Abstract
Objective
To characterize timing and determinants of mother-to-child transmission (MTCT) of HIV among mothers receiving single-dose nevirapine to prevent MTCT in Nigeria.
Methods
371 HIV-infected mothers and their infants were followed from birth, at 1 week, at 1-,3-,6-, and 12 months. Risks of in-utero (IU), intrapartum (IP/EPP), and postnatal (PP) transmission were quantified using conditional Cox’s regressions.
Results
Maternal viral-load was the only risk factor for IU transmission after controlling for known risk factors. Low birthweight, premature birth, mixed-feeding, and maternal viral-load were associated with IP/EPP transmission. Increased PP transmission was associated with low birthweight and mixed-feeding. At 6 months, mixed-fed infants were more likely to acquire infection than formula-fed children (Hazard ratio=5.74, 1.26-26.2).
Conclusion
Risk factors for in-utero transmission were different from those of intrapartum and postnatal transmission. Reducing mixed feeding and low birthweight delivery among HIV-infected mothers can further decrease intrapartum and postnatal transmission.
Keywords: Human immunodeficiency virus, Timing of mother-to-child transmission, Infant feeding, Nigeria
INTRODUCTION
The risk of mother-to-child transmission of human immunodeficiency virus (HIV) has been linked to severe maternal immune status[1], high maternal HIV-1 viral load[2], presence of sexually transmitted infections during pregnancy[3], prolonged duration of membrane rupture[1, 4], vaginal delivery[5], and mixed infant feeding[6-8].
In severely HIV-affected Nigeria, it is estimated that between 63,000 to 125,000 infants each year acquire HIV from their mothers[9]. It is also estimated that 315,000 to 625,000 children are born annually to HIV-infected mothers[10]. Current evidence indicates that 70-90% of women in the Nigerian Prevention of Mother-to-Child Transmission (PMTCT) program opt for replacement feeding[10].
Mother-to-child transmission can occur in uterine (in-utero), during delivery (intrapartum), or after birth (postnatally) through breastfeeding. Different factors influence HIV-1 transmission during each of these periods, and hence interventions to reduce transmission during each of these periods also require different strategies. If some risk factors differ, combining in-utero cases with intrapartum or postnatal cases could lead to an underestimation of the impact of some risk factors and perhaps failure to identify others. Herein, we used data from a defined cohort established to evaluate the PMTCT program in order to identify distinct risk factors for in-utero, intrapartum, and postnatal transmission in Nigeria.
METHODS
Study design
Between April 2004 and March 2006, 13,032 pregnant women were screened for HIV-1 at Plateau State Specialist Hospital (PSSH) antenatal clinic in Jos, Nigeria. Of the 593 HIV-infected pregnant women enrolled, 391 (65.9%) returned to the facility to receive intrapartum and postnatal care. Written informed consent was obtained from each woman prior to enrollment in accordance with approvals from the University of Maryland Institutional Review Board and the PSSH Ethics Committee.
Single-dose nevirapine was provided for all HIV-infected women (200 mg tablet) at the onset of labor and to the neonate (2 mg/kg) by 48 hours of life according to the Nigerian National guideline at the time.
Detailed medical history, physical examination, and 10ml blood were obtained by trained physicians and nurse counselor. Obstetric data were abstracted from medical records. Gestational age at birth was determined by physicians using a combination of uterine fundal height and menstrual history in addition to infant birth examination; sonography was not available. CD4+ lymphocyte count and HIV-1 ribonucleic acid (RNA) level were measured at booking/enrollment and delivery. Follow-up visits for both mother and child with 2-5ml blood draw for the child occurred at 1 week and at 1, 3, 6, and 12 months post-delivery.
For mothers who chose formula feeding, nutrition counselors provided education and training before discharge and again at 1 week after delivery on cleaning, sterilizing, and storing feeding and preparation equipments for both bottle-feeding and cup-feeding methods. A 6-month supply of commercial infant formula was offered free. For mothers who chose exclusive breastfeeding, counselors provided education on the importance of early weaning before 4-6 months.
Questionnaires on feeding practices in the past 24 hours, in the past one week, and since the last visit were collected at each visit. Cumulative feeding patterns over visits from birth to the first 6 months of age were then summarized. Exclusive breastfeeding was defined as the infant receiving only breastmilk from birth to 6 months of age from his/her mother and no other liquids or solids, with the exception of drops or syrups consisting of vitamins, mineral supplements, or drugs. Formula feeding was defined as provision of infant formula and the exclusion of all breastfeeding during the first 6 months of age. Mixed breastfeeding was defined as giving breast milk with non-human milk or solids[11] at any time during the first 6 months of age.
Because our study was initiated before the availability of pediatric ARV treatment in Nigeria, none of our infants received antiretroviral therapy for their infection.
Laboratory methods
Maternal samples at enrollment were screened for HIV-1 antibodies by enzyme-linked immunosorbent assay (ELISA; Vironostica, BioMerieux) and confirmed by Western blot (Immunetics, Boston).
Real-time early infant diagnosis was not yet available at the time. Infant HIV status was established retrospectively on stored samples using Roche Amplicor version 1.5 qualitative HIV-deoxyribonucleic acid (DNA) and quantitative HIV-RNA polymerase chain reaction (PCR) assays (Roche Diagnostics System). At month 12, the HIV-1 serostatus of the child was determined by ELISA testing, with positive results confirmed using a second ELISA or a Western blot. If HIV serology was positive at 12 months, earlier infant samples were tested by PCR to establish timing of infection. Infants were regarded as HIV-infected if two samples were positive for HIV-DNA or -RNA. If HIV-DNA or -RNA was not detectable or if HIV serology was negative at month 12, the infant was regarded as HIV-uninfected.
Statistical methods
The estimated time of HIV acquisition was taken to be the midpoint between the dates of the last negative test and the first positive test. Presumed time of transmission was categorized into the following time of transmission groups: positive between 0-7 days (in-utero-IU), positive between >7-30 days (mostly intrapartum may include early postnatal-IP/EPP), and positive after >30 days (postnatal-PP).
In the analysis for risk of in-utero transmission, data from all mother-infant pairs were considered. In the analysis for risk of IP/EPP, the analysis was conditioned on HIV-free survival at 7 days of age (i.e. excluding in-utero infected infant). In the analysis for PP transmission, the analysis was conditioned on HIV-free survival at 1 month by excluding both in-utero infected and intrapartum/early post-natal infected infants.
Cumulative transmission in the first 6 and 12 months of life was assessed by Kaplan-Meier analysis and association with maternal and infant variables was quantified in a Cox regression analysis. Cox regression analysis was used in model fittings and in examining for differential loss to follow-up. The model included covariates significant at p≤ 0.1 in univariate analyses and known risk factors for MTCT regardless of their level of significance. A backward elimination procedure was used to create the most parsimonious model. To control for confounding, variables that alter any significant relative hazards by ≥ 20% was retained. We assessed the proportional hazards assumption with log-log plots and regression of the Schoenfeld residuals; the goodness of fit was assessed by log-likelihood test.
RESULTS
Study population
Of 391 deliveries, there were 380 live births and 11 stillbirths (2.8%). A total of 389 deliveries were spontaneous vaginal deliveries. There were 9 live-born twins pairs. We based our analysis on 371 first singleton live-births. Mean maternal age at enrollment was 27.3 years. Mean gestational age at enrollment was 25.9 weeks with 72.1% of the women enrolled before 32 weeks of gestation.
Infant feeding
At the time of discharge, 263 (71.7%) mothers opted for exclusive formula feeding and 104 (28.3%) mothers chose exclusive breastfeeding. By 6 months, 74 (71.1%) mothers reported maintaining exclusive breastfeeding, 211 (80.2%) mothers reported maintaining formula feeding, and 82 mothers reported mixed feeding. Of the 82 mixed feeding mothers, 52 (63.4%) intended to formula feed and 30 (37.6%) intended to exclusively breastfeed at the time of discharge. Differences between the feeding groups are summarized in Table 1.
Table 1.
Maternal and neonatal characteristics and mode of infant feeding summarized during the first 6 months of life (N=371).
| Infant feeding summarized during the first 6 months |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total N=371* |
Formula-fed N=211 |
Exclusive Breastfed N=74 |
Mixed Feeding N=82 |
P Comparing between formula- fed and exclusive breastfed |
P Comparing between formula- fed and mixed-fed |
|||||
| Maternal age | ||||||||||
| Mean (SD) | 27.3 | (5.0) | 27.9 | (5.2) | 25.7 | (5.0) | 27.0 | (3.9) | 0.001 | 0.089 |
| Religion | ||||||||||
| Christian | 325 | (87.6%) | 192 | (91.0%) | 62 | (83.8%) | 67 | (81.7%) | 0.136a | 0.012 |
| Muslim | 37 | (10.0%) | 13 | (6.2%) | 10 | (13.5%) | 14 | (17.1%) | ||
| Others/Traditional | 9 | (2.4%) | 6 | (2.8%) | 2 | (2.7%) | 1 | (1.2%) | ||
| Marital status | ||||||||||
| Married/cohabitating | 338 | (91.1%) | 193 | (91.5%) | 66 | (89.2%) | 76 | (92.7%) | 0.558 | 0.734 |
| Single | 33 | (8.9%) | 18 | (8.5%) | 8 | (10.8%) | 6 | (7.3%) | ||
| Highest education attained | ||||||||||
| <Primary | 107 | (28.8%) | 57 | (27.0%) | 25 | (33.8%) | 23 | (28.1%) | 0.397 | 0.957 |
| <Secondary | 185 | (49.9%) | 107 | (50.7%) | 37 | (50.0%) | 40 | (48.8%) | ||
| Secondary and above | 79 | (21.3%) | 47 | (22.3%) | 12 | (16.2%) | 10 | (23.1%) | ||
| Maternal CD4-cell countb | ||||||||||
| Mean (SD) | 234.0 | (149.6) | 213 | (141.3) | 287 | (161.1) | 240 | (149.0) | ||
| ≤ 200 | 176 | (48.6%) | 110 | (52.9%) | 23 | (32.4%) | 41 | (51.9%) | 0.003 | 0.881 |
| > 200 | 186 | (51.4%) | 98 | (47.1%) | 48 | (67.6%) | 38 | (48.1%) | ||
| Maternal plasma HIV-1 RNAb | ||||||||||
| Mean (SD) | 4.15 | (0.97) | 4.11 | (0.98) | 4.04 | (0.97) | 4.31 | (0.97) | 0.663 | 0.174 |
| Gestational age of infant | ||||||||||
| Mean (SD) | 36.5 | (3.3) | ||||||||
| ≥ 37 weeks | 278 | (90.6%) | 159 | (89.8%) | 53 | (91.4%) | 63 | (92.7%) | 0.731 | 0.499 |
| < 37 weeks | 29 | (9.4%) | 18 | (10.2%) | 5 | (8.6%) | 5 | (7.3%) | ||
| Birthweight of infant | ||||||||||
| Mean (SD) | 2.85 | (0.49) | ||||||||
| ≥ 2.5 kg | 278 | (80.6%) | 160 | (77.7%) | 56 | (77.8%) | 59 | (93.7%) | 0.985 | 0.004 |
| < 2.5 kg | 67 | (19.4%) | 46 | (22.3%) | 16 | (22.2%) | 4 | (6.3%) | ||
| Maternal receipt of NVP | ||||||||||
| No | 67 | (18.1%) | 43 | (20.4%) | 10 | (13.5%) | 13 | (16.1%) | 0.192 | 0.400 |
| Yes | 303 | (81.9%) | 168 | (79.6%) | 64 | (86.5%) | 68 | (83.9%) | ||
| Neonatal receipt of NVP | ||||||||||
| No | 8 | (2.2%) | 3 | (1.4%) | 3 | (4.1%) | 2 | (2.6%) | 0.175 | 0.500 |
| Yes | 353 | (97.8%) | 205 | (98.6%) | 70 | (95.9%) | 74 | (97.4%) | ||
| Aware of husband’s HIV | ||||||||||
| No | 277 | (78.9%) | 146 | (74.1%) | 62 | (89.9%) | 66 | (81.5%) | 0.006 | 0.189 |
| Yes | 74 | (21.1%) | 51 | (25.9%) | 7 | (10.1%) | 15 | (18.5%) | ||
| First pregnancy | ||||||||||
| No | 275 | (74.3%) | 160 | (76.2%) | 45 | (60.8%) | 67 | (81.7%) | 0.010 | 0.309 |
| Yes | 95 | (25.7%) | 50 | (23.8%) | 29 | (39.2%) | 15 | (18.2%) | ||
| Knows that HIV can be transmitted from mother to child |
||||||||||
| No and Don’t Know | 14 | (3.8%) | 6 | (2.8%) | 5 | (6.9%) | 2 | (2.4%) | 0.120 | 0.849 |
| Yes | 355 | (96.2%) | 205 | (97.2%) | 67 | (93.1%) | 80 | (97.6%) | ||
| Electricity in house | ||||||||||
| No | 44 | (12.0%) | 26 | (12.4%) | 8 | (10.8%) | 10 | (12.5%) | 0.721 | 0.978 |
| Yes | 324 | (88.0%) | 184 | (87.6%) | 66 | (89.2%) | 70 | (87.5%) | ||
| Refrigerator in house | ||||||||||
| No | 236 | (64.0%) | 134 | (64.1%) | 53 | (71.6%) | 47 | (57.3%) | 0.241 | 0.282 |
| Yes | 133 | (36.0%) | 75 | (35.9%) | 21 | (28.4%) | 35 | (42.7%) | ||
| Duration of rupture membrane | ||||||||||
| <6 hours | 277 | (76.5) | … | … | … | … | … | |||
| ≥6 hours | 85 | (23.5) | … | … | … | … | … | |||
| Mode of delivery | ||||||||||
| Spontaneous vaginal | 369 | (99.5%) | … | … | … | … | … | |||
| Emergent Caesarean Section | 2 | (0.5%) | … | … | … | … | … | |||
| Elective Caesarean Section | 0 | (0) | … | … | … | … | … | |||
| Types of feeding selected at discharge | ||||||||||
| Exclusive Breastfeeding | 104 | (28.3%) | … | … | … | … | … | |||
| Formula Feeding | 263 | (71.7%) | … | … | … | … | … | |||
| Mixed feeding observed at c | ||||||||||
| 1 week | 8 | (2.2%) | … | … | … | … | … | |||
| 1 month | 66 | (17.8%) | … | … | … | … | … | |||
| 3 months | 73 | (19.7%) | … | … | … | … | … | |||
| 6 months | 82 | (22.1%) | … | … | … | … | … | |||
total does not sum to 371 due to missing data; of 367, 4 women had missing infant feeding information
p=0.04 when comparing Muslim vs. non-Muslim between formula-fed and exclusive breastfed groups
closest to delivery
cumulative up to each time-point
Mother-to-child transmission before 6 months of age
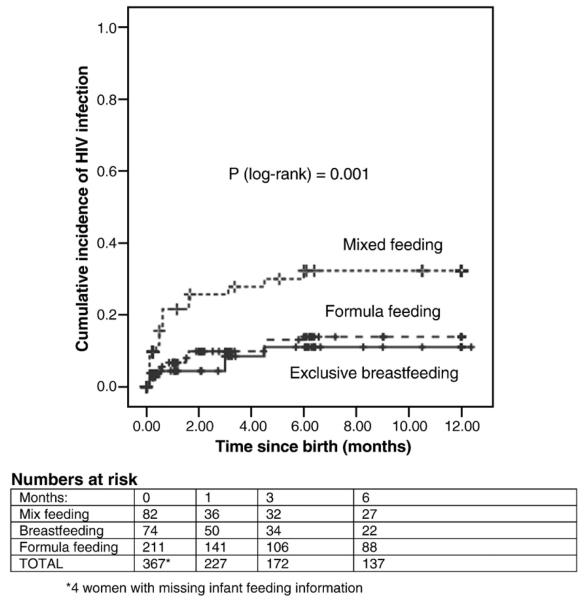
Fifty infants acquired the virus from their mothers with an overall transmission rate of 13.5%; 17 (34.0%) infants were infected in-utero, 15 (30.0%) were infected intrapartum, and 18 (36.0%) were infected postnatally. At 1 month, 32 of infants were positive by PCR, giving a transmission rate of 8.6% (95%CI, 5.7-11.4). Of 74 exclusively breastfed infants, 8.1% (95%CI, 1.9-14.3) were infected by 6 months. For infants who were continuously formula-fed, a transmission rate of 9.5% (95% CI, 5.6-13.4) was observed (Figure 1). Among infants who were breastfed but also received formula, transmission rates of 29.2% were observed. In univariate analyses, maternal CD4 ≤200, high maternal viral load closest to delivery, birth weight less than 2.5 kg, duration of membrane rupture for more than 6 hours, and mixed feeding were associated with increased transmission. Maternal and neonatal single-dose nevirapine were not associated with transmission. Transmission rate among 46 mixed-fed infants whose mothers had CD4 counts <200 was 34.1% (14 of 41) compared with 12.7% (14 of 110) among mothers with CD4 counts <200 who used formula (p=0.001). For those mothers with CD4 >200, there was no association between mixed feeding compared to formula feeding and transmission (7.9% vs. 8.2%, respectively, p=0.68).
Fig. 1.
Kaplan-Meier curves for time to HIV-1 infection during the first 6 months of life for infants who were breastfed (solid line), formula-fed (large dash line), and mixed fed (small dash line). Vertical lines denote censored observations.
Risk factors for in-utero transmission (17/371; 4.5%)
In-utero transmission was significantly associated (Table 2) with maternal CD4<200 (6.9% vs. 1.6%, p=0.013) and high maternal viral load (ptrend=0.024). In addition, there was a borderline association with prolonged duration of ruptured membrane (8.2% for ≥6 hours vs. 3.6% for <6 hours, p=0.079) and low birthweight (p=0.062). In the multivariate analysis (Table 3) controlling for viral load, maternal CD4 counts were no longer significantly associated with in-utero transmission.
Table 2.
Characteristics associated with the timing of MTCT of HIV-1
| Rate of HIV infection from birth to 1 week of life, n=17 (presumed in- utero transmission) |
Rate of HIV infection from 1 week to 1 month of life, n=15* (presumed intrapartum transmission and early post-natal transmission) |
Rate of HIV infection from 1 month to 6 months of life, n=18** (presumed postnatal transmission) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | n | % (95%CI) | P | N | n | % (95%CI) | P | N | n | % (95%CI) | P | |
| Maternal age at delivery | ||||||||||||
| < 30 | 262 | 12 | 4.6 (2.1 – 7.1) | 0.984 | 250 | 10 | 4.0 (1.6 – 6.4) | 0.773 | 240 | 10 | 4.2 (1.7 – 6.7) | 0.138 |
| ≥ 30 | 108 | 5 | 4.5 (0.6 – 8.4) | Ref | 105 | 5 | 4.9 (0.8 – 9.0) | Ref | 98 | 8 | 8.2 (2.8 – 13.6) | Ref |
| Maternal CD4-cell count b | ||||||||||||
| ≤ 200 | 175 | 12 | 6.9 (3.1 – 10.7) | 0.013 | 163 | 9 | 5.5 (2.0 – 9.0) | 0.429 | 154 | 9 | 5.8 (2.1 – 9.5) | 0.586 |
| > 200 | 186 | 3 | 1.6 (0.0 – 3.4) | Ref | 183 | 6 | 3.3 (0.1 – 5.9) | Ref | 177 | 8 | 4.5 (1.4 – 7.6) | Ref |
| Maternal plasma HIV-1 RNA b | ||||||||||||
| <1000 | 43 | 0 | 0 | 43 | 0 | 0 | 43 | 3 | 7.0 (0.0 – 14.6) | |||
| 1001 – 10,000 | 73 | 3 | 4.1 (0.2 – 8.6) | 70 | 2 | 2.9 (0.0 – 6.8) | 67 | 1 | 1.5 (0.0 – 4.4) | |||
| 10,001 – 100,000 | 116 | 6 | 5.2 (1.1 – 9.2) | 110 | 7 | 6.4 (1.8 – 11.0) | 103 | 7 | 6.8 (1.9 – 11.7) | |||
| 100,001 + | 59 | 5 | 8.5 (1.4 – 15.6) | 0.024 # | 54 | 4 | 7.4 (0.4 – 14.4) | 0.046 # | 50 | 3 | 6.0 (0.0 – 12.6) | 0.668# |
| Gestational age of infant | ||||||||||||
| ≥ 37 weeks | 277 | 11 | 4.0 (1.7 – 6.3) | Ref | 266 | 9 | 3.4 (1.2 – 5.6) | Ref | 257 | 17 | 6.6 (3.6 – 9.6) | Ref |
| < 37 weeks | 29 | 2 | 6.9 (0.0 – 16.1) | 0.457 | 27 | 4 | 14.8 (1.4 – 28.2) | 0.023 | 23 | 1 | 4.3 (0.0 – 12.6) | 1.00 |
| Birthweight of infant | ||||||||||||
| ≥ 2500 g | 277 | 10 | 3.6 (1.4 – 5.8) | Ref | 267 | 10 | 3.7 (1.4 – 6.0) | Ref | 257 | 11 | 4.3 (2.3 – 7.7) | Ref |
| < 2500 g | 67 | 6 | 9.0 (2.1 – 15.9) | 0.062 | 61 | 5 | 8.2 (1.3 – 15.1) | 0.167 | 56 | 5 | 8.9 (1.4 – 16.4) | 0.177 |
| Duration of ruptured membrane | ||||||||||||
| <6 hours | 276 | 10 | 3.6 (1.4 – 5.8) | Ref | 266 | 8 | 3.0 (1.0 – 5.1) | Ref | 258 | 13 | 5.0 (2.3 – 7.7) | Ref |
| 6+ hours | 85 | 7 | 8.2 (2.4 – 14.0) | 0.079 | 78 | 7 | 9.0 (2.6 – 15.3) | 0.023 | 71 | 4 | 5.6 (0.2 – 10.9) | 0.768 |
| Mode of infant feeding | ||||||||||||
| Formula | 211 | 8 | 3.8 (1.2 – 6.4) | Ref | 203 | 5 | 2.4 (0.3 – 4.5) | Ref | 198 | 7 | 3.5 (0.9 – 6.1) | Ref |
| Exclusive breast milk | 74 | 2 | 2.7 (0.0 – 6.4) | 0.661 | 72 | 1 | 1.4 (0.0 – 4.1) | 1.00 | 71 | 3 | 4.2 (0.0 – 8.9) | 0.726 |
| Mixed | 82 | 7 | 8.5 (2.5 – 14.5) | 0.136 | 75 | 9 | 12.0 (4.6 – 19.4) | 0.003 | 66 | 8 | 12.1 (4.2 – 20.0) | 0.015 |
excluded in-utero infected infants
excluded in-utero and intrapartum infected infants
N, total at-risk infants
n, number of HIV-positive infants
total does not sum to 371 due to missing data
closest to delivery
Ref, referent group in the comparison
p for trend
Table 3.
Results of multivariate Cox’s regression analysis of risk of infection.
| IU |
IP/EPP | PP | ||||
|---|---|---|---|---|---|---|
| Hazard ratio, HR (95%CI) |
P | Hazard ratio, HR (95%CI) |
P | Hazard ratio, HR (95%CI) |
P | |
| Low birthweight delivery | 1.54 (0.32 – 7.39) | 0.591 | 9.82 (1.23 – 78.5) | 0.031 | 5.02 (1.26 – 19.9) | 0.022 |
| Mixed feeding (vs. formula feeding) | 2.02 (0.37 – 10.9) | 0.413 | 36.5 (5.16 – 258.1) | 0.003 | 5.74 (1.26 – 26.2) | 0.024 |
| Maternal HIV-1 viral load (per 1 log increase) | 2.78 (1.05 – 7.39) | 0.040 | 2.78 (1.38 – 4.18) | 0.012 | 0.85 (0.46 – 1.58) | 0.600 |
| Maternal CD4 cell count <200 | 2.13 (0.81 – 11.0) | 0.367 | 3.95 (0.72 – 21.7) | 0.115 | 1.40 (0.43 – 4.53) | 0.578 |
| Premature birth | 1.45 (0.17 – 12.4) | 0.740 | 8.39 (1.58 – 44.6) | 0.013 | 0.95 (0.12 – 7.73) | 0.959 |
| Duration of ruptured membrane ≥ 6 hours | 1.27 (0.25 – 6.50) | 0.778 | 1.16 (0.22 – 6.07) | 0.862 | 0.98 (0.25 – 3.76) | 0.975 |
Risk factors for intrapartum/early postnatal transmission (15/354; 4.2%)
High maternal viral load (ptrend=0.046), infant gestational age <37 weeks (14.8% vs. 3.4%, p=0.023), and prolonged duration of ruptured membrane (9.0% vs. 3.0%, p=0.023) were strongly associated with risk of IP/EPP transmission (Table 2). Higher IP/EPP transmission was observed for 66 women who were mixed feeding within the first 30 days compared to women who either formula fed or exclusively breastfed their babies (12.0% vs. 2.2%, p=0.014). In the multivariate analysis (Table 3), risk of transmission was strongly associated with mixed feeding (HR=36.5) compared to formula feeding), low birthweight (HR=9.8), and premature birth (HR=8.4).
Risk factors for postnatal transmission (18/339; 5.3%)
Increased risk of postnatal transmission was associated with mixed feeding compared to formula feeding (Table 2, 12.1% vs. 3.5%, p=0.015). The rates of in-utero, intrapartum, and postnatal transmission for mothers who used formula remained similar, ranging from 3.8% to 3.5%, whereas postnatal transmission rate for mothers who breastfed increased significantly from 1.4% in the intrapartum/early postnatal period to 4.2% in the postnatal period. In the multivariate analysis (Table 3), mixed-feeding and low birthweight were independently associated with increased postnatal transmission.
DISCUSSION
To our knowledge, findings from the Nigerian PMTCT program – one of the largest PMTCT programs globally – are limited. Our current findings, which were derived from a cohort established to monitor and evaluate the Nigerian program, provide important insights into the timing-specific determinants of mother-to-child transmission of HIV that are important for prevention strategies for both mother and child. Even in the setting where breastfeeding is universal and intention to formula feed is high, our finding shows that mixed feeding is prevalent and is associated with a higher risk of transmission throughout the intrapartum and postnatal periods. Although sample size in the exclusively breastfed group was small, we found no difference in transmission risk between formula-fed and exclusively breastfed infants.
The negative impact of mixed feeding on increased transmission was seen as early as 7 days post-delivery and is consistent with finding from two other studies[7, 8]. We also found that transmission in the first 6 months of infant’s life was highest among the mothers with CD4 ≤200 who mixed fed. Mothers who chose to breastfeed but became sicker postnatally often defaulted to mixed-feeding. This practice carries the highest risk of transmission and highlights the importance of ensuring linkage to HIV treatment program while reinforcing support for the feeding choice by the mothers. For women who initially chose formula feeding but mixed fed, social stigma, family pressure and lack of partner support were often reported. From our data, 19.3% “failed” to comply with formula feeding practice at 6 months. In setting where breastfeeding is considered the norm but exclusive breastfeeding is not common, formula feeding is seen as a behavior of a mother who is sick or infected with HIV and highlights the need to have on-going support system.
Given the provision of ARV prophylaxis as the standard of care, our study was not designed nor powered to examine the effectiveness of the prophylaxis provided. Combination short-course antiretroviral (ARV) prophylaxis was adopted by the Nigerian Government in January 2007[10]. Two randomized trials[12, 13] were recently reported that definitively demonstrated the safety and efficacy of extended infant prophylaxis to reduce postnatal transmission. Although these findings highlight further decrease in transmission associated with breastfeeding that can be achieved with longer prophylaxis, further analyses are warranted to examine the elevated risk associated with mixed feeding within these settings since the damages to the intestinal mucosa from early introduction of other foods or non-human milk can still lead to increased gut permeability for viral entry and increased immune activation.
One in 10 infants in our study was born preterm. The association between preterm delivery and IP/EPP transmission and not in-utero transmission after adjusting for other risk factors observed in our study has also been reported by others[14-16], suggesting increased susceptibility of premature infants to HIV-infection that could result from immature immune system[17, 18], immature mucosal barriers[19], and low levels of maternal neutralizing antibodies[20]. We also found transmission risk associated with low birthweight that persisted into the postnatal period after controlling for maternal viral load and is independent from the effect of feeding, underscoring the important role of early and continuing antenatal care for good pregnancy outcomes for HIV-seropositive women.
A limitation to our study is our definition of in-utero and intrapartum transmission, which used the 7-day cutoff from the referent time of delivery. Sensitivity of HIV-1 DNA PCR increases rapidly in the first week of life[21, 22] and shorter time intervals using the 2 day cutoff, as proposed by Bryson et al.[23], is better able to distinguish intrauterine from intrapartum infection within the context of timing-specific risk factors[14, 15]. Nonetheless, our findings are consistent with current knowledge of the mechanisms of mother-to-child transmission. Factors that relate to gestation development (e.g. severe maternal immune status) affected only in-utero transmission, whereas factors that relate to the labor and delivery period (e.g. membrane rupture and prematurity) affected only intrapartum transmission. It is possible that mothers, who were identified as having formula-fed, may have mixed fed but did not disclose the practice to the nurse counselors due to social undesirability. However, because of our retrospective testing for HIV infection, this misclassification would have been non-differential with respect to transmission. Lastly, the small sample sizes for the breastfed and mixed-fed groups could limit the precision of our risk estimates.
In summary, our study confirms the importance of recognizing the timing of MTCT. In-utero transmission appears largely to be a function of maternal viral load while intrapartum/early postnatal transmission suggests that both viral and host factors play a role. To prevent in-utero transmission, initiation of antiretroviral prophylaxis earlier in pregnancy—especially for women who require antiretrovirals for their own clinical indications—is crucial. At the time of labor and delivery, provision of antiretrovirals and the reduction of exposure time to membrane rupture are important. Reducing preterm and low birth weight deliveries through improved prenatal could also be effective in reducing transmission in the intrapartum and early postnatal periods. Reinforcing exclusive feeding, either breastmilk or formula, through effective counseling support and minimizing mixed feeding in early months of life while increasing the potency and duration of neonatal antiretroviral prophylaxis is of paramount importance during the postnatal period.
Synopsis.
Characterizing timing-specific risk factors for mother-to-child transmission in Nigeria and their relations to infant feeding practices
Acknowledgements and Financial Support
We also acknowledge the Harvard School of Public Health’s Bill and Melinda Gates-funded AIDS Prevention in Nigeria (APIN) program (Dr. Phyllis Kanki, Principal Investigator) for funding this study and the Fogarty AIDS International Training Research Program (D43 TW001041-09) for providing research training support during the study implementation.
References
- 1.Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. 2002. pp. 484–494. [DOI] [PubMed]
- 2.Garcia PM, Kalish LA, Pitt J, et al. Maternal Levels of Plasma Human Immunodeficiency Virus Type 1 RNA and the Risk of Perinatal Transmission. 1999. pp. 394–402. [Record as suppl. [DOI] [PubMed]
- 3.Drake AL, John-Stewart GC, Wald A, et al. Herpes simplex virus type 2 and risk of intrapartum human immunodeficiency virus transmission. Obstet Gynecol. 2007;109:403–9. doi: 10.1097/01.AOG.0000251511.27725.5c. [DOI] [PubMed] [Google Scholar]
- 4.Mandelbrot L, Mayaux MJ, Bongain A, et al. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am J Obstet Gynecol. 1996;175:661–7. doi: 10.1053/ob.1996.v175.a75478. [DOI] [PubMed] [Google Scholar]
- 5.Read JS. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1: a Meta-analysis of 15 prospective cohort studies. 1999. pp. 977–87. [DOI] [PubMed]
- 6.Coutsoudis A. Influence of infant feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa. 2000. pp. 136–144. [DOI] [PubMed]
- 7.Coovadia HM, Rollins NC, Bland RM, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007;369:1107–16. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 8.Iliff PJ, Piwoz EG, Tavengwa NV, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. Aids. 2005;19:699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 9.UNICEF Children and HIV and AIDS. 2007.
- 10.Federal Government of Nigeria Ministry of Health; Nigeria National Guidelines on Prevention of Mother-To-Child Transmission (PMTCT) of HIV. 2007
- 11.WHO HIV and Infant Feeding: New evidence and programmatic experience. The World Health Organization; Geneva: Report of a Technical Consultation. 2006
- 12.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended Antiretroviral Prophylaxis to Reduce Breast-Milk HIV-1 Transmission. N Engl J Med. 2008 doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 13.Bedri A, Gudetta B, Isehak A, et al. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–13. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 14.Jourdain G, Mary JY, Coeur SL, et al. Risk factors for in utero or intrapartum mother-to-child transmission of human immunodeficiency virus type 1 in Thailand. J Infect Dis. 2007;196:1629–36. doi: 10.1086/522009. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn L, Steketee RW, Weedon J, et al. Distinct risk factors for intrauterine and intrapartum human immunodeficiency virus transmission and consequences for disease progression in infected children. Perinatal AIDS Collaborative Transmission Study. J Infect Dis. 1999;179:52–8. doi: 10.1086/314551. [DOI] [PubMed] [Google Scholar]
- 16.Magder LS, Mofenson L, Paul ME, et al. Risk factors for in utero and intrapartum transmission of HIV. J Acquir Immune Defic Syndr. 2005;38:87–95. doi: 10.1097/00126334-200501010-00016. [DOI] [PubMed] [Google Scholar]
- 17.Xanthou M, Mandyla-Sfagou H, Economou-Mavrou C, Matsaniotis N. Cytotoxicity of lymphocytes in the newborn. Arch Dis Child. 1981;56:377–81. doi: 10.1136/adc.56.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juretic E, Uzarevic B, Petrovecki M, Juretic A. Two-color flow cytometric analysis of preterm and term newborn lymphocytes. Immunobiology. 2000;202:421–8. doi: 10.1016/S0171-2985(00)80101-1. [DOI] [PubMed] [Google Scholar]
- 19.Brandtzaeg P. Development and basic mechanisms of human gut immunity. Nutr Rev. 1998;56:S5–18. doi: 10.1111/j.1753-4887.1998.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 20.Hobbs JR, Davis JA. Serum gamma-G-globulin levels and gestational age in premature babies. Lancet. 1967;1:757–9. doi: 10.1016/s0140-6736(67)91369-4. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn L, Abrams EJ, Chinchilla M, Tsai WY, Thea DM. Sensitivity of HIV-1 DNA polymerase chain reaction in the neonatal period. The New York City Perinatal HIV Transmission Collaborative Study Group. Aids. 1996;10:1181–2. [PubMed] [Google Scholar]
- 22.Chouquet C, Burgard M, Richardson S, Rouzioux C, Costagliola D. Timing of mother-to-child HIV-1 transmission and diagnosis of infection based on polymerase chain reaction in the neonatal period by a non-parametric method. Aids. 1997;11:1183–4. doi: 10.1097/00002030-199709000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Bryson YJ, Luzuriaga K, Sullivan JL, Wara DW. Proposed definitions for in utero versus intrapartum transmission of HIV-1. N Engl J Med. 1992;327:1246–7. doi: 10.1056/NEJM199210223271718. [DOI] [PubMed] [Google Scholar]