Abstract
Development and validation of a method for simultaneous identification and quantification of Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), cannabinol (CBN), and metabolites 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THCCOOH) in oral fluid. Simultaneous analysis was problematic due to different physicochemical characteristics and concentration ranges. Neutral analytes, such as THC and CBD, are present in ng/mL, rather than pg/mL concentrations, as observed for the acidic THCCOOH biomarker in oral fluid. THCCOOH is not present in cannabis smoke, definitively differentiating cannabis use from passive smoke exposure. THC, 11-OH-THC, THCCOOH, CBD, and CBN quantification was achieved in a single oral fluid specimen collected with the Quantisal™ device. One mL oral fluid/buffer solution (0.25mL oral fluid and 0.75mL buffer) was applied to conditioned CEREX® Polycrom™ THC solid phase extraction (SPE) columns. After washing, THC, 11-OH-THC, CBD, and CBN were eluted with hexane/acetone/ethyl acetate (60:30:20, v/v/v), derivatized with N, O-bis-(trimethylsilyl) trifluoroacetamide and quantified by two-dimensional gas chromatography electron ionization mass spectrometry (2D-GCMS) with cold trapping. Acidic THCCOOH was separately eluted with hexane/ethyl acetate/acetic acid (75:25:2.5, v/v/v), derivatized with trifluoroacetic anhydride and hexafluoroisopropanol, and quantified by the more sensitive 2D-GCMS–electron capture negative chemical ionization (NCI-MS). Linearity was 0.5-50ng/mL for THC, 11-OH-THC, CBD and 1-50ng/mL for CBN. The linear dynamic range for THCCOOH was 7.5–500pg/mL. Intra-and inter-assay imprecision as percent RSD at three concentrations across the linear dynamic range were 0.3%-6.6%. Analytical recovery was within 13.8% of target. This new SPE 2D-GCMS assay achieved efficient quantification of five cannabinoids in oral fluid, including pg/mL concentrations of THCCOOH by combining differential elution, 2D-GCMS with electron ionization and negative chemical ionization. This method will be applied to quantification of cannabinoids in oral fluid specimens from individuals participating in controlled cannabis and Sativex® (50% THC and 50% CBD) administration studies, and during cannabis withdrawal.
Keywords: Tetrahydrocannabinol, Cannabinoids, Oral fluid, Two-dimensional chromatography, 11-Nor-9-carboxy-tetrahydrocannabinol
1. Introduction
Cannabis (marijuana) is the most widely used illegal substance in the world [1,2]. Humans smoke or ingest cannabis for its psychotropic effects [3-5]. Δ9-tetrahydrocannabinol (THC) is the primary psychoactive constituent among several hundred chemical compounds present in cannabis [6]. THC undergoes extensive metabolism, primarily in the liver by microsomal hydroxylation and oxidation catalyzed by enzymes of the cytochrome P450 complex. Phase 1 metabolism forms psychoactive 11-hydroxy-THC (11-OH-THC), followed by further oxidation to the inactive 11-nor-9-carboxy-THC (THCCOOH) [7,8]. Additional hydroxy and dihydroxy metabolites also are formed. Phase II metabolism produces more hydrophilic THC, 11-OH-THC and THCCOOH glucuronide and sulfate conjugates to improve elimination in urine [4]. Cannabidiol (CBD) and cannabinol (CBN), two other natural cannabinoids found in relatively high concentrations in cannabis, have their own pharmacological profiles but contribute little to cannabis' psychotropic activity [9]. Sensitive and specific analytical methods for determining cannabinoid concentrations in biological tissues are needed due to the importance of cannabinoids as performance-impairing drugs, and more recently, as pharmacotherapies. Sativex® is a new cannabinoid pharmacotherapy containing approximately 50% THC and 50% CBD for analgesia for cancer and neuropathic pain, as an anti-spasmodic in multiple sclerosis, and numerous other indications. Analytical methods for quantifying cannabinoids and metabolites in plasma [10-12], blood [13], urine [13,14], hair [15], sweat [16], and oral fluid [17] are available for pharmacokinetic and pharmacodynamic studies as well as for forensic applications.
There are advantages and disadvantages for monitoring drug use with each biological matrix. Disadvantages of urine testing include ease of adulteration, dilution by increasing fluid intake, need for same-sex collectors, and embarrassment during collection. Blood collection is more invasive, painful and requires trained personnel. Oral fluid is an increasingly important alternative matrix due to its safe, non-invasive collection under direct observation, and reduced potential for dilution and adulteration. Oral fluid testing is now the specimen of choice for monitoring driving under the influence of drugs (DUID) [18]. The analysis of cannabinoids for workplace drug testing and DUID is critically important, as this class of drugs represents the highest number of positive tests [18,19]. Due to increasing interest in oral fluid drug testing, multiple sample collection devices are commercially available. Oral fluid is collected when the device is placed between the gum and teeth. Different devices collect different amounts of oral fluid and with different precision. Some devices have an adequacy volume indicator when sufficient oral fluid is collected. The pad is placed in an elution buffer to improve drug recovery. Performance varies greatly between devices. We selected the Quantisal™ device for this research, due to its advantages described in the methods section. Cannabinoid analysis in oral fluid has been problematic due to adherence of cannabinoids to the collection device reducing sensitivity, measurement of low concentrations in minimal specimen volume, and the potential for environmental contamination of oral fluid from smoked and oral drug administration.
Oral fluid contains predominantly THC rather than 11-OH-THC or THCCOOH metabolites due to contamination of the oral mucosa and oral fluid during cannabis smoking or oral ingestion of the drug. Initially, THC only was detected approximately 2h after smoking because of low sensitivity with available instrumentation [20]. Kauert et al. [21] reported detection of THC in oral fluid with the Intercept™ collection device in concentrations from 18-1041ng/mL by GCMS. Laloup et al. [22] developed a liquid-liquid extraction followed by LCMSMS for THC, also collected with the Intercept™ collection device, with linearity of 0.1-10ng/mL in 500μL oral fluid. Moore et al. [23] reported THC concentrations of 0.7-93ng/mL with the Quantisal™ collection device, but also included CBD, CBN and 2-carboxy-THC. CBD was not detected in oral fluid specimens, but CBN, a THC oxidation product, was detectable up to 2h after smoking and maximum concentration of 4.1ng/mL.
The source of THC in oral fluid also could be from passive exposure to cannabis smoke. Detection of THC metabolites, 11-OH-THC or THCCOOH, could provide evidence of active smoking, but quantification of these analytes requires a highly sensitive method capable of detection of pg/mL concentrations. Day et al. [24] first identified THCCOOH in oral fluid up to 142pg/mL with gas chromatography tandem mass spectrometry (GCMSMS). Recently, Moore et al. [25] developed a 2D-GC-NCI-MS assay specifically for THCCOOH alone in oral fluid with a limit of quantification of 2pg/mL.
Ability of analytical methods to identify cannabinoids in oral fluid varies with oral fluid collection procedure [25-27], number of simultaneously analyzed compounds [23,26-28], and analytical instrumentation [22,24,29]. Our approach was to develop a new solid phase extraction (SPE) procedure to simultaneously quantify THC, 11-OH-THC, THCCOOH, CBD and CBN from a single oral fluid specimen collected with the Quantisal™ device, with differential extraction, chromatography, and detection according to required sensitivities. This assay was developed to support our controlled oral THC and Sativex® administration studies. Cannabinoid concentrations after oral administration are expected to be lower compared to levels observed after cannabis smoking. Method development emphasized achieving enhanced analytical sensitivity with improved signal-to-noise (S/N) and lower detection limits by 2D-GCMS with cold trapping for THC, 11-OH-THC, CBD and CBN, and negative chemical ionization for THCCOOH.
2. Material and methods
2.1. Chemicals and reagents
THC, 11-OH-THC, THCCOOH, CBD, CBN (1mg/mL) and internal standards THC-d3, 11-OH-THC-d3, THCCOOH-d3 and CBD-d3 (100μg/mL) were purchased from Cerilliant Corporation (Round Rock, TX, USA). Potential interferents including acetaminophen, acetylsalicylic acid, ibuprofen, caffeine, nicotine, cotinine, norcotinine, buprenorphine, norbuprenorphine, cocaine, norcocaine, benzoylecgonine, norbenzoylecgonine, ecgonine ethyl ester, ecgonine methyl ester, anhydroecgonine methyl ester, ecgonine, m-OH-cocaine, p-OH-cocaine, m-OH-benzoylecgonine, p-OH-benzoylecgonine, methadone, EDDP, EMDP, amphetamine, methamphetamine, MDMA, MDA, codeine, norcodeine, morphine, normorphine, morphine-3-glucoronide, morphine-3-glucoronide, 6-acetylmorphine, 6-acetylcodeine, hydrocodone, hydromorphone, oxycodone, diazepam, lorazepam, oxazepam, alprazolam, imipramine, clomipramine, fluoxetine, norfluoxetine, clonidine, pentazocine and phencyclidine also were obtained from Cerilliant. N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) containing 1% trimethylchlorosilane (TMCS) and trifluoroacetic anhydride (TFAA) were from Thermo Fisher Scientific Inc. (Rockford, IL, USA). Hexafluoroisopropanol (HFIP) was supplied by Campbell Science (Rockton, IL, USA) and CEREX® Polycrom™ THC (3cc/35mg) extraction columns were from SPEware Corporation (Baldwin Park, CA, USA). Acetone, acetonitrile, hexane, and ethyl acetate were purchased from Sigma-Aldrich (Milwaukee, WI, USA), and ammonium hydroxide (28-30%), glacial acetic acid, and methanol were from Mallinckrodt Baker (Phillipsburg, NJ, USA). All chemicals were ACS reagent grade and organic solvents were HPLC grade. Quantisal™ devices for the collection of oral fluid specimens and Quantisal™ transport buffer for diluting calibrator standards were obtained from Immunalysis Corporation (Pomona, CA, USA).
2.2. Oral fluid collection procedure
The Quantisal™ collection device consists of an absorptive cellulose pad with a polypropylene stem and plastic tube containing a transport buffer solution. The collection pad has a volume adequacy indicator that turns blue when 1.0±0.1 mL oral fluid is collected. During specimen collection, the collection pad is placed into the mouth, and when the indicator window turns blue, the pad is removed and placed into the collection/transport tube containing 3mL of buffer. The buffer solution in the collection device [pH 6.6] inhibits bacterial growth, stabilizes drug content and improves elution of drugs from the collection pad, but also dilutes authentic oral fluid concentrations by a factor of four. A total specimen volume of 4mL (1mL oral fluid + 3mL buffer) is available for analysis, allowing confirmation of multiple drugs of abuse within the same specimen.
2.3. Calibrator and quality control solutions
1mg/mL solutions of THC, 11-OH-THC, CBD and CBN were diluted with methanol to prepare a mixed 10μg/mL stock solution that was stored at -20°C. Working calibrators (2.5-250ng/mL) were prepared by dilution of the stock calibrator with methanol. 50μL working calibrator was added to 1mL blank oral fluid-Quantisal™ buffer mixture to create daily calibration curves of 0.5, 1, 2.5, 5, 10, 25, and 50ng/mL containing THC, 11-OH-THC, CBD and CBN.
Working THCCOOH calibrators were prepared separately from 1mg/mL standards in methanol. A 10μg/mL intermediate stock in methanol was utilized to create working calibrators from 0.0375-2.5ng/mL. 50μL of each THCCOOH working calibrator was added to 1mL blank human oral fluid-Quantisal™ buffer mixture to produce daily THCCOOH calibration curves of 7.5, 10, 25, 50, 100, 250, and 500pg/mL. A ×4 dilution factor converted concentrations to approximate oral fluid concentrations due to the dilution of oral fluid with buffer.
Quality control (QC) methanolic solutions were prepared from different lots than those used for calibrators. Combined low (5ng/mL), medium (25ng/mL) and high (100ng/mL) working control solutions containing THC, 11-OH-THC, CBD and CBN and individual THCCOOH low (0.05ng/mL), medium (0.25ng/mL) and high (1ng/mL) controls were prepared in methanol. Addition of 75μL of appropriate working control solutions to 1mL blank oral fluid-Quantisal™ buffer mixture produced three QC across the dynamic linear range for THC, 11-OH-THC, CBD, CBN, 1.5, 7.5, 30ng/mL, and for THCCOOH, 15, 75, 300pg/mL, respectively.
The working methanolic internal standard solution contained deuterated analogs for all analytes of interest at 80ng/mL for THC-d3, 11-OH-THC-d3 and CBD-d3 and 0.8ng/mL for THCCOOH-d3.
2.4. Solid-phase extraction and derivatization
One mL blank oral fluid-Quantisal™ buffer mixture containing fortified calibrators, QC samples or authentic clinical specimens was combined with 25μL working internal standard and gently vortexed. Proteins were precipitated by addition of 1mL ice-cold acetonitrile, followed by vortexing. Tubes were centrifuged at 1855×g for 7min to pellet protein, and supernatants were decanted onto CEREX® Polycrom™ THC extraction columns preconditioned with methanol (1mL). Columns were washed with 3mL distilled water/acetonitrile/ammonium hydroxide (84:15:1, v/v/v) and dried under full vacuum (15 in Hg) for 15min. THC, 11-OH-THC, CBD and CBN were eluted with hexane/acetone/ethyl acetate (60:30:20, v/v/v; 3mL) into conical glass centrifuge tubes. Extraction columns were dried for 1min, and THCCOOH was eluted into separate conical glass centrifuge tubes with 3mL hexane/ethyl acetate/glacial acetic acid (75:25:2.5, v/v/v). Eluates were evaporated to dryness under a stream of nitrogen at 35°C in a Zymark TurbovapLV® evaporator.
Extracted residues from the first elution solvent (THC, 11-OH-THC, CBD, CBN) were reconstituted with 20μL of BSTFA and derivatized at 65°C for 40min. Trimethylsilyl (TMS) derivatives were cooled, centrifuged at 1855×g for 3min, and transferred to autosampler vials, for GCMS analysis.
THCCOOH derivatization was achieved by adding 40μL TFAA and 20μL HFIP to the second elution residue and incubating at 65°C for 40min. Fluorinated derivatives were cooled, evaporated to dryness and reconstituted in 20μL of toluene before GC-NCI-MS analysis.
2.5. Instrumentation
Sensitive quantification of THC, 11-OH-THC, CBD, CBN and THCCOOH in oral fluid was achieved by separate injections on two analytical systems utilizing different ionization techniques. Both systems were configured with a Deans switch, flame ionization detector (FID), 7683 autosampler, and 6890N gas chromatograph (GC) interfaced to an 5973 mass selective detector (MSD) (Agilent Technologies, Wilmington, DE). Both GCs also were equipped with a cryogenic focusing trap, mounted inside the GC oven (Joint Analytical Systems, Marlton, NJ) at the head of the second GC column that was cooled with compressed air. The Deans switch connected two capillary chromatographic columns with a pneumatic valve directing output of the primary column to either the FID or the inlet of the secondary column. The inlet end of the secondary column was inserted through the cryogenic trap and the outlet directed to the MSD. Thus, heart cuts of the flow from the first GC column containing the analyte of interest were diverted and cold trapped at the head of the second GC column, eliminating most matrix from reaching the second column and the mass spectrometer. This technique, 2D-GCMS, utilizes the power of separation of two GC columns, termed here GC-GC, but should be distinguished from GCXGC, a technique where two chromatographic columns are placed in series and effluent passes fully through both columns. The first instrument was operated in electron ionization (EI) mode, and the second was operated in NCI mode with ammonia as reagent gas. Operating parameters are listed in Table 1.
Table 1.
Gas chromatography, Deans switch, flame ionization detector and back inlet (cold trap) method parameters for the detection and quantification of Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC), 11-nor-9-carboxy-THC (THCCOOH), cannabidiol (CBD), and cannabinol (CBN) in human oral fluid.
| Deans switch | EI | NCI | Back inlet | EI | NCI |
|---|---|---|---|---|---|
| FID restrictor L. | 300 cm | 205 cm | Initial T | 100 °C | 280 °C |
| FID restrictor i.d. | 0.180 mm | 0.180 mm | Initial time | 10.40 min | 4.50 min |
| Aux 3 pressure | 16.3 psi | 14.7 psi | Ramp #1: | 700°C/ min | 700°C/ min |
| Final T;time | 225°C;4.8 min | 150°C;1.3 min | |||
| Front inlet | Ramp #2: | 700°C/ min | 700°C/ min | ||
| Flow mode | Constant press. | Constant press. | Final T;time | 100°C;0.8 min | 280°C;0.0 min |
| Inlet T | 275°C | 275°C | Ramp #3: | 700°C/ min; | |
| Injection mode | Pulsed-splitless | Pulsed-splitless | Final T;time | 275°C;0.0 min | |
| Pulse pressure | 45 psi | 45 psi | Oven | ||
| Pulse time | 0.80 min | 1.00 min | Initial oven T | 185 °C | 150 °C |
| Purge flow | 25 mL/min | 50 mL/min | Initial oven hold | 0.5 min | 0.5 min |
| Purge time | 0.80 min | 0.98 min | Ramp #1: | 45°C/min | 40°C/min |
| Pressure | 31.60 psi | 21.4 psi | Final T;time | 225°C;3.0 min | 250°C;1.3 min |
| Total flow | 29.9 mL/min | 55.4 mL/min | Ramp #2: | 15 °C/min | 15°C/min |
| Injection volume | 4 μL | 4 μL | Final T;time | 275°C;1.58 min | 268°C;0.0 min |
| Liner type | Single-taper | Single-taper | Ramp #3: | 80°C/min | 120°C/min |
| FID and MSD | Final T;time | 195°C;2.3 min | 200 °C;0.0 min | ||
| FID T | 275°C | 275°C | Ramp #4: | 10 °C/min | 10°C/min |
| Hydrogen flow (OFF) | 30 mL/min | 30 mL/min | Final T;time | 230°C;0.0 min | 255°C;0.0 min |
| Air flow (OFF) | 400 mL/min | 400 mL/min | Ramp #5: | 25°C/min | |
| MSD reagent gas | Vacuum | Ammonia | Final T;time | 275°C;1.0 min | |
| MSD transfer line T | 280°C | 280°C | Post T | 300°C | 300°C |
| MS source T | 230°C | 150°C | Post time | 2 min | 1 min |
| MS quad T | 150°C | 150°C | Column 1 press. | 4.0 psi | 1.0 psi |
| EM Offset (Total) | 400 (2370) | 1200 (2420) | Column 2 press. | 90.0 psi | 90.0 psi |
EI – electron ionization; EM – electron multiplier; FID – flame ionization detector; L. - length; MS – mass spectrometry; MSD – mass selective detector; NCI – negative chemical ionization; press.-pressure; T – temperature.
2.5.1. Electron Ionization GCMS
The first group of analytes (THC, 11-OH-THC, CBD, CBN) was separated on a GC-GC equipped with ZB-50 (Phenomenex, Torrance, CA) primary column (30m × 0.25mm i.d., 0.25μm film thickness) and a DB-1MS (Agilent Technologies, Wilmington, DE) secondary column (15m × 0.25mm i.d., 0.25μm film thickness). 4μL TMS derivatives were introduced to the primary column in pulsed-splitless injection mode. Analyte elution times from the primary column were determined by injection of high concentration standards with the Deans switch regulator directing effluent via the restrictor to the FID. The cryogenic trap was maintained at 100°C to capture CBD, THC and CBN. Immediately after the last analyte was cold-trapped, the oven temperature was lowered to 195°C, the cryogenic trap ramped at 700°C/min and analytes re-vaporized for migration and separation through the secondary column. The later eluting compound (11-OH-THC) was refocused with a second cold-trap before being independently released onto the secondary column, during a slow oven temperature ramp.
The MSD was operated in EI selected ion monitoring (SIM) mode for THC, 11-OH-THC, CBD and CBN. Three ions for each analyte and two for each internal standard were acquired. Target and qualifier ions are presented in Table 2. MS interface, source and quadrupole temperatures were 280, 230, and 150°C, respectively.
Table 2.
Mass selective detector parameters for Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC), 11-nor-9-carboxy-THC (THCCOOH), cannabidiol (CBD), cannabinol (CBN) and respective deuterated analogs in human oral fluid.
| Analyte | Target ion | Qualifier ions | Deans Switch cuts (min) |
|---|---|---|---|
| CBD-d3 | 393.3 | 462.3 | 6.45 - 6.80 |
| CBD | 390.3 | 458.3, 443.3 | 6.45 - 6.80 |
| THC-d3a | 374.3 | 389.3 | 8.45 - 8.75 |
| THC | 371.3 | 386.3, 303.2 | 8.45 - 8.75 |
| CBN | 367.3 | 382.3, 310.2 | 9.50 - 9.90 |
| 11-OH-THC-d3 | 374.3 | 477.3 | 16.25 - 16.60 |
| 11-OH-THC | 371.3 | 474.3, 459.4 | 16.25 - 16.60 |
| THCCOOH-d3 b | 425.3 | 593.3 | 5.08 - 5.45 |
| THCCOOH b | 422.3 | 590.3 | 5.08 - 5.45 |
THC-d 3 also is internal standard for CBN
Data from two-dimensional gas chromatography mass spectrometry (2D-GCMS) with negative chemical ionization; all other data from 2D-GCMS with electron ionization.
2.5.2. Negative Chemical Ionization GCMS
Separation of THCCOOH was achieved with a DB-1MS (Agilent Technologies, Wilmington, DE) primary column (15m × 0.25mm i.d., 0.25μm film thickness) and ZB-50 (Zebron™; Phenomenex, Torrance, CA) secondary column (30m × 0.32mm i.d., 0.25μm film thickness). 4μL fluorinated derivatives were injected in pulsed-splitless injection mode. Analytes were introduced directly onto the primary column with Deans switch valve programming to divert a “cut” of the analyte elution band to the secondary column through the cryogenic trap to achieve additional chromatographic resolution. The MSD for THCCOOH was operated in NCI SIM mode. Pure ammonia (99.999%) was the reagent gas with a flow control setting of 35 (1.8×10-4Torr). The MS ion source and quadrupole were held at 150°C, the transfer line at 280°C, and operated at 1200eV relative to the daily autotune parameter. Four ions (two for THCCOOH and two for THCCOOH-d3) acquired in a single group were monitored. Target and qualifier ions are presented in Table 2.
2.6. Data analysis and method validation
Daily calibration was performed with Agilent MSD Chemstation software version D.01.00. Analytes were identified by comparing retention times (±0.15min) and qualifier ion ratios (±15%) with average values of calibrators assayed in the same batch. Quantification was based upon ratios of target ion to deuterated internal standard peak analyte areas. No commercially available deuterated analog was available for CBN; THC-d3 was utilized as internal standard. Data were fit by linear regression with 1/x weighting. Each calibrator concentration was required to be within ±15% of target (LOQ ±20%) when calculated against the full calibration curve. Calibration curves were established to encompass expected cannabinoid concentrations in oral fluid specimens.
Specificity, linearity, limits of detection [LOD] and quantification [LOQ], analytical recovery, intra- and inter-day imprecision, extraction efficiency, carryover and stability were determined. Specificity was defined as the ability to identify and quantify an analyte in the presence of potential endogenous or exogenous interferents. Drug-free oral fluid from 10 volunteers was fortified with internal standard and analyzed to document endogenous matrix effects and potential internal standard contribution. In addition, 50 potential interferents, including common drugs of abuse, co-administered drugs, metabolites, structurally similar compounds and over-the-counter medications were evaluated by adding 1000ng/mL of each potential interfering compound to low QC samples, 1.5ng/mL for all analytes of interest except THCCOOH that was present at 15pg/mL. Low QC samples were required to quantify within ±20% of target, meet ion ratio criteria and exhibit acceptable chromatographic parameters (peak shape, resolution) for all analytes in order to document no interference.
LOD and LOQ were determined in triplicate by assaying a series of decreasing concentrations of drug-fortified human oral fluid. LOD was determined to be the lowest analyte concentration with S/N ratio of at least 3 for all ions, acceptable chromatographic peak shape, retention time and qualifier ion ratios. LOQ was established as the lowest concentration with acceptable chromatographic peak shape, retention time, qualifier ion ratios, and with concentration within ±20% of target.
Fortified oral fluid samples exceeding the linear range for THC, 11-OH-THC, CBD and CBN (1000ng/mL) and THCCOOH (10ng/mL) were extracted and analyzed to evaluate carryover. Negative samples (containing only internal standard) were injected after each carryover challenge to quantify potential carryover from the previous injection.
Analytical recovery and imprecision were evaluated across the linear range with QC samples at target oral fluid concentrations of 1.5, 7.5, 30ng/mL for THC, 11-OH-THC, CBD, and CBN and 15, 75, 300pg/mL for THCCOOH. Analytical recovery was calculated as the difference between mean and target concentrations with 4 replicates in five batches (N=20), while imprecision was expressed as percent relative standard deviation (%RSD). Intra-assay imprecision was evaluated from six determinations per concentration in one batch, and inter-assay imprecision evaluated with four replicates at each QC concentration on five separate days (N=20).
Extraction efficiency was assessed at low, medium and high QC concentrations (N=4 for each) by fortifying blank oral fluid prior to and after SPE. Extraction efficiency was calculated by comparing average analyte peak areas in the samples fortified prior to SPE with peak areas in the samples fortified after SPE.
Dilution integrity was investigated by diluting quality control samples with drug free oral fluid–Quantisal™ buffer mixture (1:4). Fifty and ninety percent dilutions (v/v) were prepared in quadruplicate. Mean assayed concentrations of diluted samples were corrected by dilution factor (×2 or ×10) and compared to target concentrations.
Analyte stability was evaluated with fortified human oral fluid-Quantisal™ buffer samples at three QC concentrations. Samples (N=4) were fortified with non-deuterated analogs and stored at room temperature for 18h, 4°C for 72h, and at -20°C followed by thawing at room temperature for three freeze-thaw cycles. Samples were fortified with internal standard immediately prior to analysis. Concentrations of QC stability samples were compared to freshly prepared calibrators and controls. In addition, the stability of derivatized extracts also was examined. GC autosampler vials were left in the autosampler tray for 48h, re-injected, and concentrations compared to initial QC results.
3. Results
3.1. Method development
We developed and validated a selective and sensitive method for the simultaneous quantification of THC, 11-OH-THC, CBD, CBN and THCCOOH in oral fluid collected with the Quantisal™ collection device. It is necessary to validate all methods for each collection buffer, as ratios of oral fluid to buffer and buffer components vary by device. In order to mimic collection conditions, a drug-free oral fluid-Quantisal™ buffer mixture (1:4, v/v) was utilized as matrix for calibrators, controls and dilution integrity experiments.
Cold acetonitrile is frequently employed to precipitate proteins in plasma samples [30]. We found this technique also was appropriate for oral fluid. Equivalent volumes of acetonitrile and oral fluid buffer mixture (1:1, v/v) were vortexed and centrifuged to precipitate proteins, producing a clearer supernatant for SPE.
Despite the difference in expected THC and THCCOOH concentrations in oral fluid, our initial goal was to simultaneously extract and quantify all analytes of interest in a single GCMS injection. However, it became apparent that in order to achieve an acceptable LOQ for THCCOOH, it was necessary to employ not only two elution and derivatization techniques, but also two analytical instrumentation techniques. Although adequate sample volume was available with the Quantisal™ collection device, performing a separate extraction for THCCOOH was not cost or time-efficient. The goal was to efficiently recover all analytes with a single SPE.
Several types of SPE columns were evaluated to separate THC, 11-OH-THC, CBD and CBN from THCCOOH. Multiple SPE columns were investigated for the extraction of cannabinoids and metabolites during method development (UCT CSTHC206 200mg/6mL, SSDBX056 50mg/10mL, Biotage ISOLUTE® THC 100mg/3mL, and SPEWare Trace-B® 35mg/3cc) with varying degrees of success. Best results were achieved with the CEREX® Polycrom™ THC 35mg/3cc columns. Several published methods utilize CEREX® Polycrom™ THC columns for the extraction of THC and metabolites from urine [31], whole blood [32] and hair [33] employing varying amounts of hexane and ethyl acetate for elution.
Columns were washed with distilled water/acetonitrile/ammonium hydroxide 84:15:1, v/v/v and THC, 11-OH-THC, CBD, and CBN were eluted with the first elution solvent (hexane/acetone/ethyl acetate, 60:30:20, v/v/v). We discovered that the addition of acetone to the hexane-ethyl acetate mixture helped retain THCCOOH on the column, while THC, 11-OH-THC, CBD and CBN were efficiently eluted. THCCOOH was easily released from the extraction columns with the addition of glacial acetic acid to the hexane-ethyl acetate elution solvent.
It was possible to elute all analytes in a single step utilizing the more acidic elution solvent (hexane/ethyl acetate/glacial acetic acid, 75:25:2.5, v/v/v), however, it caused major interferences in the quantification of THC and CBN. Cleaner extracts with greater sensitivity was achieved by performing separate elution steps.
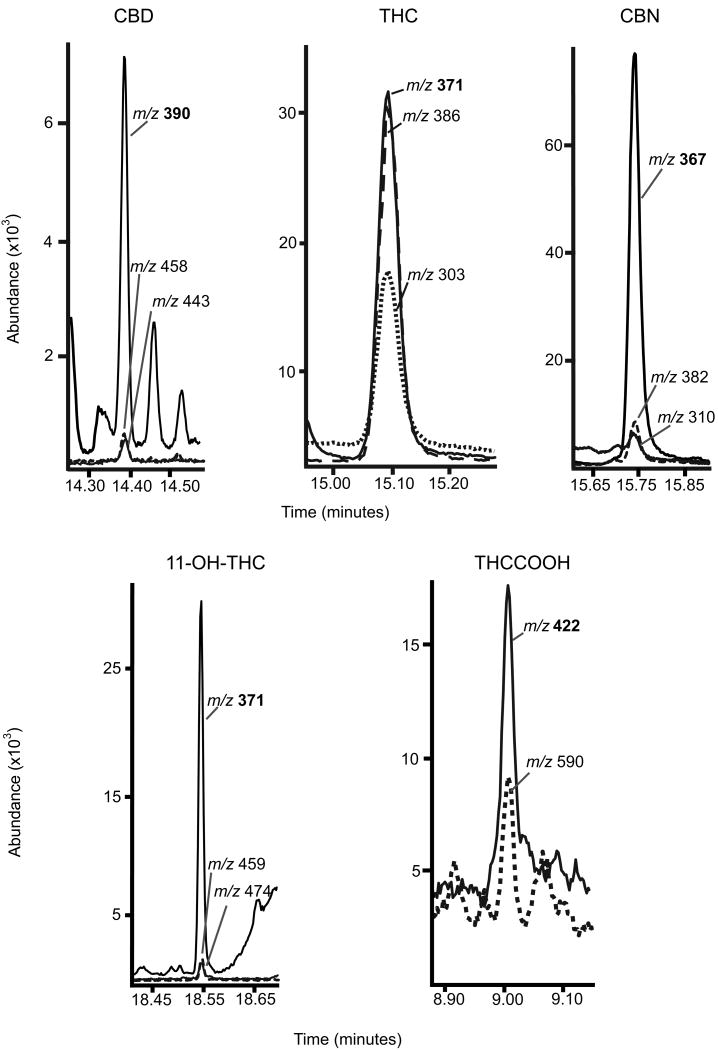
Eluents were collected and derivatized independently with BSTFA derivatizing THC, 11-OH-THC, CBD, and CBN prior to analysis by GCMS, and HFIP and TFAA derivatization of THCCOOH prior to GC-NCI-MS. Adequate sensitivity was achieved for the TMS derivatives of THC, 11-OH-THC, CBD, and CBN by injecting a larger 4μL sample in the pulsed-splitless mode. For GC-NCI-MS analysis, different combinations of derivatizing agents were evaluated to achieve the best THCCOOH sensitivity. Two chemically different derivatizing agents were needed to protect THCCOOH's carboxyl and hydroxyl groups. Four mixed derivatives were compared: hexafluorobutyric acid (HFBA) with HFIP, HFBA with pentafluoropropanol (PFPOH), pentafluoropropionic anhydride (PFPA) with PFPOH, and TFAA with HFIP. The TFAA and HFIP combination produced the highest sensitivity and cleanest derivatives, although only two confirmation ions, m/z 422 and 590, were available for monitoring. GC-NCI-MS with ammonia reagent gas provided enhanced sensitivity for fluorinated derivatives [34]. The THCCOOH LOQ (7.5pg/mL) was achieved with complex, sensitive analytical instrumentation and by modifying injection parameters to include a pulsed-splitless injection volume of 4μL. In addition, increasing the EM voltage helped improve sensitivity to the pg/mL range. Representative extracted ion chromatograms for analytes in oral fluid at the LOQ are presented in Figure 1.
Figure 1.
Extracted ion chromatograms for Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC), 11-nor-9-carboxy-THC (THCCOOH), cannabidiol (CBD), and cannabinol (CBN) in oral fluid fortified at the limit of quantification (LOQ) for each analyte. LOQ is 0.5ng/mL for THC, 11-OH-THC, CBD and 1ng/mL for CBN for two-dimensional gas chromatography mass spectrometry (2D-GCMS); and 7.5pg/mL for THCCOOH for 2D-GCMS with negative chemical ionization (NCI). Quantification ions are in bold.
Routine preventative maintenance consisted of replacing the septa and liner, and regularly clipping the head of the analytical column. Post-run temperatures, on both analytical systems, were increased and carrier gas flow reversed to back flush capillary columns. This procedure helped prolong column life and reduce the frequency of inlet, column and source maintenance. In addition, mass axis defects were evaluated before every run, and optimized ion signal (m/z) updated in the acquisition method.
3.2. Method validation
Endogenous matrix effects were evaluated in oral fluid fortified with internal standards collected from ten drug-free volunteers. There were no endogenous signal contributions for any analyte of interest. In addition, during each analytical run, a negative sample (blank oral fluid and internal standard) failed to show any interference, demonstrating that the internal standard did not contribute to measured concentrations. Exogenous interferences were assessed by fortifying low QC samples with 1000ng/mL of fifty potential interfering compounds. Quality control concentrations were within 17.3% of target and met ion ratio criteria for all analytes.
Separate elution, derivatization and characterization on independent analytical instrumentation produced two calibration curves. Calibrations were determined over six assays. Dynamic ranges were determined with seven calibrators (0.5-50ng/mL) for THC, 11-OH-THC and CBD, and six calibrators (1-50ng/mL) for CBN. Seven calibrators (7.5-500pg/mL) were employed for THCCOOH quantification. Characteristic calibration data, including LOD and LOQ for each analyte are presented in Table 3. Calibration curve R2 always exceeded 0.993. Quantification limits of 0.5ng/mL for THC, 11-OH-THC, CBD and 1ng/mL for CBN were achieved. The lower limit of quantification for THCCOOH (7.5pg/mL) was attained with GC-NCI-MS. Concentrations of all calibrators were within ±15% of target and ±20% for LOQ when calculated against the full calibration curve in all analytical batches. Negative samples injected immediately after samples containing 1000ng/mL of THC, 11-OH-THC, CBD, CBN and 10ng/mL of THCCOOH showed no evidence of carryover >LOD of the method.
Table 3.
Limits of detection (LOD) and quantification (LOQ), and mean (± standard deviation SD), slope, intercept and coefficient for determination (R2) for calibrations curves (N=6) for Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC), 11-nor-9-carboxy-THC (THCCOOH), cannabidiol (CBD), and cannabinol (CBN) in oral fluid.
| Analytes | LOD (ng/mL) | LOQ (ng/mL) | Linear range (ng/mL) | Slope (mean ± SD) | Intercept (mean ± SD) | R2 minimal |
|---|---|---|---|---|---|---|
| CBD | 0.5 | 0.5 | 0.5 - 50 | 0.111 (0.003) | 0.001 (0.001) | 0.9947 |
| THC | 0.5 | 0.5 | 0.5 - 50 | 0.118 (0.004) | -0.012 (0.004) | 0.9971 |
| CBN | 1 | 1 | 1 -50 | 0.574 (0.028) | -0.038 (0.017) | 0.9961 |
| 11-OH-THC | 0.4 | 0.5 | 0.5 - 50 | 0.114 (0.005) | -0.009 (0.002) | 0.9977 |
| THCCOOHa | 0.006 | 0.0075 | 0.0075 - 0.5 | 0.011 (0.001) | 0.047 (0.013) | 0.9933 |
Data from two-dimensional gas chromatography mass spectrometry (2D-GCMS) with negative chemical ionization; all other data from 2D-GCMS with electron ionization.
Analytical recovery and method imprecision were evaluated at three QC concentrations over the linear dynamic range of each curve. Analytical recovery and data from intra-assay (N=6) and inter-assay (N=20) imprecision are summarized in Table 4. Inter-assay imprecision (%RSD) ranged from 2.2-6.6% on four different days, while intra-assay imprecision was less than 5.2% (N=6). Evaluation of inter-assay variability by single-factor analysis of variance (ANOVA) with day as grouping variable, demonstrated differences between days (p<0.05) at medium and high QC concentrations. However, differences in daily mean analyte concentrations for these controls did not exceed 13.8% and were considered clinically insignificant. Interestingly, there were no differences (p>0.05) at low QC concentrations for all analytes. Analytical recovery calculated as the percent difference between mean and target concentrations of each analyte (N=20) ranged from 99.1-113.8%.
Table 4.
Analytical recovery and imprecision data for quantification of Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC), 11-nor-9-carboxy-THC (THCCOOH), cannabidiol (CBD), and cannabinol (CBN) in oral fluid.
| Imprecision | ||||
|---|---|---|---|---|
| Analytes | Target (ng/mL) | Inter-assay (mean %RSD,N=20, 5 replicates, 4 assays) | Intra-assay (%RSD, N=6) | Analytical recovery (N=20) |
| CBD | 1.5 | 2.8 | 1.1 | 112.4 |
| 7.5 | 2.2 | 1.2 | 109.2 | |
| 30 | 2.6 | 0.6 | 104.3 | |
| THC | 1.5 | 3.0 | 2.2 | 111.1 |
| 7.5 | 2.4 | 1.0 | 105.8 | |
| 30 | 2.5 | 0.9 | 103.8 | |
| CBN | 1.5 | 2.8 | 2.6 | 107.9 |
| 7.5 | 3.6 | 2.1 | 99.1 | |
| 30 | 6.6 | 1.8 | 100.8 | |
| 11-OH-THC | 1.5 | 3.0 | 0.3 | 112 |
| 7.5 | 4.6 | 0.9 | 106.6 | |
| 30 | 3.7 | 0.8 | 103.6 | |
| THCCOOHa | 0.015 | 4.3 | 5.2 | 105.9 |
| 0.075 | 2.3 | 1.4 | 113.8 | |
| 0.3 | 3.6 | 3.2 | 107.8 | |
Data from two-dimensional gas chromatography mass spectrometry (2D-GCMS) with negative chemical ionization; all other data from 2D-GCMS with electron ionization.
%RSD - percent relative standard deviation.
Extraction efficiencies were calculated by comparing mean peak areas (N=4) of analytes in drug-free oral fluid fortified prior to and after SPE. Mean % extraction efficiencies ranged between 41.1-87.1% (Table 5).
Table 5.
Stability and extraction efficiency data for quantification of Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC), 11-nor-9-carboxy-THC (THCCOOH), cannabidiol (CBD), and cannabinol (CBN) in oral fluid.
| Stability (%) | ||||||
|---|---|---|---|---|---|---|
| Analytes | Target (ng/ml) | Room temp 24h | 4°C, 72h | Three freeze-thaw cycles | Autosampler 48h | Mean extraction efficiencies |
| CBD | 1.5 | -3.5 | -4.9 | -4.6 | -0.1 | 50.2 |
| 7.5 | -5.5 | -1.8 | 0.8 | -0.2 | 52.1 | |
| 30 | -2.6 | 0.7 | 1.2 | 0.0 | 55.1 | |
| THC | 1.5 | 1.5 | -4.0 | -3.6 | -1.7 | 81.0 |
| 7.5 | -2.5 | -0.4 | 1.6 | -1.1 | 83.3 | |
| 30 | 0.6 | 2.6 | 3.1 | -3.4 | 87.1 | |
| CBN | 1.5 | -1.5 | -3.6 | -6.3 | 4.1 | 78.9 |
| 7.5 | -4.8 | -15.1 | 1.8 | 1.8 | 80.6 | |
| 30 | 1.2 | 1.0 | 12.0 | -4.1 | 85.5 | |
| 11-OH-THC | 1.5 | -0.6 | -2.7 | 3.6 | -0.9 | 41.1 |
| 7.5 | -4.2 | -2.4 | 0.8 | -1.2 | 42.0 | |
| 30 | -0.6 | 2.3 | -1.7 | -0.8 | 42.9 | |
| THCCOOHa | 0.015 | -1.1 | -0.2 | 5.1 | -0.6 | 85.1 |
| 0.075 | 0.0 | -1.2 | 4.9 | -2.1 | 70.4 | |
| 0.3 | -2.5 | 4.0 | 0.9 | -2.4 | 75.2 | |
Data from two-dimensional gas chromatography mass spectrometry (2D-GCMS) with negative chemical ionization; all other data from 2D-GCMSwith electron ionization.
Due to the possibility that clinical specimens could contain analyte concentrations exceeding the methods' upper LOQ, it was necessary to verify the accuracy of diluted samples. Quality control samples (N=4) were diluted 50 and 90% (v/v) with a mixture of blank oral fluid-Quantisal™ buffer. Mean measured concentrations were 86.9-99.0% of target concentrations with individual observations within 15.5%.
Stability studies were conducted to evaluate analyte loss under various temperature storage conditions. Samples (N=4) fortified at all QC concentrations and subjected to three freeze-thaw cycles prior to extraction showed mean % differences from freshly prepared controls (N=4) of −6.3-12%. Mean % differences between freshly prepared QC samples and room temperature and refrigerated stability samples (N=4) were -15.1-4%. All analytes in derivatized extracts at room temperature were stable for up to 48h. Concentrations were within 4.1% of values obtained from the initial injection, and all samples were within ±20% of target.
3.3. Clinical specimens
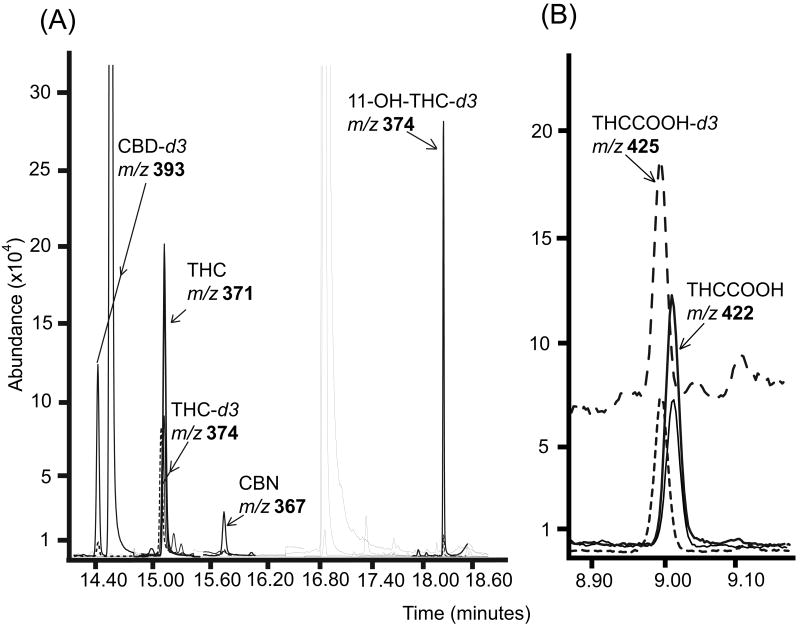
The method was employed to quantify THC, 11-OH-THC, THCCOOH, CBD, and CBN in oral fluid specimens collected with the Quantisal™ oral fluid collection device after controlled oral THC and Sativex® administration studies. The protocols were approved by the National Institute on Drug Abuse's Institutional Review Board and participants provided written informed consent. Merged ion chromatograms demonstrating two-dimensional separation of analytes from a participant's specimen containing 19.3ng/mL THC, 1.3ng/mL CBN and 83.3pg/mL THCCOOH are shown in Figure 2.
Figure 2.
Extracted ion chromatograms for (A) Δ9-tetrahydrocannabinol (THC) (19.3ng/mL), 11-hydroxy-THC (11-OH-THC), (not detected), cannabidiol (CBD) (not detected), cannabinol (CBN) (1.3ng/mL); (B) 11-nor-9-carboxy-THC (THCCOOH) (83.3pg/mL) and related deuterated analogs from a participant's oral fluid specimen. (A) Data obtained from two-dimensional gas chromatography mass spectrometry (2D-GCMS) and (B) from 2D-GCMS with negative chemical ionization (NCI). Quantification ions are in bold.
4. Discussion
Quantification of cannabinoids in oral fluid provided an analytical challenge due to the wide range of concentrations encountered, from tens to hundreds of ng/mL for THC [21,23] to a few pg/mL for THCCOOH [24,25]. Our new method allows efficient separation of THC, 11-OH-THC, CBD and CBN from THCCOOH. This separation makes possible the analysis of all major cannabinoids and metabolites in a single extraction, and takes into consideration authentic cannabinoids concentrations present in oral fluid after cannabis exposure. Utilizing two eluents, we succeeded in establishing conditions that retain THCCOOH on the SPE column, while eluting THC, 11-OH-THC, CBD and CBN. A second elution solvent containing hexane, ethyl acetate and glacial acetic acid eluted THCCOOH with high extraction efficiency values of 70-85%.
The two eluents were individually collected and derivatized. Trimethylsilyl derivatives were easily prepared with BSTFA (with 1% TMCS). THC, CBD and 11-OH-THC TMS derivatives and deuterated analogs produced ions with excellent resolution and 0.5ng/mL LOQs. The THC TMS derivative produced three strong abundant ions (371, 386, 303m/z) compared to other derivatives with secondary qualifier ions with lower abundances relative to target ions; potentially increasing the LOQ (Figure 1). However, despite a strong target and first qualifier signal for THC, the 303m/z (qualifier #2) did not provide consistent ion ratio concentrations below the established LOQ due to occasional matrix interference. The CBN TMS derivative produced two strong, clean ions (367 and 382m/z) at 0.5ng/mL, but decreased signal for the second qualifying ion (310m/z) elevated the LOQ for CBN to 1ng/mL.
GC-NCI-MS techniques are often employed to improve selectivity and sensitivity; however, one limitation of chemical ionization is fewer prominent ion peaks in the mass spectra due to the lower fragmentation energy. Kochanowski and Kala [26] presented a method for simultaneous determination of THC and THCCOOH, and detection of 11-OH-THC in a single saliva sample (collected by expectoration) using GC-NCI-MS. LOQs were reported as 0.5ng/mL for THC and THCCOOH with a linearity range of 0.5-20ng/mL. Recent application of cold trapping with 2D-GC-NCI-MS for drug quantification enhanced resolution and detection of THCCOOH only in oral fluid [25]. The detection of THCCOOH at such low concentrations is necessary in oral fluid, as it can verify cannabis ingestion and negate passive exposure as a source of positive tests. We achieved an LOQ of 7.5pg/mL for THCCOOH from the same oral fluid specimen extraction, with a second elution solvent. This low quantification was possible due to the 2D-GC-NCI-MS system with pure ammonia as a reagent gas, an increased injection volume in pulsed-splitless mode, and increased EM voltage.
One of the problems with oral fluid testing is collection of sufficient volume, especially from drug users who may have dry mouth after cannabinoid use. The Quantisal™ device collects 1±0.1mL oral fluid and dilutes the sample with 3mL of transporting buffer, adequately preventing adsorption of cannabinoids to the collection device and providing sufficient sample volume for multiple analyses. Our method utilized only 1 of 4mL from the collection device.
In conclusion, for the first time this new analytical method simultaneously identifies and quantifies THC, CBD, CBN, 11-OH-THC and THCCOOH in a single extraction of oral fluid collected with the Quantisal™ device. This validated method provides specific and accurate results over an analyte concentration range that is consistent with expected oral fluid concentrations following oral THC administration. Enhanced analytical sensitivity with improved S/N and detection limits for cannabinoids was achieved with 2D-GCMS with cold trapping. This method also is useful for quantification of cannabinoids after cannabis smoking, when parent analytes may be present in higher concentrations than after ingestion [35]; however appropriate specimen dilutions may be required.
Acknowledgments
Funding for this research was provided by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Psychopharmacology (Berl) 2002;164:61. doi: 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- 2.SAMHSA. Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2008. DHHS Publication No. SMA 08-4343, (NSDUH Series H-34. [Google Scholar]
- 3.Huestis MA, ElSohly M, Nebro W, Barnes A, Gustafson RA, Smith ML. Ther Drug Monit. 2006;28:540. doi: 10.1097/00007691-200608000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Law B, Mason PA, Moffat AC, Gleadle RI, King LJ. J Pharm Pharmacol. 1984;36:289. doi: 10.1111/j.2042-7158.1984.tb04376.x. [DOI] [PubMed] [Google Scholar]
- 5.Cone EJ, Johnson RE, Paul BD, Mell LD, Mitchell J. J Anal Toxicol. 1988;12:169. doi: 10.1093/jat/12.4.169. [DOI] [PubMed] [Google Scholar]
- 6.Ashton CH. Br J Psychiatry. 2001;178:101. doi: 10.1192/bjp.178.2.101. [DOI] [PubMed] [Google Scholar]
- 7.Huestis MA. In: Handbook of Experimental Pharmacology. Pertwee RG, editor. Vol. 168. New York: Springer. 2005. p. 657. [DOI] [PubMed] [Google Scholar]
- 8.Peat MA. In: Advances in Analytical Toxicology II. Baselt RC, editor. Year Book Medical Publishers; Chicago: 1989. p. 186. [Google Scholar]
- 9.Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. Chem Biodivers. 2007;4:1678. doi: 10.1002/cbdv.200790147. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin RS, Gustafson RA, Barnes A, Nebro W, Moolchan ET, Huestis MA. Ther Drug Monit. 2006;28:545. doi: 10.1097/00007691-200608000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Nadulski T, Sporkert F, Schnelle M, Stadelmann AM, Roser P, Schefter T, Pragst F. J Anal Toxicol. 2005;29:782. doi: 10.1093/jat/29.8.782. [DOI] [PubMed] [Google Scholar]
- 12.Gustafson RA, Moolchan ET, Barnes A, Levine B, Huestis MA. J Chromatogr B. 2003;798:145. doi: 10.1016/j.jchromb.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Musshoff F, Madea B. Ther Drug Monit. 2006;28:155. doi: 10.1097/01.ftd.0000197091.07807.22. [DOI] [PubMed] [Google Scholar]
- 14.Abraham TT, Lowe RH, Pirnay SO, Darwin WD, Huestis MA. J Anal Toxicol. 2007;31:477. doi: 10.1093/jat/31.8.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadulski T, Pragst F. J Chromatogr B. 2007;846:78. doi: 10.1016/j.jchromb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Huestis MA, Scheidweiler KB, Saito T, Fortner N, Abraham T, Gustafson RA, Smith ML. Forensic Sci Int. 2008;174:173. doi: 10.1016/j.forsciint.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Concheiro M, de Castro A, Quintela O, Cruz A, Lopez-Rivadulla M. J Chromatogr B. 2004;810:319. doi: 10.1016/j.jchromb.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Verstraete AG. Forensic Sci Int. 2005;150:143. doi: 10.1016/j.forsciint.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Raes E, Verstraete AG. Ann Pharm Fr. 2006;64:197. doi: 10.1016/s0003-4509(06)75313-5. [DOI] [PubMed] [Google Scholar]
- 20.Just WW, Filipovic N, Werner G. J Chromatogr. 1974;96:189. doi: 10.1016/s0021-9673(00)98561-3. [DOI] [PubMed] [Google Scholar]
- 21.Kauert GF, Ramaekers JG, Schneider E, Moeller MR, Toennes SW. J Anal Toxicol. 2007;31:288. doi: 10.1093/jat/31.5.288. [DOI] [PubMed] [Google Scholar]
- 22.Laloup M, Ramirez Fernandez Mdel M, Wood M, De Boeck G, Henquet C, Maes V, Samyn N. J Chromatogr A. 2005;1082:15. doi: 10.1016/j.chroma.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Moore C, Rana S, Coulter C. J Chromatogr B. 2007;852:459. doi: 10.1016/j.jchromb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Day D, Kuntz DJ, Feldman M, Presley L. J Anal Toxicol. 2006;30:645. doi: 10.1093/jat/30.9.645. [DOI] [PubMed] [Google Scholar]
- 25.Moore C, Ross W, Coulter C, Adams L, Rana S, Vincent M, Soares J. J Anal Toxicol. 2006;30:413. doi: 10.1093/jat/30.7.413. [DOI] [PubMed] [Google Scholar]
- 26.Kochanowski M, Kała M. Probl Forensic Sci. 2005;62:178. [PubMed] [Google Scholar]
- 27.Kauert GF, Iwersen-Bergmann S, Toennes SW. J Anal Toxicol. 2006;30:274. doi: 10.1093/jat/30.4.274. [DOI] [PubMed] [Google Scholar]
- 28.Cone EJ, Clarke J, Tsanaclis L. J Anal Toxicol. 2007;31:424. doi: 10.1093/jat/31.8.424. [DOI] [PubMed] [Google Scholar]
- 29.Quintela O, Andrenyak DM, Hoggan AM, Crouch DJ. J Anal Toxicol. 2007;31:157. doi: 10.1093/jat/31.3.157. [DOI] [PubMed] [Google Scholar]
- 30.Lowe RH, Karschner EL, Schwilke EW, Barnes AJ, Huestis MA. J Chromatogr A. 2007;1163:318. doi: 10.1016/j.chroma.2007.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crockett DK, Nelson G, Dimson P, Urry FM. J Anal Toxicol. 2000;24:245. doi: 10.1093/jat/24.4.245. [DOI] [PubMed] [Google Scholar]
- 32.Scurlock RD, Ohlson GB, Worthen DK. J Anal Toxicol. 2006;30:262. doi: 10.1093/jat/30.4.262. [DOI] [PubMed] [Google Scholar]
- 33.Huestis MA, Gustafson RA, Moolchan ET, Barnes A, Bourland JA, Sweeney SA, Hayes EF, Carpenter PM, Smith ML. Forensic Sci Int. 2007;169:129. doi: 10.1016/j.forsciint.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore C, Coulter C, Rana S, Vincent M, Soares J. J Anal Toxicol. 2006;30:409. doi: 10.1093/jat/30.7.409. [DOI] [PubMed] [Google Scholar]
- 35.Huestis MA, Cone EJ. J Anal Toxicol. 2004;28:394. doi: 10.1093/jat/28.6.394. [DOI] [PubMed] [Google Scholar]