Abstract
Background
CLL patients are usually only treated for progressive disease. However, the discovery of biological predictors of high risk of disease progression together with the development of newer more targeted therapies could change this paradigm. In this phase 2 study we tested the safety and efficacy of early treatment of high risk CLL patients with alemtuzumab and rituximab.
Methods
Patients were eligible for treatment if they were 1) previously untreated 2) had no NCI-Working Group 1996 criteria for treatment and 3) had at least one marker of high risk disease (17p13−, 11q22−, or combination of unmutated IgVH and CD38+/ZAP70+). Treatment consisted of subcutaneous alemtuzumab (initial dose escalation followed by 30 mg on Monday-Wednesday-Friday for 4 weeks) and intravenous rituximab (375 mg/m2/week × 4 doses). All patients received PCP and herpes virus prophylaxis and were monitored for CMV reactivation.
Results
Twenty seven of thirty (90%) patients responded to therapy with 11 (37%) complete responses (CR). Five (17%) patients with CR had no detectable minimal residual disease. Median duration of response was 14.4 months and only nine patients have required re-treatment for progressive disease to date (median follow 17.6 months). Study patients had a significantly longer time from diagnosis to first treatment for CLL using conventional indications than a comparison cohort with similar biologic risk profiles.
Conclusions
The therapy regimen was safe and effective for high-risk, early stage patients. Further studies are required to determine if this early treatment strategy decreases morbidity and mortality for high-risk CLL.
Keywords: Chronic lymphocytic leukemia, CLL, high risk, early stage, alemtuzumab, rituximab
Introduction
Chronic lymphocytic leukemia (CLL) is not yet curable with standard therapies and most patients will die from the disease or its complications1, 2. Survival from diagnosis ranges from months to many decades with a median of about 10 years2, 3. The diagnosis of CLL is now most often made early in the course of the disease with the routine use of flow cytometry and biologic parameters can be used to predict prognosis for these patients. Patients with earlier stage high risk CLL could thus be candidates for interventions designed to decrease the morbidity and mortality of their disease.
The best characterized novel prognostic parameters are specific chromosomal defects detected by using interphase fluorescent in situ hybridization (FISH), immunoglobulin mutation sequence analysis (mutation status of IgVH), and expression of the intracellular protein ZAP-70 and the membrane protein CD38. FISH analysis can detect deletions of 17q13 (17p13−) which result in loss of the p53 gene and are associated with a shorter time to initial treatment, poor response to treatment, and very poor survival4. FISH can also detect deletions of 11q22 (11q22−) which results in the loss of the ATM gene and is associated with poor prognosis4. Unmutated (UM) IgVH (<2% difference from germline sequence)5, 6, ZAP-70 expression (≥ 20% positive cells)7, and CD38 (≥ 30% expression)5, are also associated with poorer prognosis in CLL. In addition, CLL patients with UM IgVH and CD38 have a worse prognosis than CLL patients with UM IgVH and cells that do not express CD388. Although the use of these molecular prognostic markers is relatively new, sufficient progress has been made to apply this knowledge to treatment decisions in clinical trials for CLL.
Universal therapy of all early and intermediate stage (Rai9) CLL patients at diagnosis is not currently considered to be beneficial and the standard of care is to treat only patients with progressive or advanced stage disease10, 11. Delaying therapy protects patients with earlier stage indolent disease from toxicity. However, this ‘watch and wait’ approach could also unnecessarily delay therapy for those patients with inherently aggressive disease. In this subset of patients with a kinetically more active form of CLL, earlier treatment when the disease burden is low could theoretically decrease the risk of clonal evolution, which is likely an important factor in disease progression and resistance to treatment12, 13. In addition, newer and potentially less toxic therapies such as lymphocyte-targeted monoclonal antibodies (MoAb) are known to be most effective prior to the development of bulky adenopathy and splenomegaly14. In this study we therefore tested the efficacy and safety of a therapy regimen combining alemtuzumab (CAMPATH 1H, Genzyme, Cambridge MA, USA) and rituximab (Rituxan, Genentech, San Francisco CA, USA) in patients with earlier stage high risk CLL based on the biological characteristics of their disease.
The combination of alemtuzumab and rituximab was used because these MoAb have different molecular targets, could have different mechanisms of action, and are reported to have complementary activity in tissue sites involved with CLL. Alemtuzumab is specific for the CD52 antigen expressed at high level by CLL cells15, and is effective as initial16, 17 and salvage18, 19 therapy for CLL. In CLL, alemtuzumab is very effective at clearing circulating leukemic cells and has appreciable activity against malignant lymphocytes in the bone marrow (BM), but is less effective against leukemic cells in the lymph nodes16, 19. Alemtuzumab is effective therapy for many patients with 17p13− or p53 mutation who are resistant to purine analogues20. Although clinical trials have shown only limited single agent activity for rituximab in CLL21, chemoimmunotherapy (CIT) combinations of purine analogues and rituximab are highly effective for the treatment of CLL22–24. In CLL, rituximab tends to be more effective at decreasing lymphadenopathy and splenomegaly than alemtuzumab, but is less effective in clearing tumor cells from the BM14, 25. These data suggesting that the combination of alemtuzumab and rituximab could be an effective therapy for CLL are supported by a study of patients with relapsed or refractory chronic B-cell lymphoid malignancies who had a response rate of 52% with 8% complete responses (CR)14. In this study we report on the treatment of 30 patients with early-intermediate stage high risk CLL who did not meet the conventional criteria for therapy. This study is an initial step to determine if a short course alemtuzumab and rituximab therapy can achieve a clinically relevant delay in the need for conventional therapy in patients with earlier stage high risk CLL.
Materials and Methods
Patient Selection
The study was approved by the Mayo Clinic Institutional Review Board and all patients were enrolled with written informed consent. Sequential patients seen in the Division of Hematology at Mayo Clinic Rochester from January 2005 to June 2007 were evaluated for eligibility. Patients were eligible for the study if they had CLL diagnosed by flow cytometric analysis of peripheral blood, early to intermediate clinical stage disease (Rai 0 – II)9, did not fulfill criteria for treatment of their disease as defined by the NCI-Working Group criteria of 1996 (NCI-WG96)11, and had molecular markers predictive of a high risk of disease progression. The diagnosis of CLL required an absolute lymphocyte count over 5 × 109/L, monoclonal B lymphocytes having a CLL immunophenotype11, 26, and FISH analysis with a IGH probe to exclude mantle cell lymphoma. Risk of disease progression was determined using interphase FISH analysis of peripheral blood27, IgVH mutation analysis28 and expression of CD3828 and ZAP-7029 as previously described. Patients were considered to be at high risk of disease progression if they had at least one of the following: 1. 17p13− by FISH analysis, 2. 11q22− by FISH analysis, 3. UM IgVH (<2% sequence variation from germline) as well as ZAP-70 expression (≥20% cells positive on flow cytometry) and/or CD38 expression (≥30% cells positive on flow cytometry).
All patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 and adequate organ function (serum creatinine ≤ 1.5 × upper limit of normal (UNL), total bilirubin ≤ 3.0 × UNL, and serum AST ≤3.0 × UNL. Exclusion criteria included any previous treatment for CLL, evidence of active autoimmune disease and any another active primary malignancy requiring treatment or limiting expected survival to ≤2 years.
Therapy
The duration of treatment was 31 days. Patients received subcutaneous alemtuzumab with dose escalation (3 mg, 10 mg, 30 mg) over the first 3 days (Wednesday – Friday) and then received 30 mg/d Monday – Wednesday – Friday for the next 4 weeks. Rituximab therapy was started on day 8 (375 mg/m2 intravenously at a standard infusion rate) and then repeated weekly for a total of 4 doses. This regimen design ensured that the first dose of rituximab was given after the circulating lymphocyte count had been decreased by alemtuzumab therapy so as to decrease the risk of a “first dose” reaction. The first 3 doses of alemtuzumab and all doses of rituximab were premedicated with acetaminophen and diphenhydramine. Patients received allopurinol (300 mg/day) for the first 14 days of therapy. All patients received prophylaxis for PCP and herpes simplex and varicella zoster viruses during treatment and then for an additional 6 months. Patients were monitored for CMV reactivation by PCR for viral DNA weekly during treatment and then monthly for 6 months.
Response Evaluation
Patients were evaluated for the effects of treatment by physical examination and blood testing weekly during treatment, then monthly for 6 months, and then at 9 and 12 months after completion of therapy. Response to treatment was measured 2 months after completion of therapy by physical examination, complete blood count (CBC) and a BM aspirate and biopsy. Minimal residual disease (MRD) was measured in peripheral blood weekly during treatment and then monthly for 6 months and at 9 and 12 months after completion of therapy. The MRD analysis was performed by flow cytometry on the patient’s blood. Lymphocytes were distinguished from other mononuclear peripheral blood cells by forward and side scatter parameters (lymphocyte gate). This population of cells was then examined using 3 color flow cytometry for cells that co-expressed CD19 and CD5 and had dim or absent expression of CD79b. The method was capable of detecting 1:104 CLL lymphocytes but is less sensitive than the more complex 4 color MRD assays developed after the study was initiated30. Cytopenia caused by the treatment protocol was monitored weekly during treatment, then monthly for 6 months, and at 9 and 12 months after completion of treatment. The percentage of T cells (CD4+ and CD8+) and NK cells (CD16+) in the lymphocyte gate was measured by flow cytometry and absolute counts T and NK cell counts were then calculated using the absolute lymphocyte count.
The primary aim of this study was to evaluate response to treatment 2 months after the completion of therapy. All patients were initially evaluated for response using the NCI-WG96 criteria11. In patients with CR or nodular partial response (nPR), the BM biopsy was evaluated with immunohistochemical staining for evidence of residual CLL B cells. BM biopsies were stained with T-cell specific (CD3) and B-cell associated (PAX5, CD79a) antibodies to determine if the residual lymphocytes were predominantly T- or B-cells. In specimens with residual lymphocytes that were predominantly B-cells, antibodies against κ and λ light chains and antibodies to CD5 and CD23 were used to distinguish monoclonal B-cells from benign lymphocytes. Patients with evidence of residual disease were then re-classified as having a partial response (PR) and those with no residual disease as having a true CR.
Time from CLL diagnosis to initial treatment required by NCI-WG96 criteria was calculated for study patients and a comparison cohort. The comparison group, obtained from the Mayo Clinic CLL Database, had stage 0 – II CLL, FISH analysis within 3 years of diagnosis, and fulfilled the eligibility criteria for high risk disease used in the clinical trial, but had not been not enrolled in this clinical trial for logistical or other non medical reasons.
Statistical Analysis
This study was a two-stage phase II trial (Fleming design). A success was defined as a response (NCI-WG96 CR, nPR or PR) at the evaluation two months after completion of therapy. The null hypothesis was that the true response rate for this regimen is at most 50% versus the alternative hypothesis that the true response rate is 75% or greater. The study had 92% power, with a 9% Type I error rate, to detect an effective treatment if the true success rate was at least 75% versus at most 50%. A patient was considered evaluable for response if they were eligible and received treatment. A minimum of 11 and a maximum of 30 evaluable patients were required to evaluate the decision criteria. The stage one analysis was performed after the first 11 patients were evaluable for response. If 5 or fewer successes were observed then the study would be terminated and if 6 or more successes were observed then the study would continue. At final analysis, if 18 or fewer successes were observed then the regimen would be considered insufficiently active but 19 or more successes were observed then we would consider this evidence that this regimen is promising and warrants further study. Assuming that the number of responses was binomially distributed, a 95% confidence interval for the true response rate was calculated according to the approach of Duffy and Santner.
Duration of response and time to progression were evaluated. Responses were measured from the end of treatment. Duration of response was defined as the time from the date of completion of study therapy until the date of disease progression. Time to progression (TTP) was defined as the time from registration until date of disease progression. The distributions of time-to-event endpoints were estimated using the Kaplan-Meier method and patients who were event-free were censored on the date of last follow-up.
Fisher’s exact and Wilcoxon rank sum tests were used to determine if prognostic factors (age, clinical stage (Rai), and risk group using novel prognostic parameters) were similar between patients who received the study regimen and the comparison cohort. Time to treatment (TTT) was defined as the time from the date of CLL diagnosis to the date of initial treatment required by NCI-WG96 criteria. Differences between groups were evaluated using standard Kaplan-Meier methods and logrank statistics. A multivariate Cox model was used to determine whether receiving the study regimen was a significant prognostic factor for time to treatment.
Results
Patient Characteristics
Between January 2005 and June 2007 thirty eligible patients were accrued to this study at Mayo Clinic, Rochester. Patient characteristics are summarized in Table 1.
Table 1.
Patient Characteristics at Time of Registration
| Total (N=30) | |
|---|---|
| Age | |
| Median | 61.0 years |
| Range | (29.0–77.0) |
| Age Group | |
| < 70 years | 22 (73.3%) |
| ≥ 70 years | 8 (26.7%) |
| Gender | |
| Female | 10 (33.3%) |
| Male | 20 (66.7%) |
| Clinical Stage (Rai) | |
| Stage 0 | 7 (23.3%) |
| Stage I | 21 (70%) |
| Stage II | 2 (6.7%) |
| Risk group | |
| 17p− | 9 (30%) |
| 11q− | 8 (26.7%) |
| UM IgVH + ZAP-70+ +/− CD38 + | 13 (43.3%) |
| FISH | % nuclei positive |
| N = number of patients | mean (range) |
| 17p− n = 9 | 62% (15%, 94%) |
| 11q− n = 8 | 61.5% (22%, 90%) |
| Nil n = 7 | |
| 12+ n = 3 | 58% (5%, 84%) |
| 13q− n = 3 | 61% (9%, 80%) |
| IgVH Mutation | |
| Mutated (≥ 2%) | 5 (16.7%) |
| Unmutated (< 2%) | 25 (83.3%) |
| Zap 70 | |
| Negative (< 20%) | 7 (23.3%) |
| Positive (≥ 20%) | 23 (76.7%) |
| CD 38 | |
| Negative (< 30%) | 18 (60%) |
| Positive (≥ 30%) | 12 (40%) |
| Pretreatment Blood count | |
| ALC | |
| Median | 25.3 × 109/L |
| Range | (4.8–124.7) |
| HGB | |
| Median | 14.2 g/dL |
| Range | (12.0–17.7) |
| PLT | |
| Median | 178.5 × 109/L |
| Range | (100.0–312.0) |
| Time from diagnosis to treatment (Months) | |
| Median | 7.8 |
| Range | (0.7–73.1) |
| ECOG Performance Score | |
| 0 | 27 (90%) |
| 1 | 3 (10%) |
UM indicates unmutated;, ALC absolute lymphocyte count; HGB hemoglobin; PLT, platelet, Risk group is hierarchical (17p− > 11q− > UM + ZAP70 +/− CD38), FISH = highest risk abnormality in each patient (17p13− > 11q22− > 12+ > nil > 13q−)
Toxicity
All patients received the scheduled doses of treatment with no treatment delays. Most patients had asymptomatic skin erythema at their alemtuzumab injection sites for the first 2–3 days of treatment. Only 1 patient had a symptomatic “first dose” reaction to rituximab (grade 2) which responded to standard supportive care. CMV reactivation occurred in 3 (10%) patients at 13, 21, and 43 days after starting therapy. One patient required hospitalization for symptomatic CMV infection and was treated with IV foscarnet therapy for ganciclovir resistance infection which resulted in a full recovery. One patient with minor symptoms and one asymptomatic patient responded well to oral valganciclovir. Two patients had fever and rashes caused by trimethoprim/sulfamethoxazole and one of these patients required hospitalization for evaluation until the cause of fever was recognized. There were three other non-hematological grade 3 toxicities attributable to therapy (increased ALT which resolved spontaneously, skin infection responsive to oral antibiotics, and diarrhea responded to supportive care).
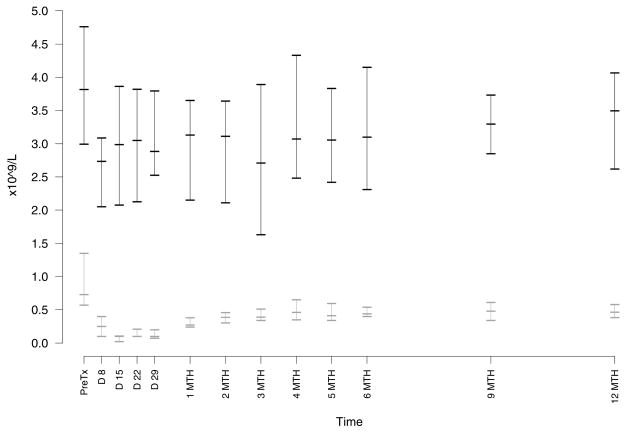
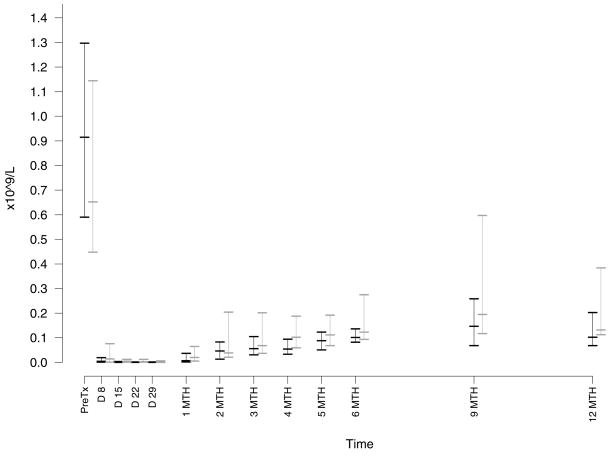
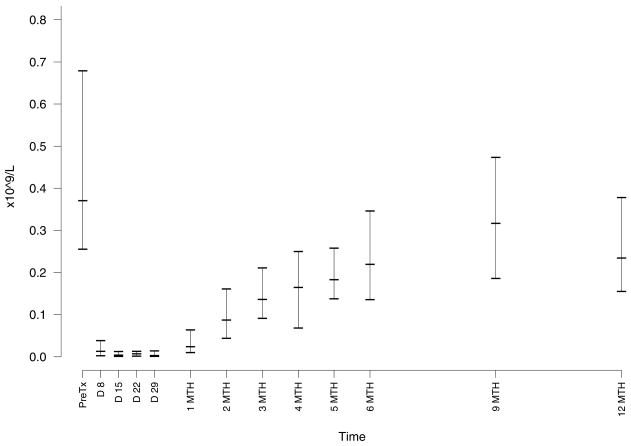
The most common adverse effect was cytopenia. Neutropenia (Figure 1) was common but only severe (grade 3 – 4) in 5 patients with no neutropenic infections. There was no grade 3–4 anemia or thrombocytopenia. All cytopenias resolved without intervention during or within one month of completion of therapy. Monocytopenia occurred in all patients in this study with a median nadir of 0.08 × 109/L (range, 0 – 0.29 (Figure 1). Patients had the expected profound decreases in their absolute lymphocyte count with a median nadir count of 0.03 × 109/L (range 0 – 0.1). The recovery of T cell counts after completion of therapy was slow with median levels below normal at 6 months for CD8+ and beyond 12 months for CD4+ cells (Figure 2). In contrast, NK cell recovered faster with a median level in the normal range by 2 months after completion of therapy (Figure 3).
Figure 1. Effect of Treatment on Neutrophil and Monocyte Counts.
Peripheral blood absolute neutrophil (ANC, upper data points, black) and monocyte (AMC, lower data points, gray) counts were done in the routine clinical laboratory. The graph shows median and 25–75 quartiles for each time point. The nadir ANC was a median count of 1.68 × 109/L (range, 0.15 – 3.65). The nadir AMC was a median count of 0.08 × 109/L (range 0 – 0.29).
Figure 2. Effect of Treatment on T Lymphocytes.
The CD4+ (black data points) and CD8+ (gray data points) T lymphocyte subsets counts were calculated from the absolute lymphocyte count and flow cytometric analysis for expression of CD3, CD4, and CD8. The median nadir count for CD4+ T cells was 0.0001 × 109/L (range 0 – 0.0034) and for CD8+ T cells was 0.0003 × 109/L (range 0 – 0.032).
Figure 3. Effect of Treatment on NK cell counts.
The NK lymphocyte counts were calculated using the absolute lymphocyte count and flow cytometric analysis of cells within the lymphocyte gate for expression of CD16. The median nadir count for NK cells was 0.0004 × 109/L (range 0 – 0.0078).
Treatment Response
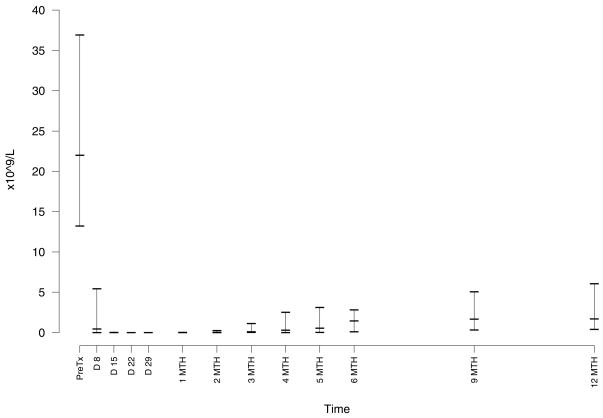
All 30 patients were evaluable with 27 responses (11 CR, 10 nPR, 6 PR) by the NCI-WG96 criteria for an overall response rate of 90% (95% CI: 77 – 97%) and a CR rate of 37% (95% CI: 20 – 56%). Resolution of lymphocytosis after initiation of treatment was rapid with a median nadir B cell count of 0.01 × 109/L (range 0 – 2.8 × 109/L) (Figure 4). When patients with nPR or CR were evaluated for residual CLL in the BM by immunohistochemical examination, 6 patients had no detectable disease. There were thus 6 true CR and 21 PR using our modified criteria for clinical response. Only six of 11 patients who achieved a CR by the NCI-WG96 criteria achieved blood MRD negative status by flow cytometry (2 negative assays at least one month apart after completion of therapy). In contrast 5 of 6 patients who achieved a CR with a negative immunohistochemical analysis BM examination were MRD negative by flow cytometry.
Figure 4. Circulating CLL B Lymphocyte Counts.
The number of circulating CLL cells was calculated after initiation of treatment from the absolute lymphocyte count and percentage of cells on flow cytometry in the lymphocyte gate defined on forward and side scatter that expressed CD19.
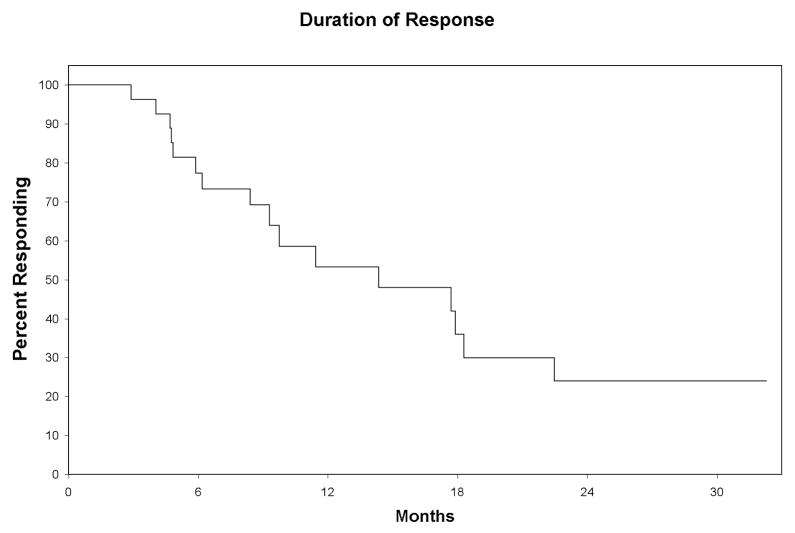
Nineteen patients have had disease progression and 1 patient has died of complications of allogeneic stem cell transplantation. Median follow-up for patients still alive was 17.6 months (range 4.7 – 33.6 months). The median duration of response in the 27 responders was 14.4 months (95% CI: 9.3 – 22.5 months) (Figure 5). Median time to progression for all patients was 12.5 months (95% CI: 7.2 – 19.3 months). Of the 5 patients who achieved a CR with negative immunohistochemical analysis and MRD tests, 4 patients are progression-free after a median of 30.2 months (range, 15.3 to 32.3 months) while one had progressive disease at 23 months but has not required subsequent treatment. Analysis of % nuclei with FISH detectable chromosomal abnormalities in patients with progressive disease (Table 2) showed no evidence of selection of aggressive clones by treatment. There was no cases clonal evolution in patients with progressive CLL.
Figure 5. Duration of response to therapy.
The median duration of response in the 27 responders was 14.4 months (95% CI: 9.3 –22.5 months)
Table 2.
Initial and Subsequent FISH Analysis on Blood in Patients with Progressive Disease
| FISH category (hierarchical) | % Peripheral Blood Cell Nuclei | ||
|---|---|---|---|
| Baseline | 2 months after completion of treatment | 12 months after completion of treatment | |
| 17p− | 58 | 0 | 34.5 |
| 39 | 15.5 | ||
| 90 | 0 | ||
| 62 | 0 | ||
| 84 | 0 | 0 | |
| 68 | 75.5 | ||
| 94 | 0 | ||
| 11q− | 39 | 0 | |
| 88 | 0 | 8.0 | |
| 69 | 8.0 | 40.5 | |
| 22 | 10 | ||
| 12+ | 73 | 0 | 21.5 |
| 53 | 30.5 | ||
| 77 | 10 | ||
| 13q− | 61 | 0 | 8.5 |
| 9 | 7 | ||
| 80 | 4.0 | 23.5 | |
Hierarchical indicates use of highest risk abnormality (17p13− > 11q22− > 12+ > 13q14−)
Nine patients have received subsequent therapy for progressive CLL and some have required more than one treatment regimen. The initial re-treatment regimens were alemtuzumab and rituximab therapy using the same schedule (n = 1); cyclophosphamide, fludarabine, alemtuzumab and rituximab (CFAR) (n = 2); pentostatin, cyclophosphamide and rituximab (PCR) (n = 4); fludarabine, cyclophosphamide and rituximab (FCR) (n=1); and rituximab, cyclophosphamide, vincristine and prednisone (R-CVP) (n = 1). For patients receiving subsequent therapies, the median time from completion of protocol therapy with alemtuzumab and rituximab to date of initiation of subsequent therapy was 8.5 months (range, 2.4 – 32.9 months). Responses to the first re-treatment regimen were: alemtuzumab and rituximab (clinical complete response (CCR) n = 1), CFAR (PR n = 1, progressive disease n=1), PCR (CR n = 1, PR n = 2, progressive disease n=1), FCR (CCR, n=1), R-CVP (CCR, n=1). Of note, two of these patients received further subsequent re-treatment with alemtuzumab and rituximab and achieved responses (PR) which were at least as good as their initial responses to this regimen.
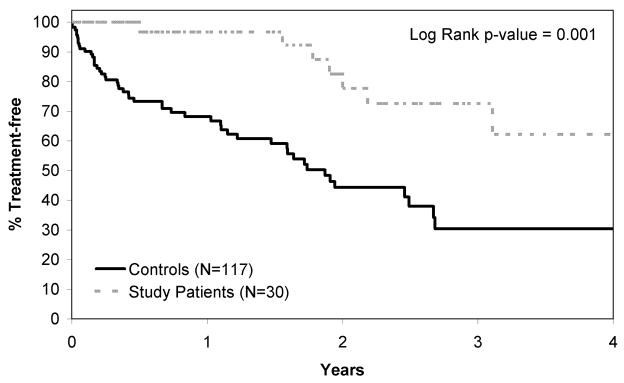
The time from diagnosis to first therapy for progressive CLL (using NCI-WG96 criteria) was compared between patients in this study and a comparison cohort. Patients in the comparison group were similar in age, clinical stage, and risk group (17p13−, 11q22−, UM IgVH and CD38+ and/or ZAP-70+) (Table 3). Median time from the date of CLL diagnosis to the date of initial treatment as required by NCI-WG96 criteria was significantly longer in patients treated with alemtuzumab and rituximab (4.4 years, 95% CI: 3.1, 6.7 years) compared to the comparison group (1.9 years, 95% CI: 1.5, 2.7 years), p=0.001 (Figure 6). In a multivariate Cox model, treatment with alemtuzumab and rituximab was a significant predictor of time from diagnosis to initial treatment required by NCI-WG96 criteria after adjusting for age, stage, and risk group (p=0.001, Table 4).
Table 3.
Characteristics of Treated and Comparison Groups
| Cases (N=30) | Comparison Group (N=117) | p-value* | |
|---|---|---|---|
| Age at diagnosis | 0.15 | ||
| Median | 61 years | 63 years | |
| Range | (28 – 76 yrs) | (42 – 89 yrs) | |
| Clinical Stage at diagnosis (Rai) | 0.58 | ||
| 0 | 17 (57%) | 55 (47%) | |
| I | 11 (37%) | 48 (41%) | |
| II | 2 (7%) | 15 (12%) | |
| Risk Group (hierarchical) | 0.24 | ||
| 17p− | 9 (30%) | 19 (16%) | |
| 11q− | 8 (27%) | 39 (33%) | |
| UM + ZAP-70+ and/or CD38+ | 13 (43%) | 59 (50%) | |
UM indicates unmutated, hierarchical indicates risk stratification with use of single highest risk prognostic factor (17p13− > 11q22− > IgVH UM and ZAP-70/CD38+).
Wilcoxon rank sum or Fisher’s exact p-value
Figure 6. Time from Diagnosis of CLL to First Treatment for Progressive Disease.
Time from diagnosis to first treatment for progressive CLL (using NCI-WG96 criteria) was plotted for patients treated on this study and a comparison cohort from the Mayo Clinic CLL Database with the same high risk features for progressive CLL who did not receive early therapy. Time to first treatment for progressive disease was significantly longer in patients who received alemtuzumab and rituximab therapy.
Table 4.
Results of Multivariate Analysis of Treated and Comparison Groups (n=147)
| Hazard Ratio (95% CI) | p-value | ||
|---|---|---|---|
| Received study regimen | 0.28 (0.13, 0.60) | 0.001 | |
| Risk group | |||
| 17p− | 2.98 (1.53, 5.80) | 0.001 | |
| 11q− | 1.38 (0.73, 2.58) | 0.32 | |
| Age ≥70 | 0.69 (0.34, 1.42) | 0.32 | |
| Rai stage I or II vs. 0 | 2.84 (1.58, 5.11) | 0.0005 | |
Discussion
We report the first study in CLL that selected patients for early treatment of their disease based on molecular prognostic markers. Early treatment with the lymphocyte directed monoclonal antibodies alemtuzumab and rituximab was effective and well tolerated. However, this treatment is non curative and its utility in the management of high risk earlier stage CLL will need further study.
The ability to accurately diagnose CLL in early stage and the discovery of biological markers that accurately predict poor prognosis allows for selection of high risk CLL patients with low disease burden for experimental therapy. We used stringent criteria to define high risk disease based on previous studies4–6 that suggested the median time from diagnosis to treatment based on the NCI-WG96 criteria for enrolled patients would be ~ 2 years. To test the accuracy of this prediction, we analyzed patients from a comparison group from our database (n = 117) not enrolled in this clinical trial. These patients had a median time to treatment of 1.9 years (95% CI: 1.5, 2.7 years) validating our selection criteria.
Recent developments have greatly expanded the therapeutic repertoire for patients with CLL and provide lower toxicity treatment options. The major toxicities of the MoAb used in this study are the “first dose” reactions and immunosuppression. In this study there was only one “first dose” reaction that required additional therapy which reflects the lower disease burden, use of subcutaneous alemtuzumab, and the reduction in CLL cell counts prior to the first administration of rituximab. In this study there were no serious long term complications and no deaths due to infectious complications. The CMV morbidity could have been decreased by monitoring and early treatment for CMV reactivation. Two patients had unanticipated and severe new allergies to prophylactic trimethoprim/sulfamethoxazole during the initial post treatment period suggesting an aberrant response by the regenerating immune system. Cytopenia is an expected complication of MoAb treatment. All patients in the study had a profound monocytopenia with slow recovery of these counts. In contrast, neutropenia was transient and had no observed clinical consequences. These data on toxicity suggest that this MoAb regimen can be safely used in patients with CLL providing there is careful monitoring for complications and a rapid response to adverse effects.
A potential concern about the use of early treatment of patients with CLL is the development of resistance to drug therapy due to selection of resistant clones. Patients with progressive disease showed no evidence of increased resistance to treatment and FISH analysis of their CLL cells did not suggest clonal selection or clonal evolution. These data suggest that the treatment regimen tested does not limit future treatment options for these patients.
This phase 2 study was designed to evaluate the safety and efficacy of the MoAb therapy and provided the data required to plan a phase 3 randomized trial comparing earlier to standard treatment. The significantly increased time from diagnosis of CLL to treatment for progressive disease using NCI-WG96 criteria among study patients relative to that of a comparison cohort is clinically important and suggests that a randomized phase 3 study is justified.
The five patients achieving MRD negative remissions had the best durations of response suggesting that the extent of response predicts its duration in patients with high risk disease. In contrast, patients not achieving a MRD negative remission had a relatively short duration of response. These patients could potentially benefit from longer treatment but this would likely also increase the risk of toxicity. Toxicity could be minimized if treatment was individualized using response assessed by clinical measurements, MRD assays and CT scans. In addition, the efficacy of the alemtuzumab and rituximab regimen could be improved by addition of other drugs with potentially additive or synergistic effects based on a better understanding of the mechanisms of action of the MoAb and the mechanism of resistance to MoAb in CLL cells.
We conclude that alemtuzumab and rituximab therapy for high risk patients with earlier clinical stage CLL is a promising new option requiring further evaluation and development. We intend to study the regimen in combination with other agents including purine analogues and newer drugs for treatment of high risk earlier stage, progressive and relapsed/refractory CLL. The data from our initial study combined with a better understanding of the mechanism of action of alemtuzumab and rituximab could result in the development of more effective and less toxic therapies for patients with CLL.
Acknowledgments
Source of Support
This work was supported by the NIH-NCI University of Iowa/Mayo Clinic SPORE Grant CA97274, R01 grant CA95241, K Award CA113408, Genentech, and Bayer Health Care Pharmaceuticals.
References
- 1.Call TG, Phyliky RL, Noel P, Habermann TM, Beard CM, O’Fallon WM, et al. Incidence of chronic lymphocytic leukemia in Olmsted County, Minnesota, 1935 through 1989, with emphasis on changes in initial stage at diagnosis. Mayo Clin Proc. 1994;69:323–28. doi: 10.1016/s0025-6196(12)62215-0. [DOI] [PubMed] [Google Scholar]
- 2.Zent CS, Ding W, Schwager SM, Reinalda MS, Hoyer JD, Jelinek DF, et al. The prognostic significance of cytopenia in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) Br J Haematol. 2008;141:615–21. doi: 10.1111/j.1365-2141.2008.07086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dighiero G, Binet J-L. When and how to treat chronic lymphocytic leukemia. N Engl J Med. 2000;343:1799–801. doi: 10.1056/NEJM200012143432410. [DOI] [PubMed] [Google Scholar]
- 4.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Kröber A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–16. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 5.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–47. [PubMed] [Google Scholar]
- 6.Hamblin T, Davis Z, Gardiner A, Oscier D, Stevenson F. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–54. [PubMed] [Google Scholar]
- 7.Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1764–75. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 8.Hamblin TJ, Orchard JA, Ibbotson RE, Davis Z, Thomas PW, Stevenson FK, et al. CD38 expression and immunoglobulin variable region mutations are independent prognostic variables in chronic lymphocytic leukemia, but CD38 expression may vary during the course of the disease. Blood. 2002;99:1023–29. doi: 10.1182/blood.v99.3.1023. [DOI] [PubMed] [Google Scholar]
- 9.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–34. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 10.Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists’ Collaborative Group. J Natl Cancer Inst. 1999;91:861–68. doi: 10.1093/jnci/91.10.861. [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O’Brien S, et al. National Cancer Institute-Sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–97. [PubMed] [Google Scholar]
- 12.Messmer BT, Messmer D, Allen SL, Kolitz JE, Kudalkar P, Cesar D, et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest. 2005;115:755–64. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanafelt TD, Witzig TE, Fink SR, Jenkins RB, Paternoster SF, Smoley SA, et al. Prospective evaluation of clonal evolution during long-term follow-up of patients with untreated early-stage chronic lymphocytic leukemia. J Clin Oncol. 2006;24:4634–41. doi: 10.1200/JCO.2006.06.9492. [DOI] [PubMed] [Google Scholar]
- 14.Faderl S, Thomas DA, O’Brien S, Garcia-Manero G, Kantarjian HM, Giles FJ, et al. Experience with alemtuzumab plus rituximab in patients with relapsed and refractory lymphoid malignancies. Blood. 2003;101:3413–15. doi: 10.1182/blood-2002-07-1952. [DOI] [PubMed] [Google Scholar]
- 15.Rossmann ED, Lundin J, Lenkei R, Mellstedt H, Osterborg A. Variability in B-cell antigen expression: implications for the treatment of B-cell lymphomas and leukemias with monoclonal antibodies. Hematol J. 2001;2:300–06. doi: 10.1038/sj.thj.6200119. [DOI] [PubMed] [Google Scholar]
- 16.Osterborg A, Fassas AS, Anagnostopoulos A, Dyer MJ, Catovsky D, Mellstedt H. Humanized CD52 monoclonal antibody Campath-1H as first-line treatment in chronic lymphocytic leukaemia. Br J Haematol. 1996;93:151–53. doi: 10.1046/j.1365-2141.1996.450989.x. [DOI] [PubMed] [Google Scholar]
- 17.Hillmen P, Skotnicki AB, Robak T, Jaksic B, Dmoszynska A, Wu J, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5553–55. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 18.Osterborg A, Dyer MJ, Bunjes D, Pangalis GA, Bastion Y, Catovsky D, et al. Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. European Study Group of CAMPATH-1H Treatment in Chronic Lymphocytic Leukemia. J Clin Oncol. 1997;15:1567–74. doi: 10.1200/JCO.1997.15.4.1567. [DOI] [PubMed] [Google Scholar]
- 19.Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–61. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 20.Lozanski G, Heerema NA, Flinn IW, Smith L, Harbison J, Webb J, et al. Alemtuzumab is an Effective Therapy for Chronic Lymphocytic Leukemia with p53 Mutations and Deletions. Blood. 2004;103:3278–81. doi: 10.1182/blood-2003-10-3729. [DOI] [PubMed] [Google Scholar]
- 21.Huhn D, von Schilling C, Wilhelm M, Ho AD, Hallek M, Kuse R, et al. Rituximab therapy of patients with B-cell chronic lymphocytic leukemia. Blood. 2001;98:1326–31. doi: 10.1182/blood.v98.5.1326. [DOI] [PubMed] [Google Scholar]
- 22.Keating MJ, O’brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–88. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 23.Kay NE, Geyer SM, Call TG, Shanafelt TD, Zent CS, Jelinek DF, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B-chronic lymphocytic leukemia. Blood. 2007;109:405–11. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrd JC, Rai K, Peterson BL, Appelbaum FR, Morrison VA, Kolitz JE, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien SM, Kantarjian H, Thomas DA, Giles FJ, Freireich EJ, Cortes J, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–70. doi: 10.1200/JCO.2001.19.8.2165. [DOI] [PubMed] [Google Scholar]
- 26.Nowakowski GS, Dewald GW, Hoyer JD, Paternoster SF, Stockero KJ, Fink SR, et al. Interphase fluorescence in situ hybridization with an IGH probe is important in the evaluation of patients with a clinical diagnosis of chronic lymphocytic leukaemia. Br J Haematol. 2005;130:36–42. doi: 10.1111/j.1365-2141.2005.05548.x. [DOI] [PubMed] [Google Scholar]
- 27.Dewald GW, Brockman SR, Paternoster SF, Bone ND, O’Fallon JR, Allmer C, et al. Chromosome anomalies detected by interphase fluorescence in situ hybridization: correlation with significant biological features of B-cell chronic lymphocytic leukaemia. Br J Haematol. 2003;121:287–95. doi: 10.1046/j.1365-2141.2003.04265.x. [DOI] [PubMed] [Google Scholar]
- 28.Jelinek DF, Tschumper RC, Geyer SM, Bone ND, Dewald GW, Hanson CA, et al. Analysis of clonal B-cell CD38 and immunoglobulin variable region sequence status in relation to clinical outcome for B-chronic lymphocytic leukaemia. Br J Haematol. 2001;115:854–61. doi: 10.1046/j.1365-2141.2001.03149.x. [DOI] [PubMed] [Google Scholar]
- 29.Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 30.Rawstron AC, Villamor N, Ritgen M, Bottcher S, Ghia P, Zehnder JL, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21:956–64. doi: 10.1038/sj.leu.2404584. [DOI] [PubMed] [Google Scholar]