Abstract
Oxidative stress is one of the hypotheses involved in the etiology of Alzheimer’s disease (AD). Considerable attention has focused on increasing the intracellular glutathione (GSH) levels in many neurodegenerative diseases, including AD. Pycnogenol® (PYC) has antioxidant properties and stabilizes intracellular antioxidant defense systems including glutathione (GSH) levels. The present study investigated the protective effects of PYC on acrolein-induced oxidative cell toxicity in cultured SH-SY5Y neuroblastoma cells. Decreased cell survival in SH-SY5Y cultures treated with acrolein correlated with oxidative stress, increased NADPH-oxidase activity, free radical production, protein oxidation/nitration (protein carbonyl, 3-nitrotyrosine) and lipid peroxidation (4-hydroxy-2-nonenal). Pretreatment with PYC significantly attenuated acrolein induced cytotoxicity, protein damages, lipid peroxidation, and cell death. A dose-response study suggested that PYC showed protective effects against acrolein toxicity by modulating oxidative stress and increasing GSH. These findings provide support that PYC may provide a promising approach for the treatment of oxidative stress related neurodegenerative diseases such as AD.
Keywords: Pycnogenol, oxidative stress, acrolein, glutathione, neurodegeneration and neuroprotection
Introduction
Intracellular oxidative stress, represented by ROS production/lipid peroxidation, is a naturally occurring physiological process that may be required in response to some cytotoxic agent as part of downstream signaling pathway [1-3]. It is well known that excessive oxidative stress can be toxic, causing membrane damage and activating pathways of cell death through protein oxidation, lipid peroxidation, and DNA cleavage processes [4-7]. Oxidative stress is associated with neurodegenerative diseases, such as Alzheimer’s disease (AD) [8, 9] and Parkinson’s disease (PD) [10-12], which depletes the antioxidant system and increases lipid peroxidation products [13-15].
Naturally occurring flavonoids possess free radical scavenging properties and neuroprotection from oxidative injury by their ability to modulate intracellular signals [16]. These flavonoids are found in fruits, vegetables and plant-derived beverages, and may have important roles as dietary components via cytoprotective actions in many organs [17, 18]. Flavonoids can act as a vasodilator [19], anti-carcinogenic, anti-inflammatory, antibacterial, immune-stimulating anti-allergic, and antiviral compounds [20]. Pycnogenol® (PYC), a patented combination of bioflavonoids extracted from the bark of French maritime pine (Pinus maritima), has a high capacity to scavenge free radicals and promote cellular health. Major constituents of PYC are polyphenols, specifically, monomeric and oligomeric units of catechin, epicathechin, and taxifolin. PYC has been shown to prevent neurotoxicity and apoptotic cell death in the presence of oxidative stressors [11, 21]. Concomitantly, PYC protects against lipid peroxidation, and pro-oxidants such as H2O2, HO− NO−, O2−, and peroxynitrite (ONOO−) [22-24]. The beneficial effects of PYC have been demonstrated previously and include inhibition of caspase-3 activation [25, 26].
Antioxidant therapy has been shown to be beneficial in neurological disorders including both AD and PD [4, 11, 13, 14, 21]. In the present study, using human neuroblastoma (SH-SY5Y) cell line, we investigated the effect of PYC, a potent antioxidant and reactive oxygen species (ROS) scavenger, on acrolein induced cytotoxicity and ROS generation. Acrolein (2-propen-1-al), the most reactive of the strongest electrophile among the unsaturated aldehydes, is a toxic compound that can be endogenously produced as a product of lipid peroxidation and from polyamine metabolism [27]. Acrolein can induce oxidative stress [28-30], react with proteins, phospholipids[28, 31], and DNA, forming stable Michael adducts [32]. This compound plays an important role in the development of oxidative damage [32] and, consequently, in the pathogenesis of selected diseases, such as AD [33]. The mechanisms by which acrolein causes oxidative damage and neurotoxicity are not completely defined, but accumulating evidence indicates that this alkenal primarily binds and depletes cellular nucleophiles, such as reduced glutathione (GSH), lipoic acid and thioredoxin [27]. Acrolein preferentially attacks free thiol (SH) groups of cysteine residues, γ-amino groups of lysine residues and histidine residues, resulting in an acrolein-amino acid adduct and introducing a carbonyl group to proteins [34] and impairs the function of selected proteins [29]. In the present study, we examined intracellular ROS production, antioxidants/oxidants level, and cell viability in SH-SY5Y neuroblastoma cells treated with both acrolein and pycnogenol.
Materials and Methods
Materials
PYC (gift from Horphag Research Ltd., Le Sen, France), acrolein (Fisher Scientific Inc. USA), dimethylsulfoxide (DMSO), 2,7-dichlorofluoresceindiacetate (DCFH-DA), 3-(3,4-dimehylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and all other chemicals were purchased from Sigma–Aldrich (St. Louis, MO, USA) unless stated otherwise. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS) were purchased from Gibicol-BRL. Hybond-polyvinylidene difluoride (PVDF) membrane was purchased from BioRad.
Cell culture and cell treatment
SH-SY5Y cells obtained from ATCC (American Type Culture Collection, Manassas, VA) were maintained in DMEM and kept at 37 °C in a humidified 5% CO2 incubator, supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and cultured in 48-well plates as well as in 100 mm culture plates. SH-SY5Y cells were exposed to different concentrations of acrolein (1, 5, 10 and 25 μM) freshly prepared in DMEM media each day before use. These concentrations were equal to 0.35, 1.75, 3.5 and 8.75 nmol/mg protein, respectively, with calculation based on the average protein concentration of the cells. The in vivo acrolein concentrations in AD brain have been reported to be 2.5 nmol/mg in amygdala and 5.0 nmol/mg in the parahippocampal gyrus [33]. The levels of acrolein used in this study are relevant to the pathological conditions observed in AD. SH-SY5Y cells were pre-incubated with different concentrations of PYC (25, 50 and 100ug/ml) for 1 h before acrolein was added. Assays for cell viability, GSH system, protein oxidation, lipid peroxidation, reduced nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase, and ROS were performed 24 h after treatments.
Estimation of Cell Viability
SH-SY5Y cells cultures were grown in 48-well plates. In order to assay cell viability, mitochondrial function was determined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] reduction assay as described by Zhao and colleagues [35]. Briefly, PYC treated/untreated SH-SY5Y cells were assessed 24 h after exposure to acrolein. Photomicrographs of each culture were taken by phase contrast microscopy prior to the MTT assay. A stock solution of MTT (prepared in identical media) was added to each well with a final concentration of 1.0 mg/ml, and incubated for 1 h. The dark blue formazan crystals formed in intact cells were extracted with 200 ml of dimethyl sulfoxide (DMSO), and absorbance at 595 nm was measured with a microplate reader (BIO-TEK). Results were expressed as the percent of MTT reduction, assuming that the absorbance of control cells was 100%.
Total ROS assay
Generation of ROS was assessed by DCFH-DA, an oxidation-sensitive fluorescent probe as previously described [35]. Intracellular H2O2 or low-molecular-weight peroxides can oxidize DCFH-DA to the highly fluorescent compound dichlorofluorscein (DCF). DCFH-DA was chosen because acrolein itself does not interfere with the fluorescence of DCFH-DA [36]. Twenty four hours after treatment with PYC and acrolein, cells were incubated with 10 μM DCFH-DA at 37 °C for 30 min, and then washed twice with PBS. The fluorescence intensity of DCF was measured in a microplate-reader at an excitation wavelength of 485 nm and an emission wavelength of 538 nm.
Estimation of Superoxide (O2·−) Production
O2·− production in SH-SY5Y cell was quantified with lucigenin-enhanced chemiluminescence, as previously described [37], with some modifications. SH-SY5Y cells were collected in 2.0 ml tubes and homogenized with an ultrasonic cell disruptor (Microson Farmingdale, NY). The sample volume was increased by the addition of 900 μL HEPES buffer pH 7.4 (NaCl, 119 mM; HEPES, 20 mM; KCl, 4.6 mM; MgSO4, 1 mM; Na2HPO4, 0.15 mM; KH2PO4, 0.4 mM; NaHCO3, 1 mM; CaCl2, 1.2 mM and glucose 5.5 mM), protease inhibitors; pepstatin A, leupeptin, aprotinin, and phenylmethylsulfonyl fluoride (PMSF), L-NAME (1 mmol/L), triethylenetetramine (1.0 mmol/L), SDS (100 μmol/L), and 10 μL lucigenin (1800 μmol/L) was added to produce a final concentration of 20 μmol/L. Samples were aliquoted in 96-well plates, allowed to dark adapt at room temperature, and the luminescence recorded. Blanks were subtracted from cell homogenate-added wells and chemiluminescence data after blank subtraction are reported as percent counts/mg protein. O2·− production was also examined in the presence of diphenyliodonium and quinacrine (100 μmol/L and 1.0 mmol/L, respectively to inhibit O2·− producing via NADPH-oxidase system), oxypurinol (100 μmol/L), rotenone (100 μmol/L), or indomethacin (10 μmol/L) to assess the effects of xanthine oxidase, mitochondrial respiration, and cyclo-oxygenase activity on the production of O2·−.
Estimation of NADPH-oxidase Activity
NADPH-oxidase activity was measured according to the method described earlier [38]. Briefly, SH-SY5Y cells were homogenized by freeze and thawing in 2.0 ml 0.1M, PBS (pH 7.4) containing HEPES, 10 mM; EDTA, 2.0 mM; EGTA, 2.0 mM; MgSO4 0.6 mM; KCl, 4.6 mM; and protease inhibitors; pepstatin A, leupeptin, aprotinin, and phenylmethylsulfonyl fluoride (PMSF). The 20 μL of homogenate was added to 900 μL HEPES–buffer (described above) containing L-NAME (1 mmol/L), triethylenetetramine (1.0 mmol/L), SDS (100 μmol/L), 20 μmol/L lucigenin, and the samples were aliquoted in 96-well plate in the dark at room temperature. Plates were placed in the luminometer (Synergy HT, BIO-TEK), allowance was made for basal luminescence, and enzyme activity was initiated by the addition of NADPH (0.2 mmol/L), and readings taken for 15 min. Blanks were subtracted from cell homogenate-added wells and chemiluminescence data after blank subtraction are reported as percent counts/min/mg protein. Samples were also assayed in the presence of diphenyliodonium and quinacrine (100 μmol/L and 1.0 mmol/L, respectively) to inhibit the NADPH oxidases producing O2·−.
Estimation of GSH
Determination of GSH was performed as described previously [4]. The reaction mixture contained 0.1 M sodium phosphate buffer (pH 8.0), 5.0 mM EDTA, 20 μl o-phthaldehyde (1.0 mg/ml), and 20 μl of above mentioned sample. After incubation for 15 min at room temperature, fluorescence at emission 420 nm and excitation at 350 nm was recorded.
Estimation of GSSG
The estimation of GSSG was performed as described previously [4]. The samples were incubated first with 0.04 M N-ethyleimide (NEM) for 30 min to interact with GSH present in the sample. The reaction mixture contained 0.1 N NaOH, 5.0 mM EDTA, 20 μl o-phthaldehyde (1.0 mg/ml), and 20 μl of above mentioned sample. After incubation for 15 min at room temperature, fluorescence at emission 420 nm and excitation at 350 nm was recorded.
Estimation of Protein Carbonyls
Protein carbonyls are an index of protein oxidation and were determined as described previously [4]. Briefly, the cell extract (5mg of protein) was derivatized with 10 mM 2,4-dinitrophenylhydrazine in the presence of 5ml of 12% SDS for 20 min at room temperature. The samples were neutralized with 7.5ml of the neutralization solution (2 M Tris in 30% glycerol). Derivatized protein samples were blotted onto nitrocellulose membranes with a slot-blot apparatus (250 ng/slot). The membrane was then washed with wash buffer [10 mM Tris–HCl (pH 7.5), 150 mM NaCl, 0.05% Tween 20], blocked by incubation in the presence of 5% BSA, followed by incubation with rabbit polyclonal anti-DNPH antibody as primary antibody for 1 h. The membranes were washed with buffer and further incubated with alkaline phosphatase (ALP)-conjugated goat anti-rabbit antibody as secondary antibody for 1 h. Blots were developed using Sigma Fast tablets (BCIP/NBT) and quantified using Scion Image (PC version of Macintosh compatible NIH Image) software.
Estimation of 3-nitrotyrosine (3-NT)
The content of 3-NT was determined by incubating the above sample with Laemmli sample buffer (0.125 M Trizma base, pH 6.8, 4% SDS, 20% glycerol) for 20 min. Then 250 ng of protein were blotted onto the nitrocellulose paper using a slot-blot apparatus and immunochemical method as described above for protein carbonyls. The mouse anti-nitrotyrosine antibody was used as primary antibody and ALP- conjugated anti-mouse secondary antibody was used for detection. Densitometric analysis of bands in images of the blots was used to calculate levels of 3-NT.
Estimation of 4-HNE conjugated proteins
Levels of HNE were quantified by slot-blot analysis as described previously [4]. Anti-HNE antibody raised in rabbit was used as the primary antibody. Controls in which the primary antibody was reacted with free HNE resulted in faint, non-specific binding of the antibody (data not shown). However, since both acrolein and acrolein + PYC-treated samples used the same antibody, background correction was identical in both samples.
Western-blots
NADPH-oxidase subunits- gp91Phox, p67Phox, p47Phox, p40Phox, and p22Phox were studied by Western-blot as described previous [4], with some modifications. The cells were lysed using an ultrasonic cell disruptor (Microson Farmingdale, NY) on ice in lysis buffer (50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1% Triton X-100, 10% glycerol including above mentioned protease inhibitors) and centrifuged at 1000 × g for 10 min. Cytosolic and membrane fractions were prepared with standard methods [39]. Protein (50μg) was loaded with the appropriate marker (Bio-Rad) on a gradient gel (4-20% Tris-HCl), followed by a transfer to a polyvinylidene fluoride membrane using a semi-dry transfer system (Bio-Rad) in transfer buffer (25 mM Tris, 150 mM glycine, 20% MeOH) 2 h at 15 volt. The membrane was blocked with 5% milk or bovine serum albumin (BSA) in Tris/saline buffer-Tween (TBST). Primary antibodies for gp91Phox, p67Phox, p47Phox, p40Phox, and p22Phox, including beta actin, Na+/K+-ATPase and GAPDH were added at a concentration of 1:1,000 and incubated overnight at 4°C. The blot was then washed thee times in TBST and incubated for 1 h with alkaline phosphatase conjugated secondary antibodies in a 1:8000 dilution. The membrane was washed three times in TBST for 5 min and developed in Sigma Fast tablets (BCIP/NBT substrate). Blots were dried, scanned with Adobe Photoshop, and quantified with Scion Image (PC version of Macintosh-compatible NIH Image).
Protein Estimation
Total protein concentrations were measured using the Pierce BCA method (Sigma, St. Louis, MO).
Statistical Analysis
Dose-dependent differences in oxidative/nitrosative stress markers, cell viabilities, and differences in NADPH oxidase activity are reported as mean ± SD. Possible differences between group means were evaluated with a one-way ANOVA coupled with a Student Newman-Keuls post-hoc test (StatView 5.0, SAS Institute). For significant differences, alpha was set at 0.05.
Results
Effect of PYC on Acrolein-Induced Cytotoxicity
Exposure of SH-SY5Y cell cultures to acrolein for 24 h reduced cell viability in a dose dependent fashion [F (15, 80) = 27.00, p < 0.0001] (Fig. 1 & 2). PYC alone has no affect on cell (thus cells can tolerate relatively high doses up to 200 μM). When acrolein is applied to cells pretreated with PYC for 1 h, the amount of cell death is significantly reduced suggesting that PYC plays a protective role. Regardless of whether the cells are pretreated with 50 and 100 μg/ml of PYC, the level of protection is the same, suggesting that more than one mechanism is involved in acrolein-related cell death. Pretreatment of cells with PYC at either low doses (5, 10 and 25 μg/ml), or high doses (150 and 200 μg/ml), did not provide significant (p > 0.05) protection (data not shown). PYC attenuated acrolein-induced cell loss at both 50 and 100μg/ml. Since 100μg/ml of PYC failed to show protection greater than 50μg/ml dose, following 1 or 25μM acrolein treatment, the high dose of PYC was not pursued further.
Fig. 1.
PYC prevented loss of SH-SY5Y cell viability. The viability was measured by the reduction of MTT. Acrolein caused loss of cell viability compared with control. 1 h pretreatment with PYC (50 and 100 μg/ml) and incubation for 24 h protected SH-SY5Y cells from acrolein cytotoxicity compared with group acrolein only. Each bar represents the group mean ± SD of six cultures/group. *p < 0.05 and **p < 0.001 versus control, #p < 0.05 and ##p < 0.001 versus acrolein only.
Fig. 2.
Phase contrast microphotographs of SH-SY5Y cells treated with acrolein with and without PYC. Acrolein treatment resulted in significant cell death with some membrane blobbing, but no other morphological changes in surviving SH-SY5Y cells. PYC (50 and 100μg/ml) significantly has reduced cell death, even at high acrolein levels. (A) Control; (B) treated with acrolein 1 μM; (C) acrolein 5 μM; (D) acrolein 10 μM; (E) acrolein 25 μM; (F) treated with PYC 50 μg/ml; (G) acrolein 1 μM + PYC 50 μg/ml; (H) acrolein 5 μM + PYC 50 μg/ml; (I) acrolein 10 μM + PYC 50 μg/ml; (J) acrolein 25 μM + PYC 50 μg/ml; (K) treated with PYC 100 μg/ml; (L) acrolein 1 μM + PYC 100 μg/ml; (M) acrolein 5 μM + PYC 100 μg/ml; (N) acrolein 10 μM + PYC 100 μg/ml; (O) acrolein 25 μM + PYC 100 μg/ml. Microphotographs were taken at 40 X and calibration bar was 10μ.
Effect of PYC on Acrolein-Induced ROS
As shown in Fig. 3, exposure of SH-SY5Y cell cultures to acrolein significantly increased ROS levels is a dose dependent fashion [F (13, 70) = 69.00, p < 0.0001]. 1 μM acrolein treatment did not significantly increase ROS levels and PYC alone has no affect on ROS production. Pretreating cells with PYC significantly reduced acrolein-induced ROS levels. A 1 h pretreatment of PYC at either 50 and 100μg/ml and subsequent acrolein exposure significantly decreased ROS levels compared to corresponding acrolein alone treatment. At the higher concentrations of acrolein (10 and 25 μM), while PYC did offer significant reductions in ROS, values were still significantly elevated compared to control. The highest concentration of PYC (100μg/ml) did not provide significantly greater antioxidant activity than 50μg/ml.
Fig. 3.
(A) PYC reduces ROS production in SH-SY5Y cells. ROS level was measured by the oxidation of DCFH-DA. Acrolein treatment increased ROS production in SH-SY5Y cells compared with control. 1 h pretreatment with PYC (50 and 100 μg/ml) and incubation for 24 h reduced ROS production by acrolein treatment in SH-SY5Y cell compared the culture treated with acrolein only. Each bar represents the group mean ± SD of six cultures/group. *p < 0.05 and **p < 0.001 versus control, #p < 0.05 and ##p < 0.001 versus acrolein only. PYC depletes O2·− production in SH-SY5Y cells. (B) O2·− level and (C) O2·− producing enzyme NADPH-oxidase activity was increased with acrolein treatment in SH-SY5Y cells compared with control. 1 h pretreatment with PYC (50 and 100 μg/ml) and incubation for 24 h reduced O2·− production by acrolein treatment in cell cultures compared with acrolein only. Each bar represents the group mean ± SD of six cultures/group. *p < 0.05 and **p < 0.001 versus control, #p <0.05 and ##p < 0.001 versus acrolein only.
Effect of PYC on Acrolein-Induced O2·− Production
Exposure of SH-SY5Y cell cultures to acrolein significantly [F (13, 70) = 27.00, p < 0.0001] increased O2·− production [Fig. 4(A)] in a dose dependent fashion. 1 μM acrolein treatment did not significantly increase O2·− levels. Pre-exposure with PYC for 1 h at either 50 or 100μg/ml followed by treatment with acrolein significantly decreased O2·− levels compared to corresponding acrolein treatment alone. The higher dose of PYC (100μg/ml) was not significantly more effective than 50μg/ml.
Fig. 4.
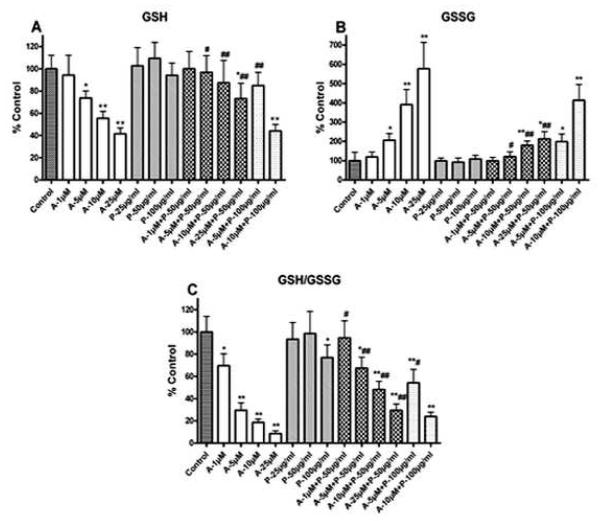
Level of GSH system was alleviated with PYC treatment in SH-SY5Y cells. Acrolein caused loss of GSH (A) increased GSSG (B) and discrepancy of GSH/GSSG (C) in SH-SY5Y cells compared with control. 1 h pretreatment with PYC (50 and 100 μg/ml) and incubation for 24 h protected cells from acrolein induced adverse effect on GSH system compared with group acrolein only. Each bar represents the group mean ± SD of six cultures/group. *p < 0.05 and **p < 0.001 versus control, #p < 0.05 and ##p < 0.001 versus acrolein only.
NADPH-oxidase Activity
Exposure of SY5Y cell cultures to acrolein significantly increased NADPH-oxidase activity [F (13, 70) = 19.80, p < 0.0001] [Fig. 4(B)] in a dose dependent fashion. 1 μM acrolein treatment or PYC alone had no significant effect on NADPH-oxidase activation. Pretreatment of SH-SY5Y cells with PYC 50 or 100μg/ml and subsequently treated with acrolein showed significant depletion in NADPH-oxidase activity. At 25 μM acrolein and treatment with PYC 50μg/ml, NADPH-oxidase activity was significantly reduced as compared to acrolein alone.
Effect of PYC on Acrolein-Induced GSH Depletion
The level of internal antioxidant tri-peptide molecule GSH was measured in SH-SY5Y cell cultures [Fig. 5(A)]. Analysis of GSH levels demonstrated a significant [F (13, 70) = 17.00, p < 0.0001] dose dependent decrease with acrolein. Acrolein treated cells when pretreated with either 50 or 100μg/ml of PYC significantly attenuated GSH declines compared to corresponding acrolein alone treatment. Pre-treatment with PYC at 100μg/ml was not effective in maintaining GSH levels when cells were subjected to 10μM of acrolein. Higher concentrations of PYC (100μg/ml) were not significantly more effective than 50μg/ml.
Fig. 5.
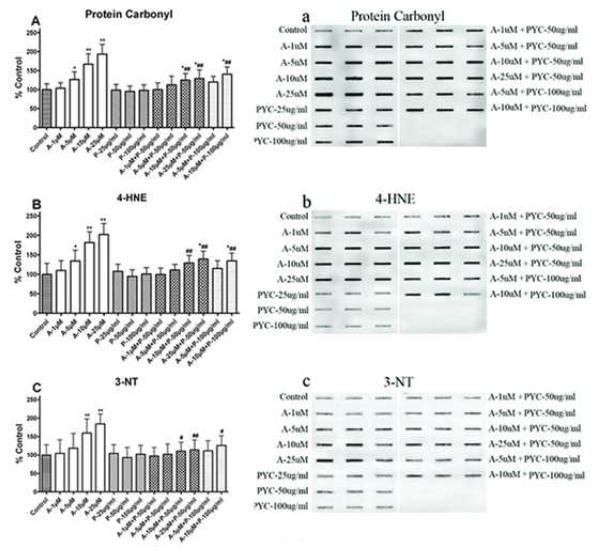
PYC treatment protects SH-SY5Y cells from protein and lipid damages. Acrolein increased protein oxidation (protein carbonyl formation A & a), lipid peroxidation (4-HNE formation B & b), and protein nitration (3-NT formation C & c) in SH-SY5Y cells compared from acrolein induced protein damages compared with group acrolein only. Blot represents one sample of each group and each bar represents the group mean ± SD of six cultures/group. *p < 0.05 and **p < 0.001 versus control, #p < 0.05 and ##p < 0.001 versus acrolein only.
Effect of PYC on Acrolein-Induced GSSG Elevation
Analysis of GSSG levels in SY5Y cell cultures demonstrated a significant dose dependent increase with acrolein treatment [F (13, 70) = 45.07, p < 0.0001] [Fig. 5(B)]. Pretreatment with PYC (50 μg/ml) and subsequent acrolein exposure significantly decreased GSSG levels as compared to acrolein alone. PYC alone has no affect on GSSG whether cells are treated at 25, 50 or 100 μg/ml. SH-SY5Y cells pretreated with 100 μg/ml PYC and subsequently exposed to 10μM acrolein, failed to significantly lower GSSG levels and were significantly higher than all other pretreatment groups.
Effect of PYC on Acrolein-Induced Changes in GSH/GSSG ratio
As shown in Fig. 5(C), exposure of SH-SY5Y cell cultures to acrolein for 24 h significantly reduced the GSH/GSSG ratio [F (13, 70) = 51.27, p < 0.0001]. Pretreatment with PYC for 1 h significantly attenuated acrolein-induced declines in the GSH/GSSG ratio. Pre-treatment with 50 μg/ml of PYC increased GSH/GSSG ratio in all acrolein exposure groups, although levels did not reach control values. Although pre-treatment with100 μg/ml of PYC alone resulted in a decline, it was capable of offsetting a decline normally observed with 5 μM acrolein treatment alone. At 10 μM acrolein exposure, pre-treatment of 100 μg/ml PYC failed to significantly elevate the GSH/GSSG ratio.
Effect of PYC on Acrolein-Induced Protein Carbonylation
Fig. 6(A) shows the levels of protein carbonyls in SH-SY5Y cell cultures treated with acrolein. The levels of protein carbonyls were significantly increased [F (13, 70) = 23.00, p < 0.0001] with acrolein in a dose dependent fashion. Pretreatment with either 50 or 100μg/ml of PYC significantly reduced protein carbonyl levels compared to acrolein treatment alone, although the levels were significantly higher compared to control. Higher concentration of PYC (100μg/ml) was not more effective than 50μg/ml.
Fig. 6.
NADPH-oxidase subunit proteins in SH-SY5Y cells were significantly affected with acrolein exposure. Cells were processed for immunobloting followed by Western-blot. The levls of p67Phox (B), p47Phox (C), and p40Phox (D) demonstrated increase in membrane and p67Phox decreased in cytosol (G). The effect of acrolein exposure was attenuated with PYC (50 μg/ml ). The gp91Phox (A) and p22Phox (E) failed to demonstrate significant changes. Na+/K+-ATPase (F) and GAPDH (G) show equal proteins.
Effect of PYC on Acrolein-Induced lipid peroxidation (4–HNE bound proteins)
The levels of 4-HNE bound proteins were significantly increased [F (13, 70) = 21.00, p < 0.0001] in SH-SY5Y cell cultures exposed to acrolein as shown in Fig. 6(B). The lower concentration of acrolein (1 μM) treatment had no significant effect on 4-HNE formation. Pretreatment with either 50 or 100 μg/ml PYC significantly decreased 4-HNE levels. Even at the highest treatment of acrolein (25 μM), PYC 50 μg/ml demonstrated a significant reduction in 4HNE levels. There were no significant differences between pretreatment of PYC at 50 and 100 μg/ml.
Effect of PYC on Acrolein-Induced Protein Nitration (3-NT, Formation)
Acrolein exposure significantly increased the levels of 3-NT in the SH-SY5Y cell culture [F (13, 70) = 17.70, p < 0.0001] as shown in Fig. 6(C). The levels of 3-NT were only affected by the highest doses of acrolein (10 μM, and 25 μM). Pretreatment with PYC significantly decreased acrolein induced 3-NT formation. Increasing the concentration of PYC from 50 to 100 μg/ml did not have a significant difference.
Effect of PYC on Acrolein-induced NADPH-oxidase activation
Western-blot analysis shows acrolein-induced changes in NADPH-oxidase complex proteins (gp91Phox, p67Phox, p47Phox, p40Phox, and p22Phox) in SH-SY5Y cells (Fig. 7) and PYC significantly reduce active complex formation.
Fig. 7.
Cytosolic protein subunits of NADPH-oxidase p67Phox (A & B), levels in the SH-SY5Y cells increased in membrane and declined in cytosol with acrolein exposure were significantly attenuated by PYC (50 μg/ml). In same fashion p47Phox (C), and p407Phox (D) also show increase with acrolein exposre and significantly decreased with PYC treatment. Each bar represents the group mean ± SD of six cultures/group. *p < 0.05 and **p < 0.001 versus control, #p < 0.05 and ##p < 0.001 versus acrolein only.
The cytosolic component of NADPH-oxidase p67Phox, p47Phox, and p40Phox, showed a significant dose-dependent increase [F (7, 40) = 15.43, p > 0.0001] in membrane (Fig. 8A,C & D) with acrolein treatment. Like p67Phox and p47Phox, p40Phox also significantly decreased [F (7, 40) = 7.955, p > 0.0001] in the membrane with PYC treatment. In reverse fashion, p67Phox subunit significantly declines [F (7, 40) = 17.44, p > 0.0001] in the cytosol (Fig. 8B), indicate a shift of the subunit to the membrane, which is attenuated by PYC significantly (Fig. 7D).
Fig. 8.
Membrane protein subunits of NADPH-oxidase gp91Phox (A) and p22Phox (B) show non significant elevation with acrolien exposure. PYC attenuates the levels of both subunits in membrane.
A major membrane subunits of NADPH-oxidase, gp91Phox and p22Phox, showed no significant change [F (7, 40) = 1.3429, p < 0.2559] with acrolein treatment (Fig. 9A & B).
Fig. 9.
Schematic presentation of proposed mechanism of protective effects of pycnogenal (PYC) on acrolein-induced cytotoxicity in SH-SY5Y cells. The thiol (-SH) – reactive property of acrolein activates NADPH-oxidase complex, leading to the production of superoxide (O2−.). Normally superoxide dismutase (SOD) converts O2−. into hydrogen peroxide (H2O2). O2−. activates nitric oxide synthase (iNOS) leading to the production of nitric oxide (NO) and the formation of peroxynitrites (ONOO−), which ultimately causes oxidative/nitrosative stress. Increased oxidative/nitrosative stress causes mitochondrial dysfunction, lipid peroxidation, protein and DNA damage, which can result in cell death. Lipid peroxidation further produces toxic aldehyde products including elevated acrolein that further aggravates degenerative process. ONOO− is known to activate poly(ADP-ribrosyl) polymerase (PARP) leading to ATP depletion and cell death. PYC prevents assembling of NADPH-oxidase subunits (both in cytosol and the membrane), and has protective effects on the beginning of acrolein-induced deleterious cascade. PYC prevents O2−. production from NADPH-oxidase activation and NO from iNOS. PYC scavenges O2−., NO, ONOO−, H2O2 and hydroxyl radicals (OH.) to prevent oxidative/nitosative stress, and elevates internal antioxidant glutathione (GSH) defenses that can enhance cell survival.
Discussion
The present set of in vitro experiments characterized the effects of Pycnogenol® on a variety of oxidative stress markers. PYC, a patented combination of bioflavonoids, scavenges oxidants and thus promotes cellular health. PYC is a mixture of polyphenols, that decrease toxic byproducts of lipid peroxidation such as H2O2, HO−., O2−. and O2− [22, 23]. Previous studies have reported increased SH-SY5Y cell survival with PYC [21, 25, 26]. The present study demonstrates that PYC can protect SH-SY5Y cells from cytotoxicity induced by acrolein in part by (1) reducing ROS production; (2) remediating suppressed NADPH-oxidase, and (3) enhancing the GSH system [25, 26]. It is been suggested that PYC may not only suppress the generation of ROS, but also attenuate apoptotic neuronal death in Aβ-induced animal models of AD, possibly by decreasing free radical generation [11]. Data from this present study shows that treatment with acrolein results in a significant increase of ROS level in a dose-dependent manner. This is consistent with previous descriptions of acrolein-induced oxidative stress [33, 40, 41].
GSH protects cultured neurons against oxidative damage resulting from acrolein, and 4-HNE [33]. GSH can also protect the brain from damage by peroxynitrite, hydroxyl free radicals, or reactive alkenals [42]. These findings suggest that these changes, are a common feature of various types of oxidative and nitrosative stress, which may be associated with the protein damages, modification of redox regulation and cellular function [43]. There is evidence that oxidative stress, including free radicals, plays a key role in AD and PD [8, 9, 44]. Brain membrane lipids are rich in polyunsaturated fatty acids, which are especially sensitive to free radical-induced lipid peroxidation [45, 46].
Several studies have reported that increased endogenous antioxidant levels, resulting from dietary or pharmacological intake of antioxidants precursors or substrates, can protect GSH from oxidative depletion and consequently protect the brain against oxidative stress [47-49]. PYC has been used as a nutritional supplement to be taken orally, and studies have indicated that this phytochemical is indeed being absorbed and metabolized by humans [50, 51]. Other studies indicate that PYC pretreatment is associated with the protection of cells undergoing oxidative stress associated death, with potential applications in neurodegenerative diseases [24].
Acrolein is the strongest electrophilic aldehyde in nature [29] that occurs as a ubiquitous pollutant in the environment. It is also formed in vivo from cellular lipid peroxidation [52, 53], and reacts with various biomolecules including proteins, DNA, and phospholipids, with the potential to disrupt their function especially in neurodegeneration [29, 33, 40, 41, 54]. The mechanisms of how acrolein inflicts tissue damage are not fully established, but data suggests that it can induce oxidative stress [54, 55] (Fig. 3). It has been reported that the acrolein-induced damaging molecules strongly react with GSH, reducing levels and subsequently induces the production of ROS [40, 41, 56, 57]. Depletion in the GSH defense favors an increase in apoptosis and a decrease in cell survival [36, 55, 58, 59] (Fig. 4). In the presence of acrolein, NADPH-oxidase can also be an important source of ROS over-production and protein modification, leading to neuronal oxidative stress. It is known that the thiol (-SH)-reactive property of acrolein are responsible for the activation of NADPH-oxidase complex and the inducible nitric oxide synthase (iNOS) [60]. In the present set of experiments, acrolein significantly increased ROS production, O2−. levels and activation of O2−. producing enzyme NADPH-oxidase (Fig. 6 & 7). Such events have been linked to apoptotic cell death via caspase-3 activation, DNA fragmentation, and poly (ADP-ribose) polymerase (PARP) cleavage [11]. PYC suppressed both ROS and NADPH oxidase activation (assembling of cytosolic subunits (p67Phox, p47Phox and p40Phox) to the membrane subunits (gp91Phox and p22Phox)), while also attenuating GSH depletion, protein damages, and acrolein-induced cell death.
The results shown in this in vitro study demonstrate that PYC probably acts as an antioxidant at the maximal dose 50 μg/ml. We demonstrated that PYC protected SH-SY5Y cells from acrolein-induced oxidative stress by decreasing the percentage of ROS production and as well as reducing NADPH-oxidase activation. In addition, we also found that PYC attenuated the reduction in GSH levels, by the decline in oxidative/nitrosative burden, which was a normal consequence of acrolein exposure. But the elevation in GSH was not due to its synthesis; since PYC treatment was not effective even at 200 μg/ml. Lower concentrations, were not enough to remove acrolein induced oxidative stress, while high concentrations of PYC, being a xenobiotic for the system, might itself be creating a burden, that would favor oxidative stress in the presence of other oxidants. This may explain why, at higher concentrations (100μg/ml) PYC did not provide sufficient antioxidant defenses. Data showing PYC protects SH-SY5Y cells from acrolein cytotoxicity, suggests that the mechanism of acrolein neurotoxicity involves oxidative stress, and that the neuroprotective effects of PYC are associated with its antioxidant properties. We also observed that acrolein may have destructive effects through mechanisms other than oxidative stress, since pretreatment with PYC never resulted in values equivalent to that of controls. PYC could be a key component for the development of therapeutics for neurodegenerations including AD, but case in its effective concentrations and timings for treatment must be observed.
Acknowledgments
This research was supported in parts by NIH grants AG21981 and AG27219.
Abbreviations used
- DCFH-DA
2,7-dichlorofluoresceindiacetate
- DMSO
dimethylsulfoxide
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- 4-HNE
4-hydroxynonenal
- L-NAME
Nω-Nitro-L-arginine methyl ester hydrochloride
- MTT
3-(3,4-dimehylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
- NEM
N-ethyleimide
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- 3-NT
3-nitrotyrosine
- PBS
phosphate-buffered saline
- PMSF
phenylmethylsulfonyl fluoride
- PMS
post-mitochondrial supernatant
- PVDF
polyvinylidene difluoride
- PC
protein carbonyl
- PYC
Pycnogenol®
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kondo M, Oya-Ito T, Kumagai T, Osawa T, Uchida K. Cyclopentenone prostaglandins as potential inducers of intracellular oxidative stress. The Journal of biological chemistry. 2001;276:12076–12083. doi: 10.1074/jbc.M009630200. [DOI] [PubMed] [Google Scholar]
- [2].Naoi M, Maruyama W, Shamoto-Nagai M, Yi H, Akao Y, Tanaka M. Oxidative stress in mitochondria: decision to survival and death of neurons in neurodegenerative disorders. Molecular neurobiology. 2005;31:81–93. doi: 10.1385/MN:31:1-3:081. [DOI] [PubMed] [Google Scholar]
- [3].Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science (New York, N.Y. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- [4].Ansari MA, Joshi G, Huang Q, Opii WO, Abdul HM, Sultana R, Butterfield DA. In vivo administration of D609 leads to protection of subsequently isolated gerbil brain mitochondria subjected to in vitro oxidative stress induced by amyloid beta-peptide and other oxidative stressors: relevance to Alzheimer’s disease and other oxidative stress-related neurodegenerative disorders. Free radical biology & medicine. 2006;41:1694–1703. doi: 10.1016/j.freeradbiomed.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Greenamyre JT, Betarbet R, Sherer TB. The rotenone model of Parkinson’s disease: genes, environment and mitochondria. Parkinsonism & related disorders. 2003;9(Suppl 2):S59–64. doi: 10.1016/s1353-8020(03)00023-3. [DOI] [PubMed] [Google Scholar]
- [6].Halliwell B. Antioxidant defence mechanisms: from the beginning to the end (of the beginning) Free radical research. 1999;31:261–272. doi: 10.1080/10715769900300841. [DOI] [PubMed] [Google Scholar]
- [7].Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Annals of neurology. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- [8].Butterfield DA, Howard B, Yatin S, Koppal T, Drake J, Hensley K, Aksenov M, Aksenova M, Subramaniam R, Varadarajan S, Harris-White ME, Pedigo NW, Jr., Carney JM. Elevated oxidative stress in models of normal brain aging and Alzheimer’s disease. Life sciences. 1999;65:1883–1892. doi: 10.1016/s0024-3205(99)00442-7. [DOI] [PubMed] [Google Scholar]
- [9].Butterfield DA, Koppal T, Subramaniam R, Yatin S. Vitamin E as an antioxidant/free radical scavenger against amyloid beta-peptide-induced oxidative stress in neocortical synaptosomal membranes and hippocampal neurons in culture: insights into Alzheimer’s disease. Reviews in the neurosciences. 1999;10:141–149. doi: 10.1515/revneuro.1999.10.2.141. [DOI] [PubMed] [Google Scholar]
- [10].Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson’s disease: evidence supporting it. Annals of neurology. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- [11].Peng QL, Buz’Zard AR, Lau BH. Pycnogenol protects neurons from amyloid-beta peptide-induced apoptosis. Brain research. 2002;104:55–65. doi: 10.1016/s0169-328x(02)00263-2. [DOI] [PubMed] [Google Scholar]
- [12].Ueda T, Ueda T, Armstrong D. Preventive effect of natural and synthetic antioxidants on lipid peroxidation in the mammalian eye. Ophthalmic research. 1996;28:184–192. doi: 10.1159/000267901. [DOI] [PubMed] [Google Scholar]
- [13].Ahmad AS, Ansari MA, Ahmad M, Saleem S, Yousuf S, Hoda MN, Islam F. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacology, biochemistry, and behavior. 2005;81:805–813. doi: 10.1016/j.pbb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- [14].Ahmad M, Saleem S, Ahmad AS, Yousuf S, Ansari MA, Khan MB, Ishrat T, Chaturvedi RK, Agrawal AK, Islam F. Ginkgo biloba affords dose-dependent protection against 6-hydroxydopamine-induced parkinsonism in rats: neurobehavioural, neurochemical and immunohistochemical evidences. Journal of neurochemistry. 2005;93:94–104. doi: 10.1111/j.1471-4159.2005.03000.x. [DOI] [PubMed] [Google Scholar]
- [15].Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free radical biology & medicine. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- [16].Mercer LD, Kelly BL, Horne MK, Beart PM. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: investigations in primary rat mesencephalic cultures. Biochemical pharmacology. 2005;69:339–345. doi: 10.1016/j.bcp.2004.09.018. [DOI] [PubMed] [Google Scholar]
- [17].Paganga G, Miller N, Rice-Evans CA. The polyphenolic content of fruit and vegetables and their antioxidant activities. What does a serving constitute? Free radical research. 1999;30:153–162. doi: 10.1080/10715769900300161. [DOI] [PubMed] [Google Scholar]
- [18].Youdim KA, Joseph JA. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free radical biology & medicine. 2001;30:583–594. doi: 10.1016/s0891-5849(00)00510-4. [DOI] [PubMed] [Google Scholar]
- [19].Duarte J, Vizcaino F. Perez, Utrilla P, Jimenez J, Tamargo J, Zarzuelo A. Vasodilatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. General pharmacology. 1993;24:857–862. doi: 10.1016/0306-3623(93)90159-u. [DOI] [PubMed] [Google Scholar]
- [20].Brown JP. A review of the genetic effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutation research. 1980;75:243–277. doi: 10.1016/0165-1110(80)90029-9. [DOI] [PubMed] [Google Scholar]
- [21].Kobayashi MS, Han D, Packer L. Antioxidants and herbal extracts protect HT-4 neuronal cells against glutamate-induced cytotoxicity. Free radical research. 2000;32:115–124. doi: 10.1080/10715760000300121. [DOI] [PubMed] [Google Scholar]
- [22].Kobuchi H, Virgili F, Packer L. Assay of inducible form of nitric oxide synthase activity: effect of flavonoids and plant extracts. Methods in enzymology. 1999;301:504–513. doi: 10.1016/s0076-6879(99)01113-1. [DOI] [PubMed] [Google Scholar]
- [23].Nardini M, Scaccini C, Packer L, Virgili F. In vitro inhibition of the activity of phosphorylase kinase, protein kinase C and protein kinase A by caffeic acid and a procyanidin-rich pine bark (Pinus marittima) extract. Biochimica et biophysica acta. 2000;1474:219–225. doi: 10.1016/s0304-4165(00)00009-x. [DOI] [PubMed] [Google Scholar]
- [24].Packer L, Rimbach G, Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free radical biology & medicine. 1999;27:704–724. doi: 10.1016/s0891-5849(99)00090-8. [DOI] [PubMed] [Google Scholar]
- [25].Siler-Marsiglio KI, Paiva M, Madorsky I, Serrano Y, Neeley A, Heaton MB. Protective mechanisms of pycnogenol in ethanol-insulted cerebellar granule cells. Journal of neurobiology. 2004;61:267–276. doi: 10.1002/neu.20057. [DOI] [PubMed] [Google Scholar]
- [26].Siler-Marsiglio KI, Shaw G, Heaton MB. Pycnogenol and vitamin E inhibit ethanol-induced apoptosis in rat cerebellar granule cells. Journal of neurobiology. 2004;59:261–271. doi: 10.1002/neu.10311. [DOI] [PubMed] [Google Scholar]
- [27].Mello CF, Sultana R, Piroddi M, Cai J, Pierce WM, Klein JB, Butterfield DA. Acrolein induces selective protein carbonylation in synaptosomes. Neuroscience. 2007;147:674–679. doi: 10.1016/j.neuroscience.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4- hydroxynonenal, malonaldehyde and related aldehydes. Free radical biology & medicine. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- [29].Lovell MA, Xie C, Markesbery WR. Acrolein, a product of lipid peroxidation, inhibits glucose and glutamate uptake in primary neuronal cultures. Free radical biology & medicine. 2000;29:714–720. doi: 10.1016/s0891-5849(00)00346-4. [DOI] [PubMed] [Google Scholar]
- [30].Park YS, Misonou Y, Fujiwara N, Takahashi M, Miyamoto Y, Koh YH, Suzuki K, Taniguchi N. Induction of thioredoxin reductase as an adaptive response to acrolein in human umbilical vein endothelial cells. Biochemical and biophysical research communications. 2005;327:1058–1065. doi: 10.1016/j.bbrc.2004.12.104. [DOI] [PubMed] [Google Scholar]
- [31].Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- [32].Pocernich CB, Butterfield DA. Acrolein inhibits NADH-linked mitochondrial enzyme activity: implications for Alzheimer’s disease. Neurotoxicity research. 2003;5:515–520. doi: 10.1007/BF03033161. [DOI] [PubMed] [Google Scholar]
- [33].Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiology of aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- [34].Pocernich CB, Cardin AL, Racine CL, Lauderback CM, Butterfield DA. Glutathione elevation and its protective role in acrolein-induced protein damage in synaptosomal membranes: relevance to brain lipid peroxidation in neurodegenerative disease. Neurochemistry international. 2001;39:141–149. doi: 10.1016/s0197-0186(01)00012-2. [DOI] [PubMed] [Google Scholar]
- [35].Zhao DL, Zou LB, Lin S, Shi JG, Zhu HB. Anti-apoptotic effect of esculin on dopamine-induced cytotoxicity in the human neuroblastoma SH-SY5Y cell line. Neuropharmacology. 2007;53:724–732. doi: 10.1016/j.neuropharm.2007.07.017. [DOI] [PubMed] [Google Scholar]
- [36].Nardini M, Finkelstein EI, Reddy S, Valacchi G, Traber M, Cross CE, van der Vliet A. Acrolein-induced cytotoxicity in cultured human bronchial epithelial cells. Modulation by alpha-tocopherol and ascorbic acid. Toxicology. 2002;170:173–185. doi: 10.1016/s0300-483x(01)00540-6. [DOI] [PubMed] [Google Scholar]
- [37].McVeigh GE, Hamilton P, Wilson M, Hanratty CG, Leahey WJ, Devine AB, Morgan DG, Dixon LJ, McGrath LT. Platelet nitric oxide and superoxide release during the development of nitrate tolerance: effect of supplemental ascorbate. Circulation. 2002;106:208–213. doi: 10.1161/01.cir.0000021600.84149.78. [DOI] [PubMed] [Google Scholar]
- [38].Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. The Journal of clinical investigation. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bianca VD, Dusi S, Bianchini E, Dal Pra I, Rossi F. beta-amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer’s disease. The Journal of biological chemistry. 1999;274:15493–15499. doi: 10.1074/jbc.274.22.15493. [DOI] [PubMed] [Google Scholar]
- [40].Luo J, Robinson JP, Shi R. Acrolein-induced cell death in PC12 cells: role of mitochondria-mediated oxidative stress. Neurochemistry international. 2005;47:449–457. doi: 10.1016/j.neuint.2005.07.002. [DOI] [PubMed] [Google Scholar]
- [41].Luo J, Shi R. Acrolein induces oxidative stress in brain mitochondria. Neurochemistry international. 2005;46:243–252. doi: 10.1016/j.neuint.2004.09.001. [DOI] [PubMed] [Google Scholar]
- [42].Koppal T, Drake J, Yatin S, Jordan B, Varadarajan S, Bettenhausen L, Butterfield DA. Peroxynitrite-induced alterations in synaptosomal membrane proteins: insight into oxidative stress in Alzheimer’s disease. Journal of neurochemistry. 1999;72:310–317. doi: 10.1046/j.1471-4159.1999.0720310.x. [DOI] [PubMed] [Google Scholar]
- [43].Miyamoto Y, Koh YH, Park YS, Fujiwara N, Sakiyama H, Misonou Y, Ookawara T, Suzuki K, Honke K, Taniguchi N. Oxidative stress caused by inactivation of glutathione peroxidase and adaptive responses. Biological chemistry. 2003;384:567–574. doi: 10.1515/BC.2003.064. [DOI] [PubMed] [Google Scholar]
- [44].LeWitt PA. New drugs for the treatment of Parkinson’s disease. Pharmacotherapy. 2000;20:26S–32S. doi: 10.1592/phco.20.2.26s.34631. [DOI] [PubMed] [Google Scholar]
- [45].Ansari MA, Ahmad AS, Ahmad M, Salim S, Yousuf S, Ishrat T, Islam F. Selenium protects cerebral ischemia in rat brain mitochondria. Biological trace element research. 2004;101:73–86. doi: 10.1385/BTER:101:1:73. [DOI] [PubMed] [Google Scholar]
- [46].Islam F, Zia S, Sayeed I, Zafar KS, Ahmad AS. Selenium-induced alteration of lipids, lipid peroxidation, and thiol group in circadian rhythm centers of rat. Biological trace element research. 2002;90:203–214. doi: 10.1385/BTER:90:1-3:203. [DOI] [PubMed] [Google Scholar]
- [47].Anderson ME, Luo JL. Glutathione therapy: from prodrugs to genes. Seminars in liver disease. 1998;18:415–424. doi: 10.1055/s-2007-1007174. [DOI] [PubMed] [Google Scholar]
- [48].Drake J, Kanski J, Varadarajan S, Tsoras M, Butterfield DA. Elevation of brain glutathione by gamma-glutamylcysteine ethyl ester protects against peroxynitrite-induced oxidative stress. Journal of neuroscience research. 2002;68:776–784. doi: 10.1002/jnr.10266. [DOI] [PubMed] [Google Scholar]
- [49].Pocernich CB, La Fontaine M, Butterfield DA. In-vivo glutathione elevation protects against hydroxyl free radical-induced protein oxidation in rat brain. Neurochemistry international. 2000;36:185–191. doi: 10.1016/s0197-0186(99)00126-6. [DOI] [PubMed] [Google Scholar]
- [50].Duweler KG, Rohdewald P. Urinary metabolites of French maritime pine bark extract in humans. Die Pharmazie. 2000;55:364–368. [PubMed] [Google Scholar]
- [51].Virgili F, Pagana G, Bourne L, Rimbach G, Natella F, Rice-Evans C, Packer L. Ferulic acid excretion as a marker of consumption of a French maritime pine (Pinus maritima) bark extract. Free radical biology & medicine. 2000;28:1249–1256. doi: 10.1016/s0891-5849(00)00244-6. [DOI] [PubMed] [Google Scholar]
- [52].Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. The Journal of biological chemistry. 1998;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- [53].Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T. Protein-bound acrolein: potential markers for oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Luo J, Shi R. Acrolein induces axolemmal disruption, oxidative stress, and mitochondrial impairment in spinal cord tissue. Neurochemistry international. 2004;44:475–486. doi: 10.1016/j.neuint.2003.09.006. [DOI] [PubMed] [Google Scholar]
- [55].Liu-Snyder P, McNally H, Shi R, Borgens RB. Acrolein-mediated mechanisms of neuronal death. Journal of neuroscience research. 2006;84:209–218. doi: 10.1002/jnr.20863. [DOI] [PubMed] [Google Scholar]
- [56].Cao Z, Hardej D, Trombetta LD, Trush MA, Li Y. Induction of cellular glutathione and glutathione S-transferase by 3H-1,2-dithiole-3-thione in rat aortic smooth muscle A10 cells: protection against acrolein-induced toxicity. Atherosclerosis. 2003;166:291–301. doi: 10.1016/s0021-9150(02)00331-3. [DOI] [PubMed] [Google Scholar]
- [57].Furuhata A, Nakamura M, Osawa T, Uchida K. Thiolation of protein-bound carcinogenic aldehyde. An electrophilic acrolein-lysine adduct that covalently binds to thiols. The Journal of biological chemistry. 2002;277:27919–27926. doi: 10.1074/jbc.M202794200. [DOI] [PubMed] [Google Scholar]
- [58].Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. Journal of neurochemistry. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- [59].Ramachandran V, Watts LT, Maffi SK, Chen J, Schenker S, Henderson G. Ethanol-induced oxidative stress precedes mitochondrially mediated apoptotic death of cultured fetal cortical neurons. Journal of neuroscience research. 2003;74:577–588. doi: 10.1002/jnr.10767. [DOI] [PubMed] [Google Scholar]
- [60].Hah JM, Roman LJ, Silverman RB. Deuterium isotope effects and product studies for the oxidation of N(omega)-allyl-L-arginine and N(omega)-allyl-N(omega)-hydroxy-L-arginine by neuronal nitric oxide synthase. Bioorganic & medicinal chemistry. 2000;8:1931–1936. doi: 10.1016/s0968-0896(00)00154-1. [DOI] [PubMed] [Google Scholar]