluorescence microscope at predetermined time intervals up to 24 hours. Cell viability at 48 hours post-incubation was determined using the Trypan blue assay.
Statistical Analysis
The data were expressed as means ± SD. Statistical evaluation of the data was performed by analysis of variance (ANOVA) followed by Student’s t-test for pairwise comparison of subgroups. Differences among means were considered statistically significant at a p value of <0.05.
Results and Discussion
Preparation and Characterization of Dendrimer Hydrogels (DHs)
Photoreactive PEGylated dendrimer macromonomers of various PEG chain lengths and degrees of PEGylation were synthesized and studied for gel formation. The synthesis and characterization of PEGylated PAMAM dendrimers were detailed in our previous work.17, 20–23 According to 1H-NMR measurements, PEGylated G3.0 macromonomers G3.0–1500L or H, G3.0–6000L or H, G3.0–12000L or H, and G3.5–1500H (Table 1) were obtained and then coupled with photoreactive acrylate groups for photoinitiated hydrogel formation. Three commonly used photoinitiators, DMPA, Irgacure 2959, and the eosin Y-based photoinitiating system, were used to initiate the crosslinking reaction. Initial screening of photoinitiators was based on highly PEGylated PAMAM dendrimer G3.0 macromonomers (i.e., G3.0–1500H, G3.0–6000H, and G3.0–12000H). Linear PEG 1500-acrylate was studied as control.
Linear PEG 1500-acrylate was crosslinked to form a hydrogel network by any one of the three photoinitiators studied as confirmed by the inverted test tube method. However, highly PEGylated G3.0-PEG-acrylate, regardless of PEG length, could not be crosslinked to form hydrogels by UV-curing in the presence of DMPA or Irgacure 2959. Although both DMPA and Ciba Irgacure 2959 are efficient photoinitiators for UV-curing of most unsaturated monomers or macromonomers such as linear PEG acrylate, the inefficiency of DMPA and Irgacure 2959 in UV-curing of PEGylated PAMAM dendrimer macromonomers remains to be elucidated. It is presumably attributed to the clustering of acrylates by dendrimers and the incomplete modification of PEG with acrylate, hence spatially restricting photoreactive acrylate groups to photoinitiator molecules such as DMPA and Irgacure 2959 in the crosslinking reaction. In contrast, the application of the eosin Y-based photoinitiating system successfully initiated the crosslinking reaction of G3.0–12000H, leading to hydrogel formation as shown in Figure 2. Although the use of the eosin Y-based photoinitiating system did not succeed in forming a “no flow” gel of DH-G3.0–1500H or DH-G3.0–6000H, a significant increase in the viscosity of their macromonomer solutions was noticed.
Figure 2.
Dendrimer hydrogel G3.0-DH–12000H formed on a Teflon substrate (image taken by digital camera).
Sontjens et al. successfully applied the eosin Y-based photoinitiating system to crosslink dendritic macromonomers constituting of a poly(ethylene glycol) core and methacrylated poly(glycerol succinic acid) dendrimer terminal blocks (i.e., PEG3400-(PGLSA-MA4)2).18 Our initial screening study confirmed that the eosin Y-based photoinitiating system is an effective photoinitiator for crosslinking star-shaped photoreactive polymers including ours. Further, our results imply that PEG length affects the ability of PEGylated dendrimers to crosslink. A completely crosslinked network would be enabled by dendrimers coupled with long PEG chains, e.g., G3.0–12000H. PAMAM G3.0 tethered with short PEG chains may only be partially crosslinked with the eosin Y-based photoinitiating system, which is indicated by the increased viscosity of their macromonomer solutions after UV exposure. SEM micrographs were obtained to further analyze the structures of the crosslinked PEGylated dendrimers in comparison with the crosslinked PEG 1500-acrylate. The formation of an isotropic hydrogel network based on linear PEG 1500-acrylate is illustrated in Figure 3A. Due to the gluey property of the resultant hydrogels, it became extremely difficult to completely remove water from the dendrimer hydrogel samples even through extended freeze-drying. Thus, an attempt to visualize these hydrogels at higher magnifications was unsuccessful. Nonetheless, the microscopic difference in morphology of the dendrimer hydrogels taken at the current magnification could still be graphically ascertained and correlated to their network structures (Figure 3B, 3C, and 3D). In contrast to a loose structure of DH-G3.0–1500H as shown in Figure 3B, numerous submicron colonies are found in DH-G3.0–6000H (Figure 3C). This is likely to be the evidence of the formation of a partially or locally crosslinked network by G3.0–6000H. Impressively, DH-G3.0–12000H formed an anisotropic network without colonies, suggesting the formation of a completely crosslinked network (Figure 3D).
Figure 3.
SEM micrographs of crosslinked PEG 1500-acrylate (A), DH-G3.0–1500H (B), DH-G3.0–6000H (C), and DH-G3.0–12000H (D).
Based on the conditions employed in this work, the synthesized photoreactive G3.0-PEG-acrylate with low degrees of PEGylation were unable to form crosslinked hydrogel networks or lead to the increase in the viscosity of the UV-cured solutions (Table 1). This observation indicates that not only the PEG chain length affects the formation of crosslinked networks but the degree of PEGylation and distribution of acrylates on the dendrimer surface are instrumental in dendrimer hydrogel formation. Longer PEG chains tethered with acrylates and a higher degree of PEGylation would enhance the accessibility of acrylate to one another for crosslinking. Nonetheless, the impact of PEG length on the formation of DH-G3.5–1500H became negligible as compared with DH-G3.0–1500H. A “no-flow” gel of DH-G3.5–1500H was confirmed by the inverted test tube method. We believe the major cause of this opposite outcome was because of a better control over the coupling site of acrylate in G3.0–1500H. In order to introduce photoreactive acrylates to the dendrimer, acryloyl chloride reacts with the hydroxyl end groups of PEG chains tethered to G3.0 or G3.5 (Scheme 1 and Scheme 2). However, acryloyl chloride can also react with free amine surface groups of G3.0 (Scheme 1). Therefore, the extent of PEG chains coupled with acrylates in G3.0–1500H was less than in G3.5–1500H, resulting in its failure to form a crosslinked network. In comparison with the ease of hydrogel formation of linear PEG acrylate, hydrogel formation based on dendritic PEG acrylate is restricted by the degree of PEGylation, PEG chain length, the distribution of acrylate on the dendrimer surface as well as types of photoinitiators.
Swelling and Disintegration Behaviors
Swelling kinetics of dendrimer hydrogels was studied to elucidate the potential effect of compositions on the swelling behaviors of the formed crosslinked networks. Despite the fact that G3.0–1500H and G3.0–6000H were only partially crosslinked, they were still capable of adsorbing water. As shown in Figure 4, the maximal swelling ratio of all the dendrimer hydrogels was quickly reached in 15 minutes. This fast swelling property was enabled by the presence of a crosslinked hydrophilic PEG network. Particularly, DH-G3.0–1500H reached the maximum swelling ratio (i.e., 30%) within 15 minutes and then its swelling ratio quickly declined to about only 3% at 6 hours and remained constant thereafter. The maximum swelling ratio of DH-G3.0–6000H was 136%; however, its swelling ratio decreased to 40 % at 6 hours and remained constant thereafter. DH-G3.0–12000H displayed a maximum swelling ratio of 240%, which was highest among the tested dendrimer hydrogels. The crosslinked PEG 1500-acrylate displayed a maximum swelling ratio of 390%, higher than the swelling ratio of any synthesized dendrimer hydrogel. These results indicate that the rank of the equilibrium swelling ratios of the hydrogels is consistent with the structural stability of their networks, which is primarily dependent on the architecture of photoreactive macromonomers as discussed earlier. The fact that DH-G3.0–1500H and DH-G3.0–6000H underwent an initial increase in water uptake followed by a significant drop was attributed to the depletion of water, which coincidentally occurred while the network was destabilized during the swelling process.
Figure 4.
Effect of PEG length on the swelling kinetics of DHs based on PAMAM dendrimer G3.0.
In general, a polymeric network possessing carboxylic acid or amine pendant groups is capable of changing its swelling and disintegration behaviors in response to pH change. For instance, the ionization of carboxylic acids at high pH or amines at low pH would make the polymer more hydrophilic, resulting in quicker swelling and/or disintegration 24. Controlled drug release can also be realized through changes in environmental pH. Since DH-G3.0–12000H and DH-G3.5–1500H formed “no flow” gels, the dependence of their disintegration on pH was studied. Previous studies have demonstrated polymer chains with terminal acrylate groups are able to form bioerodible gels, which degrade by hydrolysis of ester bonds 25. The DH-G3.0–12000H network contains a number of hydrolytic ester and urethane linkages (Scheme 1). Although the urethane linkages can undergo hydrolysis, the ester linkages are believed to be the primary site of hydrolysis responsible for the chain scission-induced destruction of the network 26, 27. The DH-G3.5–1500H network owns ester linkages (Scheme 2). Therefore, the ester linkages and/or the urethane linkages in the dendrimer hydrogel networks can be readily broken to release oligo(acrylic acid) and PEG components. Oligo(acrylic acid) is water soluble and nontoxic.25
As expected, the disintegration of PEG 1500 acrylate hydrogel was not affected by pH and invariably lost its 45% of mass at the chosen pHs following 24 hours of incubation (Figure 5). It was shown that the disintegration of DH-G3.0–12000H was pH independent as well. At 24 hours, DH-G3.0–12000H constantly lost its 49% of mass at the chosen pHs. Its pH-insensitivity was due to the significant consumption of amine surface groups by a high degree of PEGylation (88% of the dendrimer surface sites) and acrylation of some of the remaining amine surface groups. As discussed earlier, a structurally adaptive feature of dendrimer hydrogels is that the amine groups on the dendrimer surface contribute to the pH sensitivity of the crosslinked dendrimer network. Therefore, modulating of the degree of PEGylation on the dendrimer surface provides a means to generate a pH-sensitive network with a better control over its disintegration, swelling kinetics, etc. In addition, a second polymer may be required to formulate semi-interpenetrating networks with PEGylated dendrimers for structural stability enhancement. In contrast, the disintegration ratio of DH-G3.5–1500H showed pH-dependence as shown in Figure 6. Its disintegration ratio increased with pH. More specifically, DH-G3.5–1500H had the highest disintegration ratio of 47% at pH 10 and the lowest disintegration ratio of 4% at pH 2. As a result, the disintegration ratio was reduced by 91% as pH dropped from pH 10 to pH 2. According to our characterization, DH-G3.5–1500H has one third of the carboxylate groups remained, which are responsible for the pH-dependent disintegration of the gel.
Figure 5.
Effect of pH on the disintegration of DH-G3.0–12000H and cross-linked PEG 1500-acrylate.
Figure 6.
Effect of pH on the disintegration of DH-G3.5–1500H.
Cytotoxicity and Cellular Uptake
The cytotoxicity and cellular uptake of uncrosslinked and crosslinked G3.0–12000H-acrylate (FITC-labeled) were evaluated using the RAW264.7 mouse macrophages. Consistent with our previous results, uncrosslinked G3.0–12000H-acrylate displayed dose-dependent cytotoxicity. It has a negligible toxic effect on RAW264 mouse macrophages at concentrations of 0.2 µM or below (Figure 7). The cytotoxicity level of crosslinked G3.0–12000H (i.e., DH-G3.0–12000H) was similar to that of uncrosslinked G3.0–12000H. DH-G3.0–12000H did not show improved cytocompatibility possibly because of the quick disintegration of the network, thus generating internalizable degradation products in amounts equivalent to G3.0–12000H. The higher the concentration of G3.0–12000H and DH-G3.0–12000H, the more the potential of their internalization by cells. Consequently, the increased toxicity of G3.0–12000H and DH-G3.0–12000H was observed at higher concentrations. It must be noted that the photoinitiator used for crosslinking shall be nontoxic as it may leech out of the hydrogel network. The eosin Y-based photoinitiating system has been reported to be nontoxic 9. Its nontoxicity was confirmed in this work as the extent of the toxicity of crosslinked dendrimer hydrogels was similar to that of uncrosslinked PEGylated dendrimers within an exposure period of 48 hours.
Figure 7.
Cytotoxicity of G3.0–12000H and DH-G3.0–12000H.
The cellular uptake of G3.0–12000H and crosslinked G3.0–12000H (i.e., DH-G3.0–12000H) by the RAW264.7 macrophages was studied using fluorescence microscopy. In this work, the same cell seeding condition was applied throughout the experiment. Accordingly, the cell density and confluency were nearly identical across all the treatment groups, based on which the amount of fluorescence probe-labeled dendrimer nanoparticles and the number of cells taking up the nanoparticles were qualitatively analyzed and compared among the treatment groups. G3.0–12000H was quickly taken up by the macrophages within 30 minutes and more of G3.0–12000H nanoparticles were internalized at 2 µM (Figure 8B) than at 0.2 µM (Figure 8A). Further, higher concentrations and longer incubation periods interchangeably resulted in an increased cellular uptake of G3.0–12000H. A strong cellular uptake was observed at 24 hours when the nanoparticle concentration was 20 µM (Figure 8C). In contrast, crosslinking markedly reduced nonspecific cellular uptake of G3.0–12000H, which was only sparsely observed starting at 1 hour post-incubation. Even at 20 µM, the cellular uptake of DH-G3.0–12000H was still low (Figure 8D).
Figure 8.
Representative fluorescence images of cellular uptake of G3.0–12000H and DH-G3.0–12000H at various concentrations with various incubation times (A: 30 min/0.2 µM/ G3.0–12000H; B: 30 min/2 µM/G3.0–12000H; C: 24 h/20 µM/G3.0–12000H; D: 24 h/20 µM/DH-G3.0–12000H) (original magnification, 100×)
Conclusions
A novel versatile PAMAM dendrimer hydrogel platform was developed on the basis of PAMAM dendrimers G3.0 and G3.5. A completely crosslinked network (DH-G3.0–12000H) with a high water swelling ratio was successfully by UV-curing of PAMAM dendrimer G3.0 coupled with 28 PEG 12000 chains in the presence of the eosin Y-based photoinitiating system. With PAMAM dendrimer G3.5 being the underlying carrier, the dendrimer modified with 43 PEG 1500 chains was able to form a completely crosslinked network (DH-G3.5–1500H). The newly constructed dendrimer hydrogels possess high structural flexibility, high water absorptivity, good cytocompatibility, and controlled swelling and disintegration through the modulation of dendrimer surface charges and modification. PAMAM dendrimer hydrogels uniquely express the structural characteristics of both PEG hydrogel and PAMAM dendrimer and have potential for various applications in tissue engineering and drug delivery.
Acknowledgment
This research was supported in part by The Jeffress Memorial Trust (J-873), Office of Technology Transfer of Virginia Commonwealth University, and the Wallace H. Coulter Foundation. Dr. Xianjun Fang (Department of Biochemistry & Molecular Biology, Virginia Commonwealth University) provided the RAW264.7 macrophages. Dr. W. Andrew Yeudall (Philips Institute of Oral and Craniofacial Molecular Biology, Virginia Commonwealth University) provided assistance with cell culture. Irgacure 2959 was kindly given by Ciba. SEM microscopy was performed at the Virginia Commonwealth University Department of Neurobiology & Anatomy Microscopy Facility, supported, in part, with funding from NIH-NINDS Center core grant (5P30NS047463).
References and notes
- 1.Yang H, Kao WJ. Dendrimers for pharmaceutical and biomedical applications. J Biomater Sci Polym Ed. 2006;17(1–2):3–19. doi: 10.1163/156856206774879171. [DOI] [PubMed] [Google Scholar]
- 2.Khopade AJ, Caruso F. Stepwise Self-Assembled Poly(amidoamine) Dendrimer and Poly(styrenesulfonate) Microcapsules as Sustained Delivery Vehicles. Biomacromolecules. 2002;3(6):1154–1162. doi: 10.1021/bm025562k. [DOI] [PubMed] [Google Scholar]
- 3.Majoros IJ, Myc A, Thomas T, Mehta CB, Baker JR., Jr PAMAM dendrimer-based multifunctional conjugate for cancer therapy: synthesis, characterization, and functionality. Biomacromolecules. 2006;7(2):572–579. doi: 10.1021/bm0506142. [DOI] [PubMed] [Google Scholar]
- 4.Thomas TP, Patri AK, Myc A, Myaing MT, Ye JY, Norris TB, Baker JR., Jr In vitro targeting of synthesized antibody-conjugated dendrimer nanoparticles. Biomacromolecules. 2004;5(6):2269–2274. doi: 10.1021/bm049704h. [DOI] [PubMed] [Google Scholar]
- 5.Thomas TP, Shukla R, Kotlyar A, Liang B, Ye JY, Norris TB, Baker JR., Jr Dendrimer-epidermal growth factor conjugate displays superagonist activity. Biomacromolecules. 2008;9(2):603–609. doi: 10.1021/bm701185p. [DOI] [PubMed] [Google Scholar]
- 6.Woller EK, Cloninger MJ. Mannose Functionalization of a Sixth Generation Dendrimer. Biomacromolecules. 2001;2(3):1052–1054. doi: 10.1021/bm015560k. [DOI] [PubMed] [Google Scholar]
- 7.Degoricija L, Bansal PN, Sontjens SH, Joshi NS, Takahashi M, Snyder B, Grinstaff MW. Hydrogels for osteochondral repair based on photocrosslinkable carbamate dendrimers. Biomacromolecules. 2008;9(10):2863–2872. doi: 10.1021/bm800658x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carnahan MA, Middleton C, Kim J, Kim T, Grinstaff MW. Hybrid Dendritic-Linear Polyester-ethers for in Situ Photopolymerization. Journal of the American Chemical Society. 2002;124(19):5291–5293. doi: 10.1021/ja025576y. [DOI] [PubMed] [Google Scholar]
- 9.Degoricija L, Johnson CS, Wathier M, Kim T, Grinstaff MW. Photo crosslinkable Biodendrimers as ophthalmic adhesives for central lacerations and penetrating keratoplasties. Invest Ophthalmol Vis Sci. 2007;48(5):2037–2042. doi: 10.1167/iovs.06-0957. [DOI] [PubMed] [Google Scholar]
- 10.Velazquez AJ, Carnahan MA, Kristinsson J, Stinnett S, Grinstaff MW, Kim T. New dendritic adhesives for sutureless ophthalmic surgical procedures: in vitro studies of corneal laceration repair. Arch Ophthalmol. 2004;122(6):867–870. doi: 10.1001/archopht.122.6.867. [DOI] [PubMed] [Google Scholar]
- 11.Duan X, Sheardown H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: mechanical properties and corneal epithelial cell interactions. Biomaterials. 2006;27(26):4608–4617. doi: 10.1016/j.biomaterials.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Duan X, Sheardown H. Crosslinking of collagen with dendrimers. J Biomed Mater Res A. 2005;75(3):510–518. doi: 10.1002/jbm.a.30475. [DOI] [PubMed] [Google Scholar]
- 13.Joshi N, Grinstaff M. Applications of dendrimers in tissue engineering. Curr Top Med Chem. 2008;8(14):1225–1236. doi: 10.2174/156802608785849067. [DOI] [PubMed] [Google Scholar]
- 14.Guillaudeu SJ, Fox ME, Haidar YM, Dy EE, Szoka FC, Frechet JMJ. PEGylated Dendrimers with Core Functionality for Biological Applications. Bioconjugate Chemistry. 2008;19(2):461–469. doi: 10.1021/bc700264g. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y, Xu Z, Ma M, Xu T. Dendrimers as drug carriers: applications in different routes of drug administration. J Pharm Sci. 2008;97(1):123–143. doi: 10.1002/jps.21079. [DOI] [PubMed] [Google Scholar]
- 16.Dufes C, Uchegbu IF, Schaetzlein AG. Dendrimers in gene delivery. Advanced Drug Delivery Reviews. 2005;57(15):2177–2202. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Lopina ST. Penicillin V-conjugated PEG-PAMAM star polymers. J Biomater Sci Polym Ed. 2003;14(10):1043–1056. doi: 10.1163/156856203769231556. [DOI] [PubMed] [Google Scholar]
- 18.Sontjens SH, Nettles DL, Carnahan MA, Setton LA, Grinstaff MW. Biodendrimer-based hydrogel scaffolds for cartilage tissue repair. Biomacromolecules. 2006;7(1):310–316. doi: 10.1021/bm050663e. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Kao WJ. Thermoresponsive gelatin/monomethoxy poly(ethylene glycol)-poly(D,L-lactide) hydrogels: formulation, characterization, and antibacterial drug delivery. Pharm Res. 2006;23(1):205–214. doi: 10.1007/s11095-005-8417-z. [DOI] [PubMed] [Google Scholar]
- 20.Kailasan A, Yuan Q, Yang H. Synthesis and characterization of thermoresponsive polyamidoamine-polyethylene glycol-poly (D, L-lactide) (PAMAM-PEG-PDLLA) core-shell nanoparticles. Acta Biomaterialia. 2010;6(3):1131–1139. doi: 10.1016/j.actbio.2009.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar K, Yang H. Encapsulation and extended release of anti-cancer anastrozole by stealth nanoparticles. Drug Deliv. 2008;15(5):343–346. doi: 10.1080/10717540802035343. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Lopina ST. In vitro enzymatic stability of dendritic peptides. J Biomed Mater Res, Part A. 2006;76A(2):398–407. doi: 10.1002/jbm.a.30529. [DOI] [PubMed] [Google Scholar]
- 23.Yang H, Lopina ST, DiPersio LP, Schmidt SP. Stealth dendrimers for drug delivery: correlation between PEGylation, cytocompatibility, and drug payload. J Mater Sci Mater Med. 2008;19(5):1991–1997. doi: 10.1007/s10856-007-3278-0. [DOI] [PubMed] [Google Scholar]
- 24.Gillies ER, Frechet JMJ. Development of acid-sensitive copolymer micelles for drug delivery. Pure and Applied Chemistry. 2004;76(7–8):1295–1307. [Google Scholar]
- 25.Sawhney AS, Pathak CP, Hubbell JA. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(alpha -hydroxy acid) diacrylate macromers. Macromolecules. 1993;26(4):581–587. [Google Scholar]
- 26.Loh XJ, Tan KK, Li X, Li J. The in vitro hydrolysis of poly(ester urethane)s consisting of poly[(R)-3-hydroxybutyrate] and poly(ethylene glycol) Biomaterials. 2006;27(9):1841–1850. doi: 10.1016/j.biomaterials.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 27.Schoonover JR, Thompson DG, Osborn JC, Orler EB, Wrobleski DA, Marsh AL, Wang H, Palmer RA. Infrared linear dichroism study of a hydrolytically degraded poly(ester urethane) Polymer Degradation and Stability. 2001;74(1):87–96. [Google Scholar]