Abstract
Background and objective: The clinical manifestation of angioedema ranges from minor facial edema up to life-threatening swelling of mouth and throat. Hereditary defects, drugs, and food allergies may play a role in the development of angioedema. We systematically investigated the incidence of angioedema in renal allograft recipients treated with mTOR inhibitors (mTORis).
Design, setting, participants, & measurements: All patients in the authors' electronic database who had received mTORis (n = 309) between 2000 and 2008 were identified. Of these, 137 were additionally treated with angiotensin-converting enzyme inhibitors (ACEis).
Results: Nine patients (6.6%, 3.8 per 100 treatment years) developed angioedema after a mean period of 123 days under combined therapy with mTORi and ACEi. Among the remaining 172 patients on mTORi, including 119 patients treated with angiotensin-receptor blockers, only two developed angioedema (1.2%, 0.5 per 100 treatment years, P = 0.01). In patients receiving mycophenolate and ACEi (n = 462), 10 instances of angioedema were found (2.1%, 0.8 per 100 treatment years, P = 0.004).
Conclusions: This systematic investigation demonstrated a noticeable incidence of 6.6% angioedema under combined therapy with mTORi and ACEi in kidney transplant recipients. Treatment with either ACEi or mTORi alone resulted in a significantly lower incidence of angioedema, suggesting that this combination should be avoided.
Depending on its magnitude and localization, the clinical picture of angioedema varies widely from moderate self-limiting facial edema up to life-threatening swelling of lips, tongue, or throat. Underlying etiologies include hereditary defects of complement inhibitor C1, drugs, and food allergies (1,2). Increased bradykinin levels may play a role in the development of angioedema; however, the exact pathophysiology of angioedema remains unclear (3). One of the most frequent causes of angioedema is use of angiotensin-converting enzyme inhibitors (ACEis), which are estimated to be responsible for 10% to 25% of all cases of angioedema (4). ACEis are widely used in patients with hypertension, heart failure, kidney diseases, or diabetes because of their convincing efficacy. It has been suggested that ACEi increases the risk of angioedema, most likely due to vasodilatation as a consequence of reduced bradykinin degradation (3). Initial data on the occurrence of angioedema under ACEi therapy came from registration trials and pharmacovigilance registries, but only recent large prospective trials provided reliable insight on the incidence of this rare side effect.
In the randomized, double-blind OCTAVE trial with >12,000 patients, angioedema occurred in 0.68% of ACEi-treated patients (5). More recently, an overall incidence of 0.3% (n = 25 of 8576) angioedema was reported for ACEi during the ONTARGET study (6). In contrast, the use of angiotensin-receptor blockers (ARBs) was associated with a much lower risk of angioedema (0.1%; n = 10 of 8542 patients) in this trial. Thus, ARBs may be applied to patients with ACEi-induced angioedema, although 2 of 26 patients with angioedema due to ACEi therapy had also angioedema with ARBs (7). Approximately 60% of ACEi-induced angioedemas start within 1 week, but ACEi-induced angioedema may occur even after years (8).
Higher incidence of angioedema under treatment with mTOR inhibitor (mTORi) in organ-transplanted patients has been implicated in case series and several case reports (9–13). Because we were confronted with similar patients in our outpatient clinic, we initiated a systematic search in our database to investigate frequency and clinical course of angioedema in a larger cohort of kidney transplant recipients.
Materials and Methods
We conducted a retrospective, single-center analysis of all renal transplant recipients listed with their patient records in our electronic medical database Tbase (14). Starting in 1999, all medications, laboratory data, and the clinical course of all renal allograft recipients (n = 1618) treated in our department (including all 763 transplantations performed in our hospital since 1999, >1000 waitlisted patients, all 754 patients undergoing transplantation in our hospital before 1999, and all patients undergoing transplantation who visited our outpatient clinic at least once between 1999 and 2008) are compiled in the database with currently more than 2800 patient entries, corresponding to more than 3 billion lab values and 100,000 medication data.
We set January 1, 2000 (the year of sirolimus approval in Germany), as the starting point of our analysis, which ended on December 31, 2008. During this period, a total of 1111 renal transplant patients were treated in our department with their data entered in the database. We first started to identify all patients under therapy with mTORi (n = 309); either sirolimus (n = 144) or everolimus (n = 165), ACEis (n = 617), ARBs (n = 372), and mycophenolate (MPA; n = 871). ACEis and ARBs were identified with the corresponding anatomical therapeutic chemical codes. Start and end dates of each medication were captured. Next, we selected all patients with combined therapy of mTORi plus ACEi (n = 137); mTORi plus ARBs (n = 119); or MPA plus ACEi (n = 460), including start and end dates of combination therapy. Of special interest were the date and medical reason for discontinuation of mTORi, ACEi, and ARBs, because we hypothesized that the development of angioedema could be one reason to discontinue therapy. Additionally, our database was queried for the following clinical terms: “swelling,” “quincke-edema,” “angioedema,” and “laryngeal edema.” Finally, we performed a systematic review of patient medical characteristics during each event of angioedema.
Statistical Analyses
The incidences of angioedema under different treatment modalities are calculated as the number of events divided by the time of respective therapy and expressed per 100 patient years. Comparisons of incidence are performed by calculation of relative rates and confidence interval between the different groups. The effect of combined treatment on the frequency of angioedema was evaluated using χ2 test. P values <0.05 were considered to be significant.
Results
We identified a total of 309 patients with 618 treatment years after renal transplantation under immunosuppressive therapy with mTORi (everolimus: n = 165; sirolimus: n = 144). Of these, 137 patients (44.3%) received both mTORi and ACEi therapy, with a total of 240 treatment years (group A). Within this group, in 45 patients (32.8%) ACEi treatment was stopped mostly because of dry cough (n = 9), rising creatinine (n = 6), hypotension (n = 4), insufficient BP control (n = 4), and unspecific intolerance (n = 7). Treatment with mTORi therapy was discontinued in 33 (24.1%) of these patients, mainly because of proteinuria (n = 8), skin problems (n = 5), pneumonitis (n = 2), hyperlipidemia (n = 2), infections (n = 2), and unspecific intolerance (n = 8).
Of the 137 patients who received both mTORi and ACEi, nine patients (6.6%; 3.8 per 100 patient years) developed angioedema after a mean period of 123 days under combined ACEi and mTORi therapy. Demographics and important clinical characteristics of these patients are summarized in Table 1. Six patients (patients 1 to 6) who had been on ACEi for a mean of 3.17 years (range: 1.3 to 8 years) before the onset of symptoms developed angioedema after initiation of mTORi. Three patients (patients 7, 8, and 9) developed angioedema after initiation of ACEi therapy, but they had been on mTORi for more than 195 days. In three patients (patients 1, 4, and 7) complement C1-inhibitor activity was investigated showing normal values during or shortly after the event.
Table 1.
Characteristics of angioedema in combined ACEi plus mTORi therapy
| Patient No. | Age/Gender | Time Since Tx, yr | Event Time, d | Event Treatment | ACEi | Reason for ACEi Therapy | ACEi before Event, d | mTORi | mTORi before Event, d | Drug Level at Event | Concomitant ISa | Change of IS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70/M | 4.2 | 2 | ACEi stop plus P | Benazepril | BP | 1052 | Everolimus | 17 | 9.9 ng/ml | MMF 2 g | Everolimus reduced |
| 2 | 77/M | 1.5 | 1 | ACEi stop | Enalapril | BP | 486 | Everolimus | 98 | 3.7 ng/mlb | MMF 1 g, MP 6 mg | No change |
| 3 | 74/F | 18.3 | 2 | ACEi stop plus P | Enalapril | BP | 1457 | Everolimus | 44 | 5.8 ng/ml | Tacrolimus 3 mg | No change |
| 4 | 55/M | 3.9 | 1 | ACEi stop | Enalapril | BP | 1198 | Sirolimus | 99 | 14.1 ng/mlc | MMF 1 g | Sirolimus reduced |
| 5 | 55/M | 3 | 1.5 | ACEi stop | Benazepril | BP | 827 | Everolimus | 75 | 15.1 ng/ml | MP 5 mg, MPA 1.4 g | Everolimus reduced |
| 6 | 18/F | 9.3 | 1 | Everolimus stop | Ramipril | BP | 1920 | Everolimus | 730 | not done | Cyclosporin, MP 2 mg | Switch everolimus to MPA |
| 7 | 47/M | 18.8 | 3 | ACEi stop plus P | Benazepril | Proteinuria | 255 | Sirolimus | 430 | 10.5 ng/ml | MP 4 mg | Sirolimus reduced |
| 8 | 50/M | 12.3 | 1 | ACEi stop plus P | Benazepril | Proteinuria | 13 | Sirolimus | 156 | 10 ng/ml | MP 2 mgd | No change |
| 9 | 20/M | 0.33 | 1 | ACEi stop plus P | Benazepril | Proteinuria | 1 | Sirolimus | 120 | 3.1 ng/ml | MP 6 mg, Tacrolimus | No change |
Overview of the medical characteristics of seven kidney transplant recipients with angioedema under combined treatment with ACEi and mTORi. Tx, transplantation; P, prednisolone; MP, methylprednisolone (equivalent dose in case of prednisolone); MPA, mycophenolate (MMF equivalent dose in case of enteric-coated mycophenolate); IS, immunosuppression; BP, blood pressure.
Daily dosage.
Five days after event.
Five days before event.
4 mg every other day.
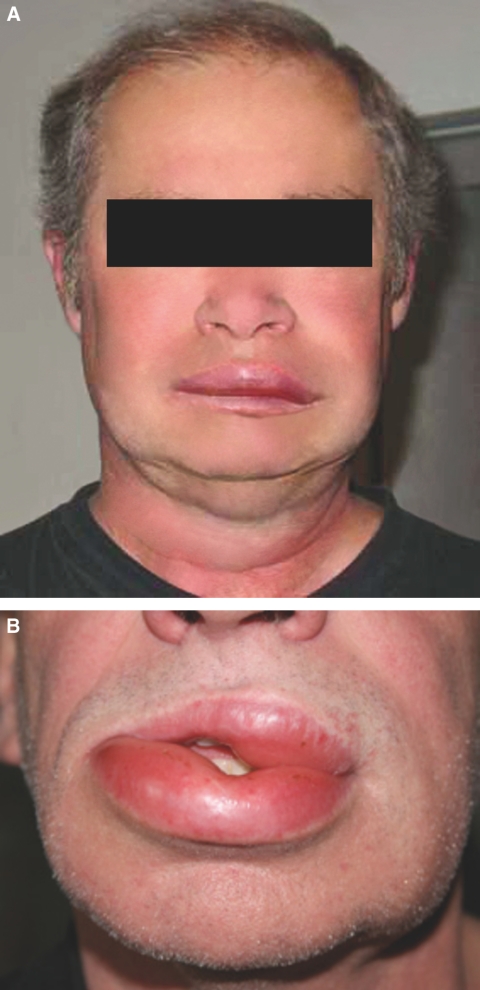
The clinical course of angioedema generally was mild to moderate, with partly fluctuating swelling of lips and face (Figure 1) in six of nine patients. By reason of angioedema, five of nine patients were hospitalized; e.g., patient 6, who presented with a more severe clinical picture of moderate tongue, lips, and eyelid edema, was hospitalized for initiation of antihistaminic and corticosteroid therapy. None of the patients were transferred to an intensive care unit; no external breathing support was necessary.
Figure 1.
Renal transplant patient no. 5 (a) and no. 7 (b) with typical lip and facial swelling during an event of angioedema under combined therapy with ACE and mTOR inhibitor.
All patients recovered rapidly after steroid bolus (n = 5) and/or stop of ACEi therapy (n = 8). In one patient (patient 6), mTORi was discontinued after the onset of angioedema. Because of high trough levels, mTORi doses were reduced in four patients (patients 1, 4, 5, and 7). For continued treatment of hypertension and/or proteinuria ARBs were initiated in eight patients with no recurrence of angioedema after discontinuation of ACEi during follow-up (mean follow-up 1298 days; range: 468 to 2919 days).
Interestingly, after the final diagnosis of angioedema was made, three patients (patients 1, 5, and 6) reported that they had experienced similar symptoms before. At the time of first symptoms, patient 1 was treated with MPA, cyclosporine, and prednisolone (5 mg/d), and he received high-dose corticosteroids in an emergency room, which led to rapid resolution of symptoms. Therapy with ACEi was continued because no one suspected a relationship with the drug. He developed similar symptoms shortly after initiation of mTORi (11 months later). When the diagnosis was made and benazepril finally was replaced by an ARB, no further signs or symptoms of angioedema were reported over the next 2.5 years despite continuous mTORi therapy.
Patient 5 noticed on several occasions over a period of 2 months varying degrees of lip swelling, with a brief improvement during concomitant prednisolone therapy of a gout attack. Every episode lasted for 2 to 3 days, and neither the patient nor his treating physician realized the association with ACEi therapy. After diagnosis of angioedema and cessation of ACEi + replacement by ARB, no further event of angioedema was seen over a period of 3 years.
Patient 6 had four episodes of angioedema under combined therapy of mTORi and ACEi over a period of 2 years, with little swelling of lips and face, until mTOR was switched to MPA because of recurrent angioedema. After another episode of angioedema 35 days after conversion, ACEi was finally stopped. Under treatment with ARB and MPA, no further episodes were observed over the last 2.5 years.
One patient with recurrent FSGS (patient 9) developed angioedema 1 day after initiation of ACEi under mTORi during a plasmapheresis session. After the current episode of angioedema, ACEi was paused for 1 month. Because of recurrent proteinuria, sirolimus was stopped, and ACEi was reintroduced without any further events of angioedema for 8 months.
For controls summarized in Table 2, we analyzed the remaining 172 patients with mTORi (378 treatment years). In this group, only two instances of angioedema were observed (1.2%; 0.5 per 100 treatment years, P = 0.01 versus group A). 119 (69%) of 172 patients received combination therapy of mTORi and ARBs, with a total of 190 treatment years. Only one patient in this group developed an episode of angioedema 21 days after initiation of sirolimus and combined therapy with ARB. (0.8%; 0.5 per 100 treatment years, P = 0.01 versus group A). Similarly, only one patient of the remaining 53 on mTORi therapy alone with 188 treatment years (1.9%; 0.5 per 100 treatment years, P = 0.2 versus group A) developed angioedema 95 days after initiation of mTORi, with no further episodes under continued (11-month) mTORi therapy.
Table 2.
General survey of diverse rates of angioedema due to patient medication
| Medication | MPA Overall | MPA Plus ACEi | mTORi Plus ACEi | mTORi Plus ARBs | mTORi Overall | mTORi Alone |
|---|---|---|---|---|---|---|
| Patients, n | 871 | 459 | 137 | 119 | 309 | 53 |
| Treatment years | 3471 | 1226 | 240 | 190 | 618 | 188 |
| Incidence of angioedema, % (n) | 1.8 (16) | 2.1 (10) | 6.6 (9) | 0.8 (1) | 3.6 (11) | 1.9 (1) |
| Angioedema per 100 patient years | 0.5 | 0.8 | 3.8 | 0.5 | 1.8 | 0.5 |
Additionally, we explored the occurrence of angioedema in all 871 patients treated with MPA (3471 treatment years). In this group, 460 patients (52.8%) received combination therapy of MPA and ACEi for a total of 1226 treatment years. In this control group, we found 10 patients (2.1%; 0.8 per 100 treatment years) with angioedema (P < 0.02 versus group A), including one patient (patient 1) who continued ACEi therapy for another year and developed recurrent angioedema 17 days after initiation of mTORi therapy (Table 1). Combination therapy with mTORi and ACEi increased the risk of developing angioedema by 3.7-fold (95% confidence interval: 1.5 to 8.9, P = 0.004) compared with combined therapy of MPA and ACEi.
Discussion
Our study is the first systematic investigation of angioedema in kidney transplant recipients under regular doses of mTORi that has a reasonable number of patients and adequate controls demonstrating a noticeable incidence of 6.6% angioedema under combination therapy with ACEi. Treatment with either ACEi or mTORi alone resulted in a significantly lower incidence of angioedema (2.2% versus 1.2%) in our observation. The clinical course of angioedema with combined therapy with ACEi and mTORi seems to be attenuated for kidney transplant patients under immunosuppressive maintenance therapy, when compared with the course described in the literature.
Stallone and colleagues (9) were the first to report five patients with angioedema in 52 kidney transplant patients taking sirolimus in combination with ramipril (5 mg/d). Similar to our observation, these patients developed non-life-threatening tongue edema within 1 month after starting ramipril, although they had taken ramipril before their transplantation without any signs of angioedema. All five patients were taking 5 mg/d sirolimus at high levels (between 16 and 20 ng/ml). After cessation of ramipril, angioedema resolved. Ramipril was restarted at lower doses (2.5 mg) in all patients at lower sirolimus levels (8 to 12 ng/ml) with no adverse effects, suggesting a dose-dependent effect of both drugs on the development of angioedema.
A similar observation was made by Fuchs (11), who described angioedema in seven heart transplant recipients 4 to 41 days after start of everolimus. Again, all patients were on combination therapy with ACEi and had high everolimus levels during the event. After cessation of ACEi and lowering everolimus levels to 3 to 8 ng/ml, the symptoms resolved in six of seven patients. Another small case series reported two kidney transplantation patients taking sirolimus and ACEi who developed nonpitting facial edema (5). In one patient, sirolimus was introduced while he had been on ACEi; in the other patient, ACEi was started, although he had been on mTORi for awhile. Symptoms resolved in both patients when the ACE inhibitor was stopped and corticosteroid therapy was increased. Another small case series described a very high incidence of angioedema in 12 (15%) of 80 renal allograft recipients on sirolimus (10). Similar to our and previous case series, the angioedema was predominantly located in the face (10 of 12 patients) with mucous membrane involvement in 7 of 12 patients. It was generally mild to moderate, although it was life threatening in one patient. In this case series, for most patients another putative cofactor was identified; 6 of 12 patients received ACEi, including one patient in whom the conversion from ARB to ACEi was the trigger for the development of angioedema. Fruit ingestion (walnut and mango) and physical activity were other triggers. Only one patient with high sirolimus levels (12 to 20 ng/ml) was on ARB, and in three patients with high sirolimus target levels (>10 ng/ml), no other obvious factor was found. The authors suggested a causal relationship between sirolimus and nonurticarial angioedema at least in 4 of 12 patients. This observation is in line with the report of Wadei et al. (13) describing three cases of sirolimus-induced angioedema in African-American renal allograft recipients. None of the patients were receiving any other drug potentially associated with angioedema.
Some reports describe a 5-fold higher incidence of angioedema for African Americans than for Caucasians (3.89 per 1000 treatment years versus 0.76 per 1000 treatment years, respectively), although this higher rate was not confirmed in a Nigerian patient population (0.54 per 1200 treatment years) (15,16). Because our investigation was limited to a Caucasian population, further studies are needed to assess whether black race increases the risk of angioedema in renal-transplanted patients treated with mTORi and/or ACEi.
In comparison with these previous studies in allograft recipients, our study more systematically assessed the incidence and frequency of angioedema in a much larger cohort with two adequate control groups. Furthermore, most of our patients were treated with lower mTORi levels compared with previous studies, because in more recent years much lower target levels for mTORi have evolved. But also, our study suggests for at least four patients a relationship between high mTORi levels and the occurrence of angioedema. In our analysis, we could find only one instance, which was attributable to mTORi therapy alone, but our target levels (historic and current) were always below 12 ng/ml for sirolimus and everolimus.
Under MPA and ACEi therapy, we found a slightly higher incidence of angioedema (2.1%) in renal allograft recipients compared with hypertensive populations (5,6). In both trials, the incidence of angioedema in ACEi-treated populations ranged between 0.3% and 0.68%. Our observation is in good agreement with previously published reports, suggesting a higher frequency of ACEi-associated angioedema (1% to 5%) in patients who underwent transplantation (9,11–13). It has been suggested that ACEis increase bradykinin levels, which could be a critical step for development of angioedema (3,15,16). Patients with hereditary deficiency of Cl-esterase inhibitor have elevated bradykinin levels and are more susceptible to angioedema. Racial differences in the kallikrein-kinin system and increased sensitivity to bradykinin may be responsible for the increased risk for black patients (9). ACE may also mediate the degradation of bradykinin; thus, ACEi may increase bradykinin levels, resulting in angioedema in vulnerable patients. In contrast, ARBs have no direct effect on the kallikrein-kinin system, but they may also increase the level of bradykinin by a yet unknown mechanism (17). Nevertheless, ARBs induce angioedema in a much lower frequency, and a few cases of ARB-induced angioedema have been described, including one patient with high sirolimus levels (10). In a small series, only 2 of 26 patients with ACEi-induced angioedema developed angioedema after conversion to ARBs. Thus, conversion to ARBs has been recommended as an overall safe option in patients with ACEi-induced angioedema, which is supported by our own experience and most sirolimus-associated cases described in the literature (7).
In vitro, sirolimus caused impaired, endothelium-dependent relaxation in response to bradykinin, suggesting that mTORis interfere with the bradykinin pathway, thereby raising susceptibility toward ACEi-induced angioedema (18). This is supported by the clinical course and the delayed onset of symptoms, which suggest that additional triggers (e.g., fruit, exercise) are necessary for the development of angioedema. Identification of this yet unknown mechanism may help to elucidate the pathophysiology of angioedema in general.
The clinical course of angioedema in sirolimus-treated patients appears to be generally mild and attenuated. Compared with hypertensive populations, who develop angioedema in more than 50% of instances within the first week after initiation of ACEi, the occurrence of angioedema varied widely (between 1 and 730 days) in our population. The delayed onset made it difficult to attribute the episode to an ACEi, similar to other case series: e.g., one patient had eight different manifestations over 3.5 years before ACEi was discontinued (19). Similar to three of our patients, another report described two patients who retrospectively recognized up to 24 episodes over 2 years (20). Not surprisingly, misdiagnoses are frequent, and both the doctor and the patient may not consider a drug-induced cause, leading to clear underreporting of this side effect.
We conclude that doctors and patients should be aware of this potentially dangerous side effect under combined therapy with mTORi and ACEi, a frequently used combination in patients with deterioration of graft function and/or proteinuria. Caution should be used when either starting an ACEi in a patient already taking mTORi or vice versa. Until more data are available, the preferential use of ARBs in patients under mTORi therapy seems advisable, because we and others observed under ARBs less angioedema, even in patients with ACEi-induced angioedema.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Donaldson VH, Evans RR: A biochemical abnormality in herediatry angioneurotic edema: Absence of serum inhibitor of C' 1-esterase. Am J Med 35: 37–44, 1963 [DOI] [PubMed] [Google Scholar]
- 2.Zuraw BL: Clinical practice. Hereditary angioedema. N Engl J Med 359: 1027–1036, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G: Nonallergic angioedema: Role of bradykinin. Allergy 62: 842–856, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Vleeming W, van Amsterdam JG, Stricker BH, de Wildt DJ: ACE inhibitor-induced angioedema. Incidence, prevention and management. Drug Saf 18: 171–188, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Kostis JB, Kim HJ, Rusnak J, Casale T, Kaplan A, Corren J, Levy E: Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 165: 1637–1642, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C: Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358: 1547–1559, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Cicardi M, Zingale LC, Bergamaschini L, Agostoni A: Angioedema associated with angiotensin-converting enzyme inhibitor use: Outcome after switching to a different treatment. Arch Intern Med 164: 910–913, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Ulmer JL, Garvey MJ: Fatal angioedema associated with lisinopril. Ann Pharmacother 26: 1245–1246, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Stallone G, Infante B, Di Paolo S, Schena A, Grandaliano G, Gesualdo L, Schena FP: Sirolimus and angiotensin-converting enzyme inhibitors together induce tongue oedema in renal transplant recipients. Nephrol Dial Transplant 19: 2906–2908, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Mahe E, Morelon E, Lechaton S, Kreis H, de Prost Y, Bodemer C: Angioedema in renal transplant recipients on sirolimus. Dermatology 214: 205–209, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Fuchs U, Zittermann A, Berthold HK, Tenderich G, Deyerling KW, Minami K, Koerfer R: Immunosuppressive therapy with everolimus can be associated with potentially life-threatening lingual angioedema. Transplantation 79: 981–983, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Burdese M, Rossetti M, Guarena C, Consiglio V, Mezza E, Soragna G, Gai M, Segoloni GP, Piccoli GB: Sirolimus and ACE-inhibitors: A note of caution. Transplantation 79: 251–252, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Wadei H, Gruber SA, El-Amm JM, Garnick J, West MS, Granger DK, Sillix DH, Migdal SD, Haririan A: Sirolimus-induced angioedema. Am J Transplant 4: 1002–1005, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Fritsche L, Horstrup J, Budde K, Reinke P, Giessing M, Tullius S, Loening S, Neuhaus P, Neumayer HH, Frei U: Old-for-old kidney allocation allows successful expansion of the donor and recipient pool. Am J Transplant 3: 1434–1439, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Ajayi AA, Adigun AQ: Angioedema and cough in Nigerian patients receiving ACE inhibitors. Br J Clin Pharmacol 50: 81–82, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown NJ, Ray WA, Snowden M, Griffin MR: Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther 60: 8–13, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Campbell DJ, Krum H, Esler MD: Losartan increases bradykinin levels in hypertensive humans. Circulation 111: 315–320, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Jeanmart H, Malo O, Carrier M, Nickner C, Desjardins N, Perrault LP: Comparative study of cyclosporine and tacrolimus vs newer immunosuppressants mycophenolate mofetil and rapamycin on coronary endothelial function. J Heart Lung Transplant 21: 990–998, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Gabb GM, Ryan P, Wing LM, Hutchinson KA: Epidemiological study of angioedema and ACE inhibitors. Aust N Z J Med 26: 777–782, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Schiller PI, Messmer SL, Haefeli WE, Schlienger RG, Bircher AJ: Angiotensin-converting enzyme inhibitor-induced angioedema: Late onset, irregular course, and potential role of triggers. Allergy 52: 432–435, 1997 [DOI] [PubMed] [Google Scholar]