Abstract
Background and objectives: Niacin administration lowers the marked hyperphosphatemia that is characteristic of renal failure. We examined whether niacin administration also reduces serum phosphorus concentrations in patients who have dyslipidemia and are free of advanced renal disease.
Design, setting, participants, & measurements: We performed a post hoc data analysis of serum phosphorus concentrations that had been determined serially (at baseline and weeks 4, 8, 12, 18, and 24) among 1547 patients who had dyslipidemia and were randomly assigned in a 3:2:1 ratio to treatment with extended release niacin (ERN; 1 g/d for 4 weeks and dose advanced to 2 g/d for 20 weeks) combined with the selective prostaglandin D2 receptor subtype 1 inhibitor laropiprant (L; n = 761), ERN alone (n = 518), or placebo (n = 268).
Results: Repeated measures analysis revealed that ERN-L treatment resulted in a net mean (95% confidence interval) serum phosphorus change comparing ERN-L with placebo treatment of −0.13 mmol/L (−0.15 to −0.13 mmol/L; −0.41 mg/dl [−0.46 to −0.37 mg/dl]). These results were consistent across the subgroups defined by estimated GFR of <60 or ≥60 ml/min per 1.73 m2, a serum phosphorus of >1.13 mmol/L (3.5 mg/dl) versus ≤1.13 mmol/L (3.5 mg/dl), the presence of clinical diabetes, or concomitant statin use.
Conclusions: We have provided definitive evidence that once-daily ERN-L treatment causes a sustained 0.13-mmol/L (0.4-mg/dl) reduction in serum phosphorus concentrations, approximately 10% from baseline, which is unaffected by estimated GFR ranging from 30 to ≥90 ml/min per 1.73 m2 (i.e., stages 1 through 3 chronic kidney disease).
Abnormalities in calcium-phosphorus homeostasis, including significant elevations in serum phosphorus concentrations, are thought to contribute to arterial stiffening, hypertension, and cardiovascular disease (CVD) risk in patients with advanced chronic kidney disease and ESRD that requires maintenance dialysis (1–6). Observational data from population-based studies suggested that even serum phosphorus concentrations within the normative range are linearly associated with measures of subclinical arteriosclerosis and the development of incident CVD outcomes (7–12). Two cross-sectional studies from patients who underwent cardiac catheterization have further indicated that serum phosphorus concentrations, primarily within the normative range, were directly associated with both the presence and the severity of angiographic coronary artery disease (13,14). Moreover, a graded, independent association between serum phosphorus concentrations (again, within the normative range) and recurrent CVD events was reported among a large clinical trial cohort of patients with a previous myocardial infarction (15).
Supplementation of calcium salts, despite their efficacy and tolerability as a phosphorus-lowering treatment in ESRD, may enhance coronary artery and aortic valve calcification (16,17). This observation highlights the need for hyperphosphatemia treatment protocols to balance potential benefits and adverse effects (18–22). Phosphorus-lowering drugs that target other cardiovascular risk factors in chronic kidney disease (CKD), simultaneously, including, for example, dyslipidemia (23), might have additive or synergistic benefits. These findings may also be relevant to populations with less advanced CKD or normal renal function.
Preliminary studies suggested that niacin administration (as niacinamide, niceritrol, or nicotinic acid) could be a useful primary or adjunctive treatment for the marked hyperphosphatemia that is characteristic of ESRD (24–30). Several reports from clinical trials of extended-release niacin (ERN) that was given to patients who had dyslipidemia and were free of clinical renal disease and hyperphosphatemia have contained limited additional data noting up to 10% reductions in the serum phosphorus concentrations of actively treated patients (31–34). These repeated clinical observations (24–34) are most plausibly explained by the direct inhibitory effect of niacin compounds on active transport-mediated phosphorus absorption in the mammalian small intestine (35–39).
Published studies of patient populations who had dyslipidemia and were receiving ERN that included phosphorus data may have failed to provide information on baseline phosphorus values (33,34), and none (31–34) performed repeated measures analyses to examine the potential effects of niacin treatment on serum phosphorus and calcium concentrations, as well as the calcium-phosphorus products.
Focused reexamination of the large, placebo-controlled clinical trial data set assembled by Maccubbin et al. (34) afforded us a unique opportunity to elucidate these and other unresolved issues regarding the impact of niacin given as the fixed-dose combination of ERN and laropiprant (ERN-L), a selective prostaglandin D2 receptor subtype 1 inhibitor that reduces niacin-induced flushing (34) or ERN alone on serum phosphorus and calcium concentrations and calcium-phosphorus products. We further evaluated whether there was evidence for significant effect modification by estimated GFR (eGFR), baseline serum phosphorus concentration, the presence of diabetes, or concurrent hepatic hydroxymethyl glutaryl–CoA reductase inhibitor (statin) use when assessing the potential impact of niacin on these routine clinical measures of calcium-phosphorus homeostasis.
Materials and Methods
The analyses described herein were performed as an ancillary study to the completed clinical trial reported by Maccubbin et al. (34). Details of the parent study design are provided in that previous publication (34). An extensive discussion of the impact of the niacin versus placebo treatments on lipid, lipoprotein, and apolipoprotein concentrations, as well adverse effects or toxicities associated with active niacin therapy, was also provided in that primary analysis (34). These findings have not been reproduced here, but we have made available Supplemental Table 1, which originally appeared in reference (34), and similar data from the niacin/laropiprant approval process are available at http://www.emea.europa.eu/humandocs/PDFs/EPAR/tredaptive/emea-combined-h889en.pdf.
Briefly, the parent study was a worldwide, multicenter, double-blind, randomized, placebo-controlled, parallel trial with a 24-week double-blind treatment period preceded by a 4-week placebo run-in period. Patients who had primary hypercholesterolemia or mixed dyslipidemia and whose serum creatinine was ≤1.7 mg/dl were assigned to initiate treatment with ERN-L, 1 g (one tablet of ERN 1 g/L 20 mg), ERN 1 g, or placebo in a 3:2:1 ratio. Study drug allocation was stratified by ongoing statin use and study site. After 4 weeks of double-blind treatment, dosages were doubled (two tablets), increasing the ERN-L dosages to 2 g/40 mg and the ERN dosage to 2 g for the remaining 20 weeks. Patients were instructed to take study therapy once daily with food, in the evening. There were nine scheduled clinic visits at weeks −4, −2, 0 (day 1), 2, 4, 8, 12, 18, and 24.
The final study protocol was reviewed and approved by the appropriate ethics committees/institutional review boards, and all patients provided written informed consent. The study was conducted under the guidelines established by the Declaration of Helsinki and Good Clinical Practice standards.
All of the serum phosphorus, creatinine, calcium, and albumin determinations were performed on fasting blood samples by a central laboratory (PPD Global Central Laboratories, Highland Heights, KY, or Zaventem, Belgium). Serum collected for these measurements was obtained from blood that was allowed to clot for 30 minutes at room temperature and then centrifuged for 15 minutes at 2000 rpm to achieve a clear serum layer over the red cell clot. The serum was immediately transferred into cryovials, refrigerated at 4°C, and shipped overnight under refrigerated conditions to the central laboratory for analyses within several hours of receipt. Concentrations of serum phosphorus, calcium, and albumin were determined using a photometric method. Serum creatinine concentrations were measured by the Jaffe kinetic method. These assays all were performed on the Roche Modular automated clinical chemistry analyzer.
Serum calcium concentration was adjusted for serum albumin concentrations <4 g/dl using the following formula: Corrected total calcium (mg/dl) = total calcium (mg/dl) + 0.8 × [4 − serum albumin (g/dl)] (40). eGFR was calculated from the creatinine-based Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula (41).
Glycemic status was determined before randomization for the parent study and designated “normal,” “impaired,” or “diabetic” on the basis of medical history, laboratory evaluations, and clinical judgment. Only the assignment of “diabetes” was used for our analyses comparing diabetic and nondiabetic strata.
Statistical Analysis
The primary objective of this post hoc analysis was to examine the 24-week phosphorus-lowering effects using the changes in serum phosphorus concentrations on the basis of measurements taken at baseline (0) and at 4, 8, 12, 18, and 24 weeks. The secondary objective was to examine whether these effects varied by prespecified subgroups, including eGFR of <60 versus ≥60 ml/min per 1.73 m2, serum phosphorus >3.5 versus ≤3.5 mg/dl, the presence of diabetes, or concomitant statin (hepatic hydroxymethyl glutaryl–CoA reductase inhibitor) use.
The primary efficacy parameter was the change from baseline in serum phosphorus concentrations averaged across weeks 12, 18, and 24. The average across weeks 12 through 24 was used in the analysis (consistent with the analysis of the primary lipid end points reported previously [34]) because it was expected that the concentrations of serum phosphorus would stabilize by week 12 (i.e., after 8 weeks on 2 g of ERN). Additional parameters included changes from baseline in serum calcium concentrations and the product of serum calcium-phosphorus concentrations, also averaged across weeks 12 through 24.
A repeated measures analysis of phosphorus concentrations was conducted using data recorded at 5 study weeks (weeks 4, 8, 12, 18, and 24), with fixed effects for treatment-by-week interaction, gender-by-week interaction, concomitant statin use-by-week interaction, baseline phosphorus-by-week interaction, country, and random patient effect. The correlation between the responses of the same patient at different time points was modeled using an unstructured covariance matrix. Separate covariance matrices were specified for each of the three treatment groups. The average differences in serum phosphorus change between treatment groups from baseline across weeks 12 through 24 was estimated using the appropriate contrasts from the model. A similar approach was used for the analyses of serum calcium concentrations and calcium-phosphorus products.
Potential treatment effect modification by eGFR of <60 versus ≥60 ml/min per 1.73 m2, serum phosphorus >3.5 versus ≤3.5 mg/dl, the presence of diabetes, or concomitant statin use was evaluated by constructing the within-subgroup estimates (with 95% confidence intervals [CIs]) of the between-treatment differences.
Baseline descriptive statistics included means ± SD, quantiles, and frequencies. Characteristics at baseline were generally comparable across the treatment arms. Changes in serum phosphorus and calcium concentrations, as well as calcium -phosphorus products, were expressed as means, with 95% CIs.
Results
As Table 1 depicts, the key baseline characteristics of the 1609 study participants did not differ comparing the ERN-L, ERN, and placebo treatment groups. Most notably, mean ± SD serum phosphorus and albumin-adjusted calcium concentrations for the three treatment arms, ERN-L, ERN, and placebo, were equivalent. Serum phosphorus concentrations were, respectively, ERN-L 1.08 ± 0.15 mmol/L (3.34 ± 0.46 mg/dl), ERN 1.08 ± 0.15 mmol/L (3.33 ± 0.47 mg/dl), and placebo 1.09 ± 0.16 mmol/L (3.38 ± 0.49 mg/dl). Serum calcium concentrations were, respectively, ERN-L 2.36 ± 0.09 mmol/L (9.41 ± 0.36 mg/dl), ERN 2.36 ± 0.09 mmol/L (9.43 ± 0.37 mg/dl), and placebo 2.36 ± 0.09 mmol/L (9.43 ± 0.37 mg/dl). The distributions of the major subgroups of interest—eGFR <60, serum phosphorus >3.5 mg/dl, presence of diabetes, and statin use—were also comparable across treatment groups. Of these 1609 patients, 62 did not have requisite mineral data available for analysis, so subsequent analyses were based on 1547 patients.
Table 1.
Key baseline characteristics
| Characteristic | ERN-L (n = 798) | ERN (n = 541) | Placebo (n = 270) |
|---|---|---|---|
| Age (years; mean ± SD) | 58.0 ± 11.0 | 57.5 ± 11.2 | 57.0 ± 11.2 |
| Male gender (n [%]) | 473 (59.3) | 348 (64.3) | 157 (58.1) |
| Diabetes (n [%]) | 136 (17.0) | 78 (14.4) | 38 (14.1) |
| Statin use (n [%]) | 533 (66.8) | 358 (66.2) | 179 (66.3) |
| Albumin (g/L [g/dl]) | |||
| mean ± SD | 441 ± 26 (4.41 ± 0.26) | 443 ± 25 (4.43 ± 0.25) | 441 ± 26 (4.41 ± 0.26) |
| 10th/50th/90th percentiles (full range) | 4.10/4.40/4.70 (3.60 to 5.40) | 4.10/4.40/4.80 (3.60 to 5.40) | 4.10/4.40/4.70 (3.50 to 5.00) |
| Calcium (mmol/L [mg/dl]) | |||
| mean ± SD | 2.35 ± 0.09 (9.41 ± 0.36) | 2.36 ± 0.09 (9.43 ± 0.37) | 2.36 ± 0.09 (9.43 ± 0.37) |
| 10th/50th/90th percentiles (full range) | 9.00/9.40/9.90 (8.40 to 10.60) | 9.00/9.40/9.90 (8.40 to 10.90) | 8.90/9.50/9.90 (8.26 to 10.90) |
| eGFR (ml/min per 1.73 m2) | |||
| mean ± SD | 76.4 ± 15.2 | 77.2 ± 15.3 | 76.7 ± 15.8 |
| 10th/50th/90th percentiles (full range) | 57.3/76.1/96.5 (31.9 to 118.3) | 58.1/77.3/97.5 (29.0 to 120.7) | 57.4/77.3/96.2 (29.6 to 111.8) |
| <60 (n [%]) | 112 (14.0) | 69 (12.8) | 38 (14.1) |
| Phosphorus (mmol/L [mg/dl]) | |||
| mean ± SD | 1.08 ± 0.15 (3.34 ± 0.46) | 1.08 ± 0.15 (3.33 ± 0.47) | 1.09 ± 0.16 (3.38 ± 0.49) |
| 10th/50th/90th percentiles (full range) | 2.70/3.30/4.00 (2.10 to 4.80) | 2.70/3.30/3.90 (2.00 to 4.90) | 2.80/3.40/4.00 (2.00 to 5.10) |
| >1.13 mmol/L (>3.5 mg/dl; n [%]) | 253 (31.7) | 168 (31.1) | 85 (31.5) |
| Calcium × phosphorus (mg2/dl2) | |||
| mean ± SD | 31.47 ± 4.70 | 31.44 ± 4.93 | 31.86 ± 4.96 |
| 10th/50th/90th percentiles (full range) | 25.52/31.35/37.62 (19.36 to 46.80) | 24.84/31.35/37.62 (18.60 to 49.92) | 25.71/31.77/38.22 (18.00 to 48.96) |
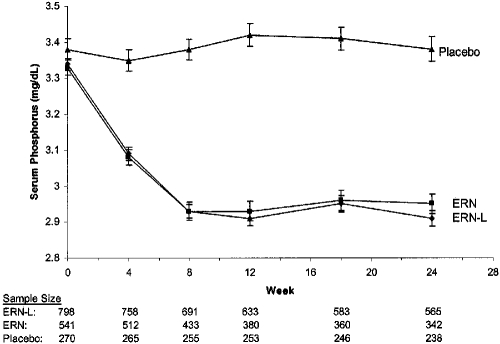
The data presented in Table 2 and Figure 1 demonstrate that ERN-L and ERN treatment both caused equivalent, sustained reductions in serum phosphorus concentrations, relative to placebo. ERN-L treatment resulted in a mean serum phosphorus change of −0.11 mmol/L (95% CI −0.13 to 0.10) (−0.35 mg/dl [95% CI 0.39 to −0.32 mg/dl]). The net mean change 95% CI in serum phosphorus concentrations, comparing ERN-L with placebo treatment (the latter caused a mean 0.06-mg/dl increase in serum phosphorus) was −0.13 mmol/L (95% CI −0.15 to −0.12 mmol/L; −0.41 mg/dl [95% CI −0.46 to −0.37 mg/dl]). ERN-L treatment also reduced the mean calcium-phosphorus products by −3.72 mg2/dl2 (95% CI −4.06 to −3.37 mg2/dl2). The corresponding net change in mean calcium-phosphorus products with ERN-L, relative to placebo, was −4.29 mg2/dl2 (95% CI −4.78 to −3.81 mg2/dl2). ERN treatment, without laropiprant, resulted in similar effects on mean serum phosphorus concentrations (Table 2) and calcium-phosphorus products. Figure 1 illustrates the time course of the observed treatment effects by ERN-L and ERN on serum phosphorus concentrations, relative to placebo. Both niacin preparations achieved their maximal impact by the eighth week of treatment, and these effects were then sustained throughout the remainder of the 24-week study.
Table 2.
Change from baseline serum phosphorus, calcium, and calcium-phosphorus product across weeks 12 through 24, with repeated measurements at weeks 4, 8, 12, 18, and 24
| Parameter | ERN-L (n = 761) | ERN (n = 518) | Placebo (n = 268) |
|---|---|---|---|
| Phosphorus | |||
| baseline (mmol/L [mg/dl]; mean ± SD) | 1.08 ± 0.15 (3.34 ± 0.46) | 1.08 ± 0.15 (3.33 ± 0.47) | 1.09 ± 0.16 (3.38 ± 0.49) |
| Δ phosphorus (mean [95% CI]) | |||
| mmol/L | −0.11 (−0.13 to −0.10) | −0.11 (−0.12 to −0.09) | 0.02 (0.01 to 0.03) |
| mg/dl | −0.35 (−0.39 to −0.32) | −0.33 (−0.37 to −0.29) | 0.06 (0.02 to 0.10) |
| Δ phosphorus versus placebo (mean [95% CI]) | |||
| mmol/L | −0.13 (−0.15 to −0.12) | −0.13 (−0.14 to −0.12) | — |
| mg/dl | −0.41 (−0.46 to −0.37) | −0.39 (−0.44 to −0.34) | — |
| P | <0.001 | <0.001 | |
| Calcium | |||
| baseline (mmol/L [mg/dl]; mean ± SD) | 2.36 ± 0.09 (9.42 ± 0.36) | 2.36 ± 0.09 (9.42 ± 0.37) | 2.36 ± 0.09 (9.43 ± 0.37) |
| Δ calcium (mean [95% CI]) | |||
| mmol/L | −0.04 (−0.04 to −0.03) | −0.04 (−0.05 to −0.03) | 0.00 (−0.01 to 0.01) |
| mg/dl | −0.14 (−0.17 to −0.12) | −0.15 (−0.18 to −0.12) | −0.01 (−0.04 to 0.02) |
| Δ calcium versus placebo (mean [95% CI]) | |||
| mmol/L | −0.03 (−0.04 to −0.03) | −0.04 (−0.05 to −0.03) | — |
| mg/dl | −0.13 (−0.17 to −0.10) | −0.14 (−0.18 to −0.10) | — |
| P | <0.001 | <0.001 |
Figure 1.
Serum phosphorus concentrations by treatment group at baseline (week 0) and at weeks 4, 8, 12, 18, and 24. Data are means ± SE, with the corresponding sample sizes at weeks 0, 4, 8, 12, 18, and 24 beneath.
Table 3 shows that within the subgroup of 215 patients whose eGFR was <60 ml/min, ERN-L treatment (n = 110) changed mean ± SD baseline serum phosphorus (3.38 ± 0.45) by −0.44 mg/dl (95% CI −0.50 to −0.37), and ERN (n = 67) treatment changed mean baseline serum phosphorus (3.41 ± 0.49) by −0.38 mg/dl (95% CI −0.47 to −0.29), whereas the placebo group (n = 38) experienced a 0.03-mg/dl mean increase (95% CI −0.09 to 0.15) from its baseline serum phosphorus (of 3.46 ± 0.45 mg/dl). These results (see Table 3) were similar to the effects (95% CI) on serum phosphorus in patients (n = 1332) with an eGFR ≥60 ml/min.
Table 3.
Change from baseline serum phosphorus and calcium-phosphorus product across weeks 12 through 24, with repeated measurements at weeks 4, 8, 12, 18, and 24, comparing eGFR <60 and eGFR ≥60 ml/min per 1.73 m2 subgroups
| Parameter | ERN-L | ERN | Placebo |
|---|---|---|---|
| eGFR <60 ml/min per 1.73 m2 (n = 215) | n = 110 | n = 67 | n = 38 |
| baseline phosphorus (mmol/L [mg/dl]; mean ± SD) | 1.09 ± 0.15 (3.38 ± 0.45) | 1.10 ± 0.16 (3.41 ± 0.49) | 1.12 ± 0.15 (3.46 ± 0.45) |
| Δ phosphorus (mean [95% CI]) | |||
| mmol/L | 0.14 (0.16 to 0.12) | 0.13 (0.15 to 0.09) | 0.01 (−0.03 to 0.05) |
| mg/dl | −0.44 (−0.50 to −0.37) | −0.38 (−0.47 to −0.29) | 0.03 (−0.09 to 0.15) |
| Δ phosphorus versus placebo (mean [95% CI]) | |||
| mmol/L | 0.15 (0.19 to −0.10) | 0.13 (0.18 to 0.08) | — |
| mg/dl | −0.46 (−0.60 to −0.32) | −0.41 (−0.55 to −0.26) | — |
| P | <0.001 | <0.001 | |
| eGFR ≥60 ml/min per 1.73 m2 (n = 1332) | n = 651 | n = 451 | n = 230 |
| baseline phosphorus (mmol/L [mg/dl]; mean ± SD) | 1.08 ± 0.15 (3.33 ± 0.47) | 1.07 ± 0.15 (3.31 ± 0.47) | 1.09 ± 0.16 (3.36 ± 0.50) |
| Δ phosphorus (mean [95% CI]) | |||
| mmol/L | 0.12 (0.13 to 0.11) | 0.11 (0.13 to 0.10) | 0.01 (−0.00 to 0.03) |
| mg/dl | −0.37 (−0.40 to −0.34) | −0.35 (−0.39 to −0.31) | 0.04 (−0.00 to 0.08) |
| Δ phosphorus versus placebo (mean [95% CI]) | |||
| mmol/L | 0.13 (0.15 to 0.12) | 0.13 (0.14 to 0.11) | — |
| mg/dl | −0.41 (−0.46 to −0.36) | −0.39 (−0.44 to −0.33) | — |
| P | <0.001 | <0.001 |
There was also no evidence of effect modification by the presence of diabetes, concomitant statin use, or baseline serum phosphorus being >3.5 versus ≤3.5 mg/dl. Among patients with diabetes, ERN-L treatment (n = 126) changed mean ± SD baseline serum phosphorus (3.42 ± 0.47) by −0.35 mg/dl (95% CI −0.43 to −0.28 mg/dl), and ERN (n = 72) treatment changed mean baseline serum phosphorus (3.48 ± 0.44) by −0.36 mg/dl (95% CI −0.45 to −0.28 mg/dl), whereas the placebo group (n = 38) experienced a 0.01-mg/dl mean increase (95% CI −0.11 to 0.14 mg/dl) from its baseline serum phosphorus (of 3.55 ± 0.51 mg/dl). Within the nondiabetic stratum, ERN-L treatment (n = 635) changed mean baseline serum phosphorus (3.32 ± 0.46) by −0.39 mg/dl (95% CI −0.42 to −0.36 mg/dl), and ERN (n = 446) treatment changed mean baseline serum phosphorus (3.30 ± 0.48) by −0.35 mg/dl (95% CI −0.39 to −0.31 mg/dl), whereas the placebo group (n = 230) experienced a 0.04-mg/dl mean increase (95% CI −0.00 to 0.08 mg/dl) from its baseline serum phosphorus (of 3.35 ± 0.48 mg/dl). Statin users who were treated with ERN-L (n = 509) experienced a mean ± SD change in their baseline serum phosphorus (3.35 ± 0.47) of −0.36 mg/dl (95% CI −0.39 to −0.32 mg/dl), and ERN (n = 344) treatment changed their mean baseline serum phosphorus (3.30 ± 0.46) by −0.36 mg/dl (95% CI −0.40 to −0.32 mg/dl), whereas the placebo group (n = 178) had a 0.03-mg/dl mean increase (95% CI −0.02 to 0.07 mg/dl) from its baseline serum phosphorus (of 3.38 ± 0.49 mg/dl). Among nonusers of statins, ERN-L treatment (n = 252) changed mean baseline serum phosphorus (3.32 ± 0.44) by −0.42 mg/dl (95% CI −0.47 to −0.37 mg/dl), and ERN (n = 174) treatment changed mean baseline serum phosphorus (3.37 ± 0.49) by −0.34 mg/dl (95% CI −0.41 to −0.28 mg/dl), whereas the placebo group (n = 90) experienced a 0.06-mg/dl mean increase (95% CI −0.00 to 0.12 mg/dl) from its baseline serum phosphorus (of 3.36 ± 0.49 mg/dl). The relative reduction in baseline serum phosphorus concentrations among those who were treated with ERN-L or ERN versus placebo also did not differ significantly comparing patients whose baseline serum phosphorus was >3.5 or ≤3.5 mg/dl. Within the stratum of serum phosphorus >3.5 mg/dl, ERN-L (n = 239) changed serum phosphorus compared with placebo (n = 85) by −0.46 mg/dl (95% CI −0.55 to −0.37 mg/dl), whereas ERN (n = 158) changed serum phosphorus by −0.41 mg/dl (95% CI −0.51 to −0.32 mg/dl) relative to placebo. For those with a serum phosphorus ≤3.5 mg/dl, ERN-L (n = 522) treatment changed serum phosphorus by −0.39 mg/dl (95% CI −0.45 to −0.34 mg/dl) compared with placebo (n = 183), whereas ERN (n = 360) changed serum phosphorus by −0.37 mg/dl (95% CI −0.44 to −0.31 mg/dl). Moreover, additional stratified analyses (data not shown) revealed no evidence of effect modification by age.
Discussion
Our detailed analyses of the phosphorus and calcium data that were collected from 1547 patients during a 24-week intervention confirmed and extended the findings from three reports published between 1998 and 2000 (31–33). We demonstrated conclusively that ERN-L and ERN alone caused a sustained approximately 11% reduction in serum phosphorus (Figure 1), accompanied by an approximately 12% lowering of the calcium-phosphorus product, without raising serum calcium concentrations. None of these effects was altered by laropiprant; neither was there any evidence of effect modification by eGFR being <60 or ≥60 ml/min, a serum phosphorus of >3.5 versus ≤3.5 mg/dl, the presence of clinical diabetes, or concomitant statin use.
Extant publications on the phosphorus-lowering impact of niacin compounds in patients with ESRD (24–30), particularly once-daily ERN (26–29), revealed the hypophosphatemic effects of niacin to be of the same magnitude achieved by calcium acetate, sevelamer, or lanthanum when these agents are administered thrice daily and timed requisitely to meals (16–21,42).
Animal model data highlight plausible mechanisms—especially fecal loss (37)—that account for the observed phosphorus-lowering effects of niacin preparations (36–40). Approximately 50% of net phosphorus absorption occurs in the duodenum and jejunum via an active transport pathway through the epithelial Na-Pi co-transporters contained in abundantly expressed, “ready to use” vesicles located within the small intestinal brush border (30,35). The energy required for this active phosphorus transport is provided by basolateral Na-K-ATPase (35). Eto et al. (38) demonstrated in a rat model of ESRD that nicotinamide inhibits small intestinal Na-Pi2b expression, reducing phosphorus absorption and preventing the progressive increase in serum phosphorus that is associated with renal failure. Another investigation in healthy rats showed, independently, that nicotinamide inhibits sodium-dependent intestinal phosphorus co-transport (36).
Abnormalities in calcium-phosphorus homeostasis, including significant elevations in serum phosphorus concentrations, are thought to contribute to arterial stiffening, hypertension, and CVD risk in patients with advanced CKD and ESRD that requires maintenance dialysis (16–18). Marked hyperphosphatemia in ESRD and the very increased phosphorus concentrations of advanced (i.e., stage 4) CKD both have been associated with the development of CVD, particularly fatal outcomes (1–6). Additional reports from populations with high CVD risk—myocardial infarction survivors (15) and patients with type 2 diabetes and hypertension (43)—have described linear associations between serum phosphorus concentrations within the normative range and arteriosclerotic outcomes, especially recurrent, fatal CVD events. Observational data from population-based studies further suggested that normative serum phosphorus concentrations are linearly associated with measures of subclinical arteriosclerosis and the development of incident CVD outcomes (7–10).
Such observational data (1–10,15,43) have engendered calls (44,45) for controlled clinical trials to test the hypothesis that serum phosphorus-lowering treatment will reduce CVD mortality, primarily. Two very prominent “blueprints” for such trials that target patients within specific eGFR ranges of stages 3 to 4 CKD (15 to 44 and 20 to 45 ml/min per 1.73 m2) were recently published (44,45). Both of these communications referenced the major commercial phosphorus binders—sevelamer, calcium acetate, and lanthanum—whereas neither trial rationalization blueprint (44,45) nor a subsequent extensive clinical practice guideline (42) referenced any of the published literature (24–34) on the phosphorus-lowering efficacy of niacin compounds.
Our extensive findings demonstrating ERN-induced phosphorus lowering contrast starkly with the very limited data available from small, brief studies of lanthanum (46), sevelamer (47), and calcium acetate (47) given to patients with stages 3 and 4 CKD. For example, both sevelamer (up to 6.4 g/d), and calcium acetate (up to 5.28 g/d) failed to lower serum phosphorus in a pilot study of 40 patients who had CKD and whose mean creatinine clearance was 36.8 ml/min and mean baseline serum phosphorus concentrations were 3.53 mg/dl (47). Moreover, given the onerous pill burden that thrice-daily phosphorus binder treatment imposes on patients with ESRD (48)—severely limiting their compliance (48)—it is an uncertain proposition that patients with less advanced, asymptomatic stages 3 to 4 CKD will comply adequately with binders.
Conclusions
In light of the secondary CVD prevention data from the crystalline niacin arm of the Coronary Drug Project (49), the established ameliorative effects of ERN on lipoprotein metabolism (31–34), and the systematic underrepresentation of patients with an eGFR <60 ml/min in randomized, controlled trials to reduce CVD outcomes (50), the data on niacin presented herein suggest that an ERN treatment arm deserves the utmost consideration for any future CVD prevention trials that target patients with stages 3 to 4 CKD.
Disclosures
D.M., D.T., O.K., and W.AH. are employees of Merck & Co., Inc., and may hold stock/stock options in the company. A.G.B. has no financial disclosures.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 1: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK: Association of elevated serum PO4, Ca X PO4 product, and parathyroid hormone with cardiac mortality risk in hemodialysis patients. J Am Soc Nephrol 12: 2131–2138, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Menon V, Greene T, Pereira AA, Wang X, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ: Relationship of phosphorus and calcium-phosphorus product with mortality in CKD. Am J Kidney Dis 46: 455–463, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Melamed ML, Eustace JA, Plantinga L, Jaar BG, Fink NE, Coresh J, Klag MJ, Powe NR: Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: A longitudinal study. Kidney Int 70: 351–357, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Levin A, Djurdjev O, Beaulieu M, Er L: Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis 52: 661–671, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Onufrak SJ, Bellasi A, Shaw LJ, Herzog CA, Cardarelli F, Wilson PW, Vaccarino V, Raggi P: Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis 199: 424–431, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA: Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 20: 397–404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ix JH, De Boer IH, Peralta CA, Adeney KL, Duprez DA, Jenny NS, Siscovick DS, Kestenbaum BR: Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol 4: 609–615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB, Gaziano JM, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Foley RN, Collins AJ, Ishani A, Kalra PA: Calcium-phosphate levels and cardiovascular disease in the community-dwelling adults: The Atherosclerotic Risk in Communities (ARIC) Study. Am Heart J 156: 556–563, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Onufrak SJ, Bellasi A, Cardarelli F, Vaccarino V, Muntner P, Shaw LJ, Raggi P: Investigation of gender heterogeneity in the association of serum phosphorus with incident coronary artery disease and all-cause mortality. Am J Epidemiol 169: 67–77, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narang R, Ridout D, Nonis C, Kooner JS: Serum calcium, phosphorus and albumin levels in relation to the angiographic severity of coronary artery disease. Int J Cardiol 60: 73–79, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Rasouli M, Mohseni-Kiasari A: Serum calcium and phosphorus associate with the occurrence and severity of angiographically documented coronary heart disease, possibly through correlation with atherogenic (apo)lipoproteins. Clin Chem Lab Med 44: 43–50, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G: Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Chertow GM, Burke SK, Raggi P: Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P: Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68: 1815–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Coladonato JA: Control of hyperphosphatemia among patients with ESRD. J Am Soc Nephrol 16 [Suppl 2]: S107–S114, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Joy MS, Finn WF: Randomized, double-blind, placebo-controlled, dose-titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: A new phosphate binder for the treatment of hyperphosphatemia. Am J Kidney Dis 42: 96–107, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Behets GJ, Dams G, Vercauteren SR, Damment SJ, Bouillon R, De Broe ME, D'Haese PC: Does the phosphate binder lanthanum carbonate affect bone in rats with chronic renal failure? J Am Soc Nephrol 15: 2219–2228, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Qunibi WY, Hootkins RE, McDowell LL, Meyer MS, Simon M, Garza RO, Pelham RW, Cleveland MV, Muenz LR, He DY, Nolan CR: Treatment of hyperphosphatemia in hemodialysis patients: The Calcium Acetate Renagel Evaluation (CARE Study). Kidney Int 65: 1914–1926 2004 [DOI] [PubMed] [Google Scholar]
- 22.Lacour B, Lucas A, Auchere D, Ruellan N, de Serre Patey NM, Drueke TB: Chronic renal failure is associated with increased tissue deposition of lanthanum after 28-day oral administration. Kidney Int 67: 1062–1069, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Pennell P, Leclercq B, Delahunty MI, Walters BA: The utility of non-HDL in managing dyslipidemia of stage 5 chronic kidney disease. Clin Nephrol 66: 336–347, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Shimoda K, Akiba T, Matsushima T, Rai T, Abe K, Hoshino M: Niceritrol decreases serum phosphate levels in chronic hemodialysis patients [in Japanese]. Nippon Jinzo Gakkai Shi 40: 1–7, 1998 [PubMed] [Google Scholar]
- 25.Takahashi Y, Tanaka, Nakamura T, Fukuwatari T, Shibata K, Shimada N, Ebihara I, Koide H: Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int 65: 1099–1104, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Sampathkumar K, Selvam M, Sooraj Y, Gowthaman S, Ajeshkumar R: Extended release nicotinic acid is a novel agent for phosphate control in dialysis. Int Urol Nephrol 38: 171–174, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Müller D, Mehling H, Otto B, Bergmann-Lips R, Luft F, Jordan J, Kettritz R: Niacin lowers serum phosphate and increases HDL cholesterol in dialysis patients. Clin J Am Soc Nephrol 2: 1249–1254, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Restrepo Valencia CA, Cruz J: Safety and effectiveness of nicotinic acid in the management of patients with chronic renal disease and hyperlipidemia associated to hyperphosphatemia [in Spanish]. Nefrologia 28: 61–66, 2008 [PubMed] [Google Scholar]
- 29.Cheng SC, Young DO: A Randomized, double blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin J Am Soc Nephrol 3: 1131–1138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampathkumar K: Niacin and analogs for phosphate control in dialysis: Perspective from a developing country. Int Urol Nephrol 41: 813–819, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Knopp RH, Alagona P, Davidson M, Goldberg AC, Kafonek SD, Kashyap M, Sprecher D, Superko HR, Jenkins S, Marcovina S: Equivalent efficacy of a time-release form of niacin (Niaspan) given once-a-night versus plain niacin in the management of hyperlipidemia. Metabolism 47: 1097–1104, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Capuzzi DM, Guyton JR, Morgan JM, Goldberg AC, Kreisberg RA, Brusco OA, Brody J: Efficacy and safety of an extended-release niacin (Niaspan): A long-term study. Am J Cardiol 82: 74U–81U, discussion 85U–86U, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Guyton JR, Blazing Ma, Hagar J, Kashyap ML, Knopp RH, McKenney JM, Nash DT, Nash SD: Extended-release niacin vs. gemfibrozil for the treatment of low levels of high-density lipoprotein cholesterol. Arch Intern Med 160: 1177–1184, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Maccubbin D, Bays HE, Olson G, Elinoff V, Elis A, Mitchel Y, Sirah W, Betteridge A, Reyes R, Yu Q, Kuznetsova O, McCrary Sisk C, Pasternak RC, Paolini JF: Lipid-modifying efficacy and tolerability of extended-release niacin/laropiprant in patients with primary hypercholesterolemia or mixed dyslipidaemia. Int J Clin Pract 62: 1959–1970, 2008. ClinicalTrials.gov registration number: NCT00269204 [DOI] [PubMed] [Google Scholar]
- 35.Debiec H, Lorenc R: Identification of Na+, Pi-binding protein in kidney and intestinal brush-border membranes. Biochem J 255: 185–191, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katai K, Tanaka H, Tatsumi S, Fukunaga Y, Genjida K, Morita K, Kuboyama N, Suzuki T, Akiba T, Miyamoto K, Takeda E: Nicotinamide inhibits sodium-dependent phosphate cotransport activity in rat small intestine. Nephrol Dial Transplant 14: 1195–1201, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Kuboyama N, Watanabe Y, Yamaguchi M, Sato K, Suzuki T, Akiba T: Effects of niceritrol on faecal and urinary phosphate excretion in normal rats. Nephrol Dial Transplant 14: 610–614, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Eto N, Miyata Y, Ohno H, Yamashita T: Nicotinamide prevents the development of hyperphosphatemia by suppressing the intestinal sodium dependent phosphate transporter in rats with adenine induced renal failure. Nephrol Dial Transplant 20: 1378–1384, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Sabbagh Y, O'Brien SP, Song W, Boulanger JH, Stockmann A, Arbeeny C, Schiavi SC. Intestinal Npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol 20: 2348–2358 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Portale AA: Blood calcium, phosphorus, and magnesium. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, edited by Favus MJ, Philadelphia, Lippincott Williams & Wilkins, 1999, pp 115–118 [Google Scholar]
- 41.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int 76 [Suppl 113]: S121–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Chonchol M, Dale R, Schrier RW, Estacio R: Serum phosphorus and cardiovascular mortality in type 2 diabetes. Am J Med 122: 380–386, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Block GA, Persky MS, Ketteler M, Kestenbaum B, Thadhani R, Kooienga L, Spiegel D, Asplin J, Ehrlich J, Dennis V, Nissenson A, Chertow GM, Wheeler DC: A randomized double-blind pilot study of serum phosphorus normalization in chronic kidney disease: A new paradigm for clinical outcomes studies in nephrology. Hemodial Int 13: 360–362, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Isakova T, Gutiérrez OM, Wolf M: A blueprint for randomized trials targeting phosphorus metabolism in chronic kidney disease. Kidney Int 76: 705–711, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Sprague SM, Abboud H, Qiu P, Dauphin M, Zhang P, Finn W: Lanthanum carbonate reduces phosphorus burden in patients with CKD stages 3 and 4: A randomized trial. Clin J Am Soc Nephrol 4: 178–185, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moysés RM: Early control of PTH and FGF23 in normophosphatemic CKD patients: A new target in CKD-MBD therapy? Clin J Am Soc Nephrol November12, 2009. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R: Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol 4: 1089–1096 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W: Fifteen year mortality in Coronary Drug Project patients: Long-term benefit with niacin. J Am Coll Cardiol 8: 1245–1255, 1986 [DOI] [PubMed] [Google Scholar]
- 50.Coca SG, Krumholz HM, Garg AX, Parikh CR: Under-representation of renal disease in randomized controlled trials of cardiovascular disease. JAMA 296: 1377–1384 2006 [DOI] [PubMed] [Google Scholar]