Abstract
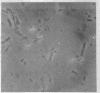
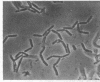
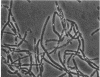
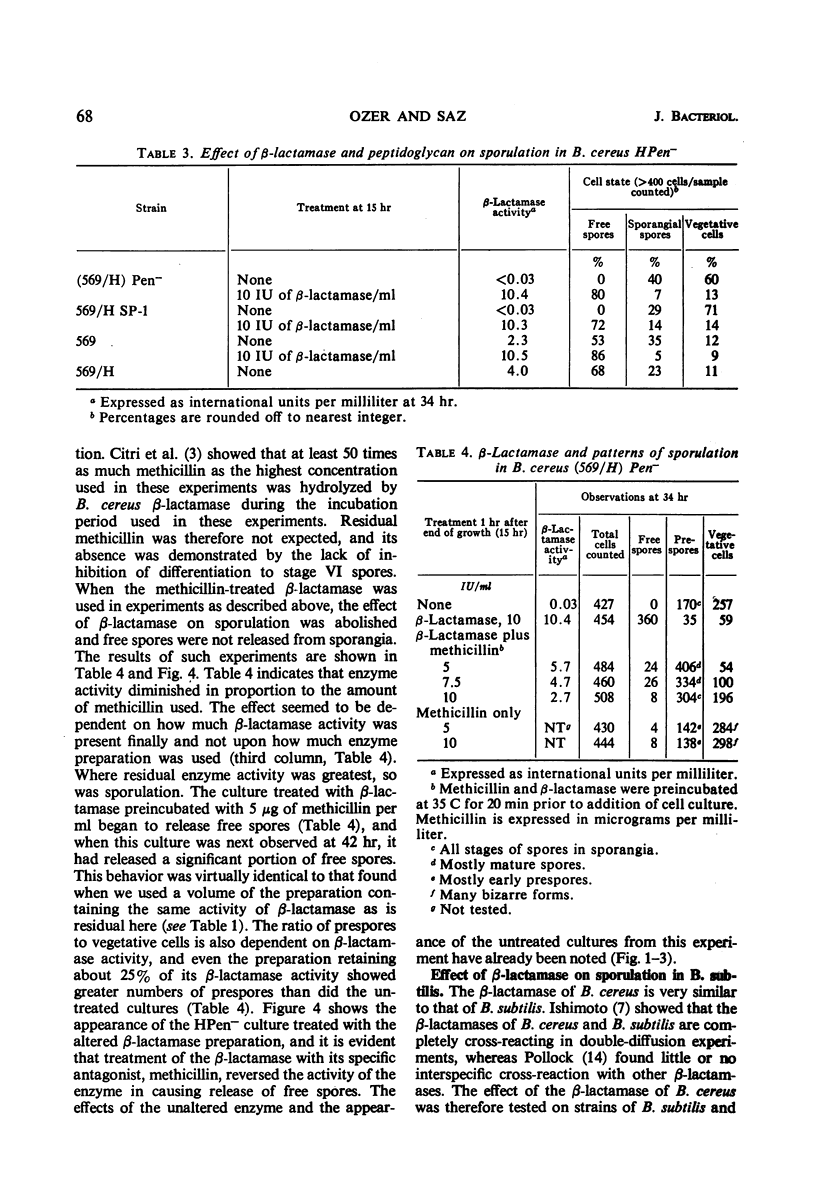
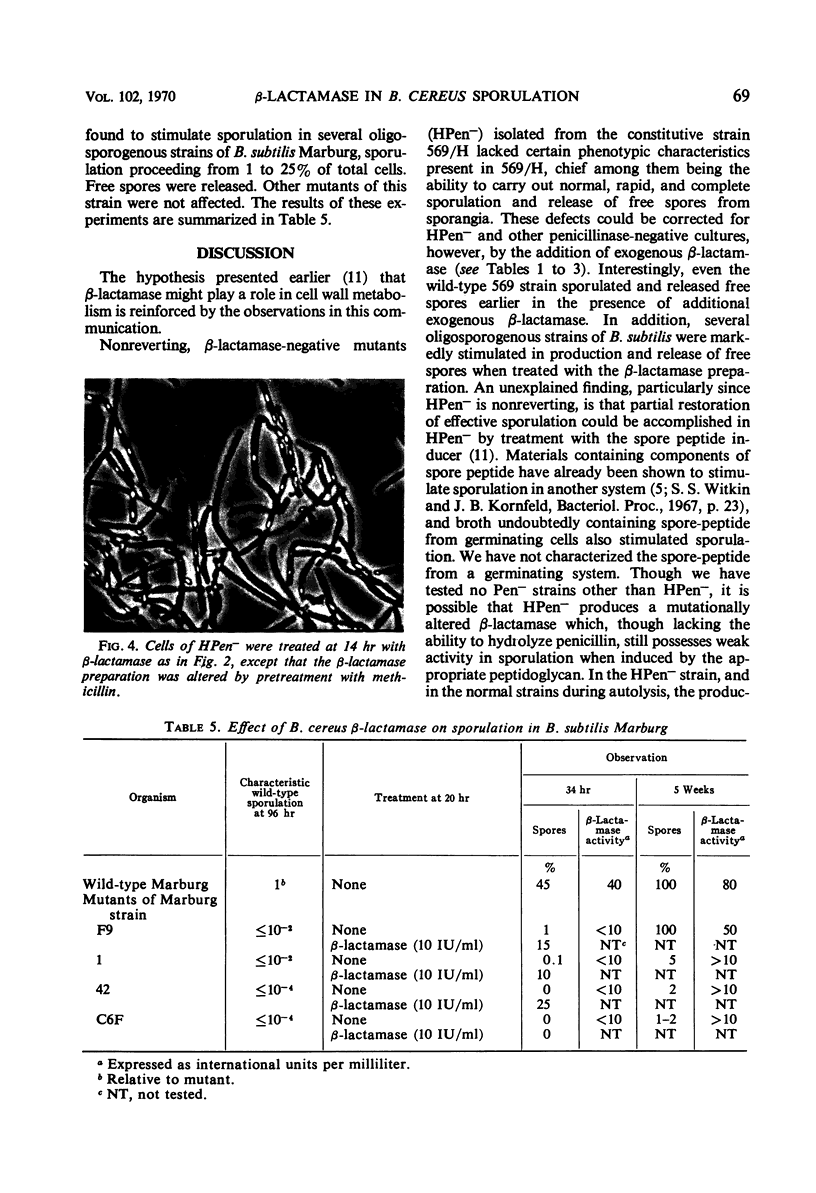
Nonreverting β-lactamase-negative strains were isolated from the β-lactamase-constitutive strain, Bacillus cereus 569 H. These strains differed from both β-lactamase-inducible and -constitutive strains not only in failure to produce β-lactamase but also in failure to autolyze on aging, delayed sporulation, and failure to release free spores from sporangia when produced. The addition of B. cereus β-lactamase of 15% purity to a final concentration of 10 IU/ml stimulates sporulation and particularly the release of free spores in culture from sporangia of strain 569 (inducible wild-type), 569/H (constitutive mutant of 569), and HPen−, a nonreverting β-lactamase strain isolated from 569/H in this laboratory. Cultures of HPen− did not release free spores without this treatment. Similar stimulation of sporulation and spore release by β-lactamase from B. cereus were observed in another β-lactamase-negative strain derived from 569/H as well as in certain sporogeny mutants of B. subtilis. The β-lactamase preparation used in these experiments was free of peptidases, proteases, and autolysins capable of solubilizing wall from vegetative cells. These results, taken with our previous finding that a soluble peptidoglycan inducer becomes available in cultures of B. cereus only at sporulation and that normal derepression of β-lactamase accompanies normal sporulation, suggest that β-lactamase in B. cereus may be involved in peptidoglycan metabolism during sporulation and possibly the breakdown of sporangial wall with the concomitant release of mature spores.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALASSA G., IONESCO H., SCHAEFFER P. PREUVE G'EN'ETIQUE D'UNE RELATION ENTRE LA PRODUCTION D'UN ANTIBIOTIQUE PAR BACILLUS SUBTILIS ET SA SPORULATION. C R Hebd Seances Acad Sci. 1963 Jul 22;257:986–988. [PubMed] [Google Scholar]
- Bernstein A., Nickerson K. W., Day R. A. Thermal penicillinasderepression and temperature dependence of penicillinase production inducible and constitutive strains of Bacillus cereus. Arch Biochem Biophys. 1967 Mar;119(1):50–54. doi: 10.1016/0003-9861(67)90427-4. [DOI] [PubMed] [Google Scholar]
- CITRI N., GARBER N., KALKSTEIN A. THE INTERACTION OF PENICILLINASE WITH PENICILLINS. 3. COMPARISON OF EXOPENICILLINASE PREPARATIONS OF VARIOUS ORIGINS. Biochim Biophys Acta. 1964 Dec 23;92:572–581. doi: 10.1016/0926-6569(64)90017-3. [DOI] [PubMed] [Google Scholar]
- DUERKSEN J. D. LOCALIZATION OF THE SITE OF FIXATION OF THE INDUCER, PENICILLIN, IN BACILLUS CEREUS. Biochim Biophys Acta. 1964 May 18;87:123–140. doi: 10.1016/0926-6550(64)90053-2. [DOI] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G. Fine structure of sporulation in Bacillus cereus grown in a chemically defined medium. J Bacteriol. 1966 Dec;92(6):1748–1764. doi: 10.1128/jb.92.6.1748-1764.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGO W. B., RUSSELL A. D. Action of penicillin on Aerobacter cloacae. J Bacteriol. 1961 Sep;82:411–417. doi: 10.1128/jb.82.3.411-417.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstadt Ozer J., Lowery D. L., Saz A. K. Derepression of beta-lactamase (penicillinase in Bacillus cereus by peptidoglycans. J Bacteriol. 1970 Apr;102(1):52–63. doi: 10.1128/jb.102.1.52-63.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIMOTO M. A STUDY OF PURIFICATION AND PROPERTIES OF BACILLUS SUBTILIS EXOPENICILLINASE. Acta Physiol Acad Sci Hung. 1963;24:35–40. [PubMed] [Google Scholar]
- KOGUT M., POLLOCK M. R., TRIDGELL E. J. Purification of penicillin-induced penicillinase of Bacillus cereus NRRL 569: a comparison of its properties with those of a similarly purified penicillinase produced spontaneously by a constitutive mutant strain. Biochem J. 1956 Mar;62(3):391–401. doi: 10.1042/bj0620391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop J., Slepecky R. A. Sporulation mutations induced by heat in Bacillus subtilis. Science. 1967 Feb 17;155(3764):838–839. doi: 10.1126/science.155.3764.838. [DOI] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. PURIFICATION AND PROPERTIES OF PENICILLINASES FROM TWO STRAINS OF BACILLUS LICHENIFORMIS: A CHEMICAL, PHYSICOCHEMICAL AND PHYSIOLOGICAL COMPARISON. Biochem J. 1965 Mar;94:666–675. doi: 10.1042/bj0940666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. Penicillinase-antipenicillinase. Ann N Y Acad Sci. 1963 May 8;103:989–1005. doi: 10.1111/j.1749-6632.1963.tb53750.x. [DOI] [PubMed] [Google Scholar]
- POWELL J. F., STRANGE R. E. Biochemical changes occurring during sporulation in Bacillus species. Biochem J. 1956 Aug;63(4):661–668. doi: 10.1042/bj0630661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL J. F., STRANGE R. E. Biochemical changes occurring during the germination of bacterial spores. Biochem J. 1953 May;54(2):205–209. doi: 10.1042/bj0540205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESTIDGE L. S., PARDEE A. B. Induction of bacterial lysis by penicillin. J Bacteriol. 1957 Jul;74(1):48–59. doi: 10.1128/jb.74.1.48-59.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogolsky M., Slepecky R. A. Elimination of a genetic determinant for sporulation of Bacillus subtilis with acriflavin. Biochem Biophys Res Commun. 1964 Jun 15;16(3):204–208. doi: 10.1016/0006-291x(64)90326-2. [DOI] [PubMed] [Google Scholar]
- SCHEPARTZ S. A., JOHNSON M. J. The nature of the binding of penicillin by bacterial cells. J Bacteriol. 1956 Jan;71(1):84–90. doi: 10.1002/path.1700710112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH H., STRANGE R. E., ZWARTOUW H. T. Alpha epsilon-Diaminopimelic acid in the peptide moiety of the cell wall polysaccharide of Bacillus anthracis. Nature. 1956 Oct 20;178(4538):865–866. doi: 10.1038/178865a0. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. Cell-wall lytic enzymes at sporulation and spore germination in Bacillus species. J Gen Microbiol. 1957 Oct;17(2):525–537. doi: 10.1099/00221287-17-2-525. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., POWELL J. F. Hexosamine-containing peptides in spores of Bacillus subtilis, B. megatherium and B. cereus. Biochem J. 1954 Sep;58(1):80–85. doi: 10.1042/bj0580080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Asporogenous mutants of Bacillus subtilis Marburg. Folia Microbiol (Praha) 1967;12(3):291–296. doi: 10.1007/BF02868746. [DOI] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J., CRAWFORD I. P. BIOCHEMICAL ASPECTS OF COMPETENCE IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. I. CHEMICAL COMPOSITION OF CELL WALLS. J Biol Chem. 1963 Sep;238:3119–3125. [PubMed] [Google Scholar]