Abstract
BACKGROUND
Obesity among pregnant women is highly prevalent worldwide and is associated in a linear manner with markedly increased risk of adverse outcome for mother and infant. Obesity in the mother may also independently confer risk of obesity to her child. The role of maternal metabolism in determining these outcomes and the potential for lifestyle modification are largely unknown.
METHODS
Relevant studies were identified by searching PubMed, the metaRegister of clinical trials and Google Scholar without limitations. Sensitive search strategies were combined with relevant medical subject headings and text words.
RESULTS
Maternal obesity and gestational weight gain have a significant impact on maternal metabolism and offspring development. Insulin resistance, glucose homeostasis, fat oxidation and amino acid synthesis are all disrupted by maternal obesity and contribute to adverse outcomes. Modification of lifestyle is an effective intervention strategy for improvement of maternal metabolism and the prevention of type 2 diabetes and, potentially, gestational diabetes.
CONCLUSIONS
Maternal obesity requires the development of effective interventions to improve pregnancy outcome. Strategies that incorporate a detailed understanding of the maternal metabolic environment and its consequences for the health of the mother and the growth of the child are likely to identify the best approach.
Keywords: pregnancy, metabolism, insulin, randomized controlled trials
Introduction
Obesity among pregnant women is highly prevalent and is associated with markedly increased risk of adverse outcome for mother and infant. Obesity in the mother may also independently confer risk of obesity to her child. In this review, we present the evidence for short- and long-term consequences of maternal obesity and gestational weight gain (GWG) and define the underlying need for an effective intervention strategy. The metabolic sequelae of maternal obesity are addressed including a review of fat deposition and location, and by detailing effects on glucose, lipid and protein metabolism, parallels with type 2 diabetes are highlighted. Lifestyle intervention strategies in type 2 diabetes and gestational diabetes are reviewed, and how these may aid in the development of an intervention for obese women is discussed. Finally, we review the relevant intervention studies, most of which are preliminary reports in women with normal and raised BMI, all of which focus on the prevention of excessive GWG. We also review the relative merits of GWG or measures of maternal insulin resistance as primary outcomes for large-scale randomized controlled trials in obese pregnant women.
Methods
To generate this review, a thorough literature search was repeatedly made in PubMed, the metaRegister of clinical trials and Google Scholar without limitations for all studies, with the last repeat performed on 30 September 2009. The Medical Subject Heading Terms used were overweight, obesity, fetus, insulin resistance, pregnancy, metabolism, lipids, amino acids, protein, physical activity, diet, diabetes, gestational diabetes, lifestyle, child, adult, body mass index, body weight, weight gain, adipose tissue, controlled clinical trial and epidemiological studies. The procedure was concluded by the perusal of the reference sections of all relevant studies or reviews, a manual search of key journals and abstracts from the major annual meetings in the field of pregnancy and endocrinology and a contact with experts on the subject, in an effort to identify relevant unpublished data. Finally, unpublished studies were also sought in the websites isrctn.org, clinicaltrials.gov, cihr.ca, action.org, UK clinical trials gateway and Wellcome Trust.
Maternal obesity
The size of the problem
In 2005, the World Health Organisation (WHO) estimated that at least 400 million adults were obese (BMI > 30 kg/m2), a figure projected to rise to over 700 million by 2015 (WHO, 2006). Among women aged 20–44, 32% were classified as obese in the USA in a survey carried out between 2003 and 2006 (WHO, 2009). The influences of obesity on reproductive health in women, particularly adverse effects on pregnancy outcome, present a significant burden to healthcare resources (Galtier-Dereure et al., 2000; Chu et al., 2008). Recent statistics from selected OECD (Organisation for Economic Co-operation and Development) countries show that in 2005, of women aged 15–64, 32.4% in the USA and 23.8% in England were obese (OECD, 2009). Among the non-pregnant women aged 12–44 in the USA, the prevalence of obesity has more than doubled since 1976 and that of severe obesity has increased dramatically; between 1979 and 2004 with Class I (30 to <35 kg/m2) and II (35 to <40 kg/m2) obesity doubling and Class III rising 3-fold (≥40 kg/m2) (Institute of Medicine, 2009). Obesity among women in many developing countries is also rising (Misra and Khurana, 2008) and is highly prevalent in the Middle East (Esmaillzadeh and Azadbakht, 2006). Few countries have reliable data documenting the incidence of obesity in pregnant women (Guelinckx et al., 2008), but cohort studies in countries such as the UK where obesity is rising show upward trajectories in parallel with those of the whole population (Kanagalingam et al., 2005; Kiran et al., 2005; Heslehurst et al., 2007, Health Survey for England 2006). In the USA, data available from nine states using the Pregnancy Risk Assessment Monitoring System (PRAMS) showed that 20% of Americans are obese at the start of pregnancy, representing a 70% increase over a decade (Kim et al., 2007).
Pregnancy complications
Prepregnancy BMI is increasingly recognized as a major determinant of pregnancy outcome, with maternal obesity associated in a linear manner with an increased risk of the majority of pregnancy complications, with the exception of spontaneous preterm labour and gastroschisis which are decreased. Several recent large cohorts, systematic reviews and meta-analyses have attempted to provide accurate risk estimates for specific complications relative to maternal obesity (O'Brien et al., 2003; Chu et al., 2007a, b, c; Smith et al., 2007; Heslehurst et al., 2008; Metwally et al., 2008; Rasmussen et al., 2008; Khashan and Kenny, 2009; Poobalan et al., 2009; Stothard et al., 2009; Torloni et al. 2009) and the point estimates from the largest and most recent datasets are summarized in Table I.
Table I.
Maternal and neonatal risks of maternal obesity
| Obese women versus normal odds ratio (95% CI) | |
|---|---|
| Congenital anomalies1 | |
| All neural tube defects | 1.87 (1.62–2.15) |
| Anencephaly | 1.39 (1.03–1.87) |
| Spina bifida | 2.24 (1.86–2.69) |
| All cardiovascular anomalies | 1.30 (1.12–1.51) |
| All septal anomalies | 1.20 (1.09–1.31) |
| Cleft palate | 1.23 (1.03–1.47) |
| Cleft lip and palate | 1.20 (1.03–1.40) |
| Anorectal atresia | 1.48 (1.12–1.97) |
| Hydrocephaly | 1.68 (1.19–2.36) |
| Limb reduction anomalies | 1.34 (1.03–1.73) |
| Gastroschisis | 0.17 ( 0.10–0.30) |
| Pregnancy complications | |
| Miscarriage2 | 1.89 (1.14–3.13) |
| Recurrent miscarrage2 | 4.68 (1.21–18.13) |
| Gestational diabetes3 | 3.01 (2.34–3.87) |
| Pre-eclampsia4 | 2.14 (1.85–2.47) |
| Venous thromboembolism5 | 2.33 (1.68–3.24) |
| Stillbirth6 | 2.07 (1.59–2.74) |
| Labour and delivery | |
| Spontaneous preterm birth <37 weeks7 | 0.5 (0.4–0.7) |
| Spontaneous preterm birth <34 weeks7 | 0.4 (0.2-0.8) |
| Spontaneous preterm birth <32 weeks7 | 0.5 (0.2–1.3) |
| Induction of labour8 | 1.88 (1.84–1.92) |
| Use of oxytocin8 | 1.59 (1.36–1.87) |
| Use of epidural8 | 1.23 (1.19–1.27) |
| Failure to progress8 | 2.31 (1.87–2.84) |
| Total Caesarean delivery9 | 2.36 (2.15–2.59) |
| Elective Caesarean delivery9 | 1.87 (1.64–2.12) |
| Emergency Caesarean section9 | 2.23 (2.07–2.42) |
| Instrumental delivery8 | 1.17 (1.13–1.21) |
| Maternal complications | |
| Duration of hospital stay (normal 2.4 days)8 | 2.71 (2.62–2.79) days |
| Maternal haemorrhage8 | 1.24 (1.24–1.28) |
| Maternal infection8 | 3.34 (2.74–4.06) |
| Neonatal complications | |
| Low Apgar score at 5 min8 | 1.57 (1.46–1.68) |
| Fetal compromise8 | 1.62 (1.54–1.70) |
| Meconium8 | 1.57 (1.42–1.73) |
| Shoulder dystocia8 | 1.04 (0.97–1.12) |
| Neonatal intensive care use8 | 1.35 (1.22–1.49) |
GWG and fat deposition
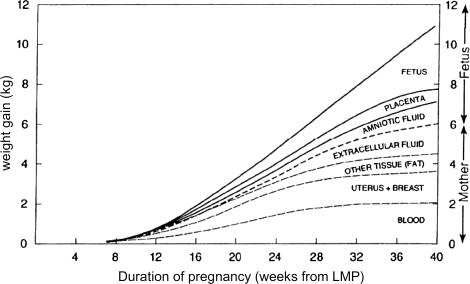
Irrespective of the degree of adiposity prior to conception, significant GWG occurs to support the functions of growth and development of the fetus (Fig. 1). However, the total amount of weight gained in normal-term pregnancies varies considerably among women and is related to the number of fetuses (singleton pregnancy 10–16.7 kg, twin pregnancy 15–22 kg, triplet pregnancy 20.5–23 kg) and inversely to the prepregnancy BMI (Institute of Medicine, 2009).
Figure 1.
Components of GWG. Reproduced with permission from nutritional support in obstetrics and gynaecology (Pitkin, 1976).
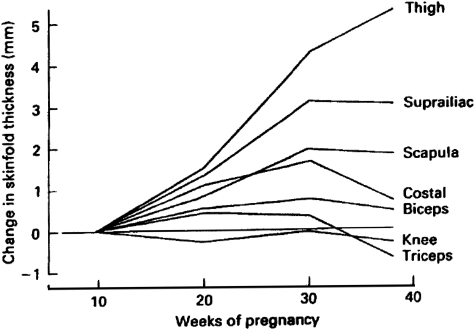
Accretion of maternal fat parallels GWG, with significant energy expended on maternal fat deposition to provide a source of energy for maintaining the fetus, and buffer short or medium-term changes in energy supply. The absolute amount of fat deposited ranges from 1.9 to 5.8 kg, potentially reflecting differences in methodology and populations as well as biological variability (Lawrence et al., 1987; Forsum et al., 1988; Goldberg et al., 1993; Sohlstrom and Forsum, 1995; Highman et al., 1998; Kopp-Hoolihan et al., 1999; Okereke et al., 2004). In obese women, this fat is primarily accumulated between 13 and 35 weeks of gestation (Okereke et al., 2004), consistent with previous skin-fold studies in healthy women (Fig. 2) (Taggart et al., 1967). The total amount of gestational fat deposited may however be reduced in obese pregnancy (Soltani and Fraser, 2000). In a prospective series of 405 women, a one unit increase in prepregnancy BMI was associated with a 0.5 kg reduction in post-partum weight retention (Kac et al., 2004). Assuming that GWG primarily reflects fat deposition, this would suggest that obese women gain less fat. Similarly, the use of a multicompartment model to measure body composition at 14 and 37 weeks of gestation in 200 women demonstrated that mean fat mass gains were 4.8, 3.9 and 2.8 kg associated with weight gains of 12.6, 12.2 and 11.0 kg in underweight, normal-weight and overweight women, respectively (Lederman et al., 1997). However, reduced fat accretion has not been universally found (Butte et al., 2003; Ehrenberg et al., 2003), which potentially reflects differences between populations and severity of obesity.
Figure 2.
Longitudinal changes in skin-fold thicknesses throughout pregnancy. Reproduced with permission from changes in skin-folds during pregnancy (Taggart et al., 1967).
Irrespective of prepregnancy BMI, gestational-related fat is predominantly accumulated centrally, with a preferential selection for central sites in obese pregnant women (Ehrenberg et al., 2003). This probably represents a combination of subcutaneous truncal fat (Sohlstrom and Forsum, 1995; Ehrenberg et al., 2003) and visceral fat (Kinoshita and Itoh, 2006). It is visceral fat which correlates strongly with metabolic risk factors such as blood pressure, insulin sensitivity and plasma lipids (Despres et al., 1990), and this relationship is maintained in pregnancy (Bartha et al., 2007). Excess central fat is also strongly associated with cardiovascular disease and diabetes in adult life (Lapidus et al., 1984; Larsson et al., 1984; Carey et al., 1996; Alberti et al., 2005; de Koning et al., 2007; Vazquez et al., 2007) and in pregnancy with glucose intolerance/gestational diabetes mellitus (GDM) (Zhang et al., 1995; Chu et al., 2007a, b, c; Martin et al., 2009) and gestational hypertension/pre-eclampsia (Sattar et al., 2001). Importantly, although pregnancy influences the site of fat deposition, it does not affect the regional functionality of adipose tissue, explaining the persistence of relationships between central fat and adverse metabolic outcomes (Lindberg et al., 1991). However, as might be anticipated from the increased fat mass, obese pregnant women demonstrate elevated circulating concentrations of leptin and raised levels of inflammatory mediators including IL-6 (Ramsay et al., 2002). Given the well recognized association between an inflammatory state and pre-eclampsia (Redman and Sargent, 2005), these could contribute to the increased risk of this disorder in obese pregnancy. In common with obesity in the non-pregnant state, others have shown that adiponectin is reduced in obese women and this could contribute to insulin resistance (Hendler et al., 2005a, b).
GWG and pregnancy outcome
Although there is a rationale for linking greater GWG with GDM from the association between fat mass and insulin resistance, the evidence, in contrast to the strong association with prepregnancy BMI, is relatively weak (Viswanathan et al., 2008). This most probably suggests a predominant influence of prepregnancy BMI than weight gain per se (Catalano et al., 1993a, b; Nohr et al., 2008) or error induced by the measurement of weight after the inception of treatment. The evidence linking GWG to the hypertensive disorders of pregnancy is also inconclusive because of inconsistent results and methodological flaws (Rasmussen et al., 2009). Similarly although there is a modest association between increased GWG and Caesarean section rates (Rasmussen et al., 2009), the contribution of GWG is modest relative to that of prepregnancy BMI. Furthermore, the relationship between GWG and the risk of large-for-gestational age (LGA) delivery is most pronounced in women with a low BMI, not those with a high BMI.
The most consistent adverse outcome for mothers with large GWG is increased post-partum weight retention which is maintained up to 3 years after the index pregnancy, independent of prepregnancy BMI (Nohr et al., 2008; Rasmussen et al., 2009). This, in turn, is likely to contribute to adverse outcome in the next pregnancy, most convincingly shown in a study of 151 000 Swedish women, in which a gain of 3 kg/m2 or more was associated with increased risk for pre-eclampsia, gestational hypertension, gestational diabetes, Caesarean delivery, stillbirth and LGA birth in the second pregnancy. The associations were linearly related to the gain in weight and were also noted in women who had a healthy prepregnancy BMI for both pregnancies (Villamor and Cnattingius, 2006).
Maternal metabolism
Lipid metabolism
Lipid metabolism undergoes major adjustment during pregnancy as, although there is no change in either basal carbohydrate oxidation or non-oxidizable carbohydrate metabolism, there is a significant 50–80% increase in basal fat oxidation during pregnancy and also in response to glucose (Okereke et al., 2004). There is also marked hyperlipidaemia in pregnancy (Knopp et al., 1973; Warth et al., 1975; Alvarez et al., 1996). Specifically, very low-density lipoprotein (VLDL) triglyceride concentrations increase 3-fold from 14-weeks gestation to term (Fahraeus et al., 1985), with concomitant decreases in the hepatic lipase activity (Alvarez et al., 1996). This increase in the plasma triglyceride concentration may drive the appearance of small dense LDL particles, particularly in late pregnancy (Sattar et al., 1997). Plasma cholesterol levels rise to a lesser degree due to an early decrease in LDL followed by a modest continuous rise in high-density lipoprotein (HDL) (particularly the HDL-2 subfraction) by over 40% after 14-weeks gestation (Fahraeus et al., 1985). HDL cholesterol exhibits a triphasic profile, rising to a peak at 25 weeks, and then declining to 32 weeks with maintenance at this level until term (Desoye et al., 1987). These changes in lipoprotein concentrations are associated with the progressive increases in estradiol, progesterone and human placental lactogen (Desoye et al., 1987), and estrogens are known to enhance VLDL production and decrease the hepatic lipase activity and may play a key role in the accumulation of triglycerides in lipoproteins of higher density than VLDL (Sacks and Walsh, 1994).
In obese pregnant women, this hyperlipidaemia is exaggerated. Total and VLDL triglycerides are increased further and plasma HDL is even lower, whereas, in contrast, LDL is unaltered (Merzouk et al., 1998; Ramsay et al., 2002; Rajasingam et al., 2009). The relative inability of insulin to suppress whole-body lipolysis leads to a marked increase in plasma free fatty acids in obese patients (Sivan et al., 1999), thereby further amplifying the already higher concentrations associated with obesity (Catalano et al., 2002). The increases in fat oxidation are also maintained even in the absence of changes to carbohydrate metabolism, with an inverse correlation between endogenous glucose production and fat oxidation from prepregnancy to early gestation (Okereke et al., 2004). Lastly, the susceptibility of LDL to oxidation, a classic associate of endothelial dysfunction, atherosclerosis and cell toxicity is exacerbated by maternal obesity (Sanchez-Vera et al., 2007). Collectively the pattern of dyslipidaemia observed in obese pregnancy is therefore similar to those observed in non-pregnant obese individuals (Sattar et al., 1998).
This dyslipidaemia may also contribute to obesity-related vascular complications including pre-eclampsia—with maternal hypertriglyceridaemia being a characteristic of women destined to develop pre-eclampsia (Potter and Nestel, 1979; Lorentzen et al., 1994; Sattar et al., 1997; Enquobahrie et al., 2004; Ramsay et al., 2004; Vadachkoria et al., 2006; Rajasingam et al., 2009). The observed changes in triglycerides in pre-eclampsia are accompanied by an almost 3-fold higher VLDL1, a 2-fold increase in VLDL2 concentration (Sattar et al., 1997; Ramsay et al., 2004), marked increases in free fatty acids, and a 3-fold increase in small dense LDL, with a reduction in large buoyant LDL subfractions (Sattar et al., 1997; Hubel et al., 1998; Ramsay et al., 2004; LLurba et al., 2005). It is these small dense LDL particles that are increased in both pre-eclampsia and obesity that are highly atherogenic and capable of promoting foam cell formation and endothelial dysfunction (Griffin et al., 1994), with further impairment of endothelial function by elevated free fatty acids. Collectively this suggests that obese women or women with excessive GWG may have sufficient pre-existing or newly acquired dyslipidaemia to facilitate the acute development of placental bed atherosis and pre-eclampsia. Furthermore, this association, in conjunction with increased inflammation, would provide a potential explanation for the strong epidemiological associations of pre-eclampsia with prepregnancy BMI and excessive GWG in non-obese women (Sebire et al., 2001; Ramsay et al., 2004; Institute of Medicine, 2009).
Amino acid metabolism
In pregnancy, the majority of amino acids are utilized for protein synthesis, with a reduction in the amount oxidized by ∼10% (Duggleby and Jackson, 2002). Although, counter-intuitively there is no increase in measured protein synthesis in the first trimester, there is an increase in the second and third trimester of 15% and 25% respectively (de Benoist et al., 1985; Jackson, 1987; Thompson and Halliday, 1992; Willommet et al., 1992). These changes are greater than can simply be accounted for by highly active protein synthesis in the fetus and placenta, implying an overall increase in protein synthesis in maternal tissues including the liver, breasts and uterus. The impact of maternal protein turnover on the fetus is striking, with a greater maternal protein synthesis in the second trimester being associated with an increase in birth length and accounts for 26% of the overall variance (Duggleby and Jackson, 2001). The proportion of amino acid metabolism, which is directed towards protein synthesis rather than oxidation, can also modify birthweight, with 34% of the variance of birthweight related to this shift (Duggleby and Jackson, 2002).
At present, the impact of obesity on amino acid metabolism is unknown. However, in non-pregnant obese women, protein synthesis is stimulated less in a hyperinsulinaemic state in comparison with lean women, with no difference in protein oxidation (Chevalier et al., 2005). Obesity is also associated with a greater supply of gluconeogenic amino acids to the liver with preference of their use over glycogen for glucose production (Chevalier et al., 2006). Lastly, visceral lean mass is positively correlated with maternal protein turnover (Duggleby and Jackson, 2001). Collectively, these data would suggest that the anabolic response to pregnancy may be impaired in obese women, raising the possibility that mechanisms may exist to limit fetal growth in a hyperinsulinaemic and glucose-rich environment.
Glucose metabolism and insulin resistance
In normal pregnancies, dynamic changes in glucose homeostasis and insulin sensitivity accompany the alterations in lipid and protein metabolism. In early pregnancies, maternal fasting glucose decreases by 2 mg/dl very early in gestation (weeks 6–10), with little further decrease by the third trimester (Mills et al., 1998). Basal hepatic glucose production increases with advancing gestation (16–30%), as does total gluconeogenesis to meet the increasing needs of the placenta and fetus (Kalhan et al., 1979, 1997; Catalano et al., 1992; Assel et al., 1993). Post-prandial glucose concentrations are also significantly elevated and the glucose peak is prolonged (Cousins et al., 1980). These increases in glucose production occur despite significant rises in fasting insulin concentrations (Catalano et al., 1992) and are also relative to maternal body weight, such that glucose production per kilogram body weight does not change throughout pregnancy (Kalhan et al., 1997). Commensurate with the increased rate of glucose production, there is an increased contribution of carbohydrate to oxidative metabolism in late pregnancy, with absolute rates of 282 g/day when compared with 210 g/day (Butte et al., 1999).
Facilitating these alterations in glucose homeostasis is marked changes in insulin secretion and sensitivity. During early pregnancies, glucose tolerance is normal or slightly improved and peripheral (muscle) sensitivity to insulin and hepatic basal glucose production is normal (Catalano et al., 1991, 1992, 1993a, b). This is accompanied by a greater-than-normal sensitivity to the blood glucose-lowering effect of exogenously administered insulin in the first trimester than in the second and third trimesters. Longitudinal studies of glucose tolerance during gestation demonstrate an increased insulin response to oral glucose in the first trimester relative to prepregnancy values (Catalano et al., 1991, 1999), with a subsequent progressive increase in nutrient-stimulated insulin responses despite only a minor deterioration in glucose tolerance, consistent with progressive insulin resistance (Catalano et al., 1991, 1999). Notably, there is also an independent effect of pregnancy on β-cell function independent of the observed changes in insulin; however, the aetiology of this effect is at present unknown, although may include the incretins GIP and GLP-1 (Meier et al., 2005; Cypryk et al., 2007). Overall, the insulin sensitivity of late normal pregnancy is reduced by 50–70% compared with normal, non-pregnant women (Ryan et al., 1985; Catalano et al., 1991, 1992, 1993a, b), with significant increases in basal insulin and the response to glucose with concomitant decreases in insulin clearance (Catalano et al., 1991, 1998a, b; Agardh et al., 1996). Consequently by the third trimester, basal and 24-h mean insulin concentrations may double and the first and second phases of insulin release are 3–3.5-fold greater in late pregnancy (Catalano et al., 1991).
The impact of obesity on these changes is substantial, in particular the decline in fasting glucose in early gestation is reduced, and glucose is not reduced at all in severely obese women (Mills et al., 1998). In late gestation, the normal reduction in peripheral insulin sensitivity of 50% (Catalano et al., 1991) is reduced in obese women as determined by the quantitative insulin sensitivity check index (Endo et al., 2006)—a validated surrogate for the direct measurement of insulin sensitivity using the euglycemic hyperinsulinemic clamp (Kirwan et al., 2001). In addition, there is marked peripheral and hepatic insulin resistance, which manifests as reduced insulin-mediated glucose disposal, a large reduction in insulin-stimulated carbohydrate oxidation and a reduction in insulin suppression of endogenous glucose production, all of which are reversed in the post-partum period (Sivan et al., 1997). Importantly, the overall effects of this impaired insulin resistance are not limited to glucose. In the post-prandial state, this obesity-related insulin resistance exaggerates the normal circulatory increases in metabolic fuels, i.e. glucose, lipids and amino acids. In fact, the fasting, post-prandial and integrated 24 h plasma concentrations of all three macronutrients are affected by enhanced insulin resistance in obese women. Consequently, the impaired glucose uptake exposes the fetus to hyperglycaemia; the inability to suppress whole body lipolysis leads to an increase in free fatty acids available for placental transfer, and the decreased ability of insulin to suppress amino acid turnover causes an elevation in maternal concentrations of branched-chain amino acids, again facilitating transfer of excess nutrients to the fetus. These alternative nutrient pathways may independently contribute to macrosomia, as maternal serum triglycerides and amino acid profiles (serine, threonine, lysine, proline, ornithine and arginine) have been associated with offspring birthweight independent of maternal glucose or prepregnancy BMI (Kalkhoff et al., 1988; Nolan et al., 1995; Di Cianni et al., 2005; Schaefer-Graf et al., 2008).
Although the precise mechanisms regulating insulin sensitivity are uncertain, it would appear that preconceptual fat mass is a major determinant. Lean women exhibit an inverse correlation between changes in insulin sensitivity and fat mass, which is not seen in obese women (Catalano et al., 1998a, b; Okereke et al., 2004). Obese women do however exhibit a negative relationship between the decrease in insulin sensitivity and accretion of fat mass from prepregnancy to late gestation (Okereke et al., 2004). Additionally, although changes in insulin sensitivity related to later pregnancy are primarily mediated at the peripheral level and secondarily at the hepatic level, elevated levels of non-esterified free fatty acids in later pregnancy may also contribute to peripheral and hepatic insulin resistance (Sivan et al., 1998; Homko et al., 2003), with adipose-derived estrogen facilitating further increases in lipids. The peripheral resistance may be mediated by reduced adipose tissue insulin receptor substrate-1 protein levels, which are 43% lower in obese women with gestational diabetes (Catalano et al., 2002). Circulating concentrations of peroxisome proliferator-activated receptor-γ (PPARγ) mRNA and protein are also lower than normal (Catalano et al., 2002), and given that PPARγ acts as an important regulator of adipose lipid storage and as a regulator of insulin sensitivity, this may further reduce the insulin suppression of lipolysis in obese pregnancy.
Longer-term consequences for the child of maternal obesity and altered metabolic state
These profound disturbances in maternal metabolism associated with obesity have obvious and immediate consequences for the growth of the developing fetus, but it is also now widely appreciated that alteration in the maternal nutritional environment may have persistent and effects for the developing child and health in adulthood. The high prevalence of maternal obesity and the emergence of obesity among even very young children have led to the suggestion that the risk of obesity in children could be acquired as a direct consequence of shared ‘obesogenic’ environment between mother and child in utero or in early post-natal life. Consistent with this is the large number of studies demonstrating a positive association of birthweight with BMI during both childhood and adulthood and the risk of overweight/obesity in later life (Fisch et al., 1975; Kramer et al., 1985; Binkin et al., 1988; Seidman et al., 1991; Rasmussen et al., 1998a, b; Hediger et al., 2009; Pietiläinen et al., 2001; Reilly et al., 2005).
Childhood obesity: the size of the problem
As maternal obesity rates have increased, so has the incidence of childhood obesity (Ogden et al., 2006). In England, 9.6% of boys aged 2–10 were obese in 1995, and 10.3% of girls; by 2004, this had risen to 15.9% and 12.8%, respectively. Very young children are also showing increasing rates of obesity, even in the first 6 months of life (Kim et al., 2006). The question arises as to whether this may represent a persistent influence of maternal obesity in utero. Certainly, increases in LGA delivery rates seem to parallel trends in maternal obesity (Surkan et al., 2004). However, although obesity and related maternal metabolic disorders are undoubtedly a cause of macrosomia (Sebire et al., 2001; Ehrenberg et al., 2004), higher birthweight and birthweight centiles can only act as surrogates for increased infant adiposity due to the potential of variable body composition for any given birthweight (McFarland et al., 1998). Fortunately, recent studies have explored the direct relationship between maternal body composition and offspring adiposity and suggest that offspring body fat is associated with maternal fat, but not with paternal fat mass (Shields et al., 2006). Neonatal fat mass is also related to the maternal glucose concentration (Shields et al., 2006). Consistent with the prepregnancy BMI data, the same group has subsequently found that maternal fat stores are independently associated with percentage fat mass in the neonate (Harvey et al., 2007). In addition, maternal triceps skin-fold thickness (a measure of maternal adiposity) showed a negative association with the percentage of neonatal lean body mass. Collectively, these data support a relationship between maternal and offspring obesity.
Maternal metabolism and neonatal adiposity
The recent Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study has highlighted the quintessentially important influence of maternal metabolic balance on offspring birthweight, demonstrating a linear relationship between maternal fasting plasma glucose, and oral glucose tolerance test (OGTT) 1- and 2-h glucose with birthweight above the 90th percentile (Metzger et al., 2008). Importantly, infant adiposity as directly measured by the sum of skin-fold thickness, exhibited a similar strong linear relationship with maternal glucose; moreover there was a strong relationship between cord blood C peptide (a surrogate marker for insulin) and neonatal adiposity, providing some of the most comprehensive evidence yet for the Pedersen hypothesis (Pedersen, 1952; HAPO Study Cooperative Research Group, 2009).
Prior to this, Catalano and colleagues had carried out direct measurement of fat mass and found that infants of women with GDM, even with a normal birthweight, had increased body fat compared with offspring of women with normal glucose tolerance (Hashimoto et al., 2002). Subsequent analysis of neonates of overweight and obese mothers has consistently demonstrated increased percentage of body fat and fat mass compared with offspring of normal weight mothers (Sewell et al., 2006; Hull et al., 2008). Maternal weight gain has also been associated with increased fetal fat free mass and fat mass (Catalano and Ehrenberg, 2006). A fatter maternal phenotype in early pregnancy and subsequently at birth for the offspring may contribute to a predetermined insulin resistant state, as fetal insulin resistance assessed by the homeostasis model of insulin resistance using cord blood demonstrated a strong positive correlation with fetal adiposity and was also strongly associated with pregravid BMI, even with adjustment for potential confounders (Catalano et al., 2009). From these studies, it is reasonable to conclude that obese mothers have not only heavier but fatter babies, but is there evidence to suggest that this association is maintained to childhood and beyond?
Maternal metabolism and obesity; relationships with long-term offspring adiposity
Higher birthweight is undoubtedly associated with a higher BMI in both boys and girls in childhood and in adulthood (Binkin et al., 1988; Curhan et al., 1996a, b; Sorensen et al., 1997; Rasmussen et al., 1998a, b; Parsons et al., 2001; Oken and Gillman, 2003; Ong, 2006).
Most observational studies in mother/child cohorts also provide evidence of an association between maternal obesity and a higher BMI in childhood and adulthood. In 8494 children from low-income families in OH, USA, the risk of obesity at 2–4 years of age was more than doubled by maternal obesity (Whitaker, 2004), and in 2626 children in the National Longitudinal Study of Youth (NLSY) aged 2–14, the risk of childhood obesity was doubled if the mother was overweight and quadrupled if the mother was obese prepregnancy (Li et al., 2005). A later report from the same cohort showed an increased risk of both early and late onset obesity in the child (Li et al., 2007). Lastly, in 3022 USA children (1982–1996), maternal prepregnancy obesity was identified as an independent factor for increased risk of early overweight and also the development of overweight with age (Salsberry and Reagan, 2005). The same relationship would appear to pertain outside the USA. In the northern Finland, birth cohort offspring of overweight or obese mothers had a higher mean BMI at birth, 1, 14 and 31 years (Laitinen et al., 2001). The UK ALSPAC cohort of children born in the 1990s has shown an association between maternal obesity prepregnancy and childhood obesity at age 7 (Reilly et al., 2005), and Swedish conscripts aged 18 years have a higher risk of obesity if their mothers were obese (Koupil and Toivanen, 2008).
These studies have largely defined childhood obesity based on a BMI at or above the 95th centile; however, in children, BMI shows a large variation with age, as does body composition (Cole et al., 2000; World Health Organization, 2000) and is usually calculated using CDC (USA) or international growth charts (Cole et al., 2000; National Center for Health Statistics, 2009). Notably, direct measurement of skin-fold thickness has suggested that these methods can fail to detect as many as 40–50% of children identified as obese (Zimmermann et al., 2004). Direct measurement of childhood adiposity has however confirmed the earlier studies measuring childhood BMI, with 3-year-old children who had been exposed to gestational diabetes having higher skin-fold thickness compared with those who had not (Wright et al., 2009). Similarly, when measurements of maternal and offspring adiposity have been determined in normal pregnancies, these have demonstrated positive relationships between maternal mid-upper arm circumference (an estimate of adiposity) and offspring body composition (Gale et al., 2007). With respect to maternal BMI, these relationships with fat mass in the child are still evident, with maternal obesity associated with increased adiposity in the 5-year-old children (Burdette et al., 2006). Consistent with this maternal overweight/obesity was an independent determinant of fat mass at the age of 7 as assessed by bioelectric impedance analysis (Blair et al., 2007) and at age 24 as assessed by dual-energy X-ray absorptiometry (Mingrone et al., 2008).
GWG and offspring adiposity
In view of the relationships between GWG and adverse maternal and neonatal outcome, the impact of GWG on childhood BMI or adiposity has also been examined. Some studies, but not all, indicate that weight gain may be a determinant of offspring obesity but the strength of the effect is generally less than that of maternal obesity per se. Specifically, Oken et al. (2007) have shown that increasing maternal weight gain was associated with increased adiposity at age 3 and at age 9–14 (Oken, 2008). Another large cohort study from the US Collaborative Perinatal Project has indicated a similar degree of association (Wrotniak et al., 2008). Maternal weight gain and infant BMI relationships have been reported in studies of Portuguese and German children (Moreira et al., 2007; Kleiser et al., 2009), although in the latter, a significant effect was only found if the mother was of normal weight at the start of pregnancy. Similar results have recently been reported from an Australian cohort, with a relationship between maternal weight gain and offspring BMI still detectable in young adulthood (21 years old) (Mamun et al., 2009). However, as previously observed, there was some evidence that the association was stronger among underweight/normal weight mothers (Mamun et al., 2009). This is not a universal finding as others have reported no relationship between maternal weight gain and offspring BMI (Catalano et al., 1995; Koupil and Toivanen, 2008).
Mechanisms linking parental and offspring adiposity
At present, given the lack of intervention studies in obese pregnant women, it is impossible to determine whether the relationships between maternal obesity or weight gain and offspring obesity are a facet of direct influences on the developing child by maternal obesity, or whether shared genetic and post-natal lifestyle influences predominate. There is good evidence for co-segregation of obesity in families, and shared dietary and physical activity behaviours (Laskarzewski et al., 1980; Simonen et al., 2002). Some of the earliest evidence to suggest a direct association between fetal and neonatal ‘overnutritional’ status and development of later obesity was derived from offspring of diabetic mothers, notably from the Pima Indians who have a very high incidence of type 2 diabetes (Pettitt et al., 1983, 1987, 1991, 1993; Dabelea et al., 2000a, b). Importantly, the analysis of siblings discordant for in utero exposure to maternal diabetes, thereby mitigating against a role for genetic factors, demonstrated that in utero exposure to maternal diabetes was associated with a mean increase in BMI of 2.6 kg/m2 (Dabelea et al., 2000a, b). Others have found a similar relationship between maternal diabetes, either pregestational type 1 and type 2 or GDM and increased childhood BMI (Silverman et al., 1998; Cho et al., 2000; Schaefer-Graf et al., 2005; Malcolm et al., 2006). Notably, the treatment of GDM has been associated with a reduction in the risk of offspring obesity, lending support to the suggestion of a direct influence of the in utero environment on later development of obesity (Hillier et al., 2007). More recently, maternal insulin resistance independent of maternal glucose tolerance status has been associated with increased infant weight gain and adiposity over the first year of life, suggesting that insulin regulation of other nutrients, e.g. lipids and amino acids, may also play a role in utero (Hamilton et al., 2009).
Many of the studies mentioned above have attempted to control for shared behaviour and potential confounders, and most describe little modification effect, but none can be complete. Accurate estimation of many of these, including diet and physical activity in children, is difficult. Duration of breast feeding is a potential cofounder (Koletzko et al., 2009), and obese women are less likely to initiate breast feeding or may breast feed for a shorter time than leaner women (Oddy et al., 2006). Rapid growth in the immediate post-natal period is another potential confounder as this is also associated with childhood obesity or increased adiposity (Monteiro and Victora, 2005; Taveras et al., 2009).
Some, but not all, reports that have addressed relationships between BMI of both parents at the time of the index pregnancy and offspring obesity have generally shown a greater effect of maternal than paternal BMI on offspring BMI (Catalano et al., 2009; Lawlor et al., 2007; Reilly et al., 2005; Salsberry and Reagan, 2005; Moschonis et al., 2008) and this could infer an acquired, as opposed to an inherited, relationship. However, one study suggests this to be explicable by inheritance of the variant of the FTO gene associated with obesity (Lawlor et al., 2008). Recently, Li et al. (2009) have shown that early life, as well as later life, weight gain in parents is associated with offspring obesity. Associations with parental early life weight gain were interpreted as being more indicative of a shared genetic component, or establishment of lifestyle pattern, but could equally reflect influences on maternal or paternal germ cell development that could influence later predisposition to obesity. The association with later life weight gain in parents is consistent with an in utero influence of maternal obesity on the offspring. Furthermore, and in line with studies of discordant sibling exposure to maternal diabetes, a report of siblings discordant for maternal obesity exposure due to a bariatric surgery demonstrated that offspring born after surgery were less obese (Kral et al., 2006). However, the family ‘nutritional’ environment may have changed between pregnancies and it is difficult to draw firm conclusions.
Intervention strategies
The escalation of obesity among women of reproductive age and the complications both short and long term for the mother and child have provided the stimulus for rapid development of an intervention to improve outcomes. To date, none has been validated for clinical use. Undoubtedly, the most successful intervention will be that which prevents the development of obesity before the reproductive years. However, the high rates of obesity among adolescent girls and the upward trends of obesity among pregnant women suggest that this is not immediately attainable. As reviewed above, we have a good understanding of the changes in metabolism accompanying obesity in pregnancy and central to these is the development of insulin resistance and its metabolic sequelae. Since there are strong similarities between the risk profile for type 2 diabetes and the hyperglycaemia, hyperinsulinaemia and dyslipidaemia that characterize maternal obesity, the extensive literature addressing interventions in type 2 diabetes and gestational diabetes may provide guidance.
Lifestyle modification as a preventative strategy for type 2 diabetes mellitus
Lifestyle intervention is now a critical component of the treatment strategy for diabetes, hypertension, cardiovascular disease and obesity in non-pregnant patients (Authors/Task Force Members, 2007; Graham et al., 2007). Importantly, effective lifestyle intervention strategies can prevent or at least delay the progression to type 2 diabetes in high-risk individuals (summarized in Table II) (Eriksson and Lindgarde, 1991; Pan et al., 1997; Diabetes Prevention Program Research, 2002; Lindstrom et al., 2003; Ramachandran et al., 2006). Notably, the Finnish Diabetes Prevention Study, in addition to the 58% reduction in the incidence of diabetes incidence, also achieved a significant reduction in weight, BMI, waist circumference, fasting plasma glucose, 2 h plasma glucose, serum triglycerides and serum total cholesterol:HDL cholesterol ratio in the intervention group within a year (Lindstrom et al., 2003). In the US Diabetes Prevention Program, attainment of weight loss was achieved by 50% of participants at 24 weeks and 74% had achieved the physical activity targets; importantly this was also accompanied by significant reductions in plasma glucose (Diabetes Prevention Program Research, 2002). These studies raise the exciting possibility that lifestyle modification in a similar form could be applied to pregnancy to prevent the onset of metabolic- and obesity-related complications.
Table II.
Summary of the four lifestyle intervention studies that aimed at preventing type 2 diabetes in non-pregnant subjects with impaired glucose tolerance
| Study | Cohort size | Intervention | Mean BMI (kg/m2) | Duration (years) | RRR (%) | ARR (%) | NNT |
|---|---|---|---|---|---|---|---|
| Malmö | 217 | Dietary and/or increased physical activity or training | 26.6 | 5 | 63 | 18 | 28 |
| DPS | 523 | Aim for ≥5% reduction in bodyweight, respectively, through diet and physical activity | 31 | 3 | 58 | 12 | 22 |
| Diet: a reduction in dietary fat to <30 proportion of total energy (E%) and saturated fat to <10%E, while increasing fibre to ≥15 g/1000 kcal. Achieved by face-to-face consultation sessions (from 30 min to 1 h) with the study nutritionist at weeks 0, 1–2 and 5–6 and at months 3, 4, 6 and 9, i.e. altogether seven sessions during the first year and every 3 months thereafter | |||||||
| Physical activity: aim of moderate physical activity of ≥30 min/day achieved through progressive, individually tailored circuit type moderate intensity resistance training sessions, exercise competitions, voluntary group walking and hiking | |||||||
| DPP | 2161a | Aim for ≥7% reduction in bodyweight, respectively, through diet and physical activity | 34 | 3 | 58 | 15 | 21 |
| Diet: a healthy low-calorie, low-fat diet | |||||||
| Physical activity: moderate intensity, such as brisk walking, for at least 150 min/week | |||||||
| Da Qing | 500 | Exercise, diet or exercise + diet | 25.8 | 6 | 46 | 27 | 25 |
| IDDP-1 | 531 | Physical activity target of moderate physical activity of >30 min/day and dietary advice including reduction in total calories, refined carbohydrates and fats, avoidance of sugar and inclusion of fibre-rich foods | 25.7 | 3 | 28 | 15 | 19 |
RRR, relative risk reduction; ARR, absolute risk reduction/1000 person-years; NNT, numbers needed to treat to prevent one case of diabetes over 12 months; DPS, The Finnish Diabetes Prevention Study; DPP, Diabetes Prevention Program; IDDP-1, Indian Diabetes Prevention Programme.
aCombined numbers for placebo and diet and exercise groups.
Safety of exercise in pregnancy
In theory, there are potential risks to the fetus during maternal exercise, including stimulation of uterine contractility (Spinnewijn et al., 1996), decreased uteroplacental flow due to preferential shunting to skeletal muscles (Kennelly et al., 2002), potentially fetal hypoglycaemia secondary to increased glucose used by skeletal muscles and reductions in circulating maternal glucose (Bonen et al., 1992) and hyperthermia from exercise. However, in reality, physical activity has not been associated with adverse obstetric complications beyond 18 weeks gestation (Madsen et al., 2007), and with respect to preterm birth may even be protective (Berkowitz et al., 1983; Hatch et al., 1998; Misra et al., 1998; Evenson et al., 2002). Furthermore, maternal hypoglycaemia, even in type 1 diabetes, has not been consistently associated with adverse neurodevelopment in the offspring, despite maternal plasma β-hydroxyburate, an index of ketosis and potentially the necessity for alternative fuel use, being negatively associated with psychomotor and intellectual development (Rizzo et al., 1991, 1995).
Given that physical activity may impact on insulin resistance, thereby reducing circulating glucose levels and decreasing the amount of glucose available for the fetus—there is the potential for an impact on fetal adiposity and birthweight. To date, three different meta-analyses have demonstrated that leisure time physical activity (LTPA) does not influence birthweight (Lokey et al., 1991; Leet and Flick, 2003; Kramer and McDonald, 2006). However, vigorous endurance exercise during the third trimester was potentially associated with a 200–500 g lower birthweight (Clapp and Dickstein, 1984; Bell et al., 1995). Should birthweight be similarly reduced across the whole range? This would be detrimental as the incidence of small-for-gestational age babies would be increased. However, if this reduction in birthweight was restricted to women who are at risk of delivering LGA infants, this would be beneficial and may reduce birth complications and the need for operative delivery. In support of this, moderate or vigorous physical activity for 2 h/week was associated with a reduced risk of delivering a LGA infant [OR 0.3 (95%CI 0.2–0.7)] but was not accompanied by an increase in small-for-gestational age infants (Alderman et al., 1998). At present, however, it is not known whether this iatrogenic growth restriction is associated with any of the classical adverse perinatal and long-term outcomes as those observed for the low birthweight growth restricted infants. Follow-up of the children in the intervention studies where birthweight has been successfully reduced will further inform this, but at present modification of birthweight has been associated with improvement in significant perinatal and neonatal metabolic sequelae (Crowther et al., 2005; Langer et al., 2005; Landon et al., 2009).
Lifestyle modification as a strategy for improving glycaemic control in GDM
Given the positive impact of lifestyle modification on type 2 diabetes and potentially birthweight, a similar approach has been applied to GDM. Initial management of affected women now consists of glucose monitoring and lifestyle modification including dietary counselling and a diet that restricts carbohydrates to 35–40% of daily calories (American Diabetes Association, 2003; Reece et al., 2009). This is based on data demonstrating that carbohydrate restriction decreases maternal glucose concentrations and improves maternal and fetal outcomes (Major et al., 1998). Furthermore, in obese women with diabetes, a 30–33% calorie restriction (to ∼25 kcal/kg actual weight per day) reduced hyperglycaemia and plasma triglycerides with no increase in ketonuria (Franz et al., 1994). However, despite diet being a core component of management, it is now recognized that dietary advice alone is insufficient for many GDM patients (Moses et al., 2009), and outcomes are improved if it is combined with pharmacological therapy including metformin (Tuffnell et al., 2003; Crowther et al., 2005; Rowan et al., 2008). Although metformin may seem an attractive therapeutic option in obese pregnancy, in one small RCT of 40 women with polycystic ovarian syndrome, it did not reduce the incidence of GDM (Fougner et al., 2008). Furthermore, metformin is not as effective as lifestyle intervention in preventing type 2 diabetes in women with a history of gestational diabetes (Ratner et al., 2008).
Regular physical activity has repeatedly been shown to improve glycaemic control in women with GDM (Avery and Walker, 2001; Brankston et al., 2004; Garcia-Patterson et al., 2001; Jovanovic-Peterson et al., 1989). Primarily due to the physiological pregnancy-related increases in insulin resistance being reduced by moderate intensity daily physical activity (Clapp and Capeless, 1991, Clapp et al., 1992). However, most of these trials studied the effects of a short-term exercise program (i.e. a single bout or only several weeks). Studies on longer-lasting exercise programs, especially those continuing into the third trimester of pregnancy, are currently lacking, and most studies have concerned the treatment, and not the prevention of GDM. However, given the positive impact on type 2 diabetes and GDM, consideration of prophylactic lifestyle modification would be appropriate.
Lifestyle modification as a strategy for preventing GDM
Epidemiological studies have suggested that physical activity prior to and during pregnancy may significantly reduce the risk of gestational diabetes (Dempsey et al., 2004a, b). Not surprisingly, the highest reduction is seen in women engaged in LTPA during both time periods (RR 0.31, 95% CI: 0.12–0.79) (Dempsey et al., 2004a, b). To date, however, there is a paucity of trial data examining the role of lifestyle modification for the prevention of gestational diabetes (Weissgerber et al., 2006). A pilot study examining controlled energy intake during pregnancy (8350 kJ/day, 200 g/day carbohydrate) combined with exercise at 30% VO2 peak demonstrated that this combination was better than mild exercise alone at controlling blood glucose concentrations, as indicated by a fasting OGTT in late pregnancy (Weissgerber et al., 2006). However, subsequent application of this combined exercise and lifestyle program was only to a small cohort of women at risk of GDM (n = 23), and although GDM was prevented, the small numbers prevent any firm conclusion (Weissgerber et al., 2006). Larger, purely physical activity based, studies are currently being undertaken; however, they are still modest in size (Oostdam et al., 2009a, b) and appear limited relative to the non-pregnant diabetes prevention studies, which aimed for a combination of modification of diet, exercise and also attainment of weight loss.
With respect to diet, the aim is to reduce post-prandial glucose levels and thereby fetal growth (Dornhorst and Frost, 2002). Low glycaemic index diets are the most frequently used instrument to achieve this, and reduce post-prandial glucose peaks as well as fasting glucose levels (Thomas and Elliott, 2009). Unfortunately, assessment of a role in the prevention of insulin resistance in pregnancy is limited. Moses et al. (2006) demonstrated that a low GI (glycaemia index) diet (n = 32) compared with a high GI diet (n = 30) was associated with a reduction in fasting glucose and a reduction in birthweight and incidence of LGA. Fraser established that a high-fibre diet (n = 13) when compared with a normal pregnancy diet (n = 12) significantly attenuated post-prandial insulin secretion (Fraser et al., 1983). Clapp combined these principles with an exercise program and demonstrated in a randomized controlled trial of 20 women that the combination of a low glycaemic diet and exercise significantly reduced birthweight, ponderal index and maternal fasting blood glucose (Clapp, 2002).
Together, these lifestyle modification trials are still too small to provide definitive conclusions (Tieu et al., 2008), but suggest that diets which are characterized by low GI and high-fibre content combined with exercise would be appropriate for obesity (Thomas et al., 2007).
Interventions for maternal obesity
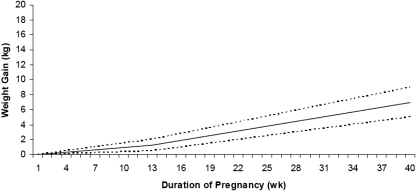
Among obese pregnant women, the primary outcome of any intervention study requires careful choice. The majority of those underway or the preliminary studies already published focus on prevention of excessive weight gain as defined by the Institute of Medicine (IOM). Observational studies undoubtedly show that women whose GWG falls within the 1990 IOM guidelines experience a better pregnancy outcome, and the new IOM 2009 guidelines provide new and more evidence-based targets for each category and, for the first time, a specific weight gain range for obese women (5–9 kg) (Rasmussen et al., 2009) (Fig. 3, Table III). However, as described above, excessive GWG is only weakly associated with several of the primary abnormalities linked to obesity including GDM and pre-eclampsia. Despite this, if observational studies are translatable to effects of intervention, prevention of excessive GWG among pregnant women could reduce the risk of LGA, Caesarean section, post-partum weight gain and potentially, childhood obesity.
Figure 3.
Recommended weight gain by week of pregnancy for obese (BMI ≥30 kg/m2) women (dashed lines represent the range of weight gain) (Institute of Medicine, 2009).
Table III.
New recommendations for total and rate of weight gain during pregnancy by prepregnancy BMI
| Prepregnancy BMI | Total weight gain |
Rates of weight gain second and third trimester |
||
|---|---|---|---|---|
| Range (kg) | Range (lbs) | Mean (range; kg/week) | Mean (range; lbs/week) | |
| Underweight (<18.5 kg/m2) | 12.5–18 | 28–40 | 0.51 (0.44–0.58) | 1 (1–1.3) |
| Normal weight (18.5–24.9 kg/m2) | 11.5–16 | 25–35 | 0.42 (0.35–0.50) | 1 (0.8–1) |
| Overweight (25.0–29.8 kg/m2) | 7–11.5 | 15–25 | 0.28 (0.23–0.33) | 0.6 (0.5–0.7) |
| Obese (≥30 kg/m2) | 5–9 | 11–20 | 0.22 (0.17–0.27) | 0.5 (0.4–0.6) |
Calculations assume a 0.5–2 kg (1.1–4.4 lbs) weight gain in the first trimester (based on Siega-Riz et al., 1994; Abrams et al., 1995; Carmichael et al., 1997).
In contrast, in view of the close association between obesity and insulin resistance as outlined above, and the proposed role that this plays in GDM, pre-eclampsia and macrosomia, maternal insulin resistance may be an alternative primary outcome in intervention studies. Dietary and exercise regimes targeted at gestational diabetes have shown some promise, and although relatively few, previous interventional strategies in GDM do provide a template for trials in obesity. Notably in practical terms, the lifestyle interventions that are offered to obese women to limit weight gain and to prevent insulin resistance, namely increased physical activity and dietary advice and individual counselling, may differ only slightly.
As described above, physical activity is a modifiable factor which reduces insulin resistance and is likely to reduce GWG. In a recent study from the Project Viva cohort, midpregnancy walking (OR 0.92; 95% CI 0.83–1.01, per 30 min/day) and vigorous physical activity (OR 0.76; 95% CI 0.60–0.97, per 30 min/day) were inversely associated with excessive GWG (Stuebe et al., 2009). Dietary advice, whether it is to reduce insulin resistance or calories, involves avoidance of simple sugars and saturated fats with adherence to a ‘healthy diet’, but the emphasis will slightly differ with each approach. Dietary energy density is also a modifiable factor which may assist pregnant women to manage weight gain, but this remains to be proven in adequately powered trials (Kramer and Kakuma, 2003; Olafsdottir et al., 2006; Deierlein et al., 2008; Stuebe et al., 2009). Importantly, although dietary glycaemic load has been found not to be associated with GWG (Deierlein et al., 2008), one study suggested that low glycaemic index diet does reduce GWG (Clapp, 2002). Lastly, focusing on prevention of weight gain, if misinterpreted by the pregnant women, has the disadvantage that it may increase the risk of inappropriate fasting or excessive caloric restriction leading to excessive ketonuria and ketonemia, and the potential to adversely impact upon neurocognitive and motor skill development of the offspring (Stehbens et al., 1977; Rizzo et al., 1991; Rizzo et al., 1995).
Perhaps the greatest challenge for obese women, however, is to achieve these behavioural changes. Understanding barriers to behavioural change using validated instruments in obese pregnant women is a prerequisite in the development of a successful intervention. Obese women are likely to have low self-esteem, and as prepregnancy weight increases, so do psychosocial measures of perceived stress, trait anxiety and depressive symptoms (Laraia et al., 2009). Furthermore, women who gain excess weight in pregnancy relative to the IOM 1990 guidelines are more likely to demonstrate symptoms of depression (Webb et al., 2009). The barriers to physical activity are also substantial, with 85% of women identifying lack of time, tiredness and the inherent physical constraints of pregnancy (Evenson et al., 2008). Socioeconomic factors are also important as pregnant women who are younger, less educated, with a higher BMI, and who have more children are more likely to eat a poor quality and energy dense diet (Rifas-Shiman et al., 2009). Pilot trials of complex interventions are an essential preliminary (Craig et al., 2008), in which psychosocial assessment is an integral part, as well as the objective measurement of dietary and physical activity before and after the intervention, in order to prove efficacy of the intervention prior to embarking on large randomized controlled trials. These feasibility studies also offer insight into the practical issues which underpin success or failure (Kinnunen et al., 2008).
The few published exploratory trials of relevant complex interventions in pregnant women are summarized in Table IV, together with ongoing studies of which we are aware. Of those completed, most have included advice on physical activity and diet, with individual counselling, and all have focused on the promotion of healthy weight gain. As all were exploratory studies, none was adequately powered to address relevant health outcomes or birthweight. When specified, control groups have generally been subjected to routine care. Not all have undertaken objective assessment of diet or physical activity, and none has reported assessment of barriers to behavioural change or changes in quality of life. The two studies to have objectively measured physical activity have shown no effect (Gray-Donald et al., 2000; Polley et al., 2002), and of the four which have assessed diet, one showed a reduction only in caffeine intake (Gray-Donald et al., 2000) and two a change towards the recommended dietary strategy (Kinnunen et al., 2008; Wolff et al., 2008). Effects on GWG have been variable, with three showing a significant reduction (Claesson et al., 2008; Wolff et al., 2008; Asbee et al., 2009), one a reduction among women of normal weight (Polley et al., 2002) and another being effective amongst low-income women only (Polley et al., 2002). Importantly, one report showed a significant improvement in plasma insulin and leptin in the intervention group (Wolff et al., 2008). The intensity of the intervention has also been variable from one study using weekly 30 min interviews and aqua aerobic classes once or twice a week (Claesson et al., 2008) and in another ten 1 h consultations with a dietician (Wolff et al., 2008), but do suggest that more intense and frequent interventions are more likely to be successful. However, interventions that are effective in one population may be inappropriate in another. Variations in healthcare practice, population demographics and access to opportunities for physical activity are all likely to influence success, and in the longer term, there will be need for evidence of health economic benefit for adoption by healthcare providers.
Table IV.
Summary of completed and ongoing lifestyle intervention studies in relation to weight gain and/or obesity
| Authors | Design | Population and sample | Intervention | Outcome |
|---|---|---|---|---|
| Completed studies | ||||
| Gray-Donald et al. (2000) | Prospective intervention study | 219 aboriginal Cree women in Quebec, Canada | Intervention: several components, e.g. exercise groups and individual counselling | GWG: no effect |
| Control: not specified | Diet: caffeine intake decreased only | |||
| PA1: no effect | ||||
| Polley et al. (2002) | Randomized controlled trial | Low-income women (USA) 61 normal BMI, 49 overweight. Control: standard care | Stepped care intervention; healthy eating and exercise advice. Newsletters on diet, physical activity biweekly, telephone contact between visits | Intervention effective in preventing excessive weight gain (IOM, 1990) only in normal weight women. No effect on diet or physical activity |
| Olson et al. (2004) | Prospective cohort with historical control group | 421 normal BMI, 139 overweight rural, primarily white women (USA). Control; historical, care not specified | Healthcare provider monitoring of weight gain; newsletters by mail with return postcards for goal setting; booklet for self-monitoring of weight gain. No visits for dietary of physical activity counselling | Significant effect in preventing excessive weight gain (IOM, 1990) in low-income women only. Diet and physical activity not assessed |
| Kinnunen et al. (2007) | Selected intervention (n = 3) and control (n = 3) maternity clinics | 105 (all BMI) primiparous women, control, standard care | Individual counselling on diet and physical activity. Control: standard care. Visits: physical activity-one primary, four boosters; diet-one primary and three boosters | No effect on excessive weight gain (IOM, 1990). Diet; significantly improved fruit and vegetable intake. No effect on physical activity. Intervention group achieved better moderate or physical activity in 3rd trimester; 46% intervention v 30% in control |
| Claesson et al. (2008) | Prospective interventional study (intervention and comparison cities) | 348 obese (BMI > 30) Swedish women. Control; standard care | Motivational sessions with midwife (individual weekly 30 min). Aqua aerobics twice a week | Significant effect on weight gain <7 kg. Diet and PA not assessed |
| Wolff et al. (2008) | Randomized controlled trial | 50 obese (BMI > 30) non-diabetic, non-smoking Danish women. Control, routine antenatal care | 10 1 h consultations with dietician to achieve energy reduction according to Danish micronutrient guidelines. Weighed food records | Significant effect on weight gain (6.6 versus 13.3 kg in control). Diet; significant reduction in energy and % of energy as fat. Carbohydrate and protein as %energy increased. Physical activity not assessed |
| Asbee et al. (2009) | Randomized controlled trial | 100 (BMI 25.5) USA women | Initial consultation with dietician. Advised to exercise 3–5 times/week. Information on IOM GWG guidelines. Weighing and advice by healthcare provider at subsequent routine appointments | Effective reduction in weight gain (mean). Routine care group significantly more Caesarean sections. Women with higher BMI less likely to adhere to IOM guidelines. Diet not assessed, physical activity not assessed |
| Guelinckx et al., submitted for publication | Randomized controlled trial | 195 (BMI > 29 kg/m2) non-diabetic Belgian women. Control group routine antenatal care, passive group given detailed information leaflet and an active group | Three group sessions at 15, 20 and 32 weeks with dietician focusing on healthy eating habits, importance of physical activity and strategies to control eating behaviour | No reduction in GWG in passive or active arm. No impact on birthweight, macrosomia, hypertensive disorders of pregnancy or Caesarean section |
| Thornton et al. (2009 | Randomized controlled trial | 257 non-diabetic obese USA women. Control unmonitored routine antenatal care with prenatal dietary management | A balanced nutritional regimen, with women asked to record in a diary all of the foods eaten during each day | Significant reduction in GWG and post-partum weight. No impact on gestational hypertension |
| Ongoing studies | ||||
| Althuizen et al. (New Life study) | Randomized controlled trial | Healthy nulliparous women (7 months pregnant) n = 300 (the Netherlands) | Tailored advice on physical activity and diet | GWG in relation to IOM guidelines BMI and skin-fold thickness |
| Brand-Miller (the CHOPP study) | Randomized controlled trial | Pregnant women (n = 1650, Sydney, Australia) | Low glycemic index diet from 12 to 16 weeks until delivery | LGA delivery; childhood obesity |
| Chasan-Taber et al. (the B.A.B.Y. study) | Randomized controlled trial | Pregnant sedentary women with GDM in a prior pregnancy (n = 364, Western Massachusetts, USA) | Tailored advice on physical activity | Incidence of gestational diabetes, physical activity levels and circulating concentrations of glucose, insulin, leptin, TNF-α, resistin, CRP, adiponectin |
| Dodd et al. (the LIMIT trial) | Randomized controlled trial | Overweight and obese pregnant women (n = 2500, Australia) | Dietary package and lifestyle advice | GWG |
| Hauner | Randomized controlled trial | Pregnant and lactating women (n = 210, Munich, Germany) | n-3 fatty acids from 15 weeks gestation until 4 months post-partum | Body mass of newborn with follow-up until age 5 |
| Ko et al. | Randomized uncontrolled | Pregnant women receiving prenatal care (WA, USA) | Vigorous physical activity | Central adiposity 6–8 weeks post-partum |
| Krummel et al. | Randomized controlled trial | Obese pregnant women (Cincinnati USA) | Dietary docosahexanoic acid (DHA) supplements from 24 to 28 weeks gestation until term | Maternal insulin sensitivity |
| Louto et al. | Cluster randomized controlled trial | Women at risk of gestational diabetes (overweight, age 40 years or older, earlier macrosomic child, diabetic first degree relatives) (Finland) | Tailored diet and physical activity counselling, five visits to public health nurse. Monthly group session with physiotherapist | Primary; gestational diabetes, birthweight. Secondary; maternal weight gain, childhood weight at 1 year; requirement for insulin treatment in pregnancy |
| Ludwig et al. | Randomized controlled trial | Overweight and obese (BMI > 25, <45) pregnant women (Boston, USA) | Low glycaemic load | Birthweight z-score |
| Parat et al. | Randomized controlled trial | Overweight or obese women (Paris, France) | Counselling on healthy eating and modest exercise | 30% reduction in rapid infancy weight gain at 2 years |
| Fit for 2 study (Oostdam et al., 2009a, b) | Randomized controlled trial | Dutch women obese (BMI > 30) or overweight with a history of macrosomia or abnormal glucose tolerance in previous pregnancy or first grade relative with type 2 GDM 2 groups of 64 subjects | Intensive exercise program (2 days of week, 60 min each) | Maternal fasting plasma glucose and relative insulin resistance. Primary neonatal outcome birthweight, QUALY |
| Poston et al. (The UPBEAT Study) | Pilot trial followed by randomized controlled trial | 2700 obese pregnant women (UK) | Tailored advice on physical activity and diet. Group sessions | Pilot study; change in dietary and physical activity behaviours, QUALY and barriers to behavioural change. RCT; maternal insulin resistance. Primary neonatal outcome birthweight, QUALY |
| Shaheta et al. | (1) Observational Study; (2) Randomized controlled trial | (1) All pregnant women delivering in District General Hospital; (2) Women with a BMI > 40 | Measurement of waist circumference at booking & 20/40. Metformin plus exercise versus metformin | Macrosomia, pre-eclampsia and GDM |
| Shen et al. | Randomized controlled trial | All BMI pregnant women (Manitoba, USA) | Community-based lifestyle intervention package (diet and exercise) during and after pregnancy | Excessive GWG |
| Vintner et al. | Randomized controlled trial | Obese (BMI > 30) pregnant women (n = 360; Odense, Denmark) | Individualized counselling on diet and physical activity | Multiple obesity-related adverse pregnancy outcomes |
Conclusions
The maternal obesity ‘epidemic’ has stimulated the need for the development of effective interventions to improve pregnancy outcome. The rationale for new interventions should incorporate a detailed understanding of the maternal metabolic environment and its consequences for the health of the mother and the child. Several randomized controlled trials are planned or underway, and using slightly different strategies should inform the best approach to effective intervention and evidence for or against adoption of the new IOM GWG guidelines. Importantly, follow-up of childhood body composition in successful studies will provide conclusive evidence for or against the developmental ‘programming’ of obesity.
Funding
The preparation of this manuscript was supported by the National Institute of Health Research (NIHR), UK, and the Chief Scientist Office (part of the Scottish Government Health Directorates).
References
- Abrams B, Selvin S. Maternal weight gain pattern and birth weight. Obstet Gynecol. 1995;86:163–169. doi: 10.1016/0029-7844(95)00118-b. [DOI] [PubMed] [Google Scholar]
- Agardh CD, Aberg A, Norden NE. Glucose levels and insulin secretion during a 75 g glucose challenge test in normal pregnancy. J Intern Med. 1996;240:303–309. doi: 10.1046/j.1365-2796.1996.52872000.x. [DOI] [PubMed] [Google Scholar]
- Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J. The metabolic syndrome-a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- Alderman BW, Zhao H, Holt VL, Watts DH, Beresford SA. Maternal physical activity in pregnancy and infant size for gestational age. Ann Epidemiol. 1998;8:513–519. doi: 10.1016/s1047-2797(98)00020-9. [DOI] [PubMed] [Google Scholar]
- Alvarez JJ, Montelongo A, Iglesias A, Lasuncion MA, Herrera E. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J Lipid Res. 1996;37:299–308. [PubMed] [Google Scholar]
- Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstet Gynecol. 2009;113:305–312. doi: 10.1097/AOG.0b013e318195baef. [DOI] [PubMed] [Google Scholar]
- Assel B, Rossi K, Kalhan S. Glucose metabolism during fasting through human pregnancy: comparison of tracer method with respiratory calorimetry. Am J Physiol Endocrinol Metab. 1993;265:E351–E356. doi: 10.1152/ajpendo.1993.265.3.E351. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2003;26:s103–s105. doi: 10.2337/diacare.26.2007.s103. [DOI] [PubMed] [Google Scholar]
- Ryden L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer M-J, Cosentino F, Jonsson B, Laakso M, et al. Authors/Task Force Members. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary: the Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- Avery MD, Walker AJ. Acute effect of exercise on blood glucose and insulin levels in women with gestational diabetes. J Matern Fetal Med. 2001;10:52–58. doi: 10.1080/714904296. [DOI] [PubMed] [Google Scholar]
- Bartha JL, Marin-Segura P, Gonzalez-Gonzalez NL, Wagner F, Aguilar-Diosdado M, Hervias-Vivancos B. Ultrasound evaluation of visceral fat and metabolic risk factors during early pregnancy. Obesity (Silver Spring) 2007;15:2233–2239. doi: 10.1038/oby.2007.265. [DOI] [PubMed] [Google Scholar]
- Bell RJ, Palma SM, Lumley JM. The effect of vigorous exercise during pregnancy on birth-weight. Aust N Z J Obstet Gynaecol. 1995;35:46–51. doi: 10.1111/j.1479-828x.1995.tb01829.x. [DOI] [PubMed] [Google Scholar]
- Berkowitz GS, Kelsey JL, Holford TR, Berkowitz RL. Physical activity and the risk of spontaneous preterm delivery. J Reprod Med. 1983;28:581–588. [PubMed] [Google Scholar]
- Binkin NJ, Yip R, Fleshood L, Trowbridge FL. Birth weight and childhood growth. Pediatrics. 1988;82:828–834. [PubMed] [Google Scholar]
- Blair NJ, Thompson JM, Black PN, Becroft DM, Clark PM, Han DY, Robinson E, Waldie KE, Wild CJ, Mitchell EA. Risk factors for obesity in 7-year-old European children: the Auckland Birthweight Collaborative Study. Arch Dis Child. 2007;92:866–871. doi: 10.1136/adc.2007.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen A, Campagna P, Gilchrist L, Young DC, Beresford P. Substrate and endocrine responses during exercise at selected stages of pregnancy. J Appl Physiol. 1992;73:134–142. doi: 10.1152/jappl.1992.73.1.134. [DOI] [PubMed] [Google Scholar]
- Brankston GN, Mitchell BF, Ryan EA, Okun NB. Resistance exercise decreases the need for insulin in overweight women with gestational diabetes mellitus. Am J Obstet Gynecol. 2004;190:188–193. doi: 10.1016/s0002-9378(03)00951-7. [DOI] [PubMed] [Google Scholar]
- Burdette HL, Whitaker RC, Hall WC, Daniels SR. Maternal infant-feeding style and children's adiposity at 5 years of age. Arch Pediatr Adolesc Med. 2006;160:513–520. doi: 10.1001/archpedi.160.5.513. [DOI] [PubMed] [Google Scholar]
- Butte NF, Hopkinson JM, Mehta N, Moon JK, Smith EOB. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. Am J Clin Nutr. 1999;69:299–307. doi: 10.1093/ajcn/69.2.299. [DOI] [PubMed] [Google Scholar]
- Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol. 2003;189:1423–1432. doi: 10.1067/s0002-9378(03)00596-9. [DOI] [PubMed] [Google Scholar]
- Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45:633–638. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- Carmichael S, Abrams B, Selvin S. The pattern of maternal weight gain in women with good pregnancy outcomes. Am J Public Health. 1997;87:1984–1988. doi: 10.2105/ajph.87.12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113:1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol. 1991;165:1667–1672. doi: 10.1016/0002-9378(91)90012-g. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Tyzbir ED, Wolfe RR, Roman NM, Amini SB, Sims EA. Longitudinal changes in basal hepatic glucose production and suppression during insulin infusion in normal pregnant women. Am J Obstet Gynecol. 1992;167:913–919. doi: 10.1016/s0002-9378(12)80011-1. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Roman NM, Tyzbir ED, Merritt AO, Driscoll P, Amini SB. Weight gain in women with gestational diabetes. Obstet Gynecol. 1993a;81:523–528. [PubMed] [Google Scholar]
- Catalano PM, Tyzbir ED, Wolfe RR, Calles J, Roman NM, Amini SB, Sims EA. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol Endocrinol Metab. 1993b;264:E60–E67. doi: 10.1152/ajpendo.1993.264.1.E60. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Drago NM, Amini SB. Maternal carbohydrate metabolism and its relationship to fetal growth and body composition. Am J Obstet Gynecol. 1995;172:1464–1470. doi: 10.1016/0002-9378(95)90479-4. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Drago NM, Amini SB. Longitudinal changes in pancreatic beta-cell function and metabolic clearance rate of insulin in pregnant women with normal and abnormal glucose tolerance. Diabetes Care. 1998a;21:403–408. doi: 10.2337/diacare.21.3.403. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Roman-Drago NM, Amini SB, Sims EA. Longitudinal changes in body composition and energy balance in lean women with normal and abnormal glucose tolerance during pregnancy. Am J Obstet Gynecol. 1998b;179:156–165. doi: 10.1016/s0002-9378(98)70267-4. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180:903–916. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Nizielski SE, Shao J, Preston L, Qiao L, Friedman JE. Downregulated IRS-1 and PPARgamma in obese women with gestational diabetes: relationship to FFA during pregnancy. Am J Physiol Endocrinol Metab. 2002;282:E522–E533. doi: 10.1152/ajpendo.00124.2001. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, Amini SB. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–1313. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32:1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasan-Taber L, Marcus BH, Stanek E, 3rd, Ciccolo JT, Marquez DX, Solomon CG, Markenson G. A randomized controlled trial of prenatal physical activity to prevent gestational diabetes: design methods. J Womens Health. 2009;18:851–859. doi: 10.1089/jwh.2008.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier S, Marliss EB, Morais JA, Lamarche M, Gougeon R. Whole-body protein anabolic response is resistant to the action of insulin in obese women. Am J Clin Nutr. 2005;82:355–365. doi: 10.1093/ajcn.82.2.355. [DOI] [PubMed] [Google Scholar]
- Chevalier SP, Burgess SC, Malloy CR, Gougeon RJ, Marliss EB, Morais JA. The greater contribution of gluconeogenesis to glucose production in obesity is related to increased whole-body protein catabolism. Diabetes. 2006;55:675–681. doi: 10.2337/diabetes.55.03.06.db05-1117. [DOI] [PubMed] [Google Scholar]
- Cho NH, Silverman BL, Rizzo TA, Metzger BE. Correlations between the intrauterine metabolic environment and blood pressure in adolescent offspring of diabetic mothers. J Pediatr. 2000;136:587–592. doi: 10.1067/mpd.2000.105129. [DOI] [PubMed] [Google Scholar]
- Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, Dietz PM. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007a;30:2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- Chu SY, Kim SY, Lau J, Schmid CH, Dietz PM, Callaghan WM, Curtis KM. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol. 2007b;197:223–228. doi: 10.1016/j.ajog.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Chu SY, Kim SY, Schmid CH, Dietz PM, Callaghan WM, Lau J, Curtis KM. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev. 2007c;8:385–394. doi: 10.1111/j.1467-789X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- Chu SY, Bachman DJ, Callaghan WM, Whitlock EP, Dietz PM, Berg CJ, O'Keeffe-Rosetti M, Bruce FC, Hornbrook MC. Association between obesity during pregnancy and increased use of health care. N Engl J Med. 2008;358:1444–1453. doi: 10.1056/NEJMoa0706786. [DOI] [PubMed] [Google Scholar]
- Claesson IM, Sydsjo G, Brynhildsen J, Cedergren M, Jeppsson A, Nystrom F, Sydsjo A, Josefsson A. Weight gain restriction for obese pregnant women: a case–control intervention study. BJOG. 2008;115:44–50. doi: 10.1111/j.1471-0528.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- Clapp JF., 3rd Maternal carbohydrate intake and pregnancy outcome. Proc Nutr Soc. 2002;61:45–50. doi: 10.1079/pns2001129. [DOI] [PubMed] [Google Scholar]
- Clapp JF, 3rd, Capeless EL. The changing glycemic response to exercise during pregnancy. Am J Obstet Gynecol. 1991;165:1678–1683. doi: 10.1016/0002-9378(91)90014-i. [DOI] [PubMed] [Google Scholar]
- Clapp JF, 3rd, Dickstein S. Endurance exercise and pregnancy outcome. Med Sci Sports Exerc. 1984;16:556–562. [PubMed] [Google Scholar]
- Clapp JF, 3rd, Rokey R, Treadway JL, Carpenter MW, Artal RM, Warrnes C. Exercise in pregnancy. Med Sci Sports Exerc. 1992;24:S294–S300. [PubMed] [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. Br Med J. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins L, Rigg L, Hollingsworth D, Brink G, Aurand J, Yen SS. The 24-hour excursion and diurnal rhythm of glucose, insulin, and C-peptide in normal pregnancy. Am J Obstet Gynecol. 1980;136:483–488. doi: 10.1016/0002-9378(80)90675-4. [DOI] [PubMed] [Google Scholar]
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new medical research council guidance. Br Med J. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS the Australian Carbohydrate Intolerance Study in Pregnant Women Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, Manson JE, Speizer FE, Stampfer MJ. Birth weight and adult hypertension and obesity in women. Circulation. 1996a;94:1310–1315. doi: 10.1161/01.cir.94.6.1310. [DOI] [PubMed] [Google Scholar]
- Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996b;94:3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- Cypryk K, Vilsbøll T, Nadel I, Smyczyńska J, Holst JJ, Lewiński A. Normal secretion of the incretin hormones glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 during gestational diabetes mellitus. Gynecol Endocrinol. 2007;23:58–62. doi: 10.1080/09513590601137004. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000a;49:2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 2000b;9:83–88. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<83::AID-MFM17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- de Benoist B, Jackson AA, Hall JS, Persaud C. Whole-body protein turnover in Jamaican women during normal pregnancy. Hum Nutr Clin Nutr. 1985;39:167–179. [PubMed] [Google Scholar]
- Deierlein AL, Siega-Riz AM, Herring A. Dietary energy density but not glycemic load is associated with gestational weight gain. Am J Clin Nutr. 2008;88:693–699. doi: 10.1093/ajcn/88.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28:850–856. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- Dempsey JC, Butler CL, Sorensen TK, Lee IM, Thompson ML, Miller RS, Frederick IO, Williams MA. A case–control study of maternal recreational physical activity and risk of gestational diabetes mellitus. Diabetes Res Clin Pract. 2004a;66:203–215. doi: 10.1016/j.diabres.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Dempsey JC, Sorensen TK, Williams MA, Lee IM, Miller RS, Dashow EE, Luthy DA. Prospective study of gestational diabetes mellitus risk in relation to maternal recreational physical activity before and during pregnancy. Am J Epidemiol. 2004b;159:663–670. doi: 10.1093/aje/kwh091. [DOI] [PubMed] [Google Scholar]
- Desoye G, Schweditsch MO, Pfeiffer KP, Zechner R, Kostner GM. Correlation of hormones with lipid and lipoprotein levels during normal pregnancy and postpartum. J Clin Endocrinol Metab. 1987;64:704–712. doi: 10.1210/jcem-64-4-704. [DOI] [PubMed] [Google Scholar]
- Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 1990;10:497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cianni G, Miccoli R, Volpe L, Lencioni C, Ghio A, Giovannitti MG, Cuccuru I, Pellegrini G, Chatzianagnostou K, Boldrini A, et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med. 2005;22:21–25. doi: 10.1111/j.1464-5491.2004.01336.x. [DOI] [PubMed] [Google Scholar]
- Dornhorst A, Frost G. The principles of dietary management of gestational diabetes: reflection on current evidence. J Hum Nutr Diet. 2002;15:145–156. doi: 10.1046/j.1365-277x.2002.00344.x. quiz 157–159. [DOI] [PubMed] [Google Scholar]
- Duggleby SL, Jackson AA. Relationship of maternal protein turnover and lean body mass during pregnancy and birth length. Clin Sci (Lond) 2001;101:65–72. [PubMed] [Google Scholar]
- Duggleby SL, Jackson AA. Higher weight at birth is related to decreased maternal amino acid oxidation during pregnancy. Am J Clin Nutr. 2002;76:852–857. doi: 10.1093/ajcn/76.4.852. [DOI] [PubMed] [Google Scholar]
- Ehrenberg HM, Huston-Presley L, Catalano PM. The influence of obesity and gestational diabetes mellitus on accretion and the distribution of adipose tissue in pregnancy. Am J Obstet Gynecol. 2003;189:944–948. doi: 10.1067/s0002-9378(03)00761-0. [DOI] [PubMed] [Google Scholar]
- Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191:964–968. doi: 10.1016/j.ajog.2004.05.052. [DOI] [PubMed] [Google Scholar]
- Endo S, Maeda K, Suto M, Kaji T, Morine M, Kinoshita T, Yasui T, Irahara M. Differences in insulin sensitivity in pregnant women with overweight and gestational diabetes mellitus. Gynecol Endocrinol. 2006;22:343–349. doi: 10.1080/09513590600724836. [DOI] [PubMed] [Google Scholar]
- Enquobahrie DA, Williams MA, Butler CL, Frederick IO, Miller RS, Luthy DA. Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am J Hypertens. 2004;17:574–581. doi: 10.1016/j.amjhyper.2004.03.666. [DOI] [PubMed] [Google Scholar]
- Eriksson KF, Lindgarde F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmo feasibility study. Diabetologia. 1991;34:891–898. doi: 10.1007/BF00400196. [DOI] [PubMed] [Google Scholar]
- Esmaillzadeh A, Azadbakht L. Whole-grain intake, metabolic syndrome, and mortality in older adults. Am J Clin Nutr. 2006;83:1439–1440. doi: 10.1093/ajcn/83.6.1439. author reply 1441–1432. [DOI] [PubMed] [Google Scholar]
- Evenson KR, Siega-Riz AM, Savitz DA, Leiferman JA, Thorp JM., Jr Vigorous leisure activity and pregnancy outcome. Epidemiology. 2002;13:653–659. doi: 10.1097/00001648-200211000-00009. [DOI] [PubMed] [Google Scholar]
- Evenson KR, Moos MK, Carrier K, Siega-Riz AM. Perceived barriers to physical activity among pregnant women. Matern Child Health J. 2008 doi: 10.1007/s10995-008-0359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahraeus L, Larsson-Cohn U, Wallentin L. Plasma lipoproteins including high density lipoprotein subfractions during normal pregnancy. Obstet Gynecol. 1985;66:468–472. [PubMed] [Google Scholar]
- Fisch RO, Bilek MK, Ulstrom R. Obesity and leanness at birth and their relationship to body habitus in later childhood. Pediatrics. 1975;56:521–528. [PubMed] [Google Scholar]
- Forsum E, Sadurskis A, Wager J. Resting metabolic rate and body composition of healthy Swedish women during pregnancy. Am J Clin Nutr. 1988;47:942–947. doi: 10.1093/ajcn/47.6.942. [DOI] [PubMed] [Google Scholar]
- Fougner KJ, Vanky E, Carlsen SM. Metformin has no major effects on glucose homeostasis in pregnant women with PCOS: results of a randomized double-blind study. Scand J Clin Lab Invest. 2008;68:771–776. doi: 10.1080/00365510802254620. [DOI] [PubMed] [Google Scholar]
- Fraser RB, Ford FA, Milner RD. A controlled trial of a high dietary fibre intake in pregnancy—effects on plasma glucose and insulin levels. Diabetologia. 1983;25:238–241. doi: 10.1007/BF00279936. [DOI] [PubMed] [Google Scholar]
- Franz MJ, Horton ES, Sr, Bantle JP, Beebe CA, Brunzell JD, Coulston AM, Henry RR, Hoogwerf BJ, Stacpoole PW. Nutrition principles for the management of diabetes and related complications. Diabetes Care. 1994;17:490–518. doi: 10.2337/diacare.17.5.490. [DOI] [PubMed] [Google Scholar]
- Gale CR, Javaid MK, Robinson SM, Law CM, Godfrey KM, Cooper C. Maternal size in pregnancy and body composition in children. J Clin Endocrinol Metab. 2007;92:3904–3911. doi: 10.1210/jc.2007-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier-Dereure F, Boegner C, Bringer J. Obesity and pregnancy: complications and cost. Am J Clin Nutr. 2000;71:1242S–1248S. doi: 10.1093/ajcn/71.5.1242s. [DOI] [PubMed] [Google Scholar]
- Garcia-Patterson A, Martin E, Ubeda J, Maria MA, de Leiva A, Corcoy R. Evaluation of light exercise in the treatment of gestational diabetes. Diabetes Care. 2001;24:2006–2007. doi: 10.2337/diacare.24.11.2006. [DOI] [PubMed] [Google Scholar]
- Goldberg GR, Prentice AM, Coward WA, Davies HL, Murgatroyd PR, Wensing C, Black AE, Harding M, Sawyer M. Longitudinal assessment of energy expenditure in pregnancy by the doubly labeled water method. Am J Clin Nutr. 1993;57:494–505. doi: 10.1093/ajcn/57.4.494. [DOI] [PubMed] [Google Scholar]
- Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De Backer G, Ebrahim S, Gjelsvik B, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J. 2007;28:2375–2414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- Gray-Donald K, Robinson E, Collier A, David K, Renaud L, Rodrigues S. Intervening to reduce weight gain in pregnancy and gestational diabetes mellitus in Cree communities: an evaluation. CMAJ. 2000;163:1247–1251. [PMC free article] [PubMed] [Google Scholar]
- Griffin BA, Freeman DJ, Tait GW, Thomson J, Caslake MJ, Packard CJ, Shepherd J. Role of plasma triglyceride in the regulation of plasma low density lipoprotein (LDL) subfractions: relative contribution of small, dense LDL to coronary heart disease risk. Atherosclerosis. 1994;106:241–253. doi: 10.1016/0021-9150(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev. 2008;9:140–150. doi: 10.1111/j.1467-789X.2007.00464.x. [DOI] [PubMed] [Google Scholar]
- Hamilton JK, Odrobina E, Yin J, Hanley AJ, Zinman B, Retnakaran R. Maternal insulin sensitivity during pregnancy predicts infant weight gain and adiposity at 1 year of age. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.231. [DOI] [PubMed] [Google Scholar]
- Harvey NC, Poole JR, Javaid MK, Dennison EM, Robinson S, Inskip HM, Godfrey KM, Cooper C, Sayer AA. Parental determinants of neonatal body composition. J Clin Endocrinol Metab. 2007;92:523–526. doi: 10.1210/jc.2006-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Wong WW, Thomas AJ, Uvena-Celebrezze J, Huston-Pressley L, Amini SB, Catalano PM. Estimation of neonatal body composition: isotope dilution versus total-body electrical conductivity. Biol Neonate. 2002;81:170–175. doi: 10.1159/000051530. [DOI] [PubMed] [Google Scholar]
- Hatch M, Levin B, Shu XO, Susser M. Maternal leisure-time exercise and timely delivery. Am J Public Health. 1998;88:1528–1533. doi: 10.2105/ajph.88.10.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Survey for England 2004: The Information Centre. 2006 www.ic.nhs.uk/pubs/healthsurvey2004ethnicfull . [Google Scholar]
- Hediger ML, Overpeck MD, McGlynn A, Kuczmarski RJ, Maurer KR, Davis WW. Growth and fatness at three to six years of age of children born small- or large-for-gestational age. Pediatrics. 1999;104:e33. doi: 10.1542/peds.104.3.e33. [DOI] [PubMed] [Google Scholar]
- Hendler I, Goldenberg RL, Mercer BM, Iams JD, Meis PJ, Moawad AH, MacPherson CA, Caritis SN, Miodovnik M, Menard KM, et al. The preterm prediction study: association between maternal body mass index and spontaneous and indicated preterm birth. Am J Obstet Gynecol. 2005a;192:882–886. doi: 10.1016/j.ajog.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Hendler I, Blackwell SC, Mehta SH, Whitty JE, Russell E, Sorokin Y, Cotton DB. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am J Obstet Gynecol. 2005b;193:979–983. doi: 10.1016/j.ajog.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Heslehurst N, Ells LJ, Simpson H, Batterham A, Wilkinson J, Summerbell CD. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. BJOG. 2007;114:187–194. doi: 10.1111/j.1471-0528.2006.01180.x. [DOI] [PubMed] [Google Scholar]
- Heslehurst N, Simpson H, Ells LJ, Rankin J, Wilkinson J, Lang R, Brown TJ, Summerbell CD. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obes Rev. 2008;9:635–683. doi: 10.1111/j.1467-789X.2008.00511.x. [DOI] [PubMed] [Google Scholar]
- Highman TJ, Friedman JE, Huston LP, Wong WW, Catalano PM. Longitudinal changes in maternal serum leptin concentrations, body composition, and resting metabolic rate in pregnancy. Am J Obstet Gynecol. 1998;178:1010–1015. doi: 10.1016/s0002-9378(98)70540-x. [DOI] [PubMed] [Google Scholar]
- Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–2292. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- Homko CJ, Cheung P, Boden G. Effects of free fatty acids on glucose uptake and utilization in healthy women. Diabetes. 2003;52:487–491. doi: 10.2337/diabetes.52.2.487. [DOI] [PubMed] [Google Scholar]
- Hubel CA, Shakir Y, Gallaher MJ, McLaughlin MK, Roberts JM. Low-density lipoprotein particle size decreases during normal pregnancy in association with triglyceride increases. J Soc Gynecol Investig. 1998;5:244–250. doi: 10.1016/s1071-5576(98)00022-7. [DOI] [PubMed] [Google Scholar]
- Hull HR, Dinger MK, Knehans AW, Thompson DM, Fields DA. Impact of maternal body mass index on neonate birthweight and body composition. Am J Obstet Gynecol. 2008;198:416.e411–416.e416. doi: 10.1016/j.ajog.2007.10.796. [DOI] [PubMed] [Google Scholar]
- HAPO. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes. 2009;58:453–459. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) Nutrition During Pregnancy. Washington, DC: National Academy Press; 1990. [Google Scholar]
- Jackson AA. Measurement of protein turnover during pregnancy. Hum Nutr Clin Nutr. 1987;41:497–498. [PubMed] [Google Scholar]
- Jovanovic-Peterson L, Durak EP, Peterson CM. Randomized trial of diet versus diet plus cardiovascular conditioning on glucose levels in gestational diabetes. Am J Obstet Gynecol. 1989;161:415–419. doi: 10.1016/0002-9378(89)90534-6. [DOI] [PubMed] [Google Scholar]
- Kac G, Benicio MHDA, Velasquez-Melendez G, Valente JG, Struchiner CJ. Gestational Weight gain and prepregnancy weight influence postpartum weight retention in a cohort of Brazilian women. J Nutr. 2004;134:661–666. doi: 10.1093/jn/134.3.661. [DOI] [PubMed] [Google Scholar]
- Kalhan S, Rossi K, Gruca L, Burkett E, O'Brien A. Glucose turnover and gluconeogenesis in human pregnancy. J Clin Invest. 1997;100:1775–1781. doi: 10.1172/JCI119704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhan SC, D'Angelo LJ, Savin SM, Adam PA. Glucose production in pregnant women at term gestation. Sources of glucose for human fetus. J Clin Invest. 1979;63:388–394. doi: 10.1172/JCI109314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoff RK, Kandaraki E, Morrow PG, Mitchell TH, Kelber S, Borkowf HI. Relationship between neonatal birth weight and maternal plasma amino acid profiles in lean and obese nondiabetic women and in type I diabetic pregnant women. Metabolism. 1988;37:234–239. doi: 10.1016/0026-0495(88)90101-1. [DOI] [PubMed] [Google Scholar]
- Kanagalingam MG, Forouhi NG, Greer IA, Sattar N. Changes in booking body mass index over a decade: retrospective analysis from a Glasgow Maternity Hospital. BJOG. 2005;112:1431–1433. doi: 10.1111/j.1471-0528.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- Kennelly MM, Geary M, McCaffrey N, McLoughlin P, Staines A, McKenna P. Exercise-related changes in umbilical and uterine artery waveforms as assessed by Doppler ultrasound scans. Am J Obstet Gynecol. 2002;187:661–666. doi: 10.1067/mob.2002.125741. [DOI] [PubMed] [Google Scholar]
- Khashan AS, Kenny LC. The effects of maternal body mass index on pregnancy outcome. Eur J Epidemiol. 2009 doi: 10.1007/s10654-009-9375-2. [DOI] [PubMed] [Google Scholar]
- Kim J, Peterson KE, Scanlon KS, Fitzmaurice GM, Must A, Oken E, Rifas-Shiman SL, Rich-Edwards JW, Gillman MW. Trends in overweight from 1980 through 2001 among preschool-aged children enrolled in a health maintenance organization. Obesity (Silver Spring) 2006;14:1107–1112. doi: 10.1038/oby.2006.126. [DOI] [PubMed] [Google Scholar]
- Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity (Silver Spring) 2007;15:986–993. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- Kinnunen TI, Pasanen M, Aittasalo M, Fogelholm M, Hilakivi-Clarke L, Weiderpass E, Luoto R. Preventing excessive weight gain during pregnancy—a controlled trial in primary health care. Eur J Clin Nutr. 2007 doi: 10.1038/sj.ejcn.1602602. [DOI] [PubMed] [Google Scholar]
- Kinnunen TI, Aittasalo M, Koponen P, Ojala K, Mansikkamaki K, Weiderpass E, Fogelholm M, Luoto R. Feasibility of a controlled trial aiming to prevent excessive pregnancy-related weight gain in primary health care. BMC Pregnancy Childbirth. 2008;8:37. doi: 10.1186/1471-2393-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Itoh M. Longitudinal variance of fat mass deposition during pregnancy evaluated by ultrasonography: the ratio of visceral fat to subcutaneous fat in the abdomen. Gynecol Obstet Invest. 2006;61:115–118. doi: 10.1159/000089456. [DOI] [PubMed] [Google Scholar]
- Kiran T, Hemmadi S, Bethal J, Evans J. Outcome of pregnancy in a woman with an increased body mass index. BJOG. 2005;112:768–772. doi: 10.1111/j.1471-0528.2004.00546.x. [DOI] [PubMed] [Google Scholar]
- Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM. Clinically useful estimates of insulin sensitivity during pregnancy: validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care. 2001;24:1602–1607. doi: 10.2337/diacare.24.9.1602. [DOI] [PubMed] [Google Scholar]
- Kleiser C, Schaffrath Rosario A, Mensink GB, Prinz-Langenohl R, Kurth BM. Potential determinants of obesity among children and adolescents in Germany: results from the cross-sectional KiGGS Study. BMC Public Health. 2009;9:46. doi: 10.1186/1471-2458-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp RH, Warth MR, Carrol CJ. Lipid metabolism in pregnancy. I. Changes in lipoprotein triglyceride and cholesterol in normal pregnancy and the effects of diabetes mellitus. J Reprod Med. 1973;10:95–101. [PubMed] [Google Scholar]
- Koletzko B, von Kries R, Monasterolo RC, Subias JE, Scaglioni S, Giovannini M, Beyer J, Demmelmair H, Anton B, Gruszfeld D, et al. Infant feeding and later obesity risk. Adv Exp Med Biol. 2009;646:15–29. doi: 10.1007/978-1-4020-9173-5_2. [DOI] [PubMed] [Google Scholar]
- Kopp-Hoolihan LE, van Loan MD, Wong WW, King JC. Fat mass deposition during pregnancy using a four-component model. J Appl Physiol. 1999;87:196–202. doi: 10.1152/jappl.1999.87.1.196. [DOI] [PubMed] [Google Scholar]
- Koupil I, Toivanen P. Social and early-life determinants of overweight and obesity in 18-year-old Swedish men. Int J Obes (Lond) 2008;32:73–81. doi: 10.1038/sj.ijo.0803681. [DOI] [PubMed] [Google Scholar]
- Kral JG, Biron S, Simard S, Hould FS, Lebel S, Marceau S, Marceau P. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118:e1644–e1649. doi: 10.1542/peds.2006-1379. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Kakuma R. Energy and protein intake in pregnancy. Cochrane Database Syst Rev (Online) 2003 doi: 10.1002/14651858.CD000032. CD000032. [DOI] [PubMed] [Google Scholar]
- Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD000180.pub2. CD000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Barr RG, Leduc DG, Boisjoly C, McVey-White L, Pless IB. Determinants of weight and adiposity in the first year of life. J Pediatr. 1985;106:10–14. doi: 10.1016/s0022-3476(85)80456-x. [DOI] [PubMed] [Google Scholar]
- Laitinen J, Power C, Jarvelin MR. Family social class, maternal body mass index, childhood body mass index, and age at menarche as predictors of adult obesity. Am J Clin Nutr. 2001;74:287–294. doi: 10.1093/ajcn/74.3.287. [DOI] [PubMed] [Google Scholar]
- Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM, Jr, et al. Eunice Kennedy Shriver National Institute of Child Health Human Development Maternal-Fetal Medicine Units Network. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol. 2005;192:989–997. doi: 10.1016/j.ajog.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 1984;289:1257–1261. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laraia BA, Siega-Riz AM, Dole N, London E. Pregravid weight is associated with prior dietary restraint and psychosocial factors during pregnancy. Obesity (Silver Spring) 2009;17:550–558. doi: 10.1038/oby.2008.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson B, Svardsudd K, Welin L, Wilhelmsen L, Bjorntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 1984;288:1401–1404. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskarzewski P, Morrison JA, Khoury P, Kelly K, Glatfelter L, Larsen R, Glueck CJ. Parent-child nutrient intake interrelationships in school children ages 6 to 19: the Princeton School District Study. Am J Clin Nutr. 1980;33:2350–2355. doi: 10.1093/ajcn/33.11.2350. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Smith GD, O'Callaghan M, Alati R, Mamun AA, Williams GM, Najman JM. Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the mater-university study of pregnancy and its outcomes. Am J Epidemiol. 2007;165:418–424. doi: 10.1093/aje/kwk030. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Timpson NJ, Harbord RM, Leary S, Ness A, McCarthy MI, Frayling TM, Hattersley AT, Smith GD. Exploring the developmental overnutrition hypothesis using parental-offspring associations and FTO as an instrumental variable. PLoS Med. 2008;5:e33. doi: 10.1371/journal.pmed.0050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M, Lawrence F, Coward WA, Cole TJ, Whitehead RG. Energy requirements of pregnancy in the Gambia. Lancet. 1987;2:1072–1076. doi: 10.1016/s0140-6736(87)91492-9. [DOI] [PubMed] [Google Scholar]
- Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN., Jr Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstet Gynecol. 1997;90:483–488. doi: 10.1016/s0029-7844(97)00355-4. [DOI] [PubMed] [Google Scholar]
- Leet T, Flick L. Effect of exercise on birthweight. Clin Obstet Gynecol. 2003;46:423–431. doi: 10.1097/00003081-200306000-00021. [DOI] [PubMed] [Google Scholar]
- Li C, Kaur H, Choi WS, Huang TT, Lee RE, Ahluwalia JS. Additive interactions of maternal prepregnancy BMI and breast-feeding on childhood overweight. Obes Res. 2005;13:362–371. doi: 10.1038/oby.2005.48. [DOI] [PubMed] [Google Scholar]
- Li C, Goran MI, Kaur H, Nollen N, Ahluwalia JS. Developmental trajectories of overweight during childhood: role of early life factors. Obesity (Silver Spring) 2007;15:760–771. doi: 10.1038/oby.2007.585. [DOI] [PubMed] [Google Scholar]
- Li L, Law C, Lo Conte R, Power C. Intergenerational influences on childhood body mass index: the effect of parental body mass index trajectories. Am J Clin Nutr. 2009;89:551–557. doi: 10.3945/ajcn.2008.26759. [DOI] [PubMed] [Google Scholar]
- Lindberg UB, Leibel RL, Silfverstolpe G, Hirsch J, Bjorntorp P, Rebuffe-Scrive M. Effects of early pregnancy on regional adipose tissue metabolism. Horm Metab Res. 1991;23:25–29. doi: 10.1055/s-2007-1003603. [DOI] [PubMed] [Google Scholar]
- Lindstrom J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, Uusitupa M, Tuomilehto J. The Finnish diabetes prevention study (DPS) Diabetes Care. 2003;26:3230–3236. doi: 10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- Llurba E, Casals E, Domínguez C, Delgado J, Mercadé I, Crispi F, Martín-Gallán P, Cabero L, Gratacós E. Atherogenic lipoprotein subfraction profile in preeclamptic women with and without high triglycerides: different pathophysiologic subsets in preeclampsia. Metabolism. 2005;54:1504–1509. doi: 10.1016/j.metabol.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Lokey EA, Tran ZV, Wells CL, Myers BC, Tran AC. Effects of physical exercise on pregnancy outcomes: a meta-analytic review. Med Sci Sports Exerc. 1991;23:1234–1239. [PubMed] [Google Scholar]
- Madsen M, Jorgensen T, Jensen ML, Juhl M, Olsen J, Andersen PK, Nybo Andersen AM. Leisure time physical exercise during pregnancy and the risk of miscarriage: a study within the Danish National Birth Cohort. BJOG. 2007;114:1419–1426. doi: 10.1111/j.1471-0528.2007.01496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major CA, Henry MJ, De Veciana M, Morgan MA. The effects of carbohydrate restriction in patients with diet-controlled gestational diabetes. Obstet Gynecol. 1998;91:600–604. doi: 10.1016/s0029-7844(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Malcolm JC, Lawson ML, Gaboury I, Lough G, Keely E. Glucose tolerance of offspring of mother with gestational diabetes mellitus in a low-risk population. Diabet Med. 2006;23:565–570. doi: 10.1111/j.1464-5491.2006.01840.x. [DOI] [PubMed] [Google Scholar]
- Mamun AA, O'Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation. 2009;119:1720–1727. doi: 10.1161/CIRCULATIONAHA.108.813436. [DOI] [PubMed] [Google Scholar]
- Martin AM, Berger H, Nisenbaum R, Lausman AY, MacGarvie S, Crerar C, Ray JG. Abdominal visceral adiposity in the first trimester predicts glucose intolerance in later pregnancy. Diabetes Care. 2009;32:1308–1310. doi: 10.2337/dc09-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland MB, Trylovich CG, Langer O. Anthropometric differences in macrosomic infants of diabetic and nondiabetic mothers. J Matern Fetal Med. 1998;7:292–295. doi: 10.1002/(SICI)1520-6661(199811/12)7:6<292::AID-MFM7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Meier JJ, Gallwitz B, Askenas M, Vollmer K, Deacon CF, Holst JJ, Schmidt WE, Nauck MA. Secretion of incretin hormones and the insulinotropic effect of gastric inhibitory polypeptide in women with a history of gestational diabetes. Diabetologia. 2005;48:1872–1881. doi: 10.1007/s00125-005-1863-7. [DOI] [PubMed] [Google Scholar]
- Merzouk H, Meghelli-Bouchenak M, el-Korso N, Belleville J, Prost J. Low birth weight at term impairs cord serum lipoprotein compositions and concentrations. Eur J Pediatr. 1998;157:321–326. doi: 10.1007/s004310050820. [DOI] [PubMed] [Google Scholar]
- Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil Steril. 2008;90:714–726. doi: 10.1016/j.fertnstert.2007.07.1290. [DOI] [PubMed] [Google Scholar]
- Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- Mills JL, Jovanovic L, Knopp R, Aarons J, Conley M, Park E, Lee YJ, Holmes L, Simpson JL, Metzger B. Physiological reduction in fasting plasma glucose concentration in the first trimester of normal pregnancy: the diabetes in early pregnancy study. Metabolism. 1998;47:1140–1144. doi: 10.1016/s0026-0495(98)90290-6. [DOI] [PubMed] [Google Scholar]
- Mingrone G, Manco M, Valera Mora ME, Guidone C, Iaconelli A, Gniuli D, Leccesi L, Chiellini C, Ghirlanda G. Influence of Maternal Obesity on Insulin Sensitivity and Secretion in the Offspring. Diabetes Care. 2008;5:5. doi: 10.2337/dc08-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93:S9–S30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- Misra DP, Strobino DM, Stashinko EE, Nagey DA, Nanda J. Effects of physical activity on preterm birth. Am J Epidemiol. 1998;147:628–635. doi: 10.1093/oxfordjournals.aje.a009503. [DOI] [PubMed] [Google Scholar]
- Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes Rev. 2005;6:143–154. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- Moreira P, Padez C, Mourao-Carvalhal I, Rosado V. Maternal weight gain during pregnancy and overweight in Portuguese children. Int J Obes (Lond) 2007;31:608–614. doi: 10.1038/sj.ijo.0803582. [DOI] [PubMed] [Google Scholar]
- Moses RG, Luebcke M, Davis WS, Coleman KJ, Tapsell LC, Petocz P, Brand-Miller JC. Effect of a low-glycemic-index diet during pregnancy on obstetric outcomes. Am J Clin Nutr. 2006;84:807–812. doi: 10.1093/ajcn/84.4.807. [DOI] [PubMed] [Google Scholar]
- Moses RG, Barker M, Winter M, Petocz P, Brand-Miller JC. Can a low glycemic index diet reduce the need for insulin in gestational diabetes mellitus? Diabetes Care. 2009;32:996–1000. doi: 10.2337/dc09-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. CDC Growth Charts, United States. 2009 http://www.cdc.gov/growthcharts/en . [Google Scholar]
- Nohr EA, Vaeth M, Baker JL, Sorensen T, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr. 2008;87:1750–1759. doi: 10.1093/ajcn/87.6.1750. [DOI] [PubMed] [Google Scholar]
- Nolan CJ, Riley SF, Sheedy MT, Walstab JE, Beischer NA. Maternal serum triglyceride, glucose tolerance, and neonatal birth weight ratio in pregnancy. Diabetes Care. 1995;18:1550–1556. doi: 10.2337/diacare.18.12.1550. [DOI] [PubMed] [Google Scholar]
- O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368–374. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- Oddy WH, Li J, Landsborough L, Kendall GE, Henderson S, Downie J. The association of maternal overweight and obesity with breastfeeding duration. J Pediatr. 2006;149:185–191. doi: 10.1016/j.jpeds.2006.04.005. [DOI] [PubMed] [Google Scholar]
- OECD. Organisation for Economic Co-operation and Development. http://www.oecd.org/ (1 June 2009, date last accessed) [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Oken E. Excess gestational weight gain amplifies risks among obese mothers. Epidemiology. 2008 doi: 10.1097/EDE.0b013e3181880ef5. [DOI] [PubMed] [Google Scholar]
- Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322. doi: 10.1016/j.ajog.2006.11.027. e321–e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke NC, Huston-Presley L, Amini SB, Kalhan S, Catalano PM. Longitudinal changes in energy expenditure and body composition in obese women with normal and impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2004;287:E472–E479. doi: 10.1152/ajpendo.00589.2003. [DOI] [PubMed] [Google Scholar]
- Olafsdottir AS, Skuladottir GV, Thorsdottir I, Hauksson A, Steingrimsdottir L. Maternal diet in early and late pregnancy in relation to weight gain. Int J Obes (Lond) 2006;30:492–499. doi: 10.1038/sj.ijo.0803184. [DOI] [PubMed] [Google Scholar]
- Olson CM, Strawderman MS, Reed RG. Efficacy of an intervention to prevent excessive gestational weight gain. Am J Obstet Gynecol. 2004;191:530–536. doi: 10.1016/j.ajog.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Ong KK. Size at birth, postnatal growth and risk of obesity. Horm Res. 2006;65(Suppl. 3):65–69. doi: 10.1159/000091508. [DOI] [PubMed] [Google Scholar]
- Oostdam N, van Poppel MN, Eekhoff EM, Wouters MG, van Mechelen W. Design of FitFor2 study: the effects of an exercise program on insulin sensitivity and plasma glucose levels in pregnant women at high risk for gestational diabetes. BMC Pregnancy Childbirth. 2009a;9:1. doi: 10.1186/1471-2393-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostdam N, van Poppel MN, Eekhoff EM, Wouters MG, van Mechelen W. Design of FitFor2 study: the effects of an exercise program on insulin sensitivity and plasma glucose levels in pregnant women at high risk for gestational diabetes. BMC Pregnancy Childbirth. 2009b;9:1. doi: 10.1186/1471-2393-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. Br Med J. 2001;323:1331–1335. doi: 10.1136/bmj.323.7325.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen J. Diabetes and Pregnancy—Blood Sugar of Newborn Infants. Copenhagan: Danish Science Press Ltd; 1952. [PubMed] [Google Scholar]
- Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med. 1983;308:242–245. doi: 10.1056/NEJM198302033080502. [DOI] [PubMed] [Google Scholar]
- Pettitt DJ, Knowler WC, Bennett PH, Aleck KA, Baird HR. Obesity in offspring of diabetic Pima Indian women despite normal birth weight. Diabetes Care. 1987;10:76–80. doi: 10.2337/diacare.10.1.76. [DOI] [PubMed] [Google Scholar]
- Pettitt DJ, Bennett PH, Saad MF, Charles MA, Nelson RG, Knowler WC. Abnormal glucose tolerance during pregnancy in Pima Indian women. Long-term effects on offspring. Diabetes. 1991;40(Suppl. 2):126–130. doi: 10.2337/diab.40.2.s126. [DOI] [PubMed] [Google Scholar]
- Pettitt DJ, Nelson RG, Saad MF, Bennett PH, Knowler WC. Diabetes and obesity in the offspring of Pima Indian women with diabetes during pregnancy. Diabetes Care. 1993;16:310–314. doi: 10.2337/diacare.16.1.310. [DOI] [PubMed] [Google Scholar]
- Pietiläinen KH, Kaprio J, Räsänen M, Winter T, Rissanen A, Rose RJ. Tracking of body size from birth to late adolescence: contributions of birth length, birth weight, duration of gestation, parents' body size, and twinship. Am J Epidemiol. 2001;154:21–29. doi: 10.1093/aje/154.1.21. [DOI] [PubMed] [Google Scholar]
- Pitkin RM. Nutritional support in obstetrics and gynecology. Clin Obstet Gynecol. 1976;19:489–513. doi: 10.1097/00003081-197609000-00002. [DOI] [PubMed] [Google Scholar]
- Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnant women. Int J Obes Relat Metab Disord. 2002;26:1494–1502. doi: 10.1038/sj.ijo.0802130. [DOI] [PubMed] [Google Scholar]
- Poobalan AS, Aucott LS, Gurung T, Smith WC, Bhattacharya S. Obesity as an independent risk factor for elective and emergency caesarean delivery in nulliparous women—systematic review and meta-analysis of cohort studies. Obes Rev. 2009;10:28–35. doi: 10.1111/j.1467-789X.2008.00537.x. [DOI] [PubMed] [Google Scholar]
- Potter JM, Nestel PJ. The hyperlipidemia of pregnancy in normal and complicated pregnancies. Am J Obstet Gynecol. 1979;133:165–170. doi: 10.1016/0002-9378(79)90469-1. [DOI] [PubMed] [Google Scholar]
- Rajasingam D, Seed PT, Briley AL, Shennan AH, Poston L. A prospective study of pregnancy outcome and biomarkers of oxidative stress in nulliparous obese women. Am J Obstet Gynecol. 2009;200:395e391–399. doi: 10.1016/j.ajog.2008.10.047. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87:4231–4237. doi: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Divergent metabolic and vascular phenotypes in pre-eclampsia and intrauterine growth restriction: relevance of adiposity. J Hypertens. 2004;22:2177–2183. doi: 10.1097/00004872-200411000-00021. [DOI] [PubMed] [Google Scholar]
- Rasmussen KM, Ann L. Committee to Reexamine IOM Pregnancy Weight Guidelines. Institute of Medicine, National Research Council; 2009. [Google Scholar]
- Rasmussen F, Johansson M. The relation of weight, length and ponderal index at birth to body mass index and overweight among 18-year-old males in Sweden. Eur J Epidemiol. 1998a;14:373–380. doi: 10.1023/a:1007411613576. [DOI] [PubMed] [Google Scholar]
- Rasmussen F, Johansson M. The relation of weight, length and ponderal index at birth to body mass index and overweight among 18-year-old males in Sweden. Eur J Epidemiol. 1998b;14:373–380. doi: 10.1023/a:1007411613576. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Chu SY, Kim SY, Schmid CH, Lau J. Maternal obesity and risk of neural tube defects: a meta-analysis. Am J Obstet Gynecol. 2008;198:611–619. doi: 10.1016/j.ajog.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, Fowler S, Kahn SE. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774–4779. doi: 10.1210/jc.2008-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- Reece EA, Leguizamón G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet. 2009;373:1789–1797. doi: 10.1016/S0140-6736(09)60515-8. [DOI] [PubMed] [Google Scholar]
- Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, Steer C, Sherriff A. Early life risk factors for obesity in childhood: cohort study. Br Med J. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc. 2009;109:1004–1011. doi: 10.1016/j.jada.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo T, Metzger BE, Burns WJ, Burns K. Correlations between antepartum maternal metabolism and child intelligence. N Engl J Med. 1991;325:911–916. doi: 10.1056/NEJM199109263251303. [DOI] [PubMed] [Google Scholar]
- Rizzo TA, Dooley SL, Metzger BE, Cho NH, Ogata ES, Silverman BL. Prenatal and perinatal influences on long-term psychomotor development in offspring of diabetic mothers. Am J Obstet Gynecol. 1995;173:1753–1758. doi: 10.1016/0002-9378(95)90422-0. [DOI] [PubMed] [Google Scholar]
- Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–2015. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- Ryan EA, O'Sullivan MJ, Skyler JS. Insulin action during pregnancy. Studies with the euglycemic clamp technique. Diabetes. 1985;34:380–389. doi: 10.2337/diab.34.4.380. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Walsh BW. Sex hormones and lipoprotein metabolism. Curr Opin Lipidol. 1994;5:236–240. doi: 10.1097/00041433-199405030-00012. [DOI] [PubMed] [Google Scholar]
- Salsberry PJ, Reagan PB. Dynamics of early childhood overweight. Pediatrics. 2005;116:1329–1338. doi: 10.1542/peds.2004-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vera I, Bonet B, Viana M, Quintanar A, Martin MD, Blanco P, Donnay S, Albi M. Changes in plasma lipids and increased low-density lipoprotein susceptibility to oxidation in pregnancies complicated by gestational diabetes: consequences of obesity. Metabolism. 2007;56:1527–1533. doi: 10.1016/j.metabol.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Sattar N, Greer IA, Louden J, Lindsay G, McConnell M, Shepherd J, Packard CJ. Lipoprotein subfraction changes in normal pregnancy: threshold effect of plasma triglyceride on appearance of small, dense low density lipoprotein. J Clin Endocrinol Metab. 1997;82:2483–2491. doi: 10.1210/jcem.82.8.4126. [DOI] [PubMed] [Google Scholar]
- Sattar N, Tan CE, Han TS, Forster L, Lean ME, Shepherd J, Packard CJ. Associations of indices of adiposity with atherogenic lipoprotein subfractions. Int J Obes Relat Metab Disord. 1998;22:432–439. doi: 10.1038/sj.ijo.0800604. [DOI] [PubMed] [Google Scholar]
- Sattar N, Clark P, Holmes A, Lean ME, Walker I, Greer IA. Antenatal waist circumference and hypertension risk. Obstet Gynecol. 2001;97:268–271. doi: 10.1016/s0029-7844(00)01136-4. [DOI] [PubMed] [Google Scholar]
- Schaefer-Graf UM, Pawliczak J, Passow D, Hartmann R, Rossi R, Buhrer C, Harder T, Plagemann A, Vetter K, Kordonouri O. Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care. 2005;28:1745–1750. doi: 10.2337/diacare.28.7.1745. [DOI] [PubMed] [Google Scholar]
- Schaefer-Graf UM, Graf K, Kulbacka I, Kjos SL, Dudenhausen J, Vetter K, Herrera E. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care. 2008;31:1858–1863. doi: 10.2337/dc08-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, Regan L, Robinson S. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;25:1175–1182. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- Seidman DS, Laor A, Gale R, Stevenson DK, Danon YL. A longitudinal study of birth weight and being overweight in late adolescence. Am J Dis Child. 1991;145:782–785. [PubMed] [Google Scholar]
- Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195:1100–1103. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Shields BM, Knight BA, Powell RJ, Hattersley AT, Wright DE. Assessing newborn body composition using principal components analysis: differences in the determinants of fat and skeletal size. BMC Pediatr. 2006;6:24. doi: 10.1186/1471-2431-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siega-Riz AM, Adair LS, Hobel CJ. Institute of Medicine maternal weight gain recommendations and pregnancy outcome in a predominantly Hispanic population. Obstet Gynecol. 1994;84:565–573. [PubMed] [Google Scholar]
- Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 1998;21(Suppl. 2):B142–B149. [PubMed] [Google Scholar]
- Simonen RL, Perusse L, Rankinen T, Rice T, Rao DC, Bouchard C. Familial aggregation of physical activity levels in the Quebec Family Study. Med Sci Sports Exerc. 2002;34:1137–1142. doi: 10.1097/00005768-200207000-00014. [DOI] [PubMed] [Google Scholar]
- Sivan E, Chen X, Homko CJ, Reece EA, Boden G. Longitudinal study of carbohydrate metabolism in healthy obese pregnant women. Diabetes Care. 1997;20:1470–1475. doi: 10.2337/diacare.20.9.1470. [DOI] [PubMed] [Google Scholar]
- Sivan E, Homko CJ, Whittaker PG, Reece EA, Chen X, Boden G. Free fatty acids and insulin resistance during pregnancy. J Clin Endocrinol Metab. 1998;83:2338–2342. doi: 10.1210/jcem.83.7.4927. [DOI] [PubMed] [Google Scholar]
- Sivan E, Homko CJ, Chen X, Reece EA, Boden G. Effect of insulin on fat metabolism during and after normal pregnancy. Diabetes. 1999;48:834–838. doi: 10.2337/diabetes.48.4.834. [DOI] [PubMed] [Google Scholar]
- Smith GC, Shah I, Pell JP, Crossley JA, Dobbie R. Maternal obesity in early pregnancy and risk of spontaneous and elective preterm deliveries: a retrospective cohort study. Am J Public Health. 2007;97:157–162. doi: 10.2105/AJPH.2005.074294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlstrom A, Forsum E. Changes in adipose tissue volume and distribution during reproduction in Swedish women as assessed by magnetic resonance imaging. Am J Clin Nutr. 1995;61:287–295. doi: 10.1093/ajcn/61.2.287. [DOI] [PubMed] [Google Scholar]
- Soltani H, Fraser RB. A longitudinal study of maternal anthropometric changes in normal weight, overweight and obese women during pregnancy and postpartum. Br J Nutr. 2000;84:95–101. doi: 10.1017/s0007114500001276. [DOI] [PubMed] [Google Scholar]
- Sorensen HT, Sabroe S, Rothman KJ, Gillman M, Fischer P, Sorensen TI. Relation between weight and length at birth and body mass index in young adulthood: cohort study. Br Med J. 1997;315:1137. doi: 10.1136/bmj.315.7116.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinnewijn WE, Lotgering FK, Struijk PC, Wallenburg HC. Fetal heart rate and uterine contractility during maternal exercise at term. Am J Obstet Gynecol. 1996;174:43–48. doi: 10.1016/s0002-9378(96)70371-x. [DOI] [PubMed] [Google Scholar]
- Stehbens JA, Baker GL, Kitchell M. Outcome at ages 1, 3, and 5 years of children born to diabetic women. Am J Obstet Gynecol. 1977;127:408–413. doi: 10.1016/0002-9378(77)90499-9. [DOI] [PubMed] [Google Scholar]
- Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301:636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol. 2009;201:58.e1–8. doi: 10.1016/j.ajog.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004;104:720–726. doi: 10.1097/01.AOG.0000141442.59573.cd. [DOI] [PubMed] [Google Scholar]
- Taggart NR, Holliday RM, Billewicz WZ, Hytten FE, Thomson AM. Changes in skinfolds during pregnancy. Br J Nutr. 1967;21:439–451. doi: 10.1079/bjn19670045. [DOI] [PubMed] [Google Scholar]
- Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123:1177–1183. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Elliott EJ. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD006296.pub2. CD006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD005105.pub2. CD005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GN, Halliday D. Protein turnover in pregnancy. Eur J Clin Nutr. 1992;46:411–417. [PubMed] [Google Scholar]
- Thornton YS, Smarkola C, Kopacz SM, Ishoof SB. Perinatal outcomes in nutritionally monitored obese pregnant women: a randomized clinical trial. J Natl Med Assoc. 2009;101:569–577. doi: 10.1016/s0027-9684(15)30942-1. [DOI] [PubMed] [Google Scholar]
- Tieu J, Crowther CA, Middleton P. Dietary advice in pregnancy for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD006674.pub2. CD006674. [DOI] [PubMed] [Google Scholar]
- Torloni MR, Betran AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, Valente O. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10:194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- Tuffnell DJ, West J, Walkinshaw SA. Treatments for gestational diabetes and impaired glucose tolerance in pregnancy. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD003395. CD003395. [DOI] [PubMed] [Google Scholar]
- Vazquez G, Duval S, Jacobs DR, Jr, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29:115–128. doi: 10.1093/epirev/mxm008. [DOI] [PubMed] [Google Scholar]
- Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368:1164–1170. doi: 10.1016/S0140-6736(06)69473-7. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Siega-Riz AM, Moos MK, Deierlein A, Mumford S, Knaack J, Thieda P, Lux LJ, Lohr KN. Outcomes of maternal weight gain. Evid Rep Technol Assess (Full Rep) 2008;168:1–223. [PMC free article] [PubMed] [Google Scholar]
- Warth MR, Arky RA, Knopp RH. Lipid Metabolism in Pregnancy. III. Altered lipid composition in intermediate, very low, low, and high-density lipoprotein fractions. J Clin Endocrinol Metab. 1975;41:649–655. doi: 10.1210/jcem-41-4-649. [DOI] [PubMed] [Google Scholar]
- Webb JB, Siega-Riz AM, Dole N. Psychosocial determinants of adequacy of gestational weight gain. Obesity (Silver Spring) 2009;17:300–309. doi: 10.1038/oby.2008.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissgerber TL, Wolfe LA, Davies GA, Mottola MF. Exercise in the prevention and treatment of maternal-fetal disease: a review of the literature. Appl Physiol Nutr Metab. 2006;31:661–674. doi: 10.1139/h06-060. [DOI] [PubMed] [Google Scholar]
- Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29–e36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- Willommet L, Schutz Y, Whitehead R, Jequier E, Fern EB. Whole body protein metabolism and resting energy expenditure in pregnant Gambian women. Am J Physiol Endocrinol Metab. 1992;263:E624–E631. doi: 10.1152/ajpendo.1992.263.4.E624. [DOI] [PubMed] [Google Scholar]
- Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes (Lond) 2008;32:495–501. doi: 10.1038/sj.ijo.0803710. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Part I: the Problem of Overweight and Obesity: Preventing and Managing the Global Epidemic. Geneva: World Health Organization Consultation on Obesity; 2000. [PubMed] [Google Scholar]
- World Health Organization. 2006 Fact Sheet No.311. [Google Scholar]
- World Health Organization. Global Database on Body Mass Index. 2009 http://apps.who.int/bmi/index.jsp . [Google Scholar]
- Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens. 2009;22:215–220. doi: 10.1038/ajh.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrotniak BH, Shults J, Butts S, Stettler N. Gestational weight gain and risk of overweight in the offspring at age 7 y in a multicenter, multiethnic cohort study. Am J Clin Nutr. 2008;87:1818–1824. doi: 10.1093/ajcn/87.6.1818. [DOI] [PubMed] [Google Scholar]
- Zhang S, Folsom AR, Flack JM, Liu K. Body fat distribution before pregnancy and gestational diabetes: findings from coronary artery risk development in young adults (CARDIA) study. Br Med J. 1995;311:1139–1140. doi: 10.1136/bmj.311.7013.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann MB, Gubeli C, Puntener C, Molinari L. Detection of overweight and obesity in a national sample of 6-12-y-old Swiss children: accuracy and validity of reference values for body mass index from the US Centers for Disease Control and Prevention and the International Obesity Task Force. Am J Clin Nutr. 2004;79:838–843. doi: 10.1093/ajcn/79.5.838. [DOI] [PubMed] [Google Scholar]