Abstract
Adolescence is characterized by a relative immaturity of the prefrontal cortex and associated cognitive control functions, which is hypothesized to be a major contributing factor to high risk behaviors. However, little is known about the role of genetic and environmental factors in frontal brain development during adolescence. Here we examined heritability of performance on the Wisconsin Card Sorting Test (WCST), an established neuropsychological measure of prefrontally-mediated executive functioning, in a longitudinal sample of adolescent twins (n=747) tested at ages 12 and 14. WSCT performance significant improved with age as indicated by a decrease in the number of perseverative errors (P<.001), which was paralleled by an increase in heritability in females (19% at age 12 and 49% at age 14) and shared environmental influences in males (non-significant at age 12 and 34% at age 14). The results suggest increasing influence of familial factors on frontal executive functioning during adolescence, as well as gender differences in the relative role of genetic and environmental factors.
Introduction
Adaptive self-regulation of goal-directed behavior requires flexible adjustment of action strategy in accordance with changing environmental demands. This process involves utilization of feedback information about action outcome, shifting cognitive set, and updating action plan. The ability for cognitive set shifting is measured by Wisconsin Card Sorting Test (WCST), an established experimental measure of executive functioning [14]. In this test, participants must derive a correct card sorting rule based on a trial-by-trial feedback (correct versus wrong). Since the sorting rule changes without warning, the participant has to modify learned response strategy using the feedback information provided. A key (inverse) indicator of cognitive flexibility is the number of “perseverative errors” that occur when the participant persists in using the old sorting strategy despite the negative feedback.
There is considerable evidence that WCST performance critically depends on the functioning of the dorsolateral prefrontal cortex (DLPFC). Early studies have shown that WCST performance is impaired by damage to the DLPFC both in humans and monkeys [24]. Subsequent studies using functional neuroimaging [5, 26] and transcranial magnetic stimulation [18] have corroborated the critical role of DLPFC activity in WCST performance. It is important to note, however, that other prefrontal areas such as posterior ventrolateral prefrontal cortex, anterior cingulate, as well as basal ganglia appear to also be involved during different stages of task performance [19, 23, 25, 36].
The WCST has been extensively used as a marker of deficits in executive cognition in schizophrenia and other neuropsychiatric disorders. Many studies have shown WCST performance deficits in schizophrenics [31] and in their healthy first degree relatives [40], although other studies failed to show such familial association [37]. Impaired WCST performance has also been reported in drug abusers [21, 33] and individuals with ADHD [6]. Studies of small groups of twins discordant for schizophrenia suggest possible involvement of both genetic and environmental factors in abnormal WCST performance [32]. Evidence for familial association of WCST measures with psychiatric disorders, although mixed, suggests that abnormal WCST performance can potentially serve as an indicator of genetic liability.
More recently, WCST has been considered as an intermediate phenotype (endophenotype) for genetic studies of psychiatric disorders such as schizophrenia [17] and ADHD [6, 13]. This approach has been implemented in a recent large-scale family study of ADHD and a linkage of WCST performance to a region on chromosome 22 has been reported [12]. Other studies reported associations of WCST performance with candidate genes, most notably the catechol-o-methyltransferase (COMT) gene, although the evidence remains mixed [2, 3].
One of the necessary attributes of an endophenotype is significant heritability, however, as of now evidence for heritability of WCST performance is very limited and mixed. Two earlier studies were based on very small samples and did not find evidence for genetic influences [8, 30]. A study by our group based on larger sample of young adult female twins has found moderate heritability of WCST performance [1]. However, two subsequent studies involving middle-age male twins[20] and a sample of male and female twins aged 18 to 83 years [38] did not find evidence for genetic influences. Thus, it remains unclear whether the WCST can serve as indicator of genetically transmitted individual characteristics of executive functioning. Furthermore, the above studies estimated heritability in adult samples, and little is known about the role of genetic factors in WCST performance in adolescence. The prefrontal cortex undergoes substantial development during the adolescent period, and its relative immaturity has been implicated in greater propensity of adolescents to engage in high-risk behaviors such as substance use and abuse [9, 35]. Therefore, for a better understanding of the etiology of such behaviors during adolescence it is important to know whether individual differences in prefrontally mediated executive functions are influenced by genetic factors.
The purpose of the present study was to examine developmental changes, long-term stability, and heritability of WCST performance in early adolescence.
Materials and Methods
Sample
Subjects were 747 adolescent twins (age at first assessment: M=12.52 years, S.D.=0.20; 47.8% females) including 166 MZ and 201 DZ pairs. Participants were recruited from the general population through a twin registry and represented three consecutive birth-year cohorts. The subjects were retested as they reached age 14 (n=482 available at the time of the analysis). Subjects with a history of serious head trauma or health conditions precluding the laboratory visit or performance of experimental tasks (e.g. severe visual impairment or mental retardation) were excluded. The study was approved by the human studies committee of the Washington University School of Medicine. A written informed assent was obtained from all participants, and a written informed consent was obtained from their parents. Zygosity was determined independently using a standard interview administered to twins' parents, research assistants' ratings of the twins' physical similarity, and a set of 7 DNA markers genotyped in 86% of the participants. The reliability of zygosity diagnosis by questionnaire has been demonstrated in previous studies [16].
Procedures
The WCST was administered using a computer-administered version of the test (WCST Research Edition, Version 3, Psychological Assessment Resources, Inc.) in accordance with a standardized WCST administration procedure [14], a detailed description of which can be found in our previous publication [1]. The number of perseverative errors, an inverse indicator of the ability to shift strategy based on feedback information, was used as the most representative WCST measure. Response scores were log-transformed to reduce skewness of the distribution, however, summary statistics are reported for raw scores.
Statistical analysis
The relative contribution of genetic and environmental factors to the total phenotypic variance of WCST performance was estimated by fitting linear structural equation models to twin data [28, 34] using the Mx program [27]. These models assume that phenotypic variance arises from the following factors: additive genetic influences (A), environmental influences shared by family members (C), and individually unique (unshared) environmental influences (E). We did not perform a separate analysis of non-additive genetic influences due to limited power.
Since our sample included both male and female twins and preliminary analyses showed differences between male and female twin correlations, we explored potential sex differences in genetic and environmental effects by fitting “sex-limitation” models [29]. The sex-limitation model allows the magnitude of genetic and environmental effects to vary independently in males and females and includes sex-specific genetic influences accounting for the possibility that the set of genes which influences a trait in males is not identical to that which influences a trait in females. Both kinds of sex differences in genetic effects can be tested by fitting sub-models of the general sex-limitation model [29]. The goodness of model fit was indicated by a -2LL (log likelihood). Different submodels were tested by dropping individual paths from the full model and comparing the goodness of fit of the restricted submodel with the goodness of fit of the more general model using a χ2 test of -2LL difference. Heritability was estimated as the percentage of the total variance of the trait attributed to genetic factors; in addition, 95% confidence intervals of the estimates were computed. A detailed description of the model fitting approach and assessment of heritability can be found elsewhere [7, 28, 34].
Results
WCST performance showed significant improvement with age as indicated by the decrease in the number of perseverative errors from age 12 to age 14 (M±SD = 12.7±9.5 and 8.2±6.1, respectively, paired t-test: t=10.5, df=480, p<0.001). There was a modest but significant correlation between WCST performance at ages 12 and 14 (r=0.37, p<0.001). There were no significant gender differences at either age. However, a significant effect of ethnicity was observed, with European Americans showing smaller number of perseverative errors compared with ethnic minorities, mainly represented by African-Americans (F(1,745)=55.5, p<0.001 and F(1,480)=42.0, p<0.001 at ages 12 and 14, respectively). Because ethnicity can potentially confound intrapair twin correlations, data were adjusted for ethnicity by computing standardized scores separately within ethnic groups. Preliminary analyses showed that different WCST measures are highly correlated (r=0.6-0.9), therefore analyses were focused on the number of perseverative errors (an inverse indicator of set shifting ability) as the most representative measure of WCST performance.
Intrapair twin correlations for WCST performance are shown in Table 1. Overall, the pattern of correlations suggests few familial (genetic or shared environmental) influences at age 12 and significant familial influences at age 14, as well as sex differences in the magnitude of genetic and environmental effects. Model fitting and comparison across nested submodels showed that all genetic influences in males could be dropped without a significant decrease of the goodness of fit.
Table 1.
Intrapair twin correlations for WCST performance (number of perseverative errors).
| Variable | rMZF (n=84) | rDZF (n=49) | rMZM (n=82) | rDZM (n=70) | rDZOS (n=82) |
|---|---|---|---|---|---|
| Age 12 | .28** | .03 | .08 | .17 | .09 |
| Age 14 | .47*** | .20 | .45*** | .38** | -.04 |
rMZF and rDZF are intrapair correlations for female MZ and DZ twins, and rMZM and rDZM are intrapair correlations for male MZ and DZ twins, respectively; rDZOS are intrapair correlations for opposite-sex twin pairs. Significance levels:
p<0.05;
p<0.01;
p<0.001.
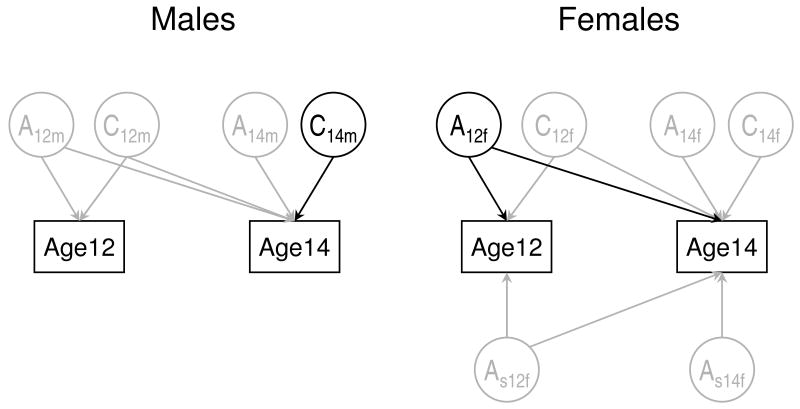
Shared environmental influences in females at both ages and in males at age 12 could be also dropped. Next, we could drop genetic influences in females specific to age 14, however, dropping the remaining female genetic paths led to a significant deterioration of fit (Δχ2=19.44, df=2, p<0.001). Likewise, dropping shared environmental influences in males at age 14 led to significant deterioration of model fit (Δχ2=12.55, df=1, p<0.001). Thus, the final and most parsimonious model (Fig. 1) included shared environmental influences in males at age 14 only and genetic influences in females that affected WCST performance at both ages, with no additional age-specific influences at age 14. Parameter estimates for this model (Table 1) indicate significant heritability in females at both ages (with increasing heritability from age 12 to age 14) and lack of heritability in males at either age.
Fig. 1.
Path diagram of the sex-limitation model in a depicted for a pair of opposite-sex DZ twins. Paths retained in the best-fitting model are shown in black, and the paths that could be dropped without significant deterioration of fit are shown in grey. Rectangulars represent the observed WCST phenotype (number of persevarative errors) measured at ages 12 and 14; circles represent the corresponding latent genetic and shared environmental factors (A and C, respectively). For simplicity, non-shared environmental influences that always contribute to the variance and therefore cannot be dropped are not shown. AS stands for female-specific genetic factors. More details can be found in Neale et al. [29].
Discussion
This study provides the first assessment of heritability of WCST performance in adolescence and thus extends our previous study performed in young adult population [1]. The present results indicate significant familial influences on the core construct of “executive functioning” measured by WCST, namely cognitive shifting, or the ability to switch to a new response strategy by utilizing performance feedback information (indexed inversely by the number of perseverative errors). However, the relative role of genetic and shared environmental factors differs as a function of sex and age. In females, the number of perseverative errors shows significant heritability that tends to increase with age (from 19% at age 12 to 49% at age 14). Importantly, the results of model fitting suggest continuity of genetic influences over the studied period of development, i.e. that same genes influence performance at both ages. In males, no evidence for heritability was found at either age, however, there was a modest but significant influence of common familial environment.
The observed pattern of sex- and age-related differences in the magnitude of genetic influences may be related to sex differences in the rate of brain maturation. Morphometric imaging studies indicate that brain volumes in the frontal lobe peak two years earlier in females [22], and functional imaging studies suggest more mature pattern of frontal activation in females [10]. If genetic factors that influence WCST performance become expressed at a certain stage of prefrontal cortical development, then these genetic influences can be expected to become penetrant earlier in females than in males. It is thus possible that boys lag behind girls with respect to age-related increases in heritability of executive function. This hypothesis is consistent with the evidence for substantial age-related increases in heritability of other cognitive and behavioral phenotypes [4, 11] and brain white matter volume [39], as well as evidence for sex differences in age-related increase in heritability: in a study of antisocial behavior females showed higher heritability in childhood, but heritability in both sexes increased over adolescence and the gap was closed by young adulthood [15]. Data from older adolescents and young adults are needed to test whether heritability of WCST performance follows a similar pattern.
The complex determination of WCST scores, in particular, dependence of heritability estimates on age and gender may also account for the discrepancies between different studies. The present study replicates our previous finding of modest heritability of WCST performance in females [1] and extends it to the adolescent population. However, two other studies that were sufficiently large to estimate heritability did not find evidence of significant influences. The study by Kremen et al. [20] was based on a male-only sample with a mean age of 48 years drawn from the Vietnam Era Twin Registry. Most WCST measures in that study showed modest but significant MZ and DZ twin correlations (ranging fron .17 to .36), but the lack of differences in the magnitude of MZ and DZ correlations suggested the absence of genetic influences. However, this pattern of correlations suggests a significant contribution of shared environmental influences (not estimated in the paper), which is highly consistent with our present finding of modest but significant shared environmental influences on WCST performance in males. The second study [38] tested twins of both sexes in a wide age range from 18 to 83 years and did not find evidence for heritability. However, because gender-specific twin correlations were not reported and male and female data were collapsed in the analysis, it is difficult to speculate whether gender differences in the pattern of twin resemblance could contribute to the negative result. A very broad age range could be another important factor, especially given that WCST performance shows non-linear (inverted U-shaped) changes over the lifespan [14]. Finally, another study [6] has reported significant sibling correlations for WCST, suggesting a modest familial influence.
Taken together, previous studies and the present findings suggest that the role of genetic and environmental factors in WCST performance is highly dependent on age and gender composition of the sample. If any, genetic influences are relatively modest, accounting for less than half of the total variance. The degree of heritability may be limited by test-retest stability of WCST performance. In the present study, the long-term stability was modest (r=.32 in males and r=.43 in females), however, it should be kept in mind that the age period studied here is characterized by substantial developmental changes in the brain, particularly its frontal lobes, and the test-retest correlation obtained in our study is likely to be the lower-bound estimate of test-retest reliability.
Relatively modest heritability of WCST performance and its dependence on age and sex diminishes potential utility of WCST as endophenotype for psychiatric disorders. Nevertheless, further investigation of developmental dynamics of genetic and environmental influences on WCST across the lifespan can help to identify which periods of brain maturation and aging are characterized by the largest genetic influences on executive function, and whether these periods differ between the sexes. This information, combined with developmental data on clinical variables such as onset of disease and individual symptoms can contribute to a better understanding of possible pathways by which genetic factors contribute to disease risk across the lifespan.
Some limitations of the present study need to be acknowledged. First, the confidence intervals of heritability assessments are rather broad, which means that “true” heritability in the general population may be greater or smaller than point estimates reported here. Second, the age range was restricted, and, given ongoing brain maturation during adolescence and changes of WCST heritability with age, the present results cannot be generalized to the whole period of adolescence; it remains to be seen how the relative role of genetic and environmental factors in executive functioning will change as the adolescents participating in their study grow older. Finally, the purpose of the study was to assess heritability in a population-based sample of subjects who were not selected for any specific condition, except the exclusion criteria mentioned above. The effect of genetic factors on WCST performance may be different in families selected for some neurological or psychiatric condition. In such groups, the range of variability in WCST scores may be increased, which may potentially result in higher or lower heritability estimates, depending on whether “extreme” scores in such groups are caused by genetic or environmental factors.
Conclusion
This study is the first to assess heritability of WCST performance in adolescents. The results show that the relative role of genetic and environmental factors varies as a function of age and sex. In females, the number of perseverative errors shows significant heritability that tends to increase with age and can therefore serve as indicators of genetically transmitted individual characteristics of executive functioning. In contrast, in males no evidence for heritability of WCST performance was found, although there was a modest but significant influence of common familial environment.
Table 2.
Heritability of WCST performance.
| Age/Sex | a2 (95% CI) | c2 (95% CI) | e2 (95% CI) |
|---|---|---|---|
| Age12: Females | .19 (.05-.35) | 0 | .81 (.65-.95) |
| Males | 0 | 0 | 1.0 |
| Age14: Females | .49 (.24-.66) | 0 | .51 (.34-.76) |
| Males | 0 | .34(.15-.49) | .66 (.51-.85) |
a2 is the proportion of total phenotypic variance explained by genetic factors (heritability), c2 and e2 are the proportions of variance shared and non-shared environmental factors, respectively (95% confidence intervals of the maximum likelihood estimates of the variance components are shown in brackets)
Acknowledgments
Supported by NIH grants DA01889 from the National Institute on Drug Abuse and the Midwest Alcoholism Research Center (P50 AA11998).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anokhin AP, Heath AC, Ralano A. Genetic influences on frontal brain function: WCST performance in twins. Neuroreport. 2003;14:1975–1978. doi: 10.1097/00001756-200310270-00019. [DOI] [PubMed] [Google Scholar]
- 2.Barnett JH, Jones PB, Robbins TW, Muller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- 3.Barnett JH, Scoriels L, Munafo MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: a meta-analysis. Twin Res Hum Genet. 2007;10:423–433. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- 5.Berman KF, Ostrem JL, Randolph C, Gold J, Goldberg TE, Coppola R, Carson RE, Herscovitch P, Weinberger DR. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: a positron emission tomography study. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- 6.Bidwell LC, Willcutt EG, Defries JC, Pennington BF. Testing for neuropsychological endophenotypes in siblings discordant for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:991–998. doi: 10.1016/j.biopsych.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nature reviews. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- 8.Campana A, Macciardi F, Gambini O, Scarone S. The Wisconsin Card Sorting Test (WCST) performance in normal subjects: a twin study. Neuropsychobiology. 1996;34:14–17. doi: 10.1159/000119284. [DOI] [PubMed] [Google Scholar]
- 9.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 10.Christakou A, Halari R, Smith AB, Ifkovits E, Brammer M, Rubia K. Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. NeuroImage. 2009;48:223–236. doi: 10.1016/j.neuroimage.2009.06.070. [DOI] [PubMed] [Google Scholar]
- 11.Davis OS, Haworth CM, Plomin R. Dramatic increase in heritability of cognitive development from early to middle childhood: an 8-year longitudinal study of 8,700 pairs of twins. Psychol Sci. 2009;20:1301–1308. doi: 10.1111/j.1467-9280.2009.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle AE, Ferreira MA, Sklar PB, Lasky-Su J, Petty C, Fusillo SJ, Seidman LJ, Willcutt EG, Smoller JW, Purcell S, Biederman J, Faraone SV. Multivariate genomewide linkage scan of neurocognitive traits and ADHD symptoms: suggestive linkage to 3q13. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1399–1411. doi: 10.1002/ajmg.b.30868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doyle AE, Willcutt EG, Seidman LJ, Biederman J, Chouinard VA, Silva J, Faraone SV. Attention-deficit/hyperactivity disorder endophenotypes. Biol Psychiatry. 2005;57:1324–1335. doi: 10.1016/j.biopsych.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Psychological Assessment Resources. Odessa, FL: 1993. Wisconsin card sorting test manual. [Google Scholar]
- 15.Jacobson KC, Prescott CA, Kendler KS. Sex differences in the genetic and environmental influences on the development of antisocial behavior. Dev Psychopathol. 2002;14:395–416. doi: 10.1017/s0954579402002110. [DOI] [PubMed] [Google Scholar]
- 16.Kasriel J, Eaves L. The zygosity of twins: further evidence on the agreement between diagnosis by blood groups and written questionnaires. J Biosoc Sci. 1976;8:263–266. doi: 10.1017/s0021932000010737. [DOI] [PubMed] [Google Scholar]
- 17.Keri S, Janka Z. Critical evaluation of cognitive dysfunctions as endophenotypes of schizophrenia. Acta Psychiatr Scand. 2004;110:83–91. doi: 10.1111/j.1600-0047.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- 18.Ko JH, Monchi O, Ptito A, Petrides M, Strafella AP. Repetitive Transcranial Magnetic Stimulation of Dorsolateral Prefrontal Cortex Affects Performance of the Wisconsin Card Sorting Task during Provision of Feedback. Int J Biomed Imaging. 2008;2008:143238. doi: 10.1155/2008/143238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konishi S, Nakajima K, Uchida I, Kameyama M, Nakahara K, Sekihara K, Miyashita Y. Transient activation of inferior prefrontal cortex during cognitive set shifting. Nat Neurosci. 1998;1:80–84. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- 20.Kremen WS, Eisen SA, Tsuang MT, Lyons MJ. Is the Wisconsin Card Sorting Test a useful neurocognitive endophenotype? Am J Med Genet B Neuropsychiatr Genet. 2007;144B:403–406. doi: 10.1002/ajmg.b.30527. [DOI] [PubMed] [Google Scholar]
- 21.Lane SD, Cherek DR, Tcheremissine OV, Steinberg JL, Sharon JL. Response perseveration and adaptation in heavy marijuana-smoking adolescents. Addict Behav. 2007;32:977–990. doi: 10.1016/j.addbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lie CH, Specht K, Marshall JC, Fink GR. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. NeuroImage. 2006;30:1038–1049. doi: 10.1016/j.neuroimage.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 24.Milner B. Effects of different brain lesions on card sorting. Arch Neurol. 1963;9:90–100. [Google Scholar]
- 25.Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event- related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagahama Y, Fukuyama H, Yamauchi H, Matsuzaki S, Konishi J, Shibasaki H, Kimura J. Cerebral activation during performance of a card sorting test. Brain. 1996;119:1667–1675. doi: 10.1093/brain/119.5.1667. [DOI] [PubMed] [Google Scholar]
- 27.Neale MC, Boker SM, Xie G, Maes HH. Mx:Statistical Modeling. Department of Psychiatry; Richmond, VA: 2002. [Google Scholar]
- 28.Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Vol. 67. Kluwer Academic Publishers; Dordrecht: 1992. [Google Scholar]
- 29.Neale MC, Roysamb E, Jacobson K. Multivariate genetic analysis of sex limitation and G × E interaction. Twin Res Hum Genet. 2006;9:481–489. doi: 10.1375/183242706778024937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicole S, Del Miglio C. Abstraction skillfulness in monozygotic and dizygotic twin pairs. Acta Genet Med Gemellol (Roma) 1997;46:57–67. doi: 10.1017/s0001566000000751. [DOI] [PubMed] [Google Scholar]
- 31.Nieuwenstein MR, Aleman A, de Haan EH. Relationship between symptom dimensions and neurocognitive functioning in schizophrenia: a meta-analysis of WCST and CPT studies. Wisconsin Card Sorting Test. Continuous Performance Test. J Psychiatr Res. 2001;35:119–125. doi: 10.1016/s0022-3956(01)00014-0. [DOI] [PubMed] [Google Scholar]
- 32.Pardo PJ, Knesevich MA, Vogler GP, Pardo JV, Towne B, Cloninger CR, Posner MI. Genetic and state variables of neurocognitive dysfunction in schizophrenia: a twin study. Schizophr Bull. 2000;26:459–477. doi: 10.1093/oxfordjournals.schbul.a033466. [DOI] [PubMed] [Google Scholar]
- 33.Pirastu R, Fais R, Messina M, Bini V, Spiga S, Falconieri D, Diana M. Impaired decision-making in opiate-dependent subjects: effect of pharmacological therapies. Drug Alcohol Depend. 2006;83:163–168. doi: 10.1016/j.drugalcdep.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3:119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- 35.Spear LP. The adolescent brain and the college drinker: biological basis of propensity to use and misuse alcohol. J Stud Alcohol Suppl. 2002:71–81. doi: 10.15288/jsas.2002.s14.71. [DOI] [PubMed] [Google Scholar]
- 36.Specht K, Lie CH, Shah NJ, Fink GR. Disentangling the prefrontal network for rule selection by means of a non-verbal variant of the Wisconsin Card Sorting Test. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stratta P, Daneluzzo E, Mattei P, Bustini M, Casacchia M, Rossi A. No deficit in Wisconsin Card Sorting Test performance of schizophrenic patients' first-degree relatives. Schizophr Res. 1997;26:147–151. doi: 10.1016/s0920-9964(97)00047-9. [DOI] [PubMed] [Google Scholar]
- 38.Taylor J. Heritability of Wisconsin Card Sorting Test (WCST) and Stroop Color-Word Test performance in normal individuals: implications for the search for endophenotypes. Twin Res Hum Genet. 2007;10:829–834. doi: 10.1375/twin.10.6.829. [DOI] [PubMed] [Google Scholar]
- 39.Wallace GL, Schmitt JEric, Lenroot R, Viding E, Ordaz S, Rosenthal MA, Molloy EA, Clasen LS, Kendler KS, Neale MC, Giedd JN. A pediatric twin study of brain morphometry. J Child Psychol Psychiatry. 2006;47:987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- 40.Wolf LE, Cornblatt BA, Roberts SA, Shapiro BM, Erlenmeyer-Kimling L. Wisconsin Card Sorting deficits in the offspring of schizophrenics in the New York High-Risk Project. Schizophr Res. 2002;57:173. doi: 10.1016/s0920-9964(01)00301-2. [DOI] [PubMed] [Google Scholar]