Abstract
Background and objectives: This study aimed to investigate the development of new-onset diabetes mellitus (NODM) in a prospective study of 97 nondiabetic uremic patients.
Design, setting, participants, & measurements: Included were 57 kidney recipients (Tx group, age 39 ± 13 years) and 40 uremic patients remaining on the waiting list for kidney transplantation (uremic controls, age 47 ± 11 years). All were examined at baseline before possible transplantation and after 12 months. The prevalence of diabetes, prediabetes, insulin sensitivity index (ISI), and insulin secretion index (Isecr) were estimated using an oral glucose tolerance test with measurements of plasma glucose and plasma insulin.
Results: One year after transplantation NODM was present in 14% (8 of 57) compared with 5% (2 of 40) in the uremic control group (P = 0.01). ISI in the Tx group deteriorated from 6.8 ± 3.9 before transplantation to 4.9 ± 2.8 at 12 months after transplantation (P = 0.005), and a slight increase in Isecr from 37 ± 19 to 46 ± 22 (P = 0.02) was seen. No significant changes occurred in the uremic controls (ISI was 7.9 ± 5 and 8.5 ± 5, and Isecr was 31 ± 17 and 28 ± 15). Using multivariate ordinal logistic regression, pre-Tx ISI and age predicted NODM (odds ratios: 0.82, P = 0.01 and 1.06, P = 0.02, respectively).
Conclusions: One year after kidney transplantation, NODM was present in 14% of patients. This was mainly caused by an increase in insulin resistance and was observed despite improvement in insulin secretion.
After successful solid organ transplantation, new-onset diabetes mellitus (NODM) develops in up to 50% of patients (1,2) and is associated with increased cardiovascular disease and decreased quality of life (3–6).
Whether NODM is related to the immunosuppressive treatment inducing insulin resistance or to impaired insulin production is a matter of debate. An impaired beta cell function has been suggested to play a major role in the development of NODM (7), and Hur et al. (8) found in Korean patients that the prevalence of NODM seen 1 year after transplantation remained high when reinvestigated after 7 years; it was mainly related to an impaired insulin secretion.
The majority of patients undergoing transplantation today receive a calcineurin inhibitor-based regimen which has been demonstrated to be toxic to the pancreatic beta cell in both pancreas- and kidney-transplanted patients (9). Thus, immunosuppressive regimens may play a major role in the development of NODM, either by reducing insulin sensitivity or secretion. This is interesting, especially because the pathophysiology behind insulin resistance also plays a role in the development of cardiovascular disease (2,10). In addition, cytomegalovirus (CMV) infection, HLA type, age, and gender (5,10–14) have also been associated with development of NODM.
The combination of triple immunosuppressive and preemptive CMV therapies has lowered the need for steroid and CMV treatment. This has been suggested as the reason for the reduction in the incidence of NODM in a recent study (15).
We prospectively studied the development of NODM by examining kidney graft recipients before and after transplantation. Furthermore, a control group of uremic patients was included to follow the natural history of diabetes in uremia. The presence of prediabetes, NODM, insulin resistance, and insulin secretion was determined by oral glucose tolerance tests (OGTTs). We hypothesized that both pretransplantation impaired glucose tolerance (IGT) and further posttransplantation insulin resistance were the main factors behind development of NODM.
Materials and Methods
This is a prospective, observational, national multicenter study including 54 (67%) of 81 patients, mostly Caucasians, with a scheduled living donor kidney transplantation in the period between January 2006 and March 2008 at four Danish transplantation centers: Copenhagen, Skejby, Odense, and Herlev University Hospitals (Tx group). A control group consisted of patients from the transplantation waiting list at Copenhagen and Herlev University Hospital (uremic controls).
All 81 patient charts were prescreened, and the following were excluded from the study: diabetes present in the chart (n = 7), diabetes diagnosed with OGTT (n = 3), prednisolone treatment above 12.5 mg/d (n = 3), and newly initiated high-dose immunosuppressive treatment as in ABO-incompatible transplantation (n = 4). Ten patients did not want to participate. The control group was established by selecting 52 patients among all 510 patients listed in the waiting list according to age under 65 years, no more than one earlier transplantation, willingness to participate, and no diagnosis of diabetes, first by chart review and then by an OGTT. During follow-up, three patients from the control group underwent transplantation with a deceased donor, and they were included in the Tx group. Four patients died in the control group, and five patients did not want to participate in the 12-month examination, leaving us with a control group of 40 uremic patients without diabetes to be reexamined after 1 year. The main reasons for nonattendance were long traveling time to the laboratory or inability to spend extra days for the examinations besides the time already used for dialysis. The patients were interviewed about their family history of diabetes, defined as a parent or siblings having diabetes type 1 or 2.
A healthy control group consisting of 14 age-, body mass index- (BMI-), and sex-matched subjects was recruited from public announcing.
The regional ethics committee (KF 01279825) and The Data Protection Agency (2006-41-5640) approved the study. All participants gave their informed written consent.
Study Procedure
All participants undergoing transplantation were examined before and at 3 and 12 months after transplantation. The uremic controls were examined at baseline and 12 months later.
The examinations were done after an overnight fast including coffee, tobacco, and exercise abstinence for 10 hours. Usual antihypertensive medication was allowed in the morning.
Hemodialysis patients were examined between the days of hemodialysis. Peritoneal dialysis patients had the last peritoneal fluid installed at 10 p.m. and drained in the morning at 6 a.m. to minimize any bias from glucose absorbed from the peritoneum. The examination began between 8 and 11 a.m., with the OGTT done at least 4 hours after draining the peritoneal fluid. After 10 minutes in the supine resting position, BP was measured in triplicate from the arm opposite to a fistula or dialysis catheter. Mean arterial BP (MAP) was based on mean of these three measurements. Fasting blood samples were drawn from an antecubital vein for the determination of glucose and insulin and HbA1c.
Plasma glucose concentrations were analyzed by the glucose-hexokinase method (Gluco-quant®; Roche Diagnostics GmbH, Mannheim, Germany), and insulin was measured using enzyme-linked immunosorbent assay kits (Elecsys; Roche Diagnostics GmbH). All assays were automated and done on a Cobas Fara robot (Roche Diagnostics GmbH). Standard laboratory methods were applied for the analysis of HbA1c. Renal function was estimated by the Cockcroft-Gault formula (estimated GFR) (16).
The examiners were blinded regarding the actual clinical and metabolic status of the patient, and all clinical information was analyzed and described after data collection was completed.
Evaluation of Glucose Tolerance
A 75-g OGTT was done according to the World Health Organization/American Diabetes Association 2007 criteria (17). We determined the prevalence of normal glucose tolerance (fasting plasma glucose <5.6 mmol/L and 2-hour postload glucose <7.8 mmol/L), impaired fasting glucose (IFG: fasting plasma glucose between 5.6 and 6.9 mmol/L and a 2-hour postload glucose <7.8 mmol/L), impaired glucose tolerance (IGT: fasting plasma glucose <6.9 mmol/L and 2-hour postload glucose between 7.8 and 11.1 mmol/L) as well as prediabetes (IFG plus IGT) and diabetes (fasting plasma glucose >7.0 mmol/L or 2-hour postload glucose >11.1 mmol/L). Plasma concentrations of glucose and insulin were measured at times −30, −15, 0, 30, 60, 90, and 120 minutes. According to Matsuda et al. (18), an insulin sensitivity index (ISI) was calculated as 10,000/square root of [fasting glucose × fasting insulin] [mean glucose × mean insulin during OGTT]. This index is highly correlated with the rate of whole-body glucose disposal during a euglycemic insulin clamp in patients with varying degrees of glucose tolerance.
The area under the curve (AUC) for insulin and glucose during the OGTT was calculated using the trapezoid rule. These variables were implemented in the insulin secretion index (Isecr), SecrAUC = AUCIns/AUCGlu (19). The difference in ISI and Isecr between baseline values and 1-year values was calculated as ΔISI and ΔISecr (Table 1).
Table 1.
Clinical data at baseline and after transplantation in relation to glycemic status 1 year after kidney transplantation
| 12-Months OGTT | No Diabetes |
NODM | OR (95% Confidence Interval) |
||
|---|---|---|---|---|---|
| NGT | Prediabetes | Crude | Adjusted | ||
| n | 32 | 17 | 8 | ||
| Age, yr | 35 ± 12 | 45 ± 12 | 44 ± 12 | 1.06 (1.02 to 1.11) | 1.06 (1.01 to 1.11) |
| Gender, male/female | 20/12 | 14/3 | 4/4 | 1.20 (0.41 to 3.52) | |
| Family history of diabetes, n | 0 | 2 | 0 | 2.99 (0.2 to 40.7) | |
| BMI, kg/m2 | 23.6 ± 3.3 | 24.9 ± 4.7 | 26.7 ± 3.4 | 1.14 (0.99 to 1.31) | |
| Change in BMI, kg/m2 | 1.8 ± 2.5 | 2.3 ± 1.4 | 1.1 ± 1.7 | 0.97 (0.76 to 1.24) | |
| MAP, mmHg | 103 ± 12 | 102 ± 14 | 109 ± 20 | 1.01 (0.98 to 1.09) | |
| Waist-hip ratio | 0.91 ± 0.09 | 0.93 ± 0.08 | 0.95 ± 0.1 | 0.88 (0.27 to 2.91) | |
| Change in waist-hip ratio | 0.0 ± 0.1 | 0.1 ± 0.2 | 0.0 ± 0.1 | 10.1 (0.1 to 891) | |
| Baseline ISI | 7.9 ± 3.3 | 5.9 ± 4.9 | 4.2 ± 1.6 | 0.82 (0.69 to 0.97) | 0.83 (0.70 to 0.98) |
| Baseline Isecr | 31.6 ± 14.4 | 42.9 ± 22.5 | 46.0 ± 19.0 | 1.03 (1.01 to 1.06) | |
| Former Tx, n (%) | 8 (25) | 3 (18) | 0 | 0.36 (0.08 to 1.55) | |
| Rejections, n (%) | 3 (9) | 4 (24) | 2 (25) | 2.63 (0.69 to 10.06) | |
| ESRD, mo, median (range) | 7 (0 to 134) | 25 (0 to 108) | 30 (5 to 48) | 1.02 (1.00 to 1.03) | |
| CMV IgG, n (%) | 21 (66) | 11 (65) | 7 (88) | 1.19 (0.40 to 3.49) | |
| Accumulated prednisolone dose within 90 days, g | 2.39 ± 1.26 | 2.93 ± 1.20 | 2.93 ± 1.65 | 1.34 (0.91 to 2.08) | |
| Use of tacrolimus at Tx, n (%) | 7 (22) | 4 (24) | 3 (37) | 1.54 (0.49 to 4.86) | |
Values are given as mean ± SD unless otherwise noted. NGT, normal glucose tolerance; NODM, new onset diabetes mellitus; Tx, kidney transplantation; CMV, cytomegalovirus.
Immunosuppression
Immunosuppression varied to some extent between the centers. Induction therapy included basiliximab (Simulect; Novartis), daclizumab (Zenapax; Roche), or antithymocyte globulin (Thymoglobulin; Genzyme B.V.).
Corticosteroids.
The majority of patients received 100 to 500 mg of intravenous methylprednisolone preoperatively, and treatment with oral prednisolone was started by 20 to 100 mg/d and was tapered to a dose of 7.5 to 10 mg at 3 months and to 5 to 7.5 mg at 9 to 12 months. In the seven patients from Odense University Hospital, no prednisolone treatment was started as part of the routine treatment regimen (20). Rejection episodes, indicated by increased plasma creatinine of 20% or greater for 2 days, or biopsy proven, were treated with intravenous methylprednisolone, 500 mg, for 3 to 5 days. For each patient the accumulated corticosteroid dose within the first 90 days was calculated and given in equivalents of prednisolone dose in grams.
Calcineurin Inhibitors.
Forty-three patients started on cyclosporine (Sandimune Neoral; Novartis) at a dose of 2.5 to 6 mg/kg twice daily tapered to a trough level of whole-blood concentration of 150 to 300 μg/L for the first 3 months and 100 to 150 μg/L thereafter. The remaining 14 patients started on tacrolimus (Prograf; Astellas) at a dose of 0.075 to 0.15 mg/kg twice daily tapered to a whole-blood concentration of 8 to 15 μg/L for the first 3 months and 5 to 10 μg/L thereafter.
A few patients (n = 8) changed from one treatment modality to another during the course of the study, and two patients were changed to rapamycin (Rapamune; Wyeth) treatment.
Other Immunosuppression
Mycophenolate mofetil (Cellcept; Roche; or Myfortic; Novartis) was used for most patients, and azathioprine (Imurel; GlaxoSmithKline Pharma) was used as an alternative drug.
Antihypertensive Treatment
Antihypertensive treatment mainly included β-blockade, calcium channel blockade, angiotensin II blockade, and diuretics. All antihypertensive medication was stopped at the time of transplantation and thereafter titrated aiming for a BP below 130/80 mmHg.
Statistical Analyses
Data analyses were done using Statistical Analysis Software (SAS®) version 9.1. Unless specified otherwise, continuous data are described as mean ± SD for normal distributions, and median and range for skewed distributions. Paired data within groups were compared by t tests for normally distributed data, and group comparisons of continuous data were done using two-sample t test for normally distributed data and nonparametric Wilcoxon rank sum test for non-normal distributed data. χ2 or Fisher exact tests were used for group comparisons between categorical data. Correlation analysis was done using Spearman rank analysis. In the Tx group, univariate and multivariate linear regression analyses were done with the difference in ISI and Isecr as dependent variables. The following independent variables were included in the multivariate regression model with stepwise backward selection (cutoff for the multivariate model was P < 0.15): age (years), gender (M/F), ESRD duration (months), former Tx (yes/no), BMI (kg/m2), waist-hip ratio (WHR) <1 (yes/no), baseline ISI and Isecr, baseline CMV serotype, IgG positive (yes/no), MAP (mmHg), use of tacrolimus after Tx (yes/no), accumulated prednisolone dose within the first 90 days (grams), acute rejection episodes (yes/no), dialysis status (yes/no), and dialysis modality (continuous ambulatory peritoneal dialysis or hemodialysis). Multivariate ordinal logistic regression using the proportional odds model with age (years), baseline ISI and Isecr, immunosuppression (use of tacrolimus after Tx (yes/no)), accumulated prednisolone dose within the first 90 days (grams), change in BMI (kg/m2), WHR, and presence of family history of diabetes was done to find risk factors for deterioration in glucose tolerance (Table 1).
P value of <0.05 was used to determine significance.
Results
The patients in the two groups were well matched according to most clinical and demographic parameters. The patients in the Tx group were younger and had a shorter duration of ESRD (P = 0.03 and P = 0.001, respectively; Table 2). Two patients (4%) in the Tx group and four patients (10%) in the uremic control group had a family history of diabetes. The prevalence of prediabetes, mainly IGT, was high in both groups (33% in the Tx group and 50% in the uremic controls, respectively; Table 3). Furthermore, both patient groups were shown to be insulin resistant compared with a well-matched healthy control group (P < 0.001; Table 2), but insulin secretion was not impaired. Estimated GFR was 77 ± 20 and 78 ± 23 ml/min at 3 and 12 months after transplantation, respectively (Table 3). At 1 year, immunosuppressive treatment of the kidney recipients was: 37% received tacrolimus, 56% cyclosporine, 91% prednisolone (average 6 mg/d), and 96% mycophenolate mofetil. Accumulated prednisolone dose at 3 months after transplantation varied between centers, with an individual range from 0 to 6455 mg. The mean daily dose of prednisolone was 13 mg at 3 months and 7 mg at 12 months. Nine patients (16%) were diagnosed with and treated for a rejection, and five were treated for CMV disease with valganciclovir for 3 to 6 months. BMI increased significantly in the Tx group compared with the uremic controls, but waist-hip ratio remained similar between the groups (Table 3).
Table 2.
Demographic and clinical data from 57 kidney recipients (Tx group), 40 uremic patients (uremic controls), and 14 healthy controls, at baseline before transplantation
| Tx Group | Uremic Controls | Healthy Controls | |
|---|---|---|---|
| n | 57 | 40 | 14 |
| Age, yr | 39 ± 13b | 47 ± 11 | 39 ± 11 |
| Gender, male/female | 38/19 | 27/13 | 9/5 |
| ESRD duration, mo | 24c (0 to 134) | 45 (1 to 168) | - |
| BMI, kg/m2 | 24 ± 4 | 24 ± 4 | 24 ± 3 |
| Waist-hip ratio | 0.92 ± 0.09 | 0.91 ± 0.08 | 0.81 ± 0.08a |
| Ever smoking, n (%) | 27 (47) | 26 (65) | 9 (64) |
| Diagnoses, n (%) | |||
| glomerulonephritis | 28 (49) | 13 (33) | - |
| hypertensive kidney disease | 5 (9) | 11 (28) | - |
| vasculitis | 1 (2) | 1 (3) | - |
| PKD | 3 (5) | 7 (18) | - |
| other/unknown | 20 (35) | 8 (20) | - |
| apoplexi | 3 (5) | 3 (8) | - |
| former Tx | 11 (19) | 10 (25) | - |
| Dialysis status, n (%) | |||
| HD | 28 (49) | 28 (70) | - |
| CAPD | 19 (33) | 9 (27) | - |
| predialysis | 10 (18) | 3 (8) | - |
| first-degree relatives with DM | 2 (4) | 4 (10) | 2 (14) |
| Fasting p-glucose, mmol/L | 5.1 ± 0.5 | 5.1 ± 0.5 | 5.0 ± 0.3 |
| p-glucose at 2 h, mmol/L | 7.4 ± 1 | 7.5 ± 2 | 5.4 ± 1a |
| HbA1c, % | 5.2 ± 0.4 | 5.2 ± 0.4 | 5.2 ± 0.2 |
| ISI | 6.8 ± 4.0 | 7.9 ± 5.1 | 14.7 ± 7.0a |
| ISecr | 36.9 ± 18.5 | 31.4 ± 17.3 | 27 ± 14 |
| SBP, mmHg | 142 ± 21 | 141 ± 24 | 118 ± 10a |
| DBP, mmHg | 85 ± 13 | 83 ± 14 | 73 ± 8a |
| Pulse | 69 ± 11 | 72 ± 11 | 61 ± 10a |
Data are presented as mean ± SD or median (range), unless otherwise noted. Wilcoxon rank sum test or two-sample t test was used where appropriate to test. HD, hemodialysis; CAPD, continuous ambulatory peritoneal dialysis; Tx, transplantation; PKD, polycystic kidney disease; DM, diabetes mellitus; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Uremic patients versus healthy controls P < 0.0005.
Tx group versus uremic controls P < 0.05.
Tx group versus uremic controls P < 0.005.
Table 3.
Metabolic and immunosuppressive data before and after kidney transplantation in 97 uremic patients without known diabetes (DM) at baseline
| Parameter | Tx Group |
Uremic Controls |
||||
|---|---|---|---|---|---|---|
| Time 0 | Time 3 mo | Time 12 mo | Time 0 | Time 12 mo | ||
| Number of patients | 57 | 57 | 57 | 40 | 40 | |
| Fasting p-glucose, mmol/L | 5.1 ± 0.5 | 5.6 ± 0.7a | 5.5 ± 0.7a | 5.05 ± 0.5 | 4.9 ± 0.9 | |
| p-glucose at 2 h, mmol/L | 7.4 ± 1.6 | 8.7 ± 3.1 | 7.8 ± 2.5 | 7.8 ± 1.7 | 7.8 ± 2.4 | |
| HbA1c, % | 5.2 ± 0.4 | 5.6 ± 0.5a | 5.6 ± 0.4a | 5.2 ± 0.4 | 5.3 ± 0.4 | |
| AUC insulin, pmol/L × 120 min | 35,358 ± 19,884 | 36,977 ± 17,023 | 45,970 ± 27,367 | 29,606 ± 17,330 | 25,745 ± 15,025 | |
| AUC glucose, mmol/L × 120 min | 948 ± 157 | 1059 ± 287 | 994 ± 237 | 965 ± 191 | 939 ± 244 | |
| ISI composite | 6.8 ± 3.9 | 4.9 ± 3.9b | 4.9 ± 2.8b | 7.9 ± 5.1 | 8.5 ± 4.9 | |
| Isecr | 36.9 ± 18.5 | 35.8 ± 17.3 | 45.8 ± 22c | 31.4 ± 17.3 | 28.4 ± 14.5 | |
| OGTT | ||||||
| DM, n (%) | 0 | 11 (19) | 8 (14)c | 0 | 2 (5) | |
| IFG/IGT, n (%) | 0/19 (33) | 3/17 (35) | 1/16 (30) | 3/18 (50) | 2/14 (40) | |
| NGT, n (%) | 38 (67) | 26 (46) | 32 (56) | 20 (50) | 22 (55) | |
| Estimated GRF, ml/min | <15 | 73.6 ± 20.3a | 78.0 ± 22.7a | <15 | <15 | |
| BMI, kg/m2 | 24.4 ± 3.9 | 25.4 ± 3.9 | 26.3 ± 4.6c | 24.1 ± 3.0 | 24.0 ± 2.7 | |
| Waist-hip ratio | 0.92 ± 0.09 | 0.94 ± 0.09 | 0.93 ± 0.09 | 0.92 ± 0.08 | 0.91 ± 0.10 | |
| MAP, mmHg | 104 ± 14 | 97 ± 11 | 98 ± 15 | 102 ± 16 | 102 ± 14 | |
| AH | 2.5 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | |
| Beta-receptor blocker, n (%) | 27 (47) | 34 (71) | 35 (61) | 16 (39) | 20 (49) | |
| Number of acute rejection episodes (%) | 0 | 8 (14) | 9 (16) | NA | NA | |
| CMV disease, n (%) | 0 | 2 (4) | 5 (9) | 0 | 0 | |
| Prednisolone, n (%) | 12 (21) | 52 (91) | 52 (91) | 5 (2) | 5 (2) | |
| Prednisolone, dose, mg | 6 | 13 | 7 | 5 | 5 | |
| Tacrolimus, n (%) | 1 (2) | 19 (33) | 21 (37) | 0 | 0 | |
| Tacrolimus, dose, mg | 0 | 2 | 2 | |||
| Cyclosporine, n (%) | 1 (2) | 36 (63) | 32 (56) | 0 | 0 | |
| Cyclosporine, dose, mg | 0 | 214 | 145 | 0 | 0 | |
| Azathioprine/sirolimus, n (%) | 0 | 2 (4) | 3 (5) | 0 | 0 | |
| MMF; Cellcept, n (%) | 4 (7) | 52 (91) | 46 (81) | 0 | 0 | |
| MMF; Myfortic, n (%) | 0 | 2 (4) | 7 (15) | 0 | 0 | |
Values are given as mean ± SD unless otherwise noted. Wilcoxon rank sum test or unpaired t test was used where appropriate to test. NGT, normal glucose tolerance; AH, mean number of antihypertensive medications; CMV, cytomegalovirus; MMF, mycophenolate mofetil.
Tx patients before versus after Tx P < 0.0005.
Tx patients before versus after Tx P < 0.005.
Tx patients before versus after Tx P < 0.05.
Eight patients (14%) developed diabetes in the Tx group compared with two patients (5%) in the uremic controls (P < 0.01; Table 3). One patient required insulin treatment for a short period, whereas the others were treated with diet alone. The transplantation patients who developed diabetes (n = 8) had a significantly lower ISI at baseline and were slightly older compared with patients with normal glucose tolerance at follow-up (P < 0.05; Table 1). Among the 14 patients receiving tacrolimus, three (21%) developed NODM compared with five (11%) of the 43 receiving cyclosporine; however, the ordinal logistic regression model could not demonstrate statistically significant difference between the two immunosuppressive regimens. The average accumulated prednisolone dose was 0.5 g (22%) higher in patients developing NODM compared with patients with normal glucose tolerance after transplantation, but these data did not reach statistical significance in the ordinal logistic regression model.
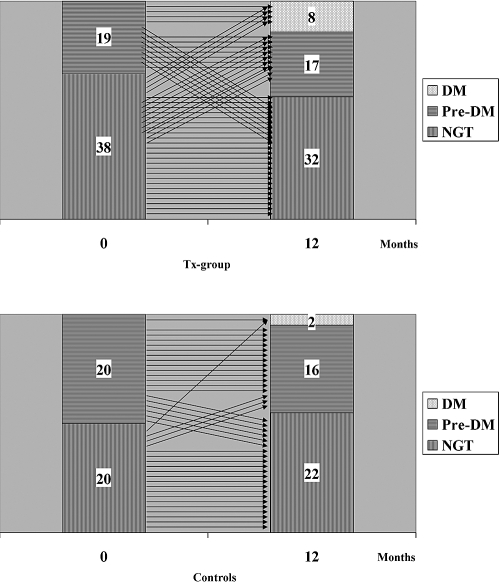
The time course for changes in glucose tolerance categories for the individual patients from baseline to follow-up for both groups is shown in Figure 1, demonstrating that patients with NODM at 1 year did not necessarily have IGT before transplantation.
Figure 1.
Individual results of OGTTs in 57 renal transplantation patients before at time 0 and 12 months after transplantation (upper panel) and in 40 uremic patients at 0 and 12 months (lower panel). DM, diabetes mellitus; NGT, normal glucose tolerance.
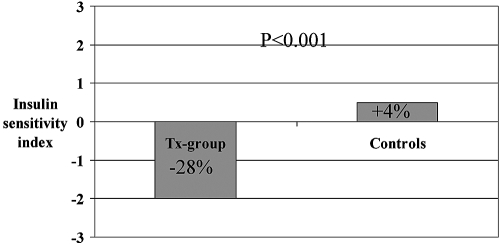
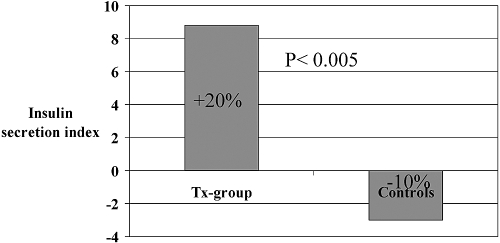
ISI deteriorated after transplantation (P < 0.005), whereas Isecr increased (P < 0.05; Figures 2 and 3 and Table 3). Both ISI and Isecr were stable in the uremic control group, and the difference between the two groups was significant (ISI, P < 0.0005 and Isecr, P < 0.005)
Figure 2.
Change in ISI after kidney transplantation.
Figure 3.
Change in Isecr after kidney transplantation.
In the Tx group, fasting plasma glucose (p-glucose) was significantly increased after 1 year, whereas it remained constant in the uremic control group (Table 3). The 2-hour glucose values were comparable, and only the 30-minute p-glucose increments were significantly different between groups (0.5 ± 0.3 versus −0.4 ± 0.2; P = 0.02). In the Tx group, the fasting p-glucose and 2-hour glucose reached their maximum at 3 months, and these levels declined at 12 months (Table 3).
Univariate linear regression with the change in ISI from baseline to 1 year as the dependent variable demonstrated a significant association with pre-Tx ISI (P < 0.0001) and pre-Tx Isecr (P = 0.0001). The two initial immunosuppressive regimens with tacrolimus or cyclosporine both reduced the ISI but with no difference between the drugs. However, when multivariate analysis was done, only high levels of pre-Tx ISI and high waist-hip ratio were associated with worsening of ISI (R2 = 0.61).
When the change in Isecr from baseline to 1 year was considered as the dependent variable, univariate linear regression showed a significant association with pre-Tx Isecr (P = 0.0002) and pre-Tx ISI (P = 0.004). Multivariate regression analysis revealed only an association between (low) baseline Isecr and an increase in Isecr (R2 = 0.24). The increase in Isecr in the patients receiving cyclosporine after transplantation was significant (P = 0.003), whereas no change was seen in the patients receiving tacrolimus (P = 0.73). The difference between the two drugs did not reach statistical significance.
Using multivariate ordinal logistic regression analysis, low pre-Tx ISI and high age predicted NODM (odds ratio (OR): 0.82, P = 0.01; and OR: 1.06, P = 0.02, respectively) but not CMV (OR: 1.19, P = 0.75) or the use of tacrolimus (OR: 1.54, P = 0.47).
Discussion
The incidence of NODM has been reported in the range between 2% and 50% (1,2). Our study is the first to include a uremic control group and at the same time use strict diagnostic criteria based on an OGTT done before and after transplantation. In this setting, we found a 12-month incidence of NODM of 14% in the transplantation patients compared with 5% in the uremic control group, despite a higher age and longer duration of ESRD in the control group. This confirms that NODM is a significant clinical problem after kidney transplantation. The high prevalence of prediabetes before transplantation (21) also underlines the fact that NODM occurs in a population of patients with an a priori high risk of diabetes.
The incidence of NODM is similar to the relatively few studies also applying strict diagnostic criteria of diabetes before and after transplantation, with incidences ranging from 18% to 24% (7,13,14). We confirmed the earlier documented high prevalence of glucometabolic changes in nondiabetic patients with stage 5 chronic kidney disease (22). In our hands, the prevalence of prediabetes (IGT plus IFG) was 41% in the combined Tx and uremic control group before transplantation. It is noteworthy that presence of prediabetes did not necessarily precede development of NODM. The independent factors predicting deterioration of glucose tolerance were low ISI and high age at baseline.
At baseline, the insulin secretion was relatively high, which may have compensated for the insulin resistance and helped to maintain a normal OGTT in the majority of patients.
Our estimations of Isecr and ISI are to some extent biased, because insulin is metabolized differently before and after transplantation, mainly because the insulin excretion is significantly decreased with decreasing GFR (23). The estimated Isecr was based on direct measurements of plasma insulin (p-insulin; AUC of p-insulin during the OGTT) and p-glucose (AUC of p-glucose during the OGTT). In the algorithm used for calculating the Isecr, the AUC insulin is in the nominator and AUC glucose in the denominator. Thus, the estimated Isecr is probably overestimated before transplantation. Our observation of an increased Isecr after transplantation is made despite a possible overestimation before transplantation, and this strongly supports our conclusion that the Isecr is increased after transplantation.
The ISI was calculated using the algorithm presented by Matsuda et al. (18): 10,000/square root ((fasting p-glucose × fasting p-insulin) × (mean OGTT glucose concentration × mean OGTT insulin concentration)). The p-insulin concentration is now in the denominator. The decreased clearance of insulin, resulting in a higher p-insulin, results in a lower estimated ISI. Our observation of a reduced ISI after transplantation corresponding to an increased insulin resistance was, again, made despite this negative bias, supporting our conclusion that the insulin resistance was increased after transplantation.
The increase in Isecr after transplantation was not sufficient to overcome the decline in ISI, resulting in deterioration of the glucose tolerance present 12 months after transplantation. The deterioration in ISI was apparent both at 3 and 12 months, whereas the increase in Isecr took place between 3 and 12 months after transplantation. This increase in Isecr was possibly related to decreased levels of CNIs. The observations made from 3 to 12 months are in keeping with the results of three previous studies (24–26), none of which included baseline data from before transplantation.
Among other risk factors to be discussed were the medications taken. The dosing of steroids did vary among centers. The average accumulated steroid dose was comparable to previous studies, and we observed an association between accumulated steroid dose and development of NODM (12,27). The average accumulated prednisolone dose was 537 mg (22%) higher in patients developing NODM compared with patients with normal glucose tolerance after transplantation, but these data did not reach statistical significance. Deterioration in insulin resistance was seen with both calcineurin inhibitors used, but the increase in insulin secretion during treatment with cyclosporine was not seen with tacrolimus. The prevalence of NODM in patients receiving tacrolimus was 21% compared with 11% in patients receiving cyclosporine. Although these findings did not reach statistical significance, they support the previous findings of an increased prevalence of NODM in patients receiving tacrolimus (12). Other drugs, like mycophenolate mofetil and azathioprine, were not associated with NODM in our study. The mode of dialysis before transplantation did not have a significant impact on the changes in ISI and Isecr. Multivariate analysis revealed that pre-Tx ISI and high WHR were significant predictors of deterioration in insulin resistance. Only pre-Tx Isecr predicted an increase in insulin secretion. Familial history of diabetes, CMV disease, acute rejection, BMI, HLA-B27 phenotype, previous transplantation, hepatitis C virus infection, and change in BMI and WHR could not be detected as risk factors for the development of insulin resistance and impaired insulin secretion in our cohort, but numbers were too small to exclude clinical significant associations.
The design of the present study was different from previous studies. We included a randomly selected uremic control group of patients from the waiting list. The transplantation patients were younger than the uremic control group but otherwise well matched and comparable by diagnoses, medication, and baseline glucometabolic status. Age was found to be a risk factor for NODM, and this was is in agreement with previous findings (13,28).
Previous studies by Nam et al. (7) in 114 Korean kidney transplantation patients with normal glucose tolerance revealed that 23.7% had NODM and 44.7% had IGT 9 to 12 months after transplantation. In this study, with no control group, Nam et al. (7) found that low insulin secretion capacity was one of the reasons for the development of NODM. In another study by Hjelmesæth et al. (13) of 167 kidney transplantation patients examined 10 weeks after transplantation, 19% of patients developed NODM, but in this study only 50% had a pretransplantation OGTT done, and there was no control group. National registry analysis suggests that 15% to 20% of renal transplant patients who do not have diabetes and receive a calcineurin inhibitor-based regimen develop NODM within 1 year of transplantation (5,29).
Our study indicates that attempts to diagnose diabetes before and after transplantation according to strict diagnostic criteria are important to identify patients who are likely to benefit from implementation of the multifactorial intervention so well documented in the treatment of diabetes. Additional studies are needed to further investigate the clinical impact of the development of prediabetes and diabetes in patients who undergo a kidney transplantation.
In conclusion, we found that at 1 year after kidney transplantation, NODM was present in 14% compared with 5% in the uremic controls. This was mainly because of a further increase in insulin resistance and was observed despite improvement in insulin secretion.
Disclosures
None.
Acknowledgments
The study was supported by an unrestricted grant from the Danish Kidney Foundation, A. P. Møller Foundation for the Advancement of Medical Science, Eva and Henry Frænkel Foundation, and the Helen Bjørnow Foundation. M.H. was supported by a fellowship from Rigshospitalet. We thank the Research Laboratory, Department of Renal Medicine C, Århus University Hospital, Skejby, and the laboratory technicians Mette Svendsen, Annette Vinding, Helle Christensen, and Andreas Haltorp for their skillful contribution to the data.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Transplant-Associated Hyperglycemia: Shedding Light on the Mechanisms,” on pages 560–562.
Access to UpToDate on-line is available for additional clinical information athttp://www.cjasn.org/
References
- 1.Boudreaux JP, McHugh L, Canafax DM, Ascher N, Sutherland DE, Payne W, Simmons RL, Najarian JS, Fryd DS: The impact of cyclosporine and combination immunosuppression on the incidence of posttransplant diabetes in renal allograft recipients. Transplantation 44: 376–381, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC: Posttransplantation diabetes: A systematic review of the literature. Diabetes Care 25: 583–592, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Gross CR, Limwattananon C, Matthees B, Zehrer JL, Savik K: Impact of transplantation on quality of life in patients with diabetes and renal dysfunction. Transplantation 70: 1736–1746, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Hjelmesaeth J, Hartmann A, Leivestad T, Holdaas H, Sagedal S, Olstad M, Jenssen T: The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int 69: 588–595, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ: Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Ojo AO: Cardiovascular complications after renal transplantation and their prevention. Transplantation 82: 603–611, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Nam JH, Mun JI, Kim SI, Kang SW, Choi KH, Park K, Ahn CW, Cha BS, Song YD, Lim SK, Kim KR, Lee HC, Huh KB: beta-Cell dysfunction rather than insulin resistance is the main contributing factor for the development of postrenal transplantation diabetes mellitus. Transplantation 71: 1417–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Hur KY, Kim MS, Kim YS, Kang ES, Nam JH, Kim SH, Nam CM, Ahn CW, Cha BS, Kim SI, Lee HC: Risk factors associated with the onset and progression of posttransplantation diabetes in renal allograft recipients. Diabetes Care 30: 609–615, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Drachenberg CB, Klassen DK, Weir MR, Wiland A, Fink JC, Bartlett ST, Cangro CB, Blahut S, Papadimitriou JC: Islet cell damage associated with tacrolimus and cyclosporine: Morphological features in pancreas allograft biopsies and clinical correlation. Transplantation 68: 396–402, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Ekstrand AV, Eriksson JG, Gronhagen-Riska C, Ahonen PJ, Groop LC: Insulin resistance and insulin deficiency in the pathogenesis of posttransplantation diabetes in man. Transplantation 53: 563–569, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Bayes B, Granada ML, Pastor MC, Lauzurica R, Salinas I, Sanmarti A, Espinal A, Serra A, Navarro M, Bonal J, Romero R: Obesity, adiponectin and inflammation as predictors of new-onset diabetes mellitus after kidney transplantation. Am J Transplant 7: 416–422, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Burroughs TE, Lentine KL, Takemoto SK, Swindle J, Machnicki G, Hardinger K, Brennan DC, Irish WD, Schnitzler MA: Influence of early posttransplantation prednisone and calcineurin inhibitor dosages on the incidence of new-onset diabetes. Clin J Am Soc Nephrol 2: 517–523, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hjelmesaeth J, Hartmann A, Kofstad J, Stenstrom J, Leivestad T, Egeland T, Fauchald P: Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation 64: 979–983, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Sato T, Inagaki A, Uchida K, Ueki T, Goto N, Matsuoka S, Katayama A, Haba T, Tominaga Y, Okajima Y, Ohta K, Suga H, Taguchi S, Kakiya S, Itatsu T, Kobayashi T, Nakao A: Diabetes mellitus after transplant: Relationship to pretransplant glucose metabolism and tacrolimus or cyclosporine A-based therapy. Transplantation 76: 1320–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Valderhaug TG, Hjelmesaeth J, Rollag H, Leivestad T, Roislien J, Jenssen T, Hartmann A: Reduced incidence of new-onset posttransplantation diabetes mellitus during the last decade. Transplantation 84: 1125–1130, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 30 [Suppl 1]: S42–S47, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Jarvinen H, Van Haeften T, Renn W, Gerich J: Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 23: 295–301, 2000 [DOI] [PubMed] [Google Scholar]
- 20.El Faramawi M, Rohr N, Jespersen B: Steroid-free immunosuppression after renal transplantation-long-term experience from a single centre. Nephrol DialTransplant 21: 1966–1973, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hornum M, Clausen P, Kjaergaard J, Hansen JM, Mathiesen ER, Feldt-Rasmussen B: Pre-diabetes and arterial stiffness in uraemic patients. Nephrol Dial Transplant 2009 [DOI] [PubMed] [Google Scholar]
- 22.Eldin WS, Ragheb A, Klassen J, Shoker A: Evidence for increased risk of prediabetes in the uremic patient. Nephron ClinPract 108: c47–c55, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Rabkin R, Simon NM, Steiner S, Colwell JA: Effect of renal disease on renal uptake and excretion of insulin in man. N Engl J Med 282: 182–187, 1970 [DOI] [PubMed] [Google Scholar]
- 24.David-Neto E, Lemos FC, Fadel LM, Agena F, Sato MY, Coccuza C, Pereira LM, de Castro MC, Lando VS, Nahas WC, Ianhez LE: The dynamics of glucose metabolism under calcineurin inhibitors in the first year after renal transplantation in nonobese patients. Transplantation 84: 50–55, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Hjelmesaeth J, Jenssen T, Hagen M, Egeland T, Hartmann A: Determinants of insulin secretion after renal transplantation. Metabolism 52: 573–578, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, Uchida K, Pescovitz MD, Marchetti P, Tuncer M, Citterio F, Wiecek A, Chadban S, El Shahawy M, Budde K, Goto N: Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant 7: 1506–1514, 2007. 17359512 [Google Scholar]
- 27.Hjelmesaeth J, Hartmann A, Kofstad J, Egeland T, Stenstrom J, Fauchald P: Tapering off prednisolone and cyclosporin the first year after renal transplantation: The effect on glucose tolerance. Nephrol Dial Transplant 16: 829–835, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Sumrani N, Delaney V, Ding Z, Davis R, Daskalakis P, Friedman EA, Butt KM, Hong JH: Posttransplant diabetes mellitus in cyclosporine-treated renal transplant recipients. Transplant Proc 23: 1249–1250, 1991 [PubMed] [Google Scholar]
- 29.Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, Woodworth TG, Brennan DC: Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant 3: 590–598, 2003 [DOI] [PubMed] [Google Scholar]