Abstract
Background and objectives: Guidelines recommend that candidates for kidney transplantation (KTx) who do not have diabetes perform a pretransplantation oral glucose tolerance test (OGTT) when fasting plasma glucose (FPG) is <110 mg/dl (<6.1 mmol/L); however, the OGTT is potentially costly and cumbersome. We studied the role of the OGTT for diagnosing diabetes and the accuracy of FPG and glycated hemoglobin (HbA1c) for predicting a diabetic OGTT before KTx.
Design, setting, participants, & measurements: In this cross-sectional study, 889 first single-kidney transplant candidates without diabetes, mainly white, performed an OGTT during the transplantation workup. Results were studied using receiver operating characteristic analysis.
Results: Of 72 (8.1%) patients with undiagnosed diabetes, only 16 (22%) had a diabetic FPG (≥126 mg/dl [≥7.0 mmol/L]). In patients with a nondiabetic FPG, diabetes (2-hour plasma glucose [2h-PG] ≥200 mg/dl [≥11.1 mmol/L]) was predicted by FPG but not by HbA1c. Performing the OGTT in patients with FPG 92 to 125 mg/dl (5.1 to 6.9 mmol/L) identified 65 (90%) patients with diabetes (16 by FPG, 49 by 2h-PG) and required seven OGTTs per patient identified. Subjecting all patients with FPG <110 mg/dl (<6.1 mmol/L) to the OGTT identified 60 (83%) patients with diabetes (16 by FPG, 44 by 2h-PG) but required 14 OGTTs per patient.
Conclusions: The OGTT was paramount in finding most cases of undiagnosed diabetes before KTx. FPG but not HbA1c predicted a diabetic OGTT. We suggest that white KTx candidates without diabetes perform a pretransplantation OGTT when FPG is 92 to 125 mg/dl (5.1 to 6.9 mmol/L).
Assessment of glycemic status before kidney transplantation (KTx) is important to enable patients with undiagnosed diabetes to be identified. In Norway, these patients have been prescribed cyclosporine rather than tacrolimus after KTx. The underlying rationale has been to lower the risk for posttransplantation hyperglycemia while maintaining short- and long-term outcomes for the kidney graft (1). In addition to undiagnosed diabetes, even nondiabetic glucose abnormalities can be important. They may threaten patient survival before KTx (2) and seem to predict new-onset diabetes after KTx (NODAT) (3–6). NODAT is associated with increased cardiovascular morbidity and reduced patient and graft survival (7–9).
According to available guidelines, the pretransplantation workup of KTx candidates seemingly without diabetes should include an oral glucose tolerance test (OGTT) when fasting plasma glucose (FPG) is <110 mg/dl (<6.1 mmol/L) (10); however, no clear explanation is provided to substantiate this recommendation. Specifically, it is unclear why patients with FPG 110 to 125 mg/dl (6.1 to 6.9 mmol/L) are not entailed. The recommended approach also suggests performing the OGTT in a large number of patients, making it time-consuming and costly in clinical practice. To select patients for a diagnostic OGTT, screening strategies based on FPG or glycated hemoglobin (HbA1c) have been proposed for patients who have undergone transplantation (11–13) and the general population (14); no such approach has been explored in KTx candidates.
Using common glycemic tests, this study aimed to identify a simple and effective strategy for detecting undiagnosed diabetes in KTx candidates. The need for an OGTT was examined, along with the accuracy of FPG and HbA1c for predicting a diabetic OGTT. FPG and HbA1c cutoffs were studied to help select patients who should proceed to a diagnostic OGTT. To these ends, pretransplantation OGTT data were studied in all patients who had ESRD but not diabetes and were referred for a first single KTx in Norway during the previous 7 yr.
Materials and Methods
Study Population and Design
This is a cross-sectional, population-based study of glucose metabolism in nondiabetic KTx candidates in Norway. All adult patients (≥18 years) who had nondiabetic ESRD and were referred for a first single KTx between September 1, 2002, and February 1, 2009, were eligible. Patients were subsequently excluded when they had (1) known diabetes despite a nondiabetic renal diagnosis or (2) incomplete OGTT data. All patients gave a written informed consent for the use of their data, and the project was performed in accordance with the Declaration of Helsinki.
Data Sources
During workup for KTx in Norway, all candidates without an established diagnosis of diabetes undergo a standard 75-g OGTT. The test result is provided on a standardized form enclosed with the KTx referral, which is forwarded to Rikshospitalet, the only transplant center in Norway. All notifications to the KTx waiting list are recorded in the hospital data system, which was used to identify eligible patients. The following data were extracted from referrals: Age, gender, ethnicity, body mass index, renal diagnosis, prednisolone use, dialysis mode and duration, hemoglobin, albumin, total cholesterol, HbA1c, FPG, and 2-hour postchallenge plasma glucose (2h-PG). The presence of known diabetes was also recorded, as defined from evidence that diabetes had been diagnosed previously (i.e., before the mandatory workup, by FPG ≥126 mg/dl [≥7.0 mmol/L] or random plasma glucose ≥200 mg/dl [≥11.1 mmol/L]).
Measurement and Classification of Glycemia
Plasma glucose and HbA1c were measured according to local practice. The American Diabetes Association criteria were used to classify patients as having diabetes (FPG ≥126 mg/dl [≥7.0 mmol/L] or 2h-PG ≥200 mg/dl [≥11.1 mmol/L]), impaired glucose tolerance (IGT; FPG <126 mg/dl [<7.0 mmol] and 2h-PG 140 to 199 mg/dl [7.8 to 11.0 mmol/L]), impaired fasting glucose (IFG; FPG 100 to 125 mg/dl [5.6 to 6.9 mmol/L] and 2h-PG <140 mg/dl [<7.8 mmol/L]), or normal glycemia (FPG <100 mg/dl [<5.6 mmol/L] and 2h-PG <140 mg/dl [<7.8 mmol/L]) (15). The World Health Organization classification was studied for comparative purposes (Table 1) and differs from the American Diabetes Association classification with respect to IFG (FPG 110 to 125 mg/dl [6.1 to 6.9 mmol/L] and 2h-PG <140 mg/dl [<7.8 mmol/L]) and normal glycemia (FPG <110 mg/dl [<6.1 mmol/L] and 2h-PG <140 mg/dl [<7.8 mmol/L]) (16). Patients who had diabetes, IGT, or IFG were defined as having hyperglycemia.
Table 1.
Fasting versus postchallenge plasma glucose classification (n = 889)
| FPG | 2h-PG (n [%]) |
||
|---|---|---|---|
| Normal (<140) | IGT (140 to 199) | Diabetes (≥200) | |
| ADA | |||
| normal (<100) | 487 (74) | 146 (22) | 29 (4) |
| IFG (100 to 125) | 100 (47) | 84 (40) | 27 (13) |
| diabetes (≥126) | 3 (19) | 2 (12) | 11 (69) |
| WHO | |||
| normal (<110) | 563 (70) | 203 (25) | 44 (5) |
| IFG (110 to 125) | 24 (38) | 27 (43) | 12 (19) |
| diabetes (≥126) | 3 (19) | 2 (12) | 11 (69) |
Glucose values in mg/dl. Weighted κ agreement 0.302 and 0.219, respectively, for American Diabetes Association (ADA) and World Health Organization (WHO) classifications.
Statistical Analysis
Mean ± SD, median (interquartile range), or frequencies (%) are given. Groups of glycemia were compared using parametric (t test, ANOVA), rank-based (Mann-Whitney, Jonckheere-Terpstra), or χ2 methods, with Fisher exact methods as appropriate. Bonferroni corrections were used for multiple comparisons. The agreement between fasting and postchallenge glycemia was assessed using weighted κ values.
Receiver operating characteristic (ROC) analyses were used for patients with FPG <126 mg/dl (<7.0 mmol/L) to assess the accuracy of FPG or HbA1c for predicting a diabetic 2h-PG. An area under the ROC curve (AUC) of 0.5 corresponds with the indiscriminate reference line, whereas 1.0 indicates perfect accuracy. Optimal cutoffs represent the point on the ROC curve yielding (1) maximum sensitivity and specificity (i.e., point closest to the top left corner) or (2) maximum vertical difference between the ROC curve and the reference line (17). The latter difference equates to (sensitivity + specificity − 1); the largest difference achieved is termed the Youden index; 1.0 represents perfect accuracy. Analyses were performed in SPSS 17.0 (SPSS, Chicago, IL). Two-sided P < 0.05 was considered statistically significant.
Sensitivity Analysis
A reference test result can influence whether a screening test is performed and/or reported. Conversely, a screening test result can influence whether the reference test is performed (verification bias) (18). Both mechanisms can potentially lead to information bias. The presence of information bias was addressed by studying patients with complete glycemic data (FPG, 2h-PG, and HbA1c) and by replacing missing data with statistically probable values using multiple imputation (19,20). This is detailed in the supplemental material.
Results
Participants
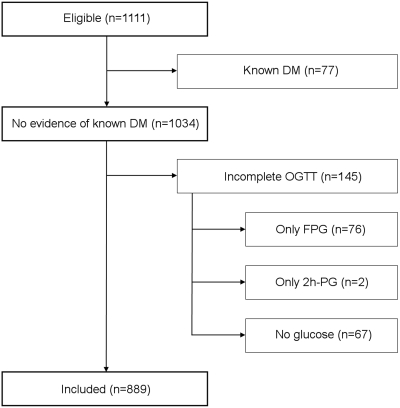
A total of 889 of 1111 eligible patients were included in the study (Figure 1). Two excluded patients without known diabetes had a diabetic FPG. Both were classified as having incomplete OGTT data, because there was only one glucose measurement and no evidence of previous diabetes. By July 15, 2009, most included patients had undergone KTx (n = 797). The remaining patients were still awaiting KTx (n = 51), had died on the waiting list (n = 32), or had permanently been taken off the list because of comorbidity (n = 9). Primary renal diagnoses were glomerulonephritis (n = 276 [31%]), nephrosclerosis (n = 204 [23%]), cystic kidney disease (n = 160 [18%]), pyelonephritis/other interstitial kidney disease (n = 107 [12%]), and other/unknown diagnoses (n = 142 [16%]).
Figure 1.
Patient disposition. All adult (≥18 years) patients who had nondiabetic ESRD and were referred for first single KTx within the study period were considered eligible (n = 1111). Upon data retrieval, patients were excluded from participation when they had (1) acknowledged diabetes before transplantation workup or (2) incomplete OGTT data. DM, diabetes mellitus.
Descriptive Data
Patient characteristics are presented in Table 2. Fifty (6%) patients were nonwhite, and 298 (34%) were female. Hyperglycemia was seen in 402 (45.2%) patients (72 diabetes [8.1%], 230 IGT [25.9%], 100 IFG [11.2%]). HbA1c was available in a nonconsecutive subset of 514 (58%) patients and correlated with FPG (r = 0.16) and 2h-PG (r = 0.18; both P < 0.001). Thirty-eight percent (n = 341) of patients were on dialysis at referral; this proportion doubled by the time of KTx (72%; 574 of 797 patients). At referral, dialysis patients had lower FPG and HbA1c as compared with patients who were predialysis (92 ± 13 versus 94 ± 11 mg/dl and 5.4 ± 0.6 versus 5.6 ± 0.5%, respectively; both P < 0.015).
Table 2.
Demographic, clinical, and laboratory results at referral for KTx
| Parameter | Normal | IFG | IGT | Diabetes | Overall | Pa |
|---|---|---|---|---|---|---|
| n | 487 | 100 | 230 | 72 | 889 | |
| Age (years; mean ± SD) | 50.7 (15.0)g,h,i | 57.3 (13.6)j | 57.4 (12.8)j | 59.2 (13.2)j | 53.9 (14.6) | <0.001 |
| Female gender (n [%]) | 187 (38)g,h,i | 20 (20)j | 72 (31)j | 19 (26)j | 298 (34) | 0.001 |
| Nonwhite ethnicity (n [%]) | 34 (7) | 4 (4) | 6 (3) | 6 (8) | 50 (6) | 0.285b |
| BMI (kg/m2; mean ± SD) | 25.1 (3.9)i | 25.9 (3.5) | 25.6 (3.9) | 26.7 (4.1)j | 25.4 (3.9) | 0.003 |
| Hemodialysis (n [%])c | 160 (33) | 26 (26) | 61 (27) | 29 (41) | 276 (31) | 0.056c |
| Peritoneal dialysis (n [%])c | 33 (7) | 9 (9) | 19 (8) | 4 (6) | 65 (7) | 0.753c |
| Not in dialysis (n [%])c | 290 (60) | 64 (65) | 149 (65) | 37 (53) | 540 (62) | 0.235c |
| Time on waiting list (weeks; median [IQR])d | 54 (30 to 94) | 60 (36 to 95) | 64 (38 to 102) | 70 (42 to 98) | 58 (35 to 96) | 0.006 |
| Time on dialysis (weeks; median [IQR]) | 0 (0 to 15) | 0 (0 to 12) | 0 (0 to 11) | 0 (0 to 17) | 0 (0 to 14) | 0.710 |
| Prednisolone use (n [%])e | 42 (13) | 3 (5) | 16 (12) | 5 (12) | 66 (12) | 0.999b |
| Hemoglobin (g/dl; mean ± SD) | 12.0 (1.5) | 12.1 (1.3) | 11.9 (1.5) | 11.9 (1.2) | 12.0 (1.4) | 0.607 |
| Total cholesterol (mg/dl; mean ± SD) | 186 (46) | 186 (39) | 183 (47) | 179 (51) | 183 (47) | 0.434 |
| Albumin (g/dl; mean ± SD) | 4.0 (0.5) | 4.0 (0.5)h | 3.9 (0.5)g | 3.9 (0.5) | 3.9 (0.5) | 0.054 |
| A1c (%; mean ± SD)f | 5.4 (0.5)i | 5.6 (0.5) | 5.6 (0.5) | 5.7 (0.6)j | 5.5 (0.5) | 0.005 |
| FPG (mg/dl; mean ± SD) | 86 (7)g,h,i | 106 (5)h,j | 95 (11)g,i,j | 108 (16)h,j | 94 (13) | <0.001 |
| 2h-PG (mg/dl; mean ± SD) | 101 (22)g,8,i | 112 (18)h,i,j | 164 (16)g,i,j | 229 (38)g,h,j | 128 (45) | <0.001 |
Tests for trend using parametric, rank-based, or χ2 tests as appropriate.
BMI, body mass index; IQR, interquartile range.
To accommodate low cell expectancies (<5 per cell), normal glucose tolerance, IFG, and IGT were collapsed for significance testing.
Data available for n = 881 patients only; the combined P value is P = 0.241.
In patients who have undergone KTx (n = 797).
Available for n = 551 patients only.
Available for n = 514 patients only.
Post hoc analyses (Bonferroni): P < 0.05 versus IFG
P < 0.05 versus IGT
P < 0.05 versus diabetes
P < 0.05 versus normal.
Classification of Hyperglycemia
Only 16 (22%) of 72 patients with diabetes had diabetes by FPG (Table 1); therefore, omitting the OGTT entirely would reclassify 56 (78%) of 72 patients with diabetes as not having diabetes. Limiting the OGTT to patients with IFG would reclassify 29 (40%), alternatively 44 (61%) of 72 patients with diabetes, depending on the IFG definition. Fasting and postchallenge diagnoses were only modestly concordant (weighted κ agreements of ≤0.3).
ROC Analyses
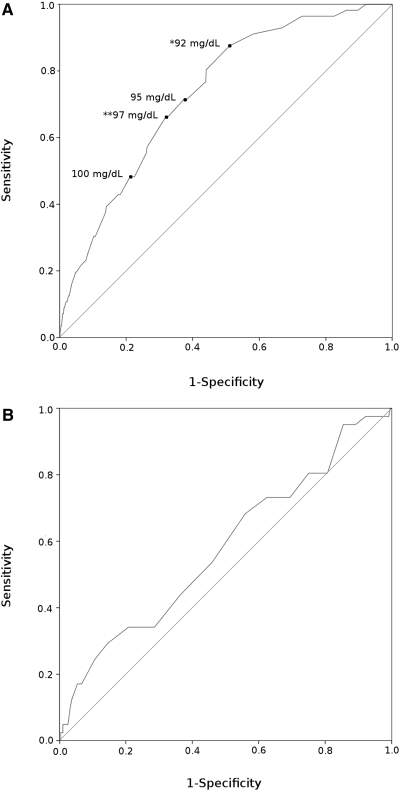
Results of the ROC analyses are shown in Figure 2, Tables 3 and 4. The AUC for FPG was 0.734 (95% confidence interval [CI] 0.674 to 0.795). When performing the OGTT for patients with FPG 92 to 125 mg/dl (5.1 to 6.9 mmol/L), 49 (88%) of 56 patients with a diabetic 2h-PG were identified. The 92-mg/dl (5.1-mmol/L) cutoff was identified as optimal by the Youden index. Overall, 65 (90%) of 72 patients with diabetes were identified by this strategy (16 by FPG, 49 by 2h-PG), which required an OGTT for 463 (53%) of 873 patients with a nondiabetic FPG. These ROC results were consistent among the predialysis patients as well as for patients on dialysis (data not shown).
Figure 2.
ROC curves. Analyses were performed on patients with FPG <126 mg/dl (<7.0 mmol/L). The ability of FPG (A; AUC 0.734 [95% CI 0.674 to 0.795]) and HbA1c (B; AUC 578 [95% CI 0.482 to 0.673]) to predict a diabetic 2h-PG (≥200 mg/dl [≥11.1 mmol/L]) parallels the area between the curved and the diagonal reference line (AUC). The top left corner of each panel represents AUC 1.0 (perfect sensitivity and specificity). *Youden index (largest vertical difference between the curve and the reference line); **point closest to the top left corner of the panel.
Table 3.
Performance of FPG in the screening for a diabetic 2h-PG (≥200 mg/dl)
| FPG (mg/dl [mmol/L]) | Diabetes Identified (n = 56) | OGTTs Required (%) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|
| ≥92 (5.1)a | 49 | 463 (53) | 0.88 (0.76 to 0.94) | 0.49 (0.45 to 0.52) |
| ≥94 (5.2) | 43 | 402 (46) | 0.77 (0.64 to 0.86) | 0.56 (0.53 to 0.59) |
| ≥95 (5.3) | 40 | 349 (40) | 0.71 (0.59 to 0.82) | 0.63 (0.59 to 0.66) |
| ≥97 (5.4)b | 37 | 297 (34) | 0.66 (0.53 to 0.77) | 0.68 (0.65 to 0.71) |
| ≥99 (5.5) | 31 | 244 (28) | 0.55 (0.42 to 0.68) | 0.74 (0.71 to 0.77) |
| ≥100 (5.6) | 27 | 210 (24) | 0.48 (0.36 to 0.61) | 0.78 (0.74 to 0.80) |
| ≥103 (5.7) | 24 | 173 (20) | 0.43 (0.31 to 0.56) | 0.82 (0.79 to 0.84) |
| ≥105 (5.8) | 22 | 137 (16) | 0.39 (0.28 to 0.52) | 0.86 (0.83 to 0.88) |
| ≥106 (5.9) | 17 | 105 (12) | 0.30 (0.20 to 0.43) | 0.89 (0.87 to 0.91) |
| ≥108 (6.0) | 14 | 83 (10) | 0.25 (0.16 to 0.37) | 0.92 (0.89 to 0.93) |
| ≥110 (6.1) | 12 | 63 (7) | 0.21 (0.13 to 0.34) | 0.94 (0.92 to 0.95) |
ROC analyses in patients with FPG <126 mg/dl (n = 873 of 889 patients). The ROC curve AUC value is 0.734 (95% CI 0.674 to 0.795).
Youden index; the FPG value with the largest vertical distance between the ROC curve and the reference line.
FPG value for which the ROC curve is closest to the upper left hand corner of the ROC curve.
Table 4.
Performance of A1c in the screening for a diabetic 2h-PG (≥200 mg/dl)
| A1c (%) | Diabetes Identified (n = 41) | OGTTs Required (%) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|
| ≥5.0 | 39 | 432 (86) | 0.95 (0.84 to 0.99) | 0.15 (0.12 to 0.18) |
| ≥5.1 | 33 | 407 (81) | 0.81 (0.66 to 0.90) | 0.19 (0.16 to 0.23) |
| ≥5.2 | 33 | 382 (76) | 0.81 (0.66 to 0.90) | 0.25 (0.21 to 0.29) |
| ≥5.3 | 30 | 351 (70) | 0.73 (0.58 to 0.84) | 0.31 (0.27 to 0.35) |
| ≥5.4 | 30 | 316 (63) | 0.73 (0.58 to 0.84) | 0.38 (0.33 to 0.42) |
| ≥5.5 | 28 | 286 (57) | 0.68 (0.53 to 0.80) | 0.44 (0.40 to 0.49) |
| ≥5.6 | 22 | 236 (47) | 0.54 (0.39 to 0.68) | 0.54 (0.49 to 0.59) |
ROC analyses in patients with FPG <126 mg/dl and available A1c data (n = 502 of 514 patients with A1c data). The ROC AUC value is 0.578 (95% CI 0.482 to 0.673).
For HbA1c, the AUC was 0.578 (95% CI 0.482 to 0.673), indicating that HbA1c could not be used as a general tool to identify patients who should proceed to a diagnostic OGTT. The accuracy improved when studied in predialysis patients alone but did not surpass that of FPG even in these patients (AUC 0.689 [95% CI 0.586 to 0.791] versus 0.710 [95% CI 0.624 to 0.795], HbA1c versus FPG, respectively). The potential gain from using HbA1c alone or combined with FPG was negligible whether patients had or had not been started on dialysis (data not shown).
Sensitivity Analysis
ROC results were almost identical for patients with complete glycemic data (n = 514), as well as in multiply imputed data sets (Supplemental Tables S1 through S9); therefore, results did not seem to be significantly influenced by information bias.
Discussion
In this study, a majority of the KTx candidates with undiagnosed diabetes required an OGTT to be identified. Despite having a poor diagnostic sensitivity in its own right, FPG seemed to be an accurate test for predicting a diabetic 2h-PG. A high detection rate for diabetes could thus be maintained when restricting the OGTT to patients having FPG within a selected range. In contrast to FPG, HbA1c did not seem useful as a general tool to predict a diabetic 2h-PG.
Only approximately 20% of patients with diabetes could be identified by FPG in our study, compared with 50% early after KTx (13) or 70% in the general population (14). The low diagnostic sensitivity of FPG may have resulted from the exclusion of patients with known diabetes. It could also be related to general glucometabolic abnormalities known to occur in ESRD. Although most patients with ESRD are insulin resistant (21), fasting glycemia is often maintained or even lowered as a result of the reduced renal clearance of insulin. Consequently, hyperglycemia in patient with ESRD may primarily be a postprandial phenomenon and thereby more easily revealed using a formal glucose challenge, such as the OGTT (22). This is consistent with two previous reports (23,24) and suggests that the OGTT is more important for detecting diabetes before as compared with after KTx. Nonetheless, the OGTT is also important after transplantation, because NODAT develops even in patients who have normal pretransplantation glucose tolerance.
In the general population, 80% of patients with undiagnosed diabetes were identified when individuals with FPG 110 to 125 mg/dl (6.1 to 6.9 mmol/L) underwent an OGTT (14). In the early posttransplantation period, this approach identified 70% of the patients with diabetes, and a cutoff of 95 mg/dl (5.3 mmol/L) was proposed to increase detection rates (13). This cutoff was chosen on the basis of the argument that, given the serious complications of diabetes, 80% sensitivity should be a minimum for detecting a diabetic 2h-PG after KTx. In our view, this detection rate is also appropriate before KTx. Our data suggest, however, that 110 mg/dl (6.1 mmol/L) and possibly even 95 mg/dl (5.3 mmol/L) are inappropriately high cutoffs for FPG before KTx, identifying only approximately 20 and 70%, respectively, of patients with a diabetic 2h-PG.
To identify ≥80% of the patients with a diabetic 2h-PG in our study, an OGTT had to be performed for patients with FPG 92 to 125 mg/dl (5.1 to 6.9 mmol/L). This identified 90% of all patients with diabetes and required an OGTT in roughly 50% of patients with a nondiabetic FPG. These results are very similar to those achieved early after KTx using a 95-mg/dl (5.3-mmol/L) cutoff (13). Importantly, this approach was also more efficient as compared with that proposed in the available guidelines (OGTT if FPG <110 mg/dl) (10). In our study, the latter identified 60 of 72 patients with diabetes (16 by FPG, 44 by 2h-PG) by subjecting 810 patients to an OGTT (Table 1; OGTT if normal FPG by World Health Organization criteria); therefore, this approach required 14 OGTTs to find one patient with diabetes (810 tests/60 diabetes), compared with only seven OGTTs when restricting the test to patients with FPG 92 to 125 mg/dl (463 tests/65 diabetes).
In individuals without kidney disease, HbA1c and FPG seem to be equally effective for detecting diabetes (25). HbA1c also seems to be useful after KTx once hematocrit has stabilized (13). In nontransplantation ESRD, however, HbA1c is difficult to interpret. Factors that increase the turnover of red blood cells, such as erythropoietin use, iron supplements, and blood loss during hemodialysis, can falsely reduce HbA1c (26–28). Moreover, the interpretation of HbA1c is confounded by an increased formation of carbamylated hemoglobin (cHb) in patients with uremia (27–30). First, HbA1c and cHb have similar isoelectric points, resulting in a tendency of certain HbA1c assays, charge-based ones in particular, to report a result that in fact is the combined concentration of HbA1c and cHb (31,32). Second, the formation of HbA1c and cHb may interact, because carbamylation and glycation occur at similar binding sites on the hemoglobin molecule (30). These factors could explain the general impression in this study that HbA1c had a limited diagnostic accuracy in KTx candidates. Although HbA1c may potentially be of some use in predialysis ESRD, only FPG was consistently accurate for predicting a diabetic 2h-PG in the population of KTx candidates as a whole.
It is debatable whether FPG, 2h-PG, or HbA1c should be used for diagnosing diabetes (33). Fasting and postprandial hyperglycemia identify different subsets of patients and may have different pathogeneses and prognostic implications (14,34). Although diabetes defined by any criterion predicts microvascular complications (33), the postprandial criteria seem more predictive of macrovascular end points (34,35). Given the major macrovascular burden in transplant patients, the OGTT could have particular relevance in KTx candidates. Postprandial criteria for diabetes were thus emphasized in our study. It is not known, however, whether KTx candidates with diabetes by FPG fare better than those diagnosed by 2h-PG, let alone at which thresholds the risk for posttransplantation complications begins to rise. The extent to which pretransplantation glycemia should guide the choice of immunosuppression is also uncertain. The potential significance of detecting diabetes by an OGTT before KTx should be interpreted with some caution until these issues are studied prospectively.
To our knowledge, this is the first study to describe a case-finding strategy for undiagnosed diabetes in KTx candidates. The large sample size was an important strength. National data were available for a 7-yr period on all patients who were referred for a first single KTx, which we believe improves the external validity of the study. Nonetheless, our study has clear limitations. First, the study population was primarily white. This reduces the generalizability of our results to nonwhite populations. Nonwhite patients were kept in the analysis to render population-based results but were too few in number to examine whether ethnicity-specific differences were present. White patients were well represented, however, and results were the same when white patients were studied separately (n = 839; data not shown). Second, differences in laboratory methods between referring centers may have influenced the results. Nonetheless, our findings can be explained by pathophysiologic mechanisms and also represent a large body of real-life data. Third, the results may have been subject to information bias as a result of missing data; however, our sensitivity analyses provided little evidence that this was the case. Fourth, glucose was measured on only one occasion for each patient. The prevalence of diabetes may have been overestimated as a result of the intraperson variability of glucose measurements (36). In the absence of unequivocal hyperglycemia, repeat testing is indicated for screening detected diabetes (15). This is probably also advisable in KTx candidates. In this population, repeat testing may also be important to find incident cases of diabetes, the rate of which seems higher as compared with the general population (37). Repeat testing may thus be particularly relevant at centers experiencing long waiting times, where undiagnosed hyperglycemia could take a toll on pretransplantation survival (2). Finally, our case-finding strategy for diagnosing diabetes is ignorant of other diabetes risk factors. The optimal care for each individual patient also requires a detailed knowledge of such factors.
Conclusions
The OGTT was of key importance in detecting undiagnosed diabetes in first single KTx candidates. Fasting glucose was helpful for predicting the occurrence of a diabetic 2h-PG. We suggest that white KTx candidates without known diabetes should undergo a pretransplantation OGTT when FPG is between 92 and 125 mg/dl (5.1 to 6.9 mmol/L). Compared with the available guidelines, this will considerably reduce the number of patients who need an OGTT and also identify a larger proportion of patients with undiagnosed diabetes. Contrary to FPG, HbA1c did not seem useful as a general tool to predict a diabetic 2h-PG among KTx candidates. Further studies are needed to verify our findings, particularly in other ethnic groups.
Disclosures
None.
Acknowledgments
This project was financed by grants from the Norwegian Foundation for Health and Rehabilitation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Transplant-Associated Hyperglycemia: Shedding Light on the Mechanisms,” on pages 560–562.
Supplemental information for this article is available online at www.cjasn.org.
References
- 1.Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, Uchida K, Pescovitz MD, Marchetti P, Tuncer M, Citterio F, Wiecek A, Chadban S, El-Shahawy M, Budde O, Goto N: Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant 7: 1506–1514, 2007. 17359512 [Google Scholar]
- 2.Lin-Tan DT, Lin JL, Wang LH, Wang LM, Huang LM, Liu L, Huang JY, Huang YL: Fasting glucose levels in predicting 1-year all-cause mortality in patients who do not have diabetes and are on maintenance hemodialysis. J Am Soc Nephrol 18: 2385–2391, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Mathew JT, Rao M, Job V, Ratnaswamy S, Jacob CK: Post-transplant hyperglycaemia: A study of risk factors. Nephrol Dial Transplant 18: 164–171, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Nam JH, Mun JI, Kim SI, Kang SW, Choi KH, Park K, Ahn CW, Cha BS, Song YD, Lim SK, Kim KR, Lee HC, Huh KB: Beta-cell dysfunction rather than insulin resistance is the main contributing factor for the development of postrenal transplantation diabetes mellitus. Transplantation 71: 1417–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Ramesh Prasad GV, Huang M, Bandukwala F, Nash MM, Rapi L, Montada-Atin T, Meliton G, Zaltzman JS: Pre-transplantation glucose testing for predicting new-onset diabetes mellitus after renal transplantation. Clin Nephrol 71: 140–146, 2009 [PubMed] [Google Scholar]
- 6.Sato T, Inagaki A, Uchida K, Ueki T, Goto N, Matsuoka S, Katayama A, Haba T, Tominaga Y, Okajima Y, Ohta K, Suga H, Taguchi S, Kakiya S, Itatsu T, Kobayashi T, Nakao A: Diabetes mellitus after transplant: Relationship to pretransplant glucose metabolism and tacrolimus or cyclosporine A-based therapy. Transplantation 76: 1320–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Hjelmesaeth J, Hartmann A, Leivestad T, Holdaas H, Sagedal S, Olstad M, Jenssen T: The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int 69: 588–595, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ: Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Lentine KL, Brennan DC, Schnitzler MA: Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol 16: 496–506, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson A, Davidson J, Dotta F, Home PD, Keown P, Kiberd B, Jardine A, Levitt N, Marchetti P, Markell M, Naicker S, O'Connell P, Schnitzler M, Standl E, Torregosa JV, Uchida K, Valantine H, Villamil F, Vincenti F, Wissing M: Guidelines for the treatment and management of new-onset diabetes after transplantation. Clin Transplant 19: 291–298, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Armstrong KA, Prins JB, Beller EM, Campbell SB, Hawley CM, Johnson DW, Isbel NM: Should an oral glucose tolerance test be performed routinely in all renal transplant recipients? Clin J Am Soc Nephrol 1: 100–108, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Hoban R, Gielda B, Temkit M, Saha C, Book BK, Baker E, Pescovitz MD: Utility of HbA1c in the detection of subclinical post renal transplant diabetes. Transplantation 81: 379–383, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Valderhaug TG, Jenssen T, Hartmann A, Midtvedt K, Holdaas H, Reisaeter AV, Hjelmesaeth J: Fasting plasma glucose and glycosylated hemoglobin in the screening for diabetes mellitus after renal transplantation. Transplantation 88: 429–434, 2009 [DOI] [PubMed] [Google Scholar]
- 14.DECODE Study Group: Is fasting glucose sufficient to define diabetes? Epidemiological data from 20 European studies. Diabetologia 42: 647–654, 1999 [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 27[ Suppl 1]: S5–S10, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation, Geneva, World Health Organization, 2006 [Google Scholar]
- 17.Perkins NJ, Schisterman EF: The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 163: 670–675, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaeschke R, Guyatt G, Sackett DL: Users' guides to the medical literature: III. How to use an article about a diagnostic test: A. Are the results of the study valid? JAMA 271: 389–391, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Horton NJ, Kleinman KP: Much ado about nothing: A comparison of missing data methods and software to fit incomplete data regression models. Am Stat 61: 79–90, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR: Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 338: b2393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J: Insulin resistance in uremia. J Clin Invest 67: 563–568, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mak RH: Impact of end-stage renal disease and dialysis on glycemic control. Semin Dial 13: 4–8, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Elbert A, Schreier L, Galli C, Beresan H, Lopez G, Traversa M, Berg G: Prevalence of impaired fasting glycemia, impaired glucose tolerance, and type 2 diabetes in hemodialyzed patients when applying new diagnostic criteria. J Ren Nutr 16: 300–303, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Rufino M, Barbero P, Hernandez D, Torres A, Lorenzo V: Importance of oral glucose overload test (OGOT) at a specific clinic for advanced CRF stages IV and V. Nefrologia 27: 30–37, 2007 [PubMed] [Google Scholar]
- 25.Bennett CM, Guo M, Dharmage SC: HbA(1c) as a screening tool for detection of Type 2 diabetes: A systematic review. Diabet Med 24: 333–343, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, Tabata T, Okamura M, Okada S, Yamakawa T, Ishimura E, Nishizawa Y: Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: Effect of anemia and erythropoietin injection. J Am Soc Nephrol 18: 896–903, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Joy MS, Cefalu WT, Hogan SL, Nachman PH: Long-term glycemic control measurements in diabetic patients receiving hemodialysis. Am J Kidney Dis 39: 297–307, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M: Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 48: 436–472, 2002 [PubMed] [Google Scholar]
- 29.Smith WG, Holden M, Benton M, Brown CB: Glycosylated and carbamylated haemoglobin in uraemia. Nephrol Dial Transplant 4: 96–100, 1989 [PubMed] [Google Scholar]
- 30.Szymezak J, Lavalard E, Martin M, Leroy N, Gillery P: Carbamylated hemoglobin remains a critical issue in HbA1c measurements. Clin Chem Lab Med 47: 612–613, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Bry L, Chen PC, Sacks DB: Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem 47: 153–163, 2001 [PubMed] [Google Scholar]
- 32.Weykamp CW, Miedema K, de Haan T, Doelman CJ: Carbamylated hemoglobin interference in glycohemoglobin assays. Clin Chem 45: 438–440, 1999 [PubMed] [Google Scholar]
- 33.Nathan DM, Balkau B, Bonora E, Borch-Johnsen K, Buse JB, Colagiuri S, Davidson MB, DeFronzo R, Genuth S, Holman RR, Ji L, Kirkman S, Knowler WC, Schatz D, Shaw J, Sobngwi E, Steffes M, Vaccaro O, Wareham N, Zinman B, Kahn R: International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care 32: 1327–1334, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett-Connor E, Ferrara A: Isolated postchallenge hyperglycemia and the risk of fatal cardiovascular disease in older women and men: The Rancho Bernardo Study. Diabetes Care 21: 1236–1239, 1998 [DOI] [PubMed] [Google Scholar]
- 35.DECODE Study Group: Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care 26: 688–696, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Selvin E, Crainiceanu CM, Brancati FL, Coresh J: Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med 167: 1545–1551, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, Woodwarth TG, Brennan DC: Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant 3: 590–598, 2003 [DOI] [PubMed] [Google Scholar]