Abstract
Memory for olfactory stimuli may be particularly affected by age-related brain changes in humans and may be an early indicator of cognitive impairment and Alzheimer's disease. Studies involving rats have offered insights into impaired cognition in aged animals, but few have examined odor memory. Therefore, it is unclear whether aged rats are a good model for possible age-related changes in odor memory in humans. Young (6 month old) and old (24 month old) rats were tested on associative learning tasks involving visual and olfactory stimuli. The first task examined age-related differences in discrimination and reversal learning for olfactory and visual stimuli; the second task utilized an associative contextual learning task involving olfactory and visual cues. Although old rats were able to perform the olfactory and visual discrimination tasks as well as young rats, old rats displayed significant age-related impairment on the reversal learning and contextual learning tasks. The results suggest that aging may have a similar deleterious effect on odor memory in rats and in humans. The findings may have important implications for the selection of memory paradigms for future research studies on aging. In addition, the use of an animal model to investigate the effects of aging on odor memory will allow researchers the ability to investigate how age-related neuroanatomical and neurochemical changes may result in impaired odor memory.
Keywords: Aging, Learning, Memory, Context, Olfactory, Visual Object
Introduction
A number of researchers have emphasized the importance of the use of animal models to examine age-related changes in cognition and behavior. However, few studies have examined age-related odor memory impairments in rodents. Older adults tend to be impaired relative to young adults on odor learning and memory tasks.(1–7) In addition, problems remembering and detecting odors may be an early indication of cognitive impairment and dementing diseases in non-demented older adults.(8–13) Therefore, additional studies are needed to examine age-related changes in olfactory learning and memory in a rodent model of aging.
Kraemer & Apfelbach (14) suggest that the modality of a to-be-remembered stimulus is critical when determining efficient learning. Rats can readily learn and maintain a high level of performance on odor memory tasks. (15–17) In addition, acquisition rates and retention of memory for olfactory stimuli may be greater than for visual stimuli in young adult rats.(18–19) However, it is not clear whether the memory superiority for olfactory stimuli is preserved later in the life of a rat, since selective pressure for olfactory function may not exist following the reproductive lifespan of the animal. Olfactory sensitivity is decreased in aged rats relative to young rats.(14,20) However, only a few studies have examined odor learning in aged rats.(14,21–24) Thus, it is unclear to what extent age-related brain changes might result in impairments in odor learning and memory in older rats.
Discrimination and Reversal Learning for Olfactory and Visual Stimuli
Although some studies have demonstrated that rats show age-related impairments on reversal learning tasks involving olfactory stimuli,(22,23) it has not been clearly demonstrated whether the learning impairment is comparable to reversal learning for stimuli presented in other modalities. Therefore, we investigated age-related differences in discrimination and reversal learning for olfactory and visual stimuli in young and aged rats in order to determine whether odor learning is as affected by aging as visual object learning in rats.(25)
Forty Fischer 344/Brown Norway (Harlan Laboratories) male rats approximately 6 months (n = 20) and 24 months (n = 20) of age were used as subjects. Each age group was evenly divided into an olfactory or visual object condition in order to provide cross modal comparisons. The testing apparatus consisted of a box with one removable guillotine door placed in the center of the box to divide the box into two separate compartments, a start chamber and a choice chamber. One row of three evenly spaced food-wells were drilled into the floor in each chamber. Olfactory stimuli consisted of powdered odorants mixed in sand and presented in plastic cups as described in previous studies.(15,16) Visual stimuli consisted of small, visually dissimilar objects mounted on small flat metal washers to stabilize the objects and to completely cover the opening of the food-wells. (16)
Discrimination was assessed using a two-choice discrimination task.(25) A total of either four odors or four objects were used in the experiment; however, each rat discriminated between only two stimuli. For each rat, one odor or object was randomly assigned as the rewarded stimulus and the other odor or object was assigned as the non-rewarded stimulus. The rat began each trial in the start chamber of the apparatus with the door to the choice chamber closed. The door was opened and the rat was allowed to choose between the two stimuli presented side-by-side in the choice chamber. If the rat selected the rewarded stimulus by either displacing the object or digging in the sand, the rat received a food reward. However, if the rat selected the non-rewarded stimulus, the rat did not receive a reward. Each rat received 12 trials per day and was tested until the animal reached a criterion of 9 correct choices out of a sliding block of 10 consecutive trials within the 12 trials.
Once each animal reached the learning criterion on the discrimination task, the reward contingencies were reversed on the following day of testing to assess reversal learning. The previously rewarded stimulus became the non-rewarded stimulus and the previously non-rewarded stimulus became the rewarded stimulus. Each rat was tested on reversal trials until the same criterion used on the discrimination trials was reached.
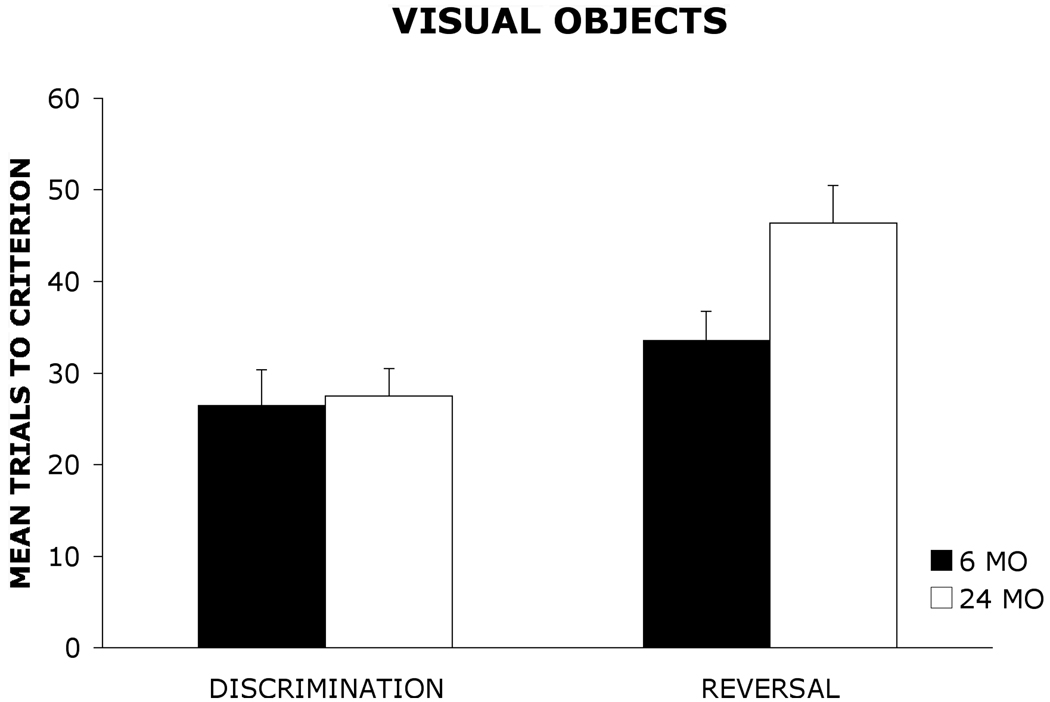
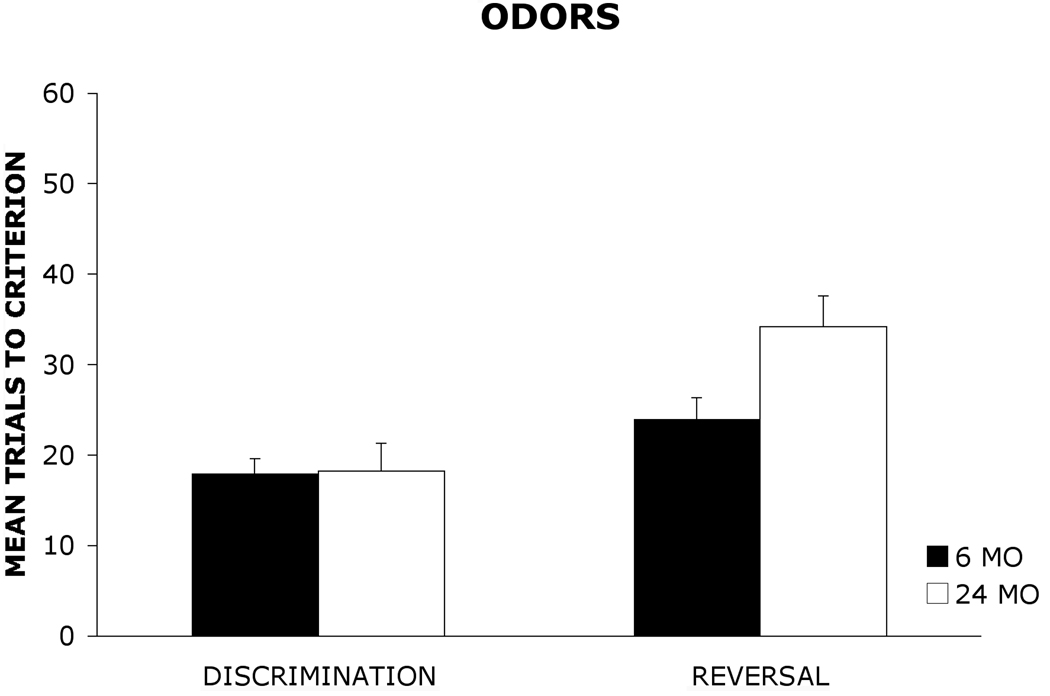
Figure 1 and Figure 2 show the mean (+ standard error) number of trials required by 6 mo and 24 mo old rats to reach the learning criterion on the olfactory and visual object discrimination and reversal tasks. A 2 × 2× 2 analysis of variance (ANOVA) with age group (6 mo, 24 mo) and stimulus (odors, visual objects) as between-group factors and task (discrimination, reversal) as a within-group factor was used to analyze the data. The results revealed a significant main effect of group, F(1, 36) = 5.87, p < .05, indicating that 6 mo old rats outperformed 24 mo old rats. There also was a significant main effect of stimulus, F(1,36) = 15.92, p < .001, suggesting that rats acquired the olfactory task more readily than the visual object task. Additionally, there was a significant main effect of task, F(1, 36) = 39.01, p < .001, indicating that rats acquired the discrimination task at a faster rate than the reversal task regardless of stimulus modality. Furthermore, the analysis revealed a significant group × task interaction, F(1, 36) = 8.16, p < .01. A Newman-Keuls post hoc comparison test of the group × task interaction revealed that there were no significant differences in the number of trials required by 6 mo and 24 mo old rats to reach the learning criterion on the olfactory (6 mo= 18 ± 1.64: 24 mo= 18.2 ± 3.12) or visual object (6 mo= 26.6 ± 3.77: 24 mo= 27.5 ± 3.03) discrimination tasks. However, 24 mo old rats required significantly more trials (p < .05) to reach the learning criterion relative to 6 mo old rats on both the olfactory reversal task (6 mo= 24 ± 2.34: 24 mo= 34.2 ± 3.37) and the visual object reversal task(6 mo= 33.6 ± 3.12: 24 mo= 46.4 ± 4.06).
Figure 1.
Mean trials to criterion (+ standard error) for 6 mo and 24 mo old rats on a visual object discrimination and reversal task.
Figure 2.
Mean trials to criterion (+ standard error) for 6 mo and 24 mo old rats on an olfactory discrimination and reversal task.
The present study represents the first direct examination of age-related differences in discrimination and reversal learning using the same paradigm for olfactory and visual stimuli to facilitate cross-modal comparisons. The similar acquisition rates on the discrimination task suggest that 24 mo old rats were able to learn the initial stimulus-reward associations as readily as 6 mo old rats. However, when the reward contingencies were reversed, 24 mo old rats required significantly more trials than 6 mo old rats to reach the learning criterion on both the olfactory and visual object reversal task. These findings suggest that 24 mo old rats show comparable impairments in reversal learning for olfactory and visual stimuli. Consistent with prior research, the data suggest that rats tend to show greater acquisition for stimuli presented in the olfactory modality than for stimuli presented in the visual modality. (18,19) Thus, olfactory-based tasks used to study learning and memory processes in aged rats may be beneficial for behavioral research given that rats may acquire tasks involving olfactory stimuli more readily than visual stimuli. The results may have important implications for the selection of memory paradigms for future research studies on aging.
Older adults show impairments on tasks that require the ability to switch cognitive sets, (26) such as a reversal-learning task. Prefrontal cortex dysfunction is suggested to result in a decreased ability to maintain and shift cognitive sets.(27) Specifically, the OFC has been shown to be important for reversal learning for olfactory stimuli.(27–29) Therefore, the age-related reversal learning deficits observed in the present study may results from age-related OFC dysfunction.
Odor-Context Associative Learning
The next experiment was designed to examine age-related changes in memory for odor-context associations. The contextual features of an episode have been shown to play a critical role in learning and memory. Research has suggested that the hippocampus may be essential for processing contextual information. Disruptions of hippocampal function have been shown to impair contextual fear conditioning(30) and appetitive conditioning to contextual stimuli.(31) Recent studies utilizing paired-associate learning paradigms also have demonstrated that hippocampal damage impairs the acquisition and retention of contextual stimuli.(32) In addition, spatial firing patterns in hippocampal neurons demonstrate significant changes when the context is altered, including changes in the spatial layout, color, and odor of an environment as well as changes in non-environmental features such as task demands.(33–34)
Similar to animals with hippocampal lesions, older rats show impaired contextual fear conditioning compared to younger rats, but normal fear conditioning to a tone.(35) However, few if any studies have investigated age-related changes in memory for contextual and olfactory associations using an appetitive-based paradigm. Therefore, we investigated memory for odor-context associations in young and aged rats.(36)
Twenty Fischer 344/Brown Norway (Harlan Laboratories) male rats 6 mo of age (n=10) and 24 mo of age (n=10) were used as test subjects. Testing was conducted in two clear Plexiglas boxes that represented two different contexts, termed Context 1 and Context 2. A context was defined as the total of all of the environmental cues in the apparatus, including floor texture, color of walls, and visual cues on the walls. In Context 1, the flooring was black and textured. The walls were black with thin white stripes (see Figure 3). In Context 2, the flooring was white and smooth. The walls were white with a different black geometric shape (i.e., star, triangle, square, circle) on each wall (see Figure 3).
Figure 3.
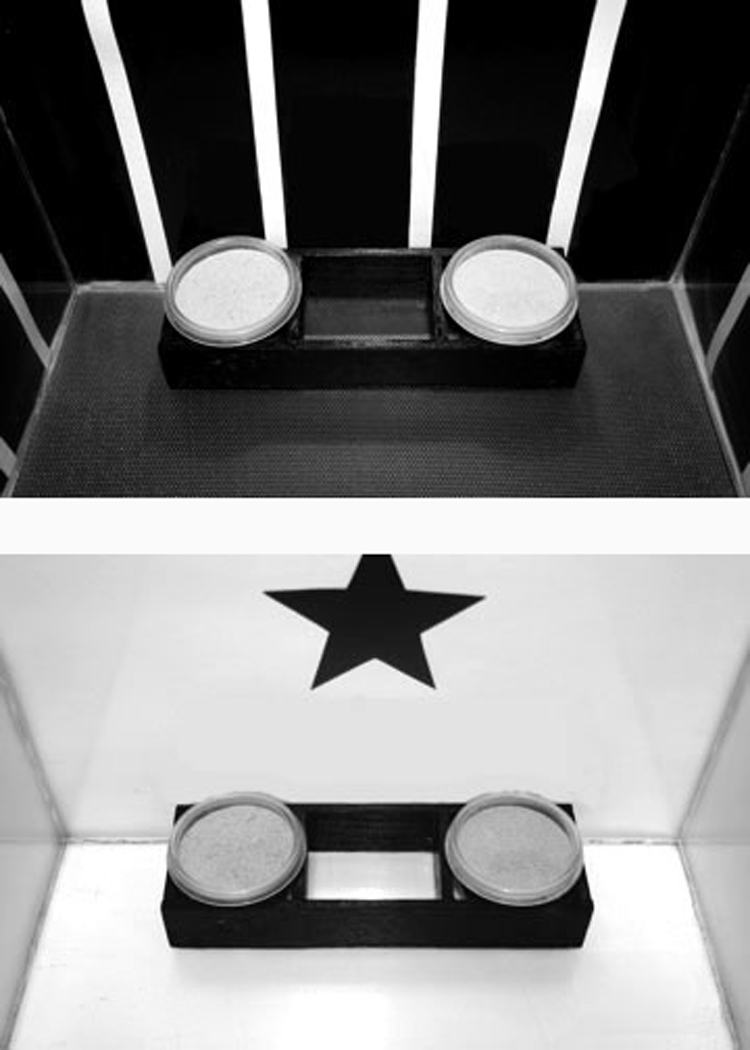
Photographs of the two contexts used in the contextual associative learning task.
Each rat was tested on a contextual learning task originally developed by Rajji and colleagues.(32) In this task, rats must learn to associate one odor with one context and a second odor with a different context. Olfactory stimuli consisted of common odors: cinnamon, cumin, ginger, and baby powder. Odors were mixed in sand and placed in small clear plastic cups as described in previous studies.(15,16) In each context, the rat was presented simultaneously with two cups, each filled with a different odorant (Odor A and Odor B). The cups were presented adjacent to one another as shown in Figure 3. In Context 1, the rat received a food reward for digging in the cup containing Odor A, but did not receive a food reward for digging in the cup containing Odor B. In Context 2, the rat received a food reward for digging in the cup containing Odor B, but did not receive a food reward for digging in the cup containing Odor A. The left/right position of each cup and the order in which rats were presented with each context followed a pseudorandom order. Each rat performed 10 trials per day with five trials in each context across eight consecutive days. The same odor problem was presented throughout all days of testing. Each trial was separated by a 15–20 min intertrial interval.
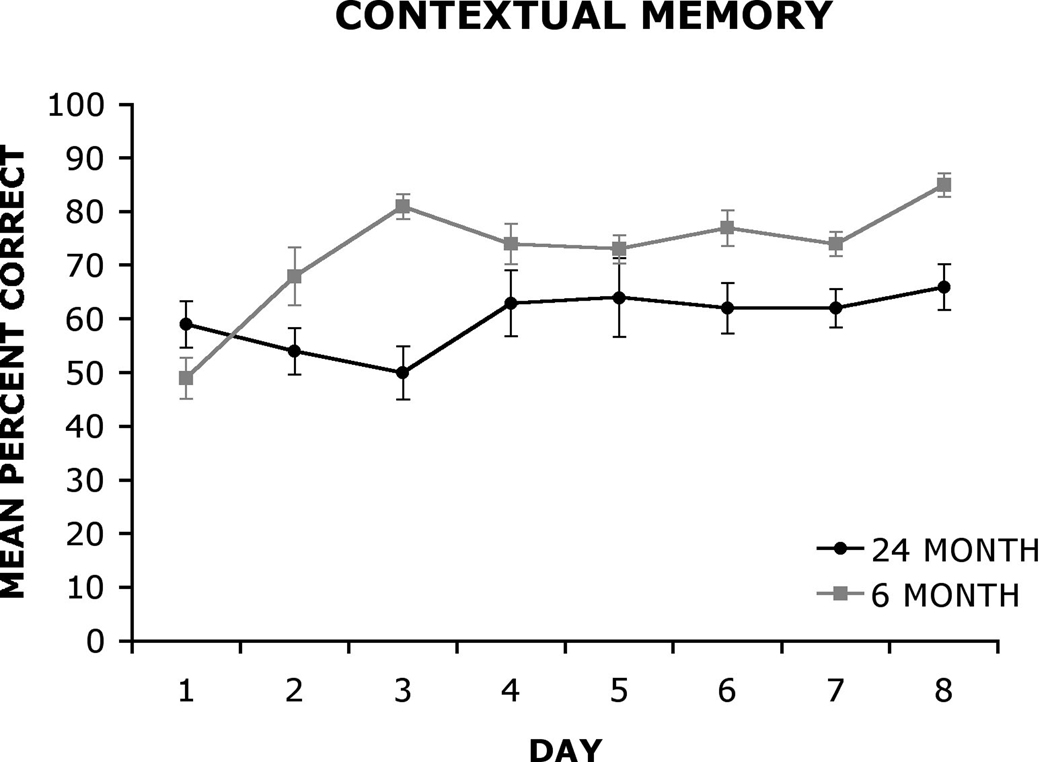
The mean (±SE) number of correct responses on the contextual learning task as a function of days (1–8) for the 6 mo and 24 mo old rats are shown in Figure 4. A 2 × 8 analysis of variance (ANOVA) with group (6 mo, 24 mo) as the between-group factor and day (days 1–8) as the within group factor was used to analyze the data. The analysis revealed a statistically significant main effect of group, F (1, 18) = 27.58, p < .001 and day F (7, 126) = 4.61, p < .001. In addition, the analysis revealed a statistically significant group × day interaction, F (7, 126) = 3.66, p < .01. A Newman-Keuls post hoc comparison test of the statistically significant group × day interaction did not detect significant differences between young and old rats on the first two days of testing. However, young rats significantly (p < .05) outperformed old rats on Day 3. Young rats continued to perform better than old rats on Days 4–7 and significantly outperformed old rats on Day 8 (p < .05). The post hoc analysis of the group × day interaction also indicated that 6 mo old rats performed significantly better on Days 3–8 compared to Day 1 (p < .05), indicating learning across the eight days of testing. However, there were no significant differences among any days of testing in the 24 mo old rats.
Figure 4.
The mean (± standard error) percent correct performance of 6 mo and 24 mo old rats on the contextual associative learning task as a function of days (1–8).
The deficits observed in 24 mo old rats in memory for odor-context associations may be due to age-related dysfunction in the medial temporal lobes and particularly in the hippocampus. Age-related functional changes have been well documented in the hippocampus(37,38) and aged rats demonstrate impairments on a variety of tasks that are considered to rely on the functional integrity of the hippocampus. (37,39,40) The present paradigm was used to examine the effects of CA3 NR1 gene deletions in the hippocampus on learning novel paired associations between olfactory cues and their context.(32) The results showed that NR1 gene deletions in less than 30% of the dorsal hippocampus CA3 subregion were sufficient to disrupt learning the contextual associations.(32) Therefore, age-related changes in the hippocampus may be responsible for the impairment found in memory for odor –context associations in the present study.
The results of the aforementioned studies provide further evidence that aged rats show deficits on olfactory learning and memory tasks that have been demonstrated to be dependent on various brain regions affected by aging. Olfactory-based tasks may be beneficial for behavioral research involving aged rats because the tasks are rapidly acquired. The results may have important implications for the selection of memory paradigms for future research studies on aging involving animal models.
Acknowledgments
The research was supported by NIH grant #AG026505 from NIA to Paul E. Gilbert. Jerlyn Tolentio was supported by a Research Supplement to Promote Diversity in Health Related Research to NIH grant #AG026505. Trinh Luu was supported by grant NIH/NIGMS SDSU MARC 5T34GM08303. We thank Caren McDonald, Danielle Fellman, Molly Moreland, Elizabeth Estes, and Chris Moreland for their assistance with data collection.
References
- 1.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Murphy C, et al. Prevalence of olfactory impairment in older adults. J. Am. Med. Assoc. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 3.Schiffman SS, Graham BG. Taste and smell perception affect appetite and immunity in the elderly. Eur. J. Clin. Nutr. 2000;3:54–63. doi: 10.1038/sj.ejcn.1601026. [DOI] [PubMed] [Google Scholar]
- 4.Wysocki CJ, Gilbert A. National Geographic Smell Survey: Effects of age are heterogeneous. Ann. N. Y. Acad. Sci. 1989;561:12–28. doi: 10.1111/j.1749-6632.1989.tb20966.x. [DOI] [PubMed] [Google Scholar]
- 5.Larsson M, Backman L. Modality memory across the adult life span: Evidence for selective age-related olfactory deficits. Exp. Aging Res. 1998;24:63–82. doi: 10.1080/036107398244364. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert PE, et al. Differential effects of normal aging on memory for odor-place and object-place associations. Exp. Aging Res. 2008 doi: 10.1080/03610730802271914. In press. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert PE, Pirogovsky E, Ferdon S, Murphy C. Differential effects of normal aging on source memory for odors and objects. J. Gerontol. 2006;61:58–60. doi: 10.1093/geronb/61.1.p58. [DOI] [PubMed] [Google Scholar]
- 8.Devanand DP, et al. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am. J. Psychiatry. 2002;157:1399–1405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- 9.Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging. 2008;25:693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert PE, Murphy C. The effect of the ApoE ε4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer’s disease, probable Alzheimer’s disease, and healthy elderly controls. J. Clin. Exp. Neuropsychol. 2004;26:779–794. doi: 10.1080/13803390490509439. [DOI] [PubMed] [Google Scholar]
- 11.Graves AB, et al. Impaired olfaction as a marker for cognitive decline. Neurology. 1999;53:1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- 12.Schiffman SS, et al. Taste, smell and neuropsychological performance of individuals at familial risk for Alzheimer’s disease. Neurobiol. Aging. 2002;23:397–404. doi: 10.1016/s0197-4580(01)00337-2. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RS, et al. The relationship between cerebral Alzheimer's disease pathology and odour identification in old age. J. Neurol. Neurosurg. Psychiatr. 2007;78:30–35. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraemer S, Apelbach R. Olfactory sensitivity, learning and cognition in young adult and aged male Wistar rats. Physiol. Behav. 2004;81:435–442. doi: 10.1016/j.physbeh.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert PE, Kesner RP. Localization of function within the dorsal hippocampus: the role of the CA3 subregion in paired-associate learning. Behav. Neurosci. 2003;117:1385–1394. doi: 10.1037/0735-7044.117.6.1385. [DOI] [PubMed] [Google Scholar]
- 17.Slotnick B, Hanford L, Hodos W. Can rats acquire an olfactory learning set? J. Exp. Psychol. Anim. Behav. Process. 2000;26:399–415. doi: 10.1037//0097-7403.26.4.399. [DOI] [PubMed] [Google Scholar]
- 18.Broadbent NJ, Squire LR, Clark RE. Rats depend on habit memory for discrimination learning and retention. Learn. Mem. 2007;14:145–151. doi: 10.1101/lm.455607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozin P, Kalat JW. In: Learning as a situation specific adaptation. Seligman MEP, Hager JL, editors. New York: Appelton-Dentruy-Crofts; 1972. pp. 66–96. [Google Scholar]
- 20.Apfelbach R, Russ D, Slotnick BM. Ontogenetic changes in odor sensitivity, olfactory receptor area, and olfactory receptor density in the rat. Chem. Senses. 1991;16:209–218. [Google Scholar]
- 21.LaSarge CL, et al. Deficits across multiple cognitive domains in a subset of aged Fischer 344 rats. Neurobiol. Aging. 2007;28:928–936. doi: 10.1016/j.neurobiolaging.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Roman FS, AlescioLautier B, Soumireu-Mourat B. Age-related learning and memory deficits in odor-reward association in rats. Neurobiol. Aging. 1996;17:31–40. doi: 10.1016/0197-4580(95)02030-6. [DOI] [PubMed] [Google Scholar]
- 23.Schoenbaum G, Nugent S, Saddoris MP, Gallagher M. Teaching old rats new tricks: Age-related impairments in olfactory reversal learning. Neurobiol. Aging. 2002;23:555–564. doi: 10.1016/s0197-4580(01)00343-8. [DOI] [PubMed] [Google Scholar]
- 24.Renteria AF, Silbaugh BC, Tolentino JC, Gilbert PE. Age-related changes in conditioned flavor preference in rats. Behav. Brain Res. 2008;188:56–61. doi: 10.1016/j.bbr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brushfield AM, et al. The effects of normal aging on odor memory using an animal model. Behav. Neurosci. 2008;122:54–62. [Google Scholar]
- 26.Mell T, et al. Effect of aging on stimulus-reward association learning. Neuropsychologia. 2005;43:554–563. doi: 10.1016/j.neuropsychologia.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;7:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol. Learn. Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolls ET, Hornak J, Wade D, McGrath J. Emotion related learning in patients with social and emotional changes associated with frontal lobe damage. J. Neurol. Neurosurg. Psychiatr. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J. Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Good M, Honey RC. Conditioning and contextual retrieval in hippocampal rats. Behav. Neurosci. 1991;105:499–509. doi: 10.1037//0735-7044.105.4.499. [DOI] [PubMed] [Google Scholar]
- 32.Rajji T, Chapman D, Eichenbaum H, Greene R. The role of CA3 hippocampal NMDA receptors in paired associate learning. J. Neurosci. 2006;26:908–915. doi: 10.1523/JNEUROSCI.4194-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith DM, Mizumori SJ. Learning-related development of context-specific neuronal responses to places and events: the hippocampal role in context processing. J. Neurosci. 2006;26:3154–3163. doi: 10.1523/JNEUROSCI.3234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 35.Houston FP, Stevenson GD, McNaughton BL, Barnes CA. Effects of age on the generalization and incubation of memory in the F344 rat. Learn. Mem. 1999;6:111–119. [PMC free article] [PubMed] [Google Scholar]
- 36.Luu TT, Pirogovsky E, Gilbert PE. Age-related changes in contextual associative learning. Neurobiol Learn Mem. 2008;89:81–85. doi: 10.1016/j.nlm.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17:13–18. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 38.Tanila H, Sipila P, Shapiro M, Eichenbaum H. Brain aging: impaired coding of novel environmental cues. J. Neurosci. 1997;17:5167–5174. doi: 10.1523/JNEUROSCI.17-13-05167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav. Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 40.Rapp PR, Amaral DG. Recognition memory deficits in a subpopulation of aged monkeys resemble the effects of medial temporal lobe damage. Neurobiol. Aging. 1991;12:481–486. doi: 10.1016/0197-4580(91)90077-w. [DOI] [PubMed] [Google Scholar]