Abstract
Background and objectives: Treatment with IFN is rarely associated with nephrotic syndrome and renal biopsy findings of minimal-change disease or FSGS.
Design, setting, participants, & measurements: We report 11 cases of collapsing FSGS that developed during treatment with IFN and improved after discontinuation of therapy.
Results: The cohort consists of seven women and four men with a mean age of 48.2 yr. Ten of the 11 patients were black. Six patients were receiving IFN-α for hepatitis C virus infection (n = 5) or malignant melanoma (n = 1), three were receiving IFN-β for multiple sclerosis, and two were treated with IFN-γ for idiopathic pulmonary fibrosis. After a mean and median duration of therapy of 4.0 and 12.6 months, respectively, patients presented with acute renal failure (mean creatinine 3.5 mg/dl) and nephrotic-range proteinuria (mean 24-hour urine protein 9.7 g). Renal biopsy revealed collapsing FSGS with extensive foot process effacement and many endothelial tubuloreticular inclusions. Follow-up was available for 10 patients, all of whom discontinued IFN. At a mean of 23.6 months, nine of 10 patients had improvement in renal function, including one with complete remission and two with partial remission. Among the seven patients with available data, mean proteinuria declined from 9.9 to 3.0 g/d. Four of the seven patients were treated with immunosuppression, and there was no detectable benefit.
Conclusions: Collapsing FSGS may occur after treatment with IFN-α, -β, or -γ and is typically accompanied by the ultrastructural finding of endothelial tubuloreticular inclusions. Optimal therapy includes discontinuation of IFN.
FSGS is the most common cause of idiopathic nephrotic syndrome in black patients and may be the most frequent cause of nephrotic syndrome in the general population (1–6). The spectrum of FSGS includes primary forms mediated by a putative circulating or permeability factor and a number of secondary forms caused by such diverse insults as hereditable mutations in podocyte genes, drugs, viral infections, and adaptive responses to reduced renal mass or other hemodynamic stress (1). A variety of histologic variants of FSGS have been identified and can be applied to both primary and secondary forms (7–9). Many secondary forms tend to manifest as particular morphologic subtypes (1).
The collapsing variant of FSGS is defined by implosive wrinkling and “collapse” of the glomerular basement membrane associated with hypertrophy and hyperplasia of overlying podocytes (10–12). Collapsing FSGS was mainly described in patients with HIV-associated nephropathy (HIVAN) (13) but also was recognized as a variant of idiopathic FSGS (11,12). Both idiopathic collapsing FSGS and HIVAN are most commonly seen in young black patients (8–12,14). Compared with the usual, most common form of FSGS with discrete segmental scars (FSGS not otherwise specified [FSGS NOS]), collapsing FSGS is distinguished by more severe nephrotic syndrome and renal insufficiency at presentation and a more rapid course to renal failure (8–12,14). Central to the morphogenesis of the collapsing variant is podocyte injury that leads to podocyte dedifferentiation, apoptosis, and proliferation, in part through dysregulation of cell cycle–related proteins (15–19). Podocyte precursor cells from the parietal cell layer may contribute to the glomerular epithelial cell proliferation (20).
HIVAN is not the only established secondary cause of collapsing FSGS. Collapsing FSGS has been reported in the setting of Parvovirus B19 infection (21) and in patients with hemophagocytic syndrome (with or without underlying lymphoma) (22). Collapsing FSGS also may follow treatment with pamidronate (23), with 15 cases reported in the medical literature (23,24). In contrast, FSGS NOS has been reported to result from treatment with lithium (25), sirolimus (26), and more recently anabolic steroids (27). Although rare cases of collapsing FSGS also have been reported after treatment with IFN-α (28–30), this therapeutic agent is more commonly associated with minimal-change disease (MCD) (31–38) and FSGS NOS (39–47). We report 11 additional cases of collapsing FSGS that developed during treatment with IFN, including six IFN-α (for hepatitis C virus [HCV] infection or melanoma), three IFN-β (for multiple sclerosis [MS]), and two IFN-γ (for idiopathic pulmonary fibrosis).
Materials and Methods
Eleven cases of collapsing FSGS after treatment with IFN-α, -β, or -γ were identified from the archives of the Renal Pathology Laboratory of Columbia University Medical Center. The 11 biopsies were received from 1999 through 2008, during which time 15,783 native renal biopsies were processed in the laboratory. The biopsies were received from 11 nephrologists at 10 hospitals in five states, for a biopsy incidence of 0.07%. All cases were processed for light microscopy, immunofluorescence, and electron microscopy according to standard techniques, and in all cases, glomeruli were available for all three modalities of evaluation.
The diagnosis of collapsing FSGS was based on a proposed morphologic classification system (7). In brief, the collapsing variant requires at least one glomerulus with the defining features of wrinkling and retraction of the glomerular basement membrane (“collapse”) and hypertrophy and hyperplasia of overlying visceral epithelial cells. Tubular atrophy and interstitial fibrosis and interstitial inflammation were graded on a scale of mild, moderate, and severe corresponding to 0 to 25%, 26 to 50%, and 51 to 100% of the cortex sampled, respectively. Similarly, the degree of vascular disease was graded as mild, moderate, and severe on the basis of the extent of luminal narrowing. For immunofluorescence, 3-μm cryostat sections were stained with FITC-conjugated rabbit anti-human IgG, IgM, IgA, C3, C1q, κ light chain, and λ light chain (Dako Corp., Carpinteria, CA). Electron microscopy was performed with a JEOL 100S or JEOL 1010 electron microscope. The degree of foot process effacement was estimated as the percentage of nonsclerosed glomerular capillary surface area exhibiting effaced foot processes in all electron microscopic fields examined. The number of endothelial tubuloreticular inclusions (TRIs) was evaluated on a scale of 0 to 3 (0, negative; 1, one TRI per glomerulus; 2, two to three TRIs per glomerulus; and 3, four or more TRIs per glomerulus).
Patient charts were reviewed retrospectively for presenting symptoms, laboratory findings, details of treatment with IFN, post-biopsy treatment, and follow-up. The following definitions were used: Nephrotic-range proteinuria (NRP; 24-hour urine protein >3 g/d); hypoalbuminemia (serum albumin <3.5 g/dl); and renal insufficiency (serum creatinine >1.2 mg/dl). Nephrotic syndrome was defined as NRP, hypoalbuminemia, and peripheral edema. Hypertension was defined as systolic BP >140 mmHg, diastolic BP >90 mmHg, or active treatment with antihypertensive agents.
For the purpose of outcomes analysis, the following definitions were applied: Stable renal function (SRF) was defined as a change in serum creatinine of ≤20% of the initial value. Complete remission (CR) was defined as a 24-hour urine protein of ≤300 mg/d and SRF at last follow-up. Partial remission (PR) was defined by the presence of a 24-hour urine protein of ≤2 g/d, a reduction in proteinuria of at least 50%, and SRF at last follow-up. This study was approved by the Institutional Review Board of Columbia University.
Results
The cohort of 11 patients with FSGS after treatment with IFN included seven women and four men with a mean age of 48.2 years (range 27 to 69 years; Table 1). Ten of the 11 patients were African-American, and one patient was of Hispanic origin. Six patients were treated with IFN-α for either HCV infection (5 patients) or malignant melanoma (1 patient). Three patients were receiving IFN-β for MS, and two patients were receiving IFN-γ for idiopathic pulmonary fibrosis. To our knowledge, the particular IFN formulation was administered to each patient at standard therapeutic dosages (IFN-α for HCV: 3 million units (MU) subcutaneously three times per week; IFN-α for melanoma: 20 MU/m2 subcutaneously for 5 d for 4 consecutive weeks followed by 10 MU/m2 three times per week; IFN-β1A: 30 μg intramuscularly once per week or 22 to 44 μg subcutaneously three times per week; IFN-β1B: 8 MU subcutaneously every other day; IFN-γ: 50 μg/m2 subcutaneously three times per week). The median and mean duration of IFN therapy at the time of renal biopsy was 4.0 and 12.6 mo, respectively. A shorter duration of treatment was seen for the majority of patients who were treated with IFN-α (mean 3.3 months). Seven of the 11 patients had a history of hypertension, and two had a history of diabetes, although neither of the patients with diabetes had a history of nephropathy or retinopathy. Medical history also included obesity in three patients, remote intravenous drug use in three patients (all with HCV infection), hypothyroidism in two patients, and rheumatoid arthritis and Raynaud phenomenon in one patient. Patient 2 was the subject of a previous report (28).
Table 1.
Clinical findings in patients with collapsing FSGS after treatment with IFN
| Parameter | Patient |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Age (years) | 46 | 56 | 41 | 64 | 69 | 36 | 27 | 33 | 37 | 67 | 54 |
| Race/ethnicity | Black | Black | Black | Hispanic | Black | Black | Black | Black | Black | Black | Black |
| Gender | Female | Female | Male | Male | Male | Female | Female | Female | Female | Male | Female |
| Hypertension | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | No |
| Diabetes | No | No | No | No | Yes | No | No | Yes | No | No | No |
| Medical history | None | Previous IVDA, hypothyroid | Previous IVDA | Previous IVDA | None | Obesity | None | Obesity | None | RA, Raynaud | Obesity, hypothyroid |
| Indication for IFN | HCV | HCV | HCV | HCV | HCV | Melanoma | MS | MS | MS | IPF | IPF |
| Duration of IFN therapy (months) | 4 | 2.5 | 3 | 2 | 3 | 5 | Unknown | 36 | 48 | 18 | 4 |
| Form of IFN | α | α | α | α | α | α | β-1A | β-1B | β-1A | γ | γ |
| SCr (mg/dl) | 3.2 | 9.6 | 1.3 | 1.5 | 6.9 | 1.4 | 0.7 | 2.6 | 2.3 | 3.5 | 3.8 |
| 24-Hour Uprot (g/d) | 10.0 | 19.4 | 23.7 | 4.5 | 3.3 | 27.0 | 16.0 | 1.9 | 2.4 | 4.0 | 26.5 |
| Serum albumin (g/dl) | 0.8 | 1.4 | 1.5 | 1.3 | 2.3 | 1.6 | 0.6 | 3.3 | 3.6 | 3.1 | 2.0 |
| Edema | No | Yes | Yes | Yes | No | Yes | Yes | No | No | No | Yes |
| Urine sediment | RBCs | RBCs | RBCs | Bland | Bland | Bland | RBCs | Bland | WBCs | Bland | RBCs |
| Follow-up duration (months) | 6 | 4 | 18 | 53 | 7 | 10 | NA | 54 | 52 | 32 | 2 |
| steroids | Pulse only | Yes | No | No | Pulse only | No | Yes | Yes | No | Yes | |
| additional immunosuppression | No | No | No | No | No | No | No | Cytoxan | No | No | |
| discontinue IFN | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| final SCr (mg/dl) | 1.2 | 1.7 | HD-dep | 1.0 | 5.6 | 1.0 | 1.7 | 1.2 | 2.1 | 1.5 | |
| final 24-hour Uprot (g/d) | 3.6 | 10.0 | Negative | NA | 4.5 | 0.5 | 1.4 | 0.65 | NA | ||
HD-dep, hemodialysis dependent; IPF, idiopathic pulmonary fibrosis; IVDA, intravenous drug use; MS, multiple sclerosis; NA, not available; RA, rheumatoid arthritis; RBCs, red blood cells; SCr, serum creatinine; Uprot, urinary protein; WBCs white blood cells.
At the time of biopsy, the mean serum creatinine was 3.5 mg/dl, and 10 of 11 patients had evidence of renal insufficiency (Table 1). The mean 24-hour urine protein was 9.7 g/d, and nine of 11 patients had NRP. Ten patients had hypoalbuminemia and six had edema, all of whom met criteria for full nephrotic syndrome. Five patients had microhematuria and one had leukocyturia, but red blood cell casts were not identified in any patient. HIV studies were negative in all seven patients who underwent testing, including all five patients with a history of HCV infection. HIV testing was not performed on the remaining four patients because of the absence of risk factors. Evidence of remote hepatitis B virus infection, without active antigenemia, was present in one patient who had co-infection with HCV. Five of 11 patients had a positive antinuclear antibody, but none of the patients had hypocomplementemia or signs or symptoms of systemic lupus erythematosus (SLE).
Clinical follow-up was available for 10 patients, all of whom discontinued treatment with IFN after the renal biopsy (Table 1). Six patients were treated with steroids, including one patient who was treated with cytoxan and steroids for MS. The median and mean duration of follow-up were 14.0 and 23.6 months, respectively. During the period of follow-up, nine of 10 patients had a decline in serum creatinine from a mean of 3.9 mg/dl to a mean of 1.9 mg/dl. Seven of the nine patients reached a final creatinine of ≤1.7 mg/dl, including four who returned to normal range. Follow-up urinary protein levels were available for seven patients, all of whom had a decline in 24-hour urine protein from a mean of 9.9 g/d to a mean of 3.0 g/d. Two of the seven patients met criteria for PR, one had a CR, and the remaining four had a decline in 24-hour urine protein from a mean of 14.7 to 4.9 g/d but did not meet criteria for CR or PR. Four of the seven patients were treated with steroids, including one with PR and three who did not meet criteria for CR or PR. Thus, beyond discontinuation of IFN, no additional benefit of immunosuppressive therapy could be detected.
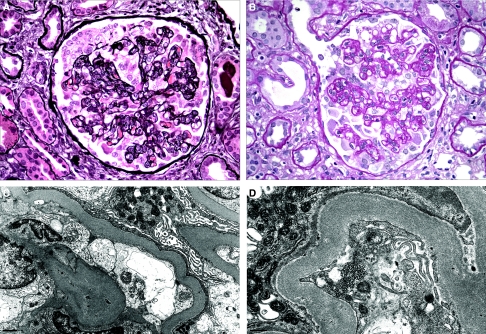
The renal biopsy findings are highlighted in Table 2. The sampling for light microscopy included a mean of 18.5 glomeruli (range 6 to 33 glomeruli). A mean of 34.2% of glomeruli per biopsy exhibited lesions of FSGS (range 4.3 to 83.3%), whereas a mean of 10.2% of glomeruli were globally sclerotic (range 0.0 to 23.1%). All 11 biopsies met defining criteria for collapsing FSGS (7) (Figure 1, A and B). In addition, all biopsies exhibited evidence of acute tubular injury, which was more prominent and diffuse in four cases. The degree of tubular atrophy and interstitial fibrosis ranged from mild (n = 3) to moderate (n = 6) to severe (n = 2). Interstitial inflammation was present in all cases and ranged from mild (n = 6) to moderate (n = 5). Tubular microcysts, a common finding in collapsing FSGS, were identified in four biopsies. Arteriosclerosis and arteriolosclerosis with hyalinosis were identified in eight of 11 biopsies and in all cases were mild to moderate in severity. Immunofluorescence revealed no evidence of immune complex deposition in any of the 11 biopsies. In six of the biopsies, focal staining for IgM and C3 was noted in glomeruli with collapse or segmental sclerosis.
Table 2.
Renal biopsy findings in patients with collapsing FSGS after treatment with IFN
| Parameter | Patient |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Light microscopy | |||||||||||
| no. of glomeruli | 7 | 6 | 13 | 33 | 29 | 12 | 21 | 23 | 30 | 11 | 19 |
| no. of GS glomeruli | 1 | 0 | 3 | 1 | 5 | 0 | 4 | 1 | 5 | 1 | 1 |
| no. of SS glomeruli | 1 | 5 | 3 | 6 | 10 | 5 | 7 | 1 | 3 | 5 | 13 |
| TA/IF | Moderate | Moderate | Mild | Mild | Moderate | Moderate | Moderate | Mild | Moderate | Severe | Severe |
| Interstitial inflammation | Mild | Mild | Mild | Mild | Moderate | Moderate | Moderate | Mild | Mild | Moderate | Moderate |
| Tubular injury | Yes | Diffuse | Yes | Yes | Diffuse | Diffuse | Yes | Yes | Yes | Yes | Diffuse |
| Tubular microcysts | No | No | No | No | Yes | Yes | No | No | No | Yes | Yes |
| Vascular disease | No | Mild | No | Mild | Moderate | Moderate | No | Mild | Mild | Moderate | Moderate |
| Electron microscopy | |||||||||||
| % FPE | 80 | 100 | 90 | 80 | 100 | 100 | 70 | 90 | 90 | 75 | 75 |
| TRIs | 2+ | 3+ | 2+ | 2+ | 2+ | 2+ | 3+ | 3+ | 3+ | 2+ | No |
| deposits | Rare mesangial | No | Rare mesangial | No | No | No | No | No | No | No | No |
FPE, foot process effacement; GS, globally sclerotic; SS, segmentally sclerotic; TA/IF, tubular atrophy and interstitial fibrosis; TRIs, endothelial tubuloreticular inclusions.
Figure 1.
Pathologic findings. (A) A glomerulus with collapsing FSGS exhibits global wrinkling and retraction of the glomerular basement membranes and diffuse swelling and proliferation of overlying visceral epithelial cells (Jones methenamine silver). (B) In this glomerulus with collapsing FSGS, some of the podocytes are detached from the glomerular basement membrane and lie free within the urinary space (periodic acid Schiff). (C) On ultrastructural evaluation, visceral epithelial cells exhibit microvillous transformation and complete foot process effacement. An endothelial TRI is present. (D) At high magnification, an endothelial TRI is seen. Magnifications: ×400 in A and B; ×5000 in C; ×8000 in D.
By electron microscopy, all biopsies revealed diffuse foot process effacement, which involved a mean of 86.4% of the glomerular capillary wall surface (range 70 to 100%; Figure 1C). Endothelial TRIs, also referred to as “IFN footprints,” were seen in 10 of the 11 biopsies and were noted to be extensive in all 10 cases (Figure 1D). Two biopsies from patients with HCV infection displayed rare small paramesangial electron densities without well-developed electron-dense deposits.
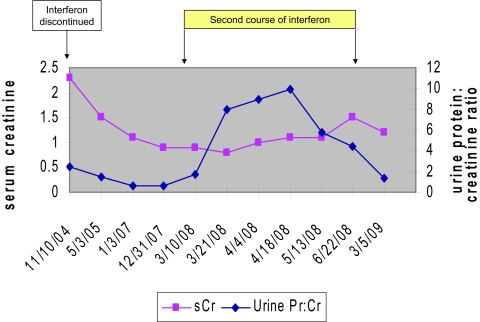
Of particular interest, patient 9 was a 37-year-old woman who had been treated with IFN-β-1A for 4 years for MS (Figure 2). After the development of proteinuria (urine protein-creatinine ratio of 2.4) and renal insufficiency (creatinine 2.3 mg/dl), IFN was discontinued, leading to normalization of renal function (creatinine 0.8 mg/dl) and a marked decline in proteinuria (urine protein-creatinine ratio 0.57). More than 3 years later, the patient received a second course of treatment with the same IFN formulation, leading to massive proteinuria (9.97 g/d) and a rise in creatinine to 1.5 mg/dl. Again, discontinuation of IFN led to reduction in proteinuria (1.4 g/d) and improvement in renal function (creatinine 1.2 mg/dl).
Figure 2.
Temporal relationship among serum creatinine, proteinuria, and use of IFN in patient 9.
Discussion
IFN was originally identified in 1957 and was named for its ability to confer viral interference (48). The nomenclature of α, β, and γ is based on the major IFN peaks observed under HPLC. The major cells of origin for α, β, and γ IFN are leukocytes (other than lymphocytes), fibroblasts, and T cells and natural killer cells, respectively. Many other cell types are also capable of synthesizing lower levels of IFN-α and -β when virally infected.
IFNs play a role in signaling between immune cells and represent an important component of the innate immune response against viral infection. IFN-α and -β are produced by virally infected cells and induce cellular changes in neighboring cells that prevent viral replication and protein synthesis. In contrast, IFN-γ acts by stimulating macrophage activation and MHC expression and does not directly block viral infection.
Recombinant DNA technology has allowed cloning and mass production of IFNs for therapeutic use in multiple medical conditions. IFN-α, including both IFN-α2a and IFN-α2b, is approved for the treatment of chronic HCV infection, hepatitis B virus infection, hairy cell leukemia, follicular lymphoma, malignant melanoma, AIDS-related Kaposi sarcoma, and condyloma acuminata. IFN-β, including both IFN-β1a and IFN-β1b, is indicated solely for the treatment of MS. IFN-γ is approved for the treatment of chronic granulomatous disease and malignant osteopetrosis. IFN-γ also has been studied extensively as treatment for idiopathic pulmonary fibrosis but is no longer recommended for this indication on the basis of the recent large, randomized, placebo-controlled INSPIRE (INternational study of Survival outcomes in idiopathic Pulmonary fibrosis with InteRfEron gamma-1b) trial, which was terminated prematurely because of the absence of efficacy and higher mortality in the treatment group (49). All forms of IFN are administered either subcutaneously or intramuscularly and are associated with significant adverse effects that frequently limit use, including acute constitutional symptoms, chronic fatigue and anorexia, neurologic toxicity, autoimmune thyroiditis, hepatotoxicity, and neutropenia. Given the frequency of these serious adverse effects, it is not surprising that the relatively infrequent occurrence of nephrotoxicity has received much less attention.
A spectrum of podocytopathies has been described in the setting of chronic treatment with IFN (Table 3). There have been eight case reports of nephrotic syndrome as a result of MCD, including six patients who were treated with INF-α and two who were treated with IFN-β. IFN was discontinued in all eight patients, three of whom also received steroids. All eight patients went into remission, including CR in six. Interestingly, IFN was subsequently reintroduced in three patients (31,32,37), followed by recurrence of nephrotic syndrome in two (31,32).
Table 3.
Published reports of FSGS or minimal-change disease after treatment with IFN
| Source | IFN Type | IFN Indication | IFN Duration | Age (years) | Gender | Race | Renal Presentation | Uprot (g/d) | Albumin (g/dl) | Creatinine (mg/dl) | Duration of Follow-up | Treated with Prednisone? | Clinical Remission? | Renal Biopsy Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Auerbach et al. (31), 1984 | α | Mycosis fungoides | 2 weeks | 52 | Female | NA | NS and ARF | 28.0 | Hypoalbuminemia | 4.1 | 3.5 months | No | CR | MCD and AIN |
| Rettmar et al. (32), 1995 | α | CML | 5 days | 32 | Male | White | NS | 17.9 | NA | 1.3 | 7 weeks | No | Yes | MCD |
| Nakao et al. (33), 2002 | β | Melanoma | 4 months | 64 | Male | NA | NS | 7.7 | 2.8 | 0.95 | 53 days | No | Yes | MCD |
| Nishimura et al. (34), 2002 | α-2b | HCV | 3 months | 57 | Female | NA | NS | 16.8 | 1.5 | 0.7 | 4 months | Yes | CR | MCD |
| Dizer et al. (35), 2003 | α | HCV | 6 months | 32 | Male | NA | NS and ARF | NA | 1.2 | 3.3 | 5 months | No | CR | MCD |
| Suresh et al. (36), 2003 | α | HCV | 27 weeks | 40 | Male | NA | NS | 2.3 | 1.5 | NA | 6 weeks | Yes | CR | MCD |
| Kumasaka et al. (37), 2006 | β-1b | MS | 22 months | 43 | Female | NA | NS | 11.4 | 1.8 | NA | 9 months | Yes | CR | MCD |
| Tovar et al. (38), 2008 | α-2b | HCV | 4 months | 50 | Female | NA | NS | 12.4 | 1.9 | Normal (CrCl 98) | 12 months | No | CR | MCD |
| Ault et al. (39), 1988 | γ | ALL | 19 days | 12 | Male | NA | NS and ARF | UA positive | 2.3 | 14.3 | 1 month | No | CR | FSGS |
| Traynor et al. (40), 1994 | α-2a | Mycosis fungoides | 11 weeks | 44 | Male | NA | NS and ARF | 11.0 | NA | 8.0 | 9 months | Yes | PR | FSGS |
| Horowitz et al. (41), 1995 | α-2b | Gastric carcinoma | 5 weeks | 47 | Male | Black | NS and ARF | 42.0 | NA | 13.5 | NA | NA | NA | FSGS |
| Coroneos et al. (42), 1996 | α | HCV | 5 weeks | 51 | Female | NA | NS and ARF | 18.0 | 1.7 | 12.0 | 5 months | Yes | No | FSGS |
| Nassar et al. (43), 1998 | α | Hypereosin syndrome | 20 months | 57 | Male | Black | NS and ARF | 4.73 | NA | 11.2 | 16 months | No | No | FSGS |
| Shah et al. (44), 1998 | α | CML | 4 weeks | 42 | Male | Black | NS and ARF | 7.4 | 2.1 | 11.4 | 12 weeks | Yes | No | FSGS |
| Shah et al. (44), 1998 | α | CML | 3 weeks | 45 | Female | Black | NS and ARF | 7.5 | 1.4 | 7.1 | 20 weeks | Yes | PR | FSGS |
| Dressler et al. (45), 1999 | α | Myeloma | 4 weeks | 46 | Male | Black | NS and ARF | 7.2 | 1.4 | 3.8 | 4 months | Yes | No | FSGS |
| Willson (46), 2002 | α | HCV | 1 year | 55 | Female | Black | NS | 6.3 | 1.1 | 0.9 | 1 year | No | PR | FSGS |
| Couto et al. (47), 2006 | α-2b | HCV | 4 months | 44 | Male | NA | NS | 16.5 | 1.2 | 0.9 | 10 months | Yes | No | FSGS |
| Stein et al. (28), 2001 | α | HCV | 10 weeks | 57 | Female | Black | NS and ARF | 19.3 | 1.4 | 9.6 | 8 weeks | Yes | No | Collapsing FSGS |
| Bremer et al. (29), 2003 | α | CML and HCV | 1 month | 40 | Male | NA | NS and ARF | 15.9 | 0.6 | 7.6 | NA | Yes | NA | Collapsing FSGS |
| Kanungo et al. (30), 2009 | α | HCV | 3 months | 52 | Female | Black | ARF | NA | NA | 9.0 | NA | NA | No | Collapsing FSGS |
AIN, acute interstitial nephritis; ALL, acute lymphoblastic leukemia; ARF, acute renal failure; CML, chronic myeloid leukemia; CR, complete remission; CrCl, creatinine clearance; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; NA, not available; NS, nephrotic syndrome; PR, partial remission; UA, urinalysis.
Reports of FSGS after treatment with IFN have included FSGS of the usual type (FSGS NOS) (10 cases) and collapsing FSGS (three cases; Table 3). Among the 13 reported cases, at least 12 had full nephrotic syndrome and 11 had acute renal failure (with information about nephrotic parameters unavailable in one case) (30). Twelve patients were receiving IFN-α, and one patient was being treated with IFN-γ. IFN was discontinued in all patients, and eight also received steroids. A decline in proteinuria and improvement in renal function were documented in nine of 10 patients with available follow-up, although only one patient had a CR (39) and only three had a PR (40,44,46). Of note, all three reported patients with collapsing FSGS were receiving IFN-α, including one (28) from our center, who also is included in this study (patient 2). Furthermore, one of the three cases of purported collapsing FSGS is complicated by the fact that the patient had a creatinine of 2.5 mg/dl and biopsy-proven diabetic glomerulosclerosis before receiving IFN-α (30). Of note, there are also reports of patients who were treated with IFN and had renal biopsy findings of thrombotic microangiopathy (50) and acute tubular necrosis (51). In addition, reversible IFN-associated nephrotic syndrome in the absence of renal biopsy has been described (52).
In contrast to the reported cases in Table 3, all 11 patients in our cohort who developed podocytopathies in the setting of treatment with IFN had biopsy findings of collapsing FSGS. Similar to the reports of collapsing FSGS and FSGS NOS in Table 3, our patients presented with nephrotic syndrome and renal insufficiency and had a strong black racial predominance. Outcomes were also similar, with most patients experiencing an improvement in renal function and reduction in proteinuria but few patients meeting criteria for CR or PR. Given the generally poor outcome in patients with idiopathic collapsing FSGS (8–12,14), the improvement after discontinuation of IFN-α, -β, and -γ argues strongly in favor of its causative role in the development of collapsing FSGS in our cohort. This causative relationship is further strengthened by the reports of recurrent nephrotic syndrome after reintroduction of IFN (patient 9) (31,32). It is interesting to note that the mean duration of IFN therapy before the development of collapsing FSGS was 2.95 months for the eight patients who were treated with IFN-α (Tables 1 and 3). In contrast, the development of collapsing FSGS occurred after 3 and 4 years of treatment with IFN-β in the two patients with available data (Table 1).
There are two important distinctions between our cohort and the reported cases in Table 3. First, we identified only cases of collapsing FSGS, whereas the majority of reports describe FSGS of the usual or NOS type. This discrepancy may relate to differences in nomenclature, because many of the reports of FSGS NOS were published around the time that collapsing FSGS was becoming a recognized entity. Furthermore, almost all of the cases in Table 3 were reported before the publication of a working proposal for the classification of FSGS (7), which requires only a single collapsing lesion to diagnose the collapsing variant. Our report is also the first to describe a high prevalence of endothelial TRIs (n in 10 of 11 patients). Endothelial TRIs are interanastamosing microtubular structures that are seen mainly within the cytoplasm of endothelial cells and most commonly encountered in the setting of SLE and HIV infection. Because TRIs can be induced in tissue culture or animal models by exposure to IFN-α, these structures have been referred to as “IFN footprints” (53,54). Our only case without detectable TRIs had suboptimal tissue for ultrastructural evaluation, leading us to conclude that TRIs are a characteristic and diagnostically helpful feature of collapsing FSGS secondary to treatment with IFN. Of course, HIV nephropathy should always be excluded in the setting of collapsing FSGS with TRIs. Even in the setting of HIV infection and collapsing FSGS, a history of treatment with IFN should be sought because of the high prevalence of HIV and HCV co-infection.
How IFN therapy causes collapsing FSGS is unknown. Possibilities include direct effects of IFN on the podocyte or indirect effects via altered cytokine milieu. IFN-α and IFN-β act via binding to the α/β receptor, whereas IFN-γ is recognized by a separate receptor. Receptor engagement leads to transcription of multiple IFN response genes that encode proteins that interfere with viral replication and assembly, suppress cellular proliferation, increase antimicrobial resistance, and alter cell metabolism. Although there is some overlap in subcellular pathways, receptor activation by IFN-γ leads to enhanced oxidative capacity of macrophages, natural killer cell activation, and increased expression of MHC genes. One or more of these subcellular events may also be operant in the podocyte, which expresses the α/β receptor and can be stimulated by IFN-γ in vitro to express MHC class II antigens and intercellular adhesion molecule 1 (55).
IFNs are known to activate adaptive immune mechanisms (including Th1 response) that promote macrophage activation. Generalized macrophage activation is the key process that underlies hemophagocytic syndrome, a condition that is associated with collapsing FSGS (22). Of interest, podocytopathies including FSGS can also occur in SLE (56), an autoimmune condition with high circulating levels of IFN. The possibility that IFN could participate in collapsing glomerulopathies related to viral infections such as HIV and parvovirus B19 deserves further study. A central role for activation of the Th1 response has been proposed for some forms of collapsing FSGS (57). It remains to be determined how IFN may alter the synthesis of other, potentially pathogenic cytokines, such as IL-6 or IL-13 family members, which have been incriminated as permeability factors in FSGS and MCD (58,59).
Conclusions
We report 11 cases of collapsing FSGS after treatment with IFN-α, -β, or -γ. This is the first report of FSGS after the use of IFN-β, the first description of collapsing FSGS in patients who were treated with IFN-γ, and the first study to emphasize the frequency and diagnostic importance of endothelial TRIs. Optimal therapy for this lesion includes discontinuation of treatment with IFN; the role of additional immunosuppressive therapy remains undefined. Similar to pamidronate, IFN should be considered a well-established cause of drug-induced collapsing FSGS.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1.D'Agati V: The spectrum of focal segmental glomerulosclerosis: New insights. Curr Opin Nephrol Hypertens 17: 271–281, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Haas M, Spargo BH, Coventry S: Increasing incidence of focal segmental glomerulosclerosis among adult nephropathies: A 20-year renal biopsy study. Am J Kidney Dis 26: 740–750, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Korbet SM, Genchi R, Borok RZ, Schwartz MM: The racial prevalence of glomerular lesions in nephrotic adults. Am J Kidney Dis 27: 647–651, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Haas M, Meehan SM, Karrision TG, Spargo BH: Changing etiologies of unexplained nephrotic adult nephrotic syndrome: A comparison of renal biopsy findings from 1976–1979 and 1995–1997. Am J Kidney Dis 30: 621–631, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Kitiyakara C, Eggers P, Kopp JB: Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis 44: 815–825, 2004 [PubMed] [Google Scholar]
- 6.Nair R, Walker PD: Is IgA nephropathy the commonest primary glomerulopathy among young adults in the USA? Kidney Int 69: 1455–1458, 2006 [DOI] [PubMed] [Google Scholar]
- 7.D'Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of FSGS: A working proposal. Am J Kidney Dis 43: 368–382, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Stokes MB, Valeri AM, Markowitz GS, D'Agati VD: Cellular focal segmental glomerulosclerosis: Clinical and pathologic features. Kidney Int 70: 1783–1792, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Thomas DB, Franceschini N, Hogan SL, ten Holder S, Jennette CE, Falk RJ, Jennette JC: Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int 69: 920–926, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Weiss MA, Daquioag E, Margolin EG, Pollak VE: Nephrotic syndrome, progressive irreversible renal failure, and glomerular “collapse”: A new clinicopathologic entity? Am J Kidney Dis 7: 20–28, 1986 [DOI] [PubMed] [Google Scholar]
- 11.Valeri A, Barisoni L, Appel GB, Seigle R, D'Agati V: Idiopathic collapsing focal segmental glomerulosclerosis: A clinicopathologic study. Kidney Int 50: 1734–1746, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Detwiler RK, Falk RJ, Hogan SL, Jennette JC: Collapsing glomerulopathy: A clinically and pathologically distinct variant of focal segmental glomerulosclerosis. Kidney Int 45: 1416–1424, 1994 [DOI] [PubMed] [Google Scholar]
- 13.D'Agati VD, Suh J, Carbone L, Appel GB: The pathology of HIV-associated nephropathy: A detailed morphologic and comparative study. Kidney Int 35: 1358–1370, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Laurinavicius A, Hurwitz S, Rennke HG: Collapsing glomerulopathy in HIV and non-HIV patients: A clinicopathological and follow-up study. Kidney Int 56: 2203–2213, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Barisoni L, Kriz W, Mundel P, D'Agati V: The dysregulated podocyte phenotype: A novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999 [DOI] [PubMed] [Google Scholar]
- 16.D'Agati VD: Podocyte injury in focal segmental glomerulosclerosis: Lessons from animal models (a play in five acts). Kidney Int 73: 399–406, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Barisoni L, Bruggeman LA, Mundel P, D'Agati VD, Klotman PE: HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int 58: 173–181, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Shankland SJ, Eitner F, Hudkins KL, Goodpaster T, D'Agati VD, Alpers CE: Differential expression of cyclin-dependent kinase inhibitors in human glomerular disease: Role in podocyte proliferation and maturation. Kidney Int 58: 674–683, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Barisoni L, Mokrzycki M, Sablay L, Nagata M, Yamase H, Mundel P: Podocyte cell cycle regulation and proliferation in collapsing glomerulopathies. Kidney Int 58: 137–143, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Dijkman HB, Weening JJ, Smeets B, Verrijp KC, van Kuppevelt TH, Assmann KK, Steenbergen EJ, Wetzels JF: Proliferating cells in HIV and pamidronate-associated collapsing focal segmental glomerulosclerosis are parietal epithelial cells. Kidney Int 70: 338–344, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Moudgil A, Nast CC, Bagga A, Wei L, Nurmamet A, Cohen AH, Jordan SC, Toyoda M: Association of parvovirus B19 infection with idiopathic collapsing glomerulopathy. Kidney Int 59: 2126–2133, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Thaunat O, Delahousse M, Fakhouri F, Martinez F, Stephan JL, Noel LH, Karras A: Nephrotic syndrome associated with hemophagocytic syndrome. Kidney Int 69: 1892–1898, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, Kuhn JA, Dratch AD, D'Agati VD: Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 12: 1164–1172, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Perazella MA, Markowitz GS: Bisphosphonate nephrotoxicity. Kidney Int 74: 1385–1393, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Markowitz GS, Radhakrishnan J, Kambham N, Valeri AM, Hines WH, D'Agati VD: Lithium nephrotoxicity: A progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol 11: 1439–1448, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Letavernier E, Bruneval P, Mandet C, Van Huyen JP, Peraldi MN, Helal I, Noel LH, Legendre C: High sirolimus levels may induce focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2: 326–333, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Herlitz LC, Markowitz GS, Farris AB, Schwimmer JA, Stokes MB, Kunis C, Colvin RB, D'Agati VD: Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol 21: 163–172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein DF, Ahmed A, Sunkhara V, Khalbuss W: Collapsing focal segmental glomerulosclerosis with recovery of renal function: An uncommon complication of interferon therapy for hepatitis C. Dig Dis Sci 46: 530–535, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Bremer CT, Lastrapes A, Alper AB, Mudad R: Interferon-alpha-induced focal segmental glomerulosclerosis in chronic myelogenous leukemia. Am J Clin Oncol 26: 262–264, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kanungo S, Tamirisa S, Gopalakrishnan R, Salinas-Madrigal L, Bastani B: Collapsing glomerulopathy as a complication of interferon therapy for hepatitis C infection. Int Urol Nephrol June4, 2009. [ epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Averbuch SD, Austin HA, Sherwin SA, Antonovych T, Bunn PA, Longo DL: Acute interstitial nephritis with the nephrotic syndrome following recombinant leukocyte a interferon therapy for mycosis fungoides. N Engl J Med 310: 32–35, 1984 [DOI] [PubMed] [Google Scholar]
- 32.Rettmar K, Kienast J, van de Loo J: Minimal change glomerulonephritis with reversible proteinuria during interferon α2A therapy for chronic myeloid leukemia. Am J Hematol 49: 355–366, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Nakao K, Sugiyama H, Makino E, Matsuura H, Ohmoto A, Sugimoto T, Ichikawa H, Wada J, Yamasaki Y, Makino H: Minimal change nephrotic syndrome developing during postoperative interferon-beta therapy for malignant melanoma. Nephron 90: 498–500, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Nishimura S, Miura H, Yamada H, Shinoda T, Kitamura S, Miura Y: Acute onset of nephrotic syndrome during interferon-α treatment for chronic active hepatitis C. J Gastroenterol 37: 854–858, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Dizer U, Beker CM, Yavuz I, Ortatatli M, Ozguven V, Pahsa A: Minimal change disease in a patient receiving INF-α therapy for chronic hepatitis C virus infection. J Interferon Cytokine Res 23: 51–54, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Suresh RL, Suryati Y, Merican I: Interferon induced glomerular disease in a patient with chronic hepatitis C. Med J Malaysia 58: 594–596, 2003 [PubMed] [Google Scholar]
- 37.Kumasaka R, Nakamura N, Shirato K, Fujita T, Murakami R, Shimada M, Nakamura M, Osawa H, Yamabe H, Okumura K: Nephrotic syndrome associated with interferon-beta-1b therapy for multiple sclerosis. Clin Exp Nephrol 10: 222–225, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Tovar JL, Buti M, Segarra A, Majo J, Esteban R: De novo nephrotic syndrome following pegylated interferon alfa 2b/ribavirin therapy for chronic hepatitis C infection. Int Urol Nephrol 40: 539–541, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Ault BH, Stapleton FB, Gaber L, Martin A, Roy S, Murphy SB: Acute renal failure during therapy with recombinant human gamma interferon. N Engl J Med 319: 1397–1400, 1988 [DOI] [PubMed] [Google Scholar]
- 40.Traynor A, Kuzel T, Samuelson E, Kanwar Y: Minimal change glomerulopathy and glomerular visceral epithelial hyperplasia associated with alpha-interferon therapy for cutaneous T-cell lymphoma. Nephron 67: 94–100, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Horowitz R, Glicklich D, Sablay LB, Wiernik PH, Wadler S: Interferon-induced acute renal failure: A case report and literature review. Med Oncol 12: 55–57, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Coroneos E, Petrusevska G, Varghese F, Truong LD: Focal segmental glomerulosclerosis with acute renal failure associated with α-interferon therapy. Am J Kidney Dis 28: 888–892, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Nassar GM, Pedro P, Remmers RE, Mohanty LB, Smith W: Reversible renal failure in a patient with the hypereosinophilia syndrome during therapy with alpha interferon. Am J Kidney Dis 31: 121–126, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Shah M, Jenis EH, Mookerjee BK, Schriber JR, Baer MR, Herzig GP, Wetzler M: Interferon-alpha-associated focal segmental glomerulosclerosis with massive proteinuria in patients with chronic myeloid leukemia following high dose chemotherapy. Cancer 83: 1938–1946, 1998 [PubMed] [Google Scholar]
- 45.Dressler D, Wright JR, Houghton JB, Kalra PA: Another case of focal segmental glomerulosclerosis in an acutely uraemic patient following interferon therapy. Nephrol Dial Transplant 14: 2049–2050, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Willson RA: Nephrotoxicity of interferon alpha-ribavirin therapy for chronic hepatitis C. J Clin Gastroenterol 35: 89–92, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Couto CA, Faria LC, Ribeiro DD, de Paula Farah K, de Melo Couto OF, de Abreu Ferrari TC: Life-threatening thrombocytopenia and nephrotic syndrome due to focal segmental glomerulosclerosis associated with pegylated interferon alpha-2b and ribavirin treatment for hepatitis C. Liver Int 26: 1294–1297, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Isaacs A, Lindenmann J: Viral interference: I. The interferon. Proc R Soc Lond B Biol Sci 147: 258–267, 1957 [PubMed] [Google Scholar]
- 49.King TE, Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, Noble PW, Sahn SA, Szwarcberg J, Thomeer M, Valeyre D, du Bois RM: Effects of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): A multicentre, randomized, placebo-controlled trial. Lancet 374: 222–228, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Jadoul M, Piessevaux H, Ferrant A, Cosyns JP, van Ypersele de Strihou C: Renal thrombotic microangiopathy in patients with chronic myelogenous leukaemia treated with interferon-alpha 2b. Nephrol Dial Transplant 10: 111–113, 1995 [PubMed] [Google Scholar]
- 51.Fahal IH, Murry N, Chu P, Bell GM: Acute renal failure during interferon therapy. BMJ 306: 973, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selby P, Kohn J, Raymond J, Judson I, McElwain T: Nephrotic syndrome during treatment with interferon. BMJ 290: 1180, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rich SA, Owens TR: Inducibility of human lupus inclusions by interferons [Abstract]. J Cell Biochem [Suppl 6]: 279A, 1982 [Google Scholar]
- 54.Feldman D, Hoar RM, Niemann WH, Valentine T, Cukierski M, Hendrickx AG: Tubuloreticular inclusions in placental chorionic villi of rhesus monkeys after maternal treatment with interferon. Am J Obstet Gynecol 155: 413–424, 1986 [DOI] [PubMed] [Google Scholar]
- 55.Baudeau C, Delarue F, He CJ, Nguyen G, Adida C, Peraldi MN, Sraer JD, Rondeau E: Induction of MHC class II molecules HLA-DR, -DP, and -DQ and ICAM1 in human podocytes by gamma-interferon. Exp Nephrol 2: 306–312, 1994 [PubMed] [Google Scholar]
- 56.Kraft SW, Schwartz MM, Korbet SM, Lewis EJ: Glomerular podocytopathy in patients with systemic lupus erythematosus. J Am Soc Nephrol 16: 175–179, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Albaqumi M, Soos TJ, Barisoni L, Nelson PJ: Collapsing glomerulopathy. J Am Soc Nephrol 17: 2854–2863, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Savin VJ, Sharma M, McCarthy ET, Sharma R, Reddy S, Dong JW, Hess S, Kopp J: Cardiotrophin like cytokine-1: Candidate for the focal glomerular sclerosis permeability factor [Abstract]. J Am Soc Nephrol 19: 59A, 2008. 18045850 [Google Scholar]
- 59.Lai KW, Wei CL, Tan LK, Tan PH, Chiang GS, Lee CG, Jordan SC, Yap HK: Overexpression of interleukin-13 induces minimal change like nephropathy in rats. J Am Soc Nephrol 18: 1476–1485, 2007 [DOI] [PubMed] [Google Scholar]