Summary
Syntaxin 4 is a component of the SNARE complex that regulates membrane docking and fusion. Using a yeast two-hybrid screen, we identify a novel interaction between syntaxin 4 and cytoplasmic murine CENPF, a protein previously demonstrated to associate with the microtubule network and SNAP-25. The binding domain for syntaxin 4 in CENPF was defined by yeast two-hybrid assay and co-immunoprecipitation. Confocal analyses in cell culture reveal a high degree of colocalization between endogenously expressed proteins in interphase cells. Additionally, the endogenous SNARE proteins can be isolated as a complex with CENPF in immunoprecipitation experiments. Further analyses demonstrate that murine CENPF and syntaxin 4 colocalize with components of plasma membrane recycling: SNAP-25 and VAMP2. Depletion of endogenous CENPF disrupts GLUT4 trafficking whereas expression of a dominant-negative form of CENPF inhibits cell coupling. Taken together, these studies demonstrate that CENPF provides a direct link between proteins of the SNARE system and the microtubule network and indicate a diverse role for murine CENPF in vesicular transport.
Keywords: CENPF, Syntaxin 4, GLUT4, Rab11a, VAMP2, SNAP-25
Introduction
Movement of membrane bound vesicles to and from the cell membrane is dependent on the cytoskeleton. Along with the actin network, microtubules serve as tracks for the efficient and directed movement of organelles and vesicles (Caviston and Holzbaur, 2006; Hehnly and Stamnes, 2007; Ishiki and Klip, 2005; Soldati and Schliwa, 2006; Vedrenne and Hauri, 2006). A wide range of studies report the importance of the microtubule network in the transport of specific cargos in polarized and non-polarized cells. For example, microtubule-based transport and dynein activity are essential for TrkA signaling to Rap1 and MAPK1/2 as inhibition of dynein activity results in altered vesicular trafficking (Miyamoto et al., 2006; Wu et al., 2007). Similarly, kinesin motors regulate vesicular cargo along microtubules (Schnapp, 2003). Furthermore, a PKA- and calcium-dependent pathway controls non-polarized macrophage secretion of apoE along the microtubule network (Kockx et al., 2007). Additionally, treatment with agents that disrupt microtubule function, such as nocodozale and rotenone, inhibit movement of proteins from the Golgi to the cell surface (Feng, 2006; Zheng et al., 2007). However, identification and characterization of proteins directly linking membrane bound vesicles to the microtubule network remain elusive.
Human centromere protein F (CENPF) (also called mitosin) was independently identified by the Yen and Lee groups utilizing auto-immune antibodies and Rb-binding properties (Rattner et al., 1993; Zhu et al., 1995b). Our group discovered the murine homolog and, although previous publications have used the name LEK1 (Ashe et al., 2004; Goodwin et al., 1999; Pooley et al., 2006; Soukoulis et al., 2005), we now refer to this protein as murine centromere protein F (hereafter referred to as murine CENPF) to more accurately describe the gene product. Murine CENPF, like other family members, is a large protein (2998 aa) and shares significant sequence and domain homology with related proteins in both humans and avians (Ashe et al., 2004; Dees et al., 2000; Goodwin et al., 1999; Liao et al., 1995; Pabon-Pena et al., 2000; Zhu et al., 1995a; Zhu et al., 1995b). The human homolog binds the kinetochore and is an important regulator of mitosis and cell division (Feng et al., 2006; Liang et al., 2004; Liao et al., 1995; Rattner et al., 1993; Yang et al., 2005; Zhu et al., 1995a; Zhu et al., 1997; Zhu et al., 1995b). In addition, both human and murine CENPF bind proteins associated with the microtubule network, including tubulin (Feng et al., 2006) and Nde1 (Soukoulis et al., 2005). The binding of murine CENPF to Nde1 is of particular interest as Nde1 interacts with Lis1 and dynein to modulate the microtubule network in regulation of cell shape and movement (Faulkner et al., 2000; Gibbons, 1996; Rattner et al., 1993; Smith et al., 2000; Zhu et al., 1997). Relevant to the current study, the Lis1 pathway functions with the Golgi network and in membrane trafficking (Kondratova et al., 2005; Liang et al., 2004). Utilizing dominant-negative protein expression and induced suppression of murine CENPF expression, we demonstrate that interference with CENPF function severely alters the microtubule network (Soukoulis et al., 2005). In addition, several studies show that membrane trafficking and positioning of organelles are dependent on interaction of the microtubule network with Nde1 and Lis1 (Banks and Heald, 2001; Faulkner et al., 2000; Gibbons, 1996; Smith et al., 2000; Terada et al., 1996; Xiang et al., 1999).
Budding and fusion events between donor and acceptor membranes are essential for vesicular transport. The SNARE (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptors) family of proteins is responsible, at least in part, for regulation of such fusion events (Chen and Scheller, 2001; Ishiki and Klip, 2005). Apposing SNARE proteins, which consist of vesicle-associated membrane proteins (VAMPs), plasma membrane-associated syntaxins, and cytoplasmic synaptosomal-associated proteins (SNAPs), form coiled-coil aggregates that are important in regulating membrane fusion events (Aikawa et al., 2006; Chen and Scheller, 2001; Ishiki et al., 2005; Martin et al., 1998; McMahon et al., 1993; Rowe et al., 1999). Plasma membrane trafficking of proteins between subcellular domains and translocation to the cell surface is mediated, in part, by SNARE proteins (Chen and Scheller, 2001; Jahn and Sudhof, 1999; Mallard et al., 2002; Sollner et al., 1993; Wilcke et al., 2000). The SNARE protein syntaxin 4 is an integral membrane protein that localizes to the plasma membrane and is essential in vesicular docking and fusion (Aikawa et al., 2006; Bajohrs et al., 2005; Band et al., 2002; Pooley et al., 2006). Specifically, syntaxins are critical in vesicular transport of GLUT4-containing vesicles in skeletal muscle, cardiomyocytes and adipose tissue after insulin stimulation (Bryant et al., 2002; Cain et al., 1992; Martin et al., 1996; Pessin et al., 1999). Determining the physical linkage of SNAREs to the microtubule network is essential for understanding the role of this cytoskeletal component in the myriad of trafficking events.
In a recent study, we reported that murine CENPF physically associates with SNAP-25 (synaptosomal-associated protein 25), and together these proteins form a complex with Rab11a, myosin Vb, and VAMP2 in the recycling endosome pathway (Pooley et al., 2006). Furthermore, disruption of endogenous murine CENPF function by dominant-negative protein expression or protein knockdown severely retarded the recycling endosome network and transferrin trafficking (Chen and Scheller, 2001). Although this is the only report of CENPF regulating vesicular transport, the family has been shown to function with the cytoskeleton (Goodwin et al., 1999; Feng et al., 2006), and from this, we postulate that CENPF may have a extensive role in controlling SNARE-mediated vesicular transport by the microtubule network. Further data is crucial to establish the roles for CENPF in the diverse processes of vesicular transport.
In the current study, using yeast two-hybrid (Y2H) and biochemical analyses, we demonstrate that syntaxin 4 and murine CENPF physically interact. These data are consistent with the hypothesis that murine CENPF is a critical component in the dynamic regulation of plasma membrane trafficking with the microtubule network through its interaction with SNARE proteins and Nde1 (Soukoulis et al., 2005). Using genetic, immunolocalization, and immunoprecipitation studies, we demonstrate that both transiently expressed and endogenous cytoplasmic CENPF directly associate with syntaxin 4 at the Golgi complex and this complex also contains VAMP2 and SNAP-25. Additionally, disruption of CENPF interferes with cell coupling in NIH3T3 fibroblasts, demonstrating inhibition of gap junction function at the cell membrane (Francis and Lo, 2006) and indicating the essential role of CENPF in membrane trafficking. Finally, we show that disruption of CENPF function inhibits GLUT4 trafficking, a system used to model syntaxin 4 function in membrane trafficking in 3T3 adipocytes. Thus, the present study establishes a physical link between murine CENPF and the SNARE complex and suggests a role for CENPF in the regulation for vesicular transport by the microtubule network.
Results
Identification of syntaxin 4 as a murine CENPF binding partner
Despite the significant amount of literature characterizing CENPF family members, most work has focused on the C terminus of the protein (Clark et al., 1997; Feng et al., 2006; Konstantinidou et al., 2003; Liao et al., 1995; Zhou et al., 2005; Zhu et al., 1995a; Zhu et al., 1997; Zhu et al., 1995b). Relatively little was known concerning potential molecular interactions and functions in the major N-terminal regions of the molecule until recently. Feng et al. identified a microtubule binding domain in the initial 385 amino acids of human CENPF (Feng et al., 2006), and our group demonstrated the interaction of murine CENPF with Nde1 and SNAP-25 (Pooley et al., 2006; Soukoulis et al., 2005). To identify binding partners in this domain, and thus, ascribe molecular function, we conducted an extensive Y2H screen using the N-terminal coiled-coil domain (amino acids 1-689; coding sequences for exons 1-6) of murine CENPF as bait. From this screen, we identified specific proteins known to regulate organelle positioning and membrane trafficking, one of which was the cytoplasmic SNARE protein SNAP-25 (Pooley et al., 2006). The screen yielded CENPF interaction with a second SNARE protein: syntaxin 4, the binding partner of SNAP proteins, which helps mediate vesicular docking and fusion. Y2H analysis was used to further define binding domains within these proteins. From this assay, the first 474 amino acids of murine CENPF (NTmCENPF) were determined to be required for syntaxin 4 interaction (Fig. 1), and further reduction of this sequence eliminated syntaxin 4 binding altogether. Interestingly, our group identified this region of CENPF for its interaction with SNAP-25, whereas Feng et al. recognized the same area for the interaction of the human homolog with tubulin (Feng et al., 2006; Pooley et al., 2006). Y2H analysis determined that the C-terminal 144 amino acids of syntaxin 4 were indispensable for CENPF binding (Fig. 1).
Fig. 1.
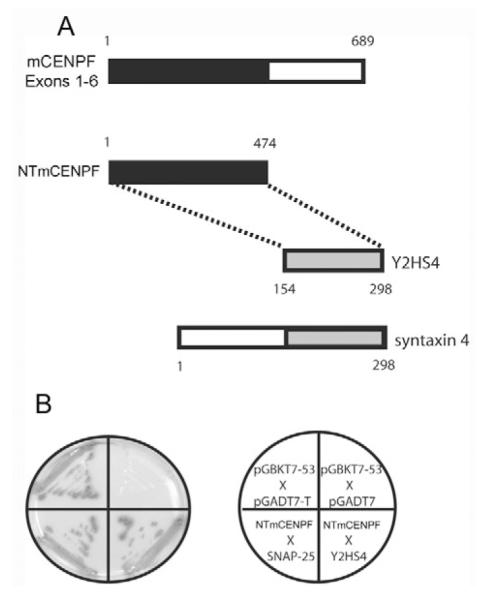
Identification of syntaxin 4 as a murine CENP-F-interacting protein. (A) A Y2H screen was conducted and described previously (Pooley et al., 2006). One of the interacting proteins with murine CENP-F (mCENP-F) was identified as syntaxin 4. The plasmid was isolated from a colony that survived on QDO media and subsequently sequenced. The resulting sequence was identified as the C-terminal 144 aa of syntaxin 4, labeled Y2HS4. The N-terminal 474 aa region of murine CENP-F (NTmCENP-F), was further characterized as being the region of CENP-F sufficient for syntaxin 4 interaction by Y2H. (B) Positive associations grew on QDO medium and exhibited blue color upon testing for β-Gal. As a positive control, growth was indicated by yeast transformed with pGBKT7-53 and pGADT7-T. The previously described interaction of NTmCENP-F with SNAP-25 was also used as a positive control (Pooley et al., 2006). The negative control with yeast co-expressing pGBTK-53 and the empty vector pGADT7 demonstrated no growth on the medium. The test interaction clearly demonstrates that NTmCENP-F does associate with the Y2HS4 portion of syntaxin 4 in yeast.
Transient protein expression within mammalian cells further confirmed the interaction of CENPF and syntaxin 4 The minimal syntaxin 4 binding domain of CENPF (myc-tagged N-terminal 474 amino acids of CENPF, termed NTmCENPF), and GFP-tagged syntaxin 4 were expressed in COS-7 cells. Lysates were prepared and co-immunoprecipitations (co-IP) were conducted with the appropriate antibodies. As seen in Fig. 2F, we were able to co-precipitate GFP-syntaxin 4 utilizing NTmCENPF, whereas all control experiments demonstrated no spurious GFP-syntaxin 4 association with CENPF. Syntaxin 4 did not react with any other sequences in CENPF except NTmCENPF (our unpublished data). Taken together, these results demonstrate that the N-terminal 474 amino acids of murine CENPF are necessary and sufficient for syntaxin 4 association.
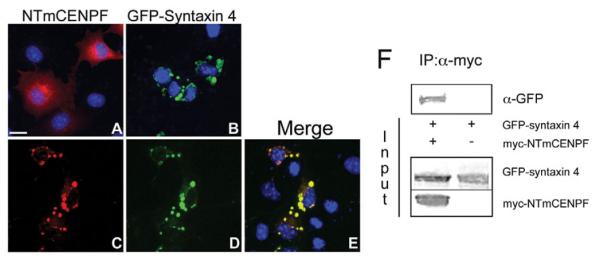
Fig. 2.
Transfected NTmCENPF redistributes in COS-7 cells expressing GFP-syntaxin 4. (A) COS-7 cells singly transfected with NTmCENPF show a cytoplasmic distribution of the protein (red) with a high perinuclear distribution. (B) Cells singly transfected with GFP-syntaxin 4 (green) show a significantly different distribution at defined foci throughout the cell. (C-E) When cells are cotransfected with syntaxin 4 and NTmCENPF, syntaxin 4 remains at the multiple intracellular foci, and NTmCENPF is redistributed to the same foci occupied by GFP-syntaxin 4, as seen in the merged image (E). DAPI (blue) was used to visualize the nuclei. (F) COS-7 cells were transfected with NTmCENPF-myc and GFP-syntaxin or with GFP-syntaxin 4 alone for a negative control. An immunoprecipitation was conducted with α-myc antibody, and blots were probed with α-GFP antibody. Input lanes show transfected protein expression in the lysate. GFP-syntaxin 4 was precipitated in the presence of NTmCENPF-myc. The singly transfected control shows there was no spurious binding of GFP-syntaxin 4 to the beads.
Transiently expressed N-terminal murine CENPF and syntaxin 4 colocalize in cells
Knowing that these proteins interacted in Y2H and co-IP analyses, we investigated the subcellular localization of these proteins. Transient expression of NTmCENPF in COS-7 cells results in strong protein localization to the perinuclear region of cells and more diffusely to the cell periphery (Fig. 2A). As previously reported for syntaxins in COS cells (Banfield et al., 1994; Quinones et al., 1999), transient expression of GFP-syntaxin 4 leads to protein localization in multiple foci located throughout the cell (Fig. 2B). This punctate perinuclear distribution is very similar to previous reports of exogenously-expressed syntaxin in COS cells (Bandfield et al., 1994; Quinones et al., 1999). However, in cells co-expressing both proteins, NTmCENPF redistributed to GFP-syntaxin 4-positive foci with a high degree of colocalization (Fig. 2C-E). Taken together, these data support our Y2H and co-IP data and demonstrate an interaction between murine CENPF and syntaxin 4.
Endogenous CENPF and syntaxin 4 associate in mammalian cells
We next examined the endogenous localization of CENPF and syntaxin 4 in murine cell lines. Two cell lines previously shown to express endogenous CENPF and syntaxin 4 were used: C2C12 myoblasts and 3T3 L1 adipocytes (Pooley et al., 2006; Soukoulis et al., 2005; Tortorella and Pilch, 2002). As seen in the myoblast line in Fig. 3A, confocal analysis demonstrated significant colocalization in the perinuclear region of the cell extending into the cell periphery. Band et al. (Band et al., 2002) have previously observed this perinuclear to peripheral distribution of endogenous syntaxin 4 in cultured NRK cells. In the present study, overlap was not absolute, as the staining pattern of CENPF extended further in the cell periphery than that of syntaxin 4. This is to be expected given that both proteins have been shown to bind other proteins and function in multiple pathways.
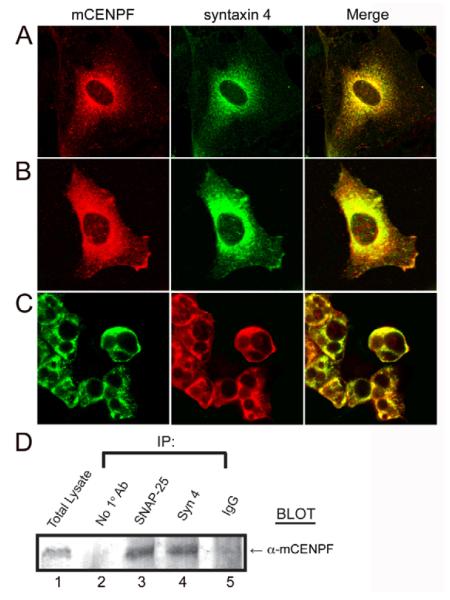
Fig. 3.
Endogenous CENPF and syntaxin 4 colocalize in murine cells. (A) There is significant overlap of endogenous murine CENPF (mCENPF) and syntaxin 4 expression throughout the cytoplasm in C2C12 myoblasts (merge). There is a high degree of colocalization in the perinuclear region, but CENPF has a broader distribution extending further into the cell periphery. (B) 3T3-L1 pre-adipocytes also demonstrate similar expression patterns of CENPF and syntaxin 4 to that seen in C2C12 cells. However, there is higher expression of both endogenous proteins at the cell periphery than in C2C12 myoblasts. (C) 3T3-L1 cells were differentiated as described in Materials and Methods. Both endogenous CENPF and syntaxin 4 appear to have high levels of expression distributed throughout the cells. All images are from confocal microscopy. Bar, 10 μm. (D) Murine CENPF forms an endogenous complex with syntaxin 4. Endogenous protein complexes were analyzed using C2C12 cell lysates for co-immunoprecipitation analysis with Sepharose beads alone, α-SNAP-25 antibody, syntaxin 4 antiserum, or IgG antibody alone. After precipitation, elution and western blotting, the blot was probed with α-CENPF antibody. Lane 1 demonstrates the presence of CENPF in the lysate. Lane 2 demonstrates the absence of precipitation with beads alone. Lane 3 is a positive control, showing that CENPF precipitates with SNAP-25. Lane 4 shows that CENPF precipitates with syntaxin 4. Lane 5 demonstrates the lack of precipitation with non-immune IgG.
Syntaxin 4 function has been studied extensively in 3T3-L1 adipocytes (Cain et al., 1992; Pessin et al., 1999; Volchuk et al., 1996). Therefore, in prelude to our functional studies of this novel interaction, we examined the subcellular distribution of CENPF and syntaxin 4 in this cell line (Fig. 3C). The intense perinuclear staining of both CENPF and syntaxin 4 mirrored the cytoplasmic expression observed in C2C12 cells, but there was more significant staining of both proteins at the cell periphery than in the myoblast cell line (Fig. 3B). This pattern of syntaxin 4 localization reflects results previously reported for this cell type (Band et al., 2002). Furthermore, differentiated 3T3-L1 adipocytes demonstrated a high degree of endogenous CENPF and syntaxin 4 colocalization (Fig. 3C). Protein localization was broader in the differentiated cells and extended to the cell periphery.
To corroborate these results, we probed for the presence of endogenous complexes containing both CENPF and syntaxin 4 using immunoprecipitation of C2C12 lysates. As seen in Fig. 3D, CENPF was readily coprecipitated with syntaxin 4 (lane 4). By contrast, precipitations with beads alone (lane 2) and non-immune IgG (lane 5) were negative. As a positive control, we co-precipitated CENPF from the same lysate with an antiserum against the CENPF binding partner SNAP-25 (Fig. 3D, lane 3). Taken together, these data, identifying endogenous colocalization and biochemical interaction through antibody co-immunoprecipitation, support the hypothesis that CENPF associates with the endogenous SNARE complex within eukaryotic cells.
Transient expression of NTmCENPF redistributes endogenous SNAP-25 and syntaxin 4
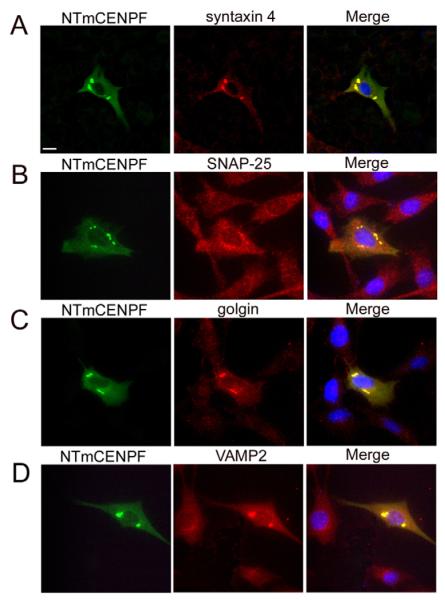
In an effort to define the role of murine CENPF in the regulation of vesicular function, we examined whether expression of its syntaxin-4-binding domain might influence subcellular localization of the trafficking apparatus in C2C12 cells. As seen in Fig. 4, forced expression of NTmCENPF resulted in the concentration of syntaxin 4 in perinuclear foci (compare Fig. 3 with Fig. 4A). SNAP-25, a known binding partner of both syntaxin 4 and CENPF also accumulated in this position but not completely, as other regions of the cytoplasm remained weakly positive for the anti-SNAP-25 antibody (Fig. 4B). Utilizing the trans-Golgi network (TGN) marker golgin-97, we determined that these NTmCENPF–syntaxin 4–SNAP-25 foci colocalized within TGN (Fig. 4C) and not with markers of the early or recycling endosome (data not shown). VAMP2, but not VAMP3, also accumulated in the TGN of cells expressing NTmCENPF (Fig. 4D). We would also note that expression of GFP-syntaxin 4 alone also results in accumulation of endogenous CENPF in the TGN in C2C12 cells (data not shown).
Fig. 4.
N-terminal CENPF expression localizes to foci containing both TGN and recycling endosome markers. NTmCENPF was transfected into C2C12 cells and markers for SNAREs, TGN and recycling endosomes were immunolabelled. (A) Syntaxin 4 did not colocalize with NTmCENPF-myc, but there was a redistribution of the protein in cells that were transfected. (B-D) SNAP-25, golgin and VAMP2 did colocalize with NTmCENPF. NTmCENPF immunofluorescence is shown in green; endogenous markers are in red. DAPI was used to visualize the nuclei (blue). Bar, 10 μm.
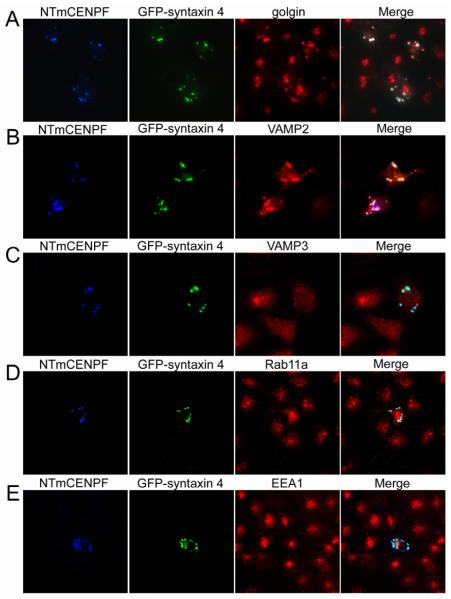
Cotransfection was used to determine whether redistribution of components was a consistent feature of transient expression of NTmCENPF and syntaxin 4. As seen in Fig. 5A, expression of exogenous proteins resulted in the same accumulation of product in the Golgi and/or TGN. It should be noted that previous studies have reported that exogenously expressed syntaxin accumulates in the Golgi (Rowe et al., 1999; Salaun et al., 2004; Takuma et al., 2002; Washbourne et al., 2001). Consistent with expression of NTmCENPF alone, VAMP2 (Fig. 5B) and SNAP-25 (data not shown) were readily seen in this compartment, whereas VAMP3, Rab11a and EEA1 were excluded from the TGN. The dramatic redistribution of syntaxin 4, VAMP2 and SNAP-25 to the TGN after expression of NT-mCENPF suggests a role for this protein in vesicular transport.
Fig. 5.
Transiently expressed NTmCENPF and syntaxin 4 colocalize at the TGN in COS-7 cells. COS-7 cells were cotransfected with NTmCENPF and GFP-syntaxin 4, and only those cells expressing both transient proteins were analyzed. (A) NTmCENPF and GFP-syntaxin 4 colocalize with the TGN marker golgin-97. (B) VAMP2 also colocalizes to a high degree with NTmCENPF and GFP-syntaxin 4. (C) VAMP3 does not demonstrate any significant redistribution to NTmCENPF–GFP-syntaxin 4 foci. (D) Rab11a, a marker of recycling endosomes, does not demonstrate noticeable colocalization. (E) Early endosomes, stained with EEA1, also show no significant redistribution to NTmCENPF–GFP-syntaxin 4 foci. Therefore, the NTmCENPF–GFP-syntaxin 4 complex is specific for localization at the TGN. NTmCENPF staining is indicated in blue, GFP-syntaxin 4 in green and the third marker, as indicated, is in red. Bar, 10 μm.
Disruption of CENPF function interferes with cell coupling
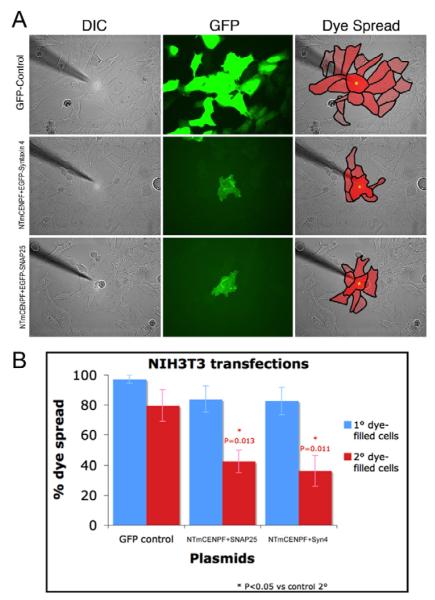
Coupling of cells is dependent, in part, on the efficient trafficking of connexin molecules to and from the cell membrane (Wei et al., 2004). Transfer of molecular dyes in cultured cells is an efficient and sensitive measure of connexin-based cell coupling (Francis and Lo, 2006). Dye coupling has the advantage of allowing the direct visualization of gap junction communication and function in a community of cells. For our purposes, 3T3 fibroblasts were transfected with GFP vector, GFP-syntaxin and NTmCENPF, or GFP-SNAP-25 and NTmCENPF. Living transfected cells were identified by GFP expression and loaded with sulforhodamine101 dye (dark red cell with yellow spot, Fig. 6A). Dye transfer from the injected cell to first tier cells (those in direct contact with the injected cell) and second tier cells (those in contact with first tier cells) were quantified using established methods (Francis and Lo, 2006). As seen in Fig. 6, dye is efficiently transferred to first and second tier cells after injection into control GFP transfected cells. In addition, this figure demonstrates that control transfection of cells in the first and second tier cells adjacent to the injected cells have no apparent affect on cell coupling. By contrast, expression of NTmCENPF with either GFP-labeled SNAP-25 or syntaxin 4 in the injected cell alone significantly inhibits the transfer of dye to neighboring cells, especially second tier cells (Fig. 6A,B). Inhibition of coupling is observed with co-expression of NTmCENPF with either syntaxin 4 or SNAP-25 even though NTmCENPF–syntaxin 4 complexes accumulate in the TGN (Fig. 5A) whereas SNAP-25–NTmCENPF concentrates in the recycling endosome (Pooley et al., 2006). Expression of NTmCENPF alone results in the same disruption of dye transfer (data not shown). These data provide direct evidence that CENPF function is important for proper cell coupling.
Fig. 6.
NTmCENPF expression interferes with cell coupling. (A) Cultured 3T3 fibroblasts were transfected with control GFP or NTmCENPF and GFP-SNAP-25 or NTmCENPF and GFP-syntaxin 4 and living, transfected cells were loaded with sulforhodamine101 dye (dark red cell with yellow spot). The first and second tier cells are outlined to quantify transfer; first tier cells touch the injected cell and second tier cells are those dye-transferred cells not touching the injected cell. (B) Percentage of dye spread was quantified according to methods described and the cells expressing NTmCENPF showed significantly less coupling at the second tier level.
Inhibition of CENPF function inhibits glucose transport
Syntaxin 4 has a critical role in GLUT4 trafficking in insulin-responsive tissues (Martin et al., 1998; Cain et al., 1992; Pessin et al., 1999; Volchuk et al., 1996). Insulin stimulates the translocation of intracellular GLUT4 vesicle pools to the plasma membrane in target tissues, which include cardiac myocytes, skeletal muscle and adipose tissue. Activation of insulin receptors triggers a large increase of GLUT4 vesicle trafficking and exocytosis as compared to basal conditions. Additionally, although studies have concentrated on the function of SNAP-23 in GLUT4 trafficking, SNAP-25 has been isolated from GLUT4-positive cells (Jagadish et al., 1996). Thus, confident that this model could directly measure murine CENPF function in vesicular transport, we analyzed whether inhibition of CENPF function would inhibit GLUT4 trafficking. Briefly, 3T3-L1 cells were differentiated and CENPF function was inhibited using MO knockdown, a method previously described by our group (Ashe et al., 2004; Soukoulis et al., 2005; Pooley et al., 2006) in conjunction with the 2-deoxy-D-glucose transport assay (Kawanishi et al., 2000). The level of GLUT 4 trafficking was compared between differentiated 3T3-L1 adipocyte cultures treated with control (SC) or those treated with CENPF MO. To determine whether CENPF MO treatment was specific for CENPF depletion, replicate SC control and experimental MO-treated cultures were processed for western blot analysis. Fig. 7A demonstrates that CENPF protein levels drop significantly with experimental but not control MO application. By contrast, levels of tubulin, syntaxin 4, GLUT4, and β-actin remain unchanged. As seen in Fig. 7B, depletion of CENPF resulted in a 53% reduction of 2-deoxy-D-[1,2-3H]glucose binding at the cell surface over that of control MO cultures (P<0.01). These results prove functional evidence that depletion of CENPF has a direct effect on GLUT4 trafficking. As a control, SC and CENPF MO-treated cultures were assayed for glucose uptake with and without insulin stimulation. As seen in Fig. 7C, strong induction of glucose uptake was observed with insulin treatment in SC MO-treated cultures and this response was statistically significant compared with no insulin treatment (P<0.01). By contrast, glucose uptake was not observed in CENPF MO-treated cultures with or without insulin treatment. These data indicate that MO treatment alone is neither stimulatory nor inhibitory to glucose uptake.
Fig. 7.
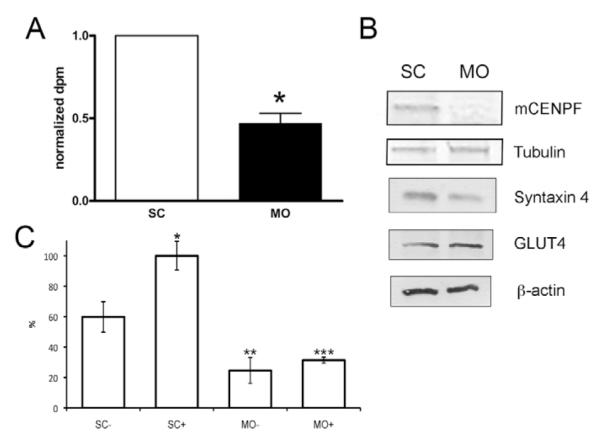
Depletion of murine CENPF alters GLUT4 trafficking. (A) 48 hours post-MO treatment, cells were processed according to described methods. Normalized to SC cell populations, cells with MO addition had a 50% decrease in radio-labeled glucose trafficking to the plasma membrane (measured as disintegration per minute; dpm). Data are normalized against the SC counts and are shown as means ± s.e.m. from three independent experiments. *One sample Student’s t-test, P<0.01 versus SC control. (B) 3T3-L1 adipocytes were differentiated and depleted of mCENPF by addition of MO. As shown previously (Ashe et al., 2004; Pooley et al., 2006; Soukoulis et al., 2005), MO addition is specific to mCENPF depletion as demonstrated by western blot. MO treatment also had no effect on tubulin, syntaxin 4, GLUT4 or β-actin levels. (C) 3T3-L1 adipocytes were differentiated, treated with either standard control or mCENPF MO, and assayed for glucose uptake as noted in the text. Control (SC) and experimental (MO) treatments were conducted in the presence (+) and absence (−) of insulin. Glucose uptake values were normalized to SC+ and shown as percentages of that value. There is a statistically significant difference between SC− and SC+ populations (P<0.01; indicated by *), SC− and MO− populations (P<0.001; indicated by **), and SC+ and MO+ populations (P=0.0001; indicated by ***). There was not a statistically significant difference between the MO− and MO+ populations.
Discussion
The CENPF family regulates diverse cellular processes
The CENPF family has diverse roles in cell cycle, division and differentiation. Although sequences in the C terminus have even been shown to regulate gene expression (Ma et al., 2006; Zhou et al., 2005), many important functions associated with CENPF are thought to be mediated through the microtubule network (Ashe et al., 2004; Dees et al., 2000; Pooley et al., 2006; Rattner et al., 1993; Soukoulis et al., 2005; Zhu et al., 1995b). For example, human CENPF binds the kinetochore and is highly expressed at the G1-S boundary (Zhu et al., 1997; Zhu et al., 1995b). The kinetochore is located at the centromere of the chromosome and serves as the site for microtubule spindle attachment during mitosis (Cleveland et al., 2003; Rieder and Salmon, 1998). Silencing of CENPF results in misalignment of chromosomes during mitosis and premature cell death (Yang et al., 2005), demonstrating a central role for this protein in microtubule regulation of mitosis and cell division. CENPF is also critical in the modulation of cell shape and motility. This protein directly interacts with Nde1, a regulator of dynein and microtubules (Soukoulis et al., 2005; Vergnolle and Taylor, 2007). Dynein, a microtubule-based motor, is also important for chromosome positioning and segregation through this interaction (Cleveland et al., 2003; Heald and Walczak, 1999; Sharp et al., 2000). In turn, Nde1 interacts with Lis1 in organelle positioning and movement, especially relating to developing neurons in the CNS (Feng et al., 2000; Feng and Walsh, 2004). Taken together, CENPF family proteins demonstrate diverse roles in organelle dynamics associated with microtubule-based processes. Although it is clear from this current study and previously reported data that CENPF family proteins are involved in organelle positioning, to date, the interacting proteins and regulators of such functions are largely unknown. Through a series of experiments to identify interacting proteins, we have now determined that murine CENPF provides a link between the MT network and vesicular transport.
Interaction with syntaxin 4 reveals a potentially broad role for murine CENPF in vesicular transport
Using genetic, biochemical and immunochemical analyses, we demonstrate that murine CENPF has a direct interaction with syntaxin 4. This interaction is mediated through sequences in the N-terminal portion of CENPF that are highly conserved in various vertebrate genomes (NCBI search) and predicted to form the same tertiary coiled coil structure. The highly conserved nature of these sequences, their broad distribution in vertebrate genomes, the ubiquitous nature of CENPF expression in developing organisms, and the diversity of putative cellular function suggests a general role for this protein in cell function.
Both transiently expressed NTmCENPF and endogenous CENPF associate with syntaxin 4 in co-IP analyses. Furthermore, NTmCENPF and syntaxin 4 colocalize specifically to the TGN. Expression of the truncated form of NTmCENPF results in accumulation of this protein and syntaxin 4 in the TGN. As seen in previous studies (Rowe et al., 1999; Salaun et al., 2004; Takuma et al., 2002; Washbourne et al., 2001), exogenous expression of syntaxin 4 leads to its accumulation in the TGN (Fig. 5). Our data demonstrate that NTmCENPF also localizes to the TGN with the SNARE proteins SNAP-25 and VAMP2, both of which are associated with vesicular movement to and from the cell membrane. This is expected as both SNAP-25 and VAMP2 interact with syntaxin 4 (Pevsner et al., 1994). This result suggests a function for CENPF in vesicular transport and provides a method to analyze its potential role in this basic cell activity.
Murine CENPF is critical in regulation of vesicular transport
Previous studies on CENPF have demonstrated its roles in mitosis and cell division (Feng et al., 2006; Hussein and Taylor, 2002; Konstantinidou et al., 2003; Liao et al., 1995; Zhou et al., 2005; Zhu et al., 1995a; Zhu et al., 1997; Zhu et al., 1995b) and even gene transcription (Ma et al., 2006; Zhou et al., 2005). Other, in vivo and in vitro, analyses of CENPF and its binding partners suggest additional roles in cell movement and organelle positioning and translocation (Sasaki et al., 2000; Soukoulis et al., 2005; Vergnolle and Taylor, 2007). Identification of mCENPF interaction with syntaxin 4 and the dramatic redistribution of these proteins with exogenous expression of these binding partners strongly suggests a potential role in regulation of vesicular transport. To test the significance of CENPF-syntaxin 4 interaction, we employed two different experimental interventions. First, GLUT4 assay was used as a model of plasma membrane trafficking, as syntaxin 4 is critical in GLUT4 vesicle trafficking (Foster and Klip, 2000; Thurmond et al., 1998; Watson and Pessin, 2001). In depleting cells of CENPF, there was a significant decrease in the amount of labeled glucose recovered from 3T3-L1 adipocyte plasma membranes as compared with control groups. This is completely consistent with its role in movement of receptors to the cell surface by vesicular transport (Foster and Klip, 2000; Thurmond et al., 1998; Watson and Pessin, 2001). In addition, the inhibition of dye transfer between cells is a model to test the integrity of cell coupling after disruption of CENPF function by directly assaying for gap junction function at the cell surface (Francis and Lo, 2006; Lauf et al., 2002; Moskalewski et al., 1994). Our analyses show that disruption of murine CENPF function significantly diminished dye transfer between treated cells and clearly demonstrates inhibition of gap junction activity at the cell surface, resulting from mis-regulated protein trafficking to the cell membrane. Taken together with our previous work, the current data predict a broad role for CENPF in vesicular transport.
Materials and Methods
Yeast two-hybrid screen
This screen was previously described by Pooley et al. (Pooley et al., 2006). Briefly, a large N-terminal region of cytoplasmic murine CENPF (amino acids 1-689, termed ‘LCR’) was utilized in the Matchmaker Y2H System 3 (BD Biosciences Clontech). Library plasmids were isolated from yeast colonies that survived on quadruple dropout medium (QDO; SD, –Ade, –His, –Leu, –Trp, X-a-Gal) and exhibited lacZ expression. The inserts were then sequenced by the Vanderbilt Sequencing Core Facility and identified using NCBI Blast (Altschul et al., 1990). For each identified protein product, false positive tests with empty vector and random protein matings were conducted to eliminate spurious interactions, according to manufacturer’s recommendations.
Cell culture, transfection and constructs
COS-7, NIH3T3-L1 and C2C12 cells (ATCC) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10, 10 and 20% FBS, respectively, 100 μg/ml penicillin-streptomycin and L-glutamine, in a 5% CO2 atmosphere at 37°C. 3T3-L1 pre-adipocytes were differentiated by treatment with insulin, dexamethasone and isobutylmethlxanthine as previously described (Frost and Lane, 1985), and cells were used for experimentation 9-12 days after initiation of differentiation. For transfection, cells were grown to 50-75% confluency and transfected with DNA using FuGENE 6 (Roche) according to manufacturer’s recommendations. Murine N-terminal CENPF (NTmCENPF) was constructed by placing the N-terminal 474 amino acids of CENPF into the CMV-myc vector and full-length syntaxin 4 was placed into the EGFP-C3 vector (BD Biosciences Clontech).
Immunostaining and microscopy
For transient and endogenous studies, cells were gently washed with 1× PBS and fixed with either 4% paraformaldehyde to visualize endogenous proteins or with methanol to visualize transient protein for 20 minutes. Subsequently, cells were washed with 1× PBS, permeabilized with 0.25% Triton X-100 in 1× PBS for 10 minutes, and blocked for at least 1 hour in 2% BSA in 1× PBS at room temperature. Primary antibodies were incubated overnight at 4°C. Cells were then washed three times in 1× PBS and secondary antibodies were added for 1 hour at room temperature. Cells were again washed three times with 1× PBS and coverslips mounted with AquaPoly/Mount (PolySciences). Cells were visualized by fluorescence microscopy with an AX70 (Olympus), or for confocal analysis, with a LSM510 (Zeiss) microscope. Images were captured using Magnafire (Olympus). All images of control and experimental cells were processed identically.
Co-immunoprecipitation using transient transfections
COS-7 cells were grown on 10 cm plates; lysates were harvested 48 hours post transfection. The ProFound Mammalian Myc Tag Co-IP Kit (Pierce) was utilized according to manufacturer’s protocol. Briefly, cells were washed once with ice-cold TBS, incubated with M-Per extraction reagent (Pierce) containing protease inhibitor (Sigma), and centrifuged at 16,000 g for 20 minutes at 4°C. Lysate protein concentration of the supernatant was determined using a bicinchoninic acid solution assay (Pierce). For 2 hours, 100 μg total lysate was incubated with gentle shaking at 4°C with 10 μl anti-myc agarose slurry. Columns were washed three times with 1× TBS-Tween. Protein was eluted with 2× non-reducing sample buffer (Pierce) at 95°C for 5 minutes. To reduce proteins for SDS-PAGE analysis and western blot analysis, 2 μl 2-mercaptoethanol was added. Total lysate supernatant (10 μl) was used to confirm protein expression. Blots were developed using NBT-BCIP (Roche) and scanned (Hewlett-Packard) to produce digital images.
Co-immunoprecipitation of endogenous protein complexes containing murine CENPF
C2C12 cells were lysed with Nonidet P-40 buffer with gentle sonication. Whole cell lysates were recovered and samples containing 2-3 mg total protein were precleared with GammaBind Plus Sepharose (Amersham Biosciences) for 20 minutes with gentle rotation at 4°C. Cell lysates were collected and incubated overnight with 3 μg polyclonal syntaxin 4 antibody (Sigma). GammaBind Plus Sepharose was added to bind the antibody-protein complex. Beads were washed 3 times with cold 1× PBS and proteins were eluted with Laemmli sample buffer at a boiling temperature for 5 minutes. Proteins were resolved on a 6% SDS-PAGE gel and analyzed by western blotting. Protein lysate (20 μg) was loaded to visualize CENPF in whole cell lysate.
MO antisense oligomer treatment
Production of and methods utilizing morpholino oligonucleotides (MO) to specifically knock down endogenous CENPF have been previously reported (Ashe et al., 2004; Pooley et al., 2006; Soukoulis et al., 2005).
2-Deoxy-D-glucose transport assay
Forty-eight hours after MO addition, 3T3-L1 adipocytes were serum-starved for 1 hour. The cells were then incubated with 100 nM insulin in KRH buffer for 20 minutes. Glucose transport was initiated by addition of 0.5 mM 2-deoxy-D-[1,2-3H]glucose (0.25 μCi). After 10 minutes, transport was terminated by washing the cells three times with cold KRH buffer. Cells were then solubilized with 0.5% SDS, and the incorporated radioactivity was measured by liquid scintillation counting. All quantitative data are representative of three separate experiments conducted over 3 days, each with n=6-8. As a control to demonstrate the insulin-dependent nature of glucose transport with morpholino inhibition, standard control and CENPF MO-treated cultures were assayed for glucose uptake with and without insulin stimulation. A one-sample Student’s t-test was used after normalization to standard control (SC) cell populations.
Cell coupling
Cultured 3T3 fibroblasts were transfected with GFP alone as a control, both NTmCENPF and GFP-SNAP-25, or both NTmCENPF and GFP-syntaxin 4. Living transfected cells were identified and to quantitatively assess dye coupling, intracellular impalement was carried out with microelectrode filled with sulforhodamine101. The fluorescent dye was injected into the impaled cells iontophoretically using a current pulse of 1-3 nA of 0.5 second duration once per second for a total duration of 2 minutes. After an additional 3 minutes, the total extent of dye spread was recorded as the number of surrounding cells containing the injected dye. Transfer of dye was quantified and outlined according to published methods in first and second tier cells (Francis and Lo, 2006).
Antibodies
Rab11a (a gift from James Goldenring, Vanderbilt University) and murine CENPF antibodies were previously described (Pooley et al., 2006; Soukoulis et al., 2005). SNAP-25, syntaxin 4, and β-tubulin antibodies were obtained from Sigma; Golgin-97 was obtained from Molecular Probes; VAMP2 and VAMP3 antibodies were purchased from StressGen; syntaxin 4, EEA1, α-myc and α-GFP antibodies were obtained from BD Bioscience; Alexa-Fluor-488- and Alexa-Fluor-568-conjugated secondary antibodies were also utilized (Molecular Probes). For triple labeled immunofluorescence studies, polyclonal anti-myc (Novus) was directly labeled with the Zenon Alexa-647 labeling kit (Molecular Probes). Alkaline phosphatase-conjugated secondary antibodies for western blot were also purchased from Sigma.
Acknowledgments
Experiments were performed in part through the use of the VUMC Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, HD15052, DK59637 and EY08126). This work was primarily funded by the NIH in a grant to D.M.B. The authors are grateful for funding from the American Diabetes Association for Research Grant support (7-04-RA-69, L.-J.M.).
References
- Aikawa Y, Xia X, Martin TF. SNAP25, but not syntaxin 1A, recycles via an ARF6-regulated pathway in neuroendocrine cells. Mol. Biol. Cell. 2006;17:711–722. doi: 10.1091/mbc.E05-05-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ashe M, Pabon-Pena L, Dees E, Price KL, Bader D. LEK1 is a potential inhibitor of pocket protein-mediated cellular processes. J. Biol. Chem. 2004;279:664–676. doi: 10.1074/jbc.M308810200. [DOI] [PubMed] [Google Scholar]
- Bajohrs M, Darios F, Peak-Chew SY, Davletov B. Promiscuous interaction of SNAP-25 with all plasma membrane syntaxins in a neuroendocrine cell. Biochem. J. 2005;392:283–289. doi: 10.1042/BJ20050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band AM, Ali H, Vartiainen MK, Welti S, Lappalainen P, Olkkonen VM, Kuismanen E. Endogenous plasma membrane t-SNARE syntaxin 4 is present in rab11 positive endosomal membranes and associates with cortical actin cytoskeleton. FEBS Lett. 2002;531:513–519. doi: 10.1016/s0014-5793(02)03605-0. [DOI] [PubMed] [Google Scholar]
- Banfield DK, Lewis MJ, Rabouille C, Warren G, Pelham HR. Localization of Sed5, a putative vesicle targeting molecule, to the cis-Golgi network involves both its transmembrane and cytoplasmic domains. J. Cell Biol. 1994;127:357–371. doi: 10.1083/jcb.127.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks JD, Heald R. Chromosome movement: dynein-out at the kinetochore. Curr. Biol. 2001;11:R128–R131. doi: 10.1016/s0960-9822(01)00059-8. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell. Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- Cain CC, Trimble WS, Lienhard GE. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J. Biol. Chem. 1992;267:11681–11684. [PubMed] [Google Scholar]
- Caviston JP, Holzbaur EL. Microtubule motors at the intersection of trafficking and transport. Trends Cell Biol. 2006;16:530–537. doi: 10.1016/j.tcb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat. Rev. Mol. Cell. Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- Clark GM, Allred DC, Hilsenbeck SG, Chamness GC, Osborne CK, Jones D, Lee WH. Mitosin (a new proliferation marker) correlates with clinical outcome in node-negative breast cancer. Cancer Res. 1997;57:5505–5508. [PubMed] [Google Scholar]
- Cleveland DW, Mao Y, Sullivan KF. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112:407–421. doi: 10.1016/s0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- Dees E, Pabon-Pena LM, Goodwin RL, Bader D. Characterization of CMF1 in avian skeletal muscle. Dev. Dyn. 2000;219:169–181. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1055>3.3.co;2-2. [DOI] [PubMed] [Google Scholar]
- Faulkner NE, Dujardin DL, Tai CY, Vaughan KT, O’Connell CB, Wang Y, Vallee RB. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat. Cell Biol. 2000;2:784–791. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- Feng J. Microtubule: a common target for parkin and Parkinson’s disease toxins. Neuroscientist. 2006;12:469–476. doi: 10.1177/1073858406293853. [DOI] [PubMed] [Google Scholar]
- Feng J, Huang H, Yen TJ. CENP-F is a novel microtubule-binding protein that is essential for kinetochore attachments and affects the duration of the mitotic checkpoint delay. Chromosoma. 2006;115:320–329. doi: 10.1007/s00412-006-0049-5. [DOI] [PubMed] [Google Scholar]
- Feng Y, Walsh CA. Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron. 2004;44:279–293. doi: 10.1016/j.neuron.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Feng Y, Olson EC, Stukenberg PT, Flanagan LA, Kirschner MW, Walsh CA. LIS1 regulates CNS lamination by interacting with mNudE, a central component of the centrosome. Neuron. 2000;28:665–679. doi: 10.1016/s0896-6273(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Foster LJ, Klip A. Mechanism and regulation of GLUT-4 vesicle fusion in muscle and fat cells. Am. J. Physiol. Cell Physiol. 2000;279:C877–C890. doi: 10.1152/ajpcell.2000.279.4.C877. [DOI] [PubMed] [Google Scholar]
- Francis RJ, Lo CW. Primordial germ cell deficiency in the connexin 43 knockout mouse arises from apoptosis associated with abnormal p53 activation. Development. 2006;133:3451–3460. doi: 10.1242/dev.02506. [DOI] [PubMed] [Google Scholar]
- Frost SC, Lane MD. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J. Biol. Chem. 1985;260:2646–2652. [PubMed] [Google Scholar]
- Gibbons IR. The role of dynein in microtubule-based motility. Cell Struct. Funct. 1996;21:331–342. doi: 10.1247/csf.21.331. [DOI] [PubMed] [Google Scholar]
- Goodwin RL, Pabon-Pena LM, Foster GC, Bader D. The cloning and analysis of LEK1 identifies variations in the LEK/centromere protein F/mitosin gene family. J. Biol. Chem. 1999;274:18597–18604. doi: 10.1074/jbc.274.26.18597. [DOI] [PubMed] [Google Scholar]
- Heald R, Walczak CE. Microtubule-based motor function in mitosis. Curr. Opin. Struct. Biol. 1999;9:268–274. doi: 10.1016/s0959-440x(99)80037-2. [DOI] [PubMed] [Google Scholar]
- Hehnly H, Stamnes M. Regulating cytoskeleton-based vesicle motility. FEBS Lett. 2007;581:2112–2118. doi: 10.1016/j.febslet.2007.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein D, Taylor SS. Farnesylation of Cenp-F is required for G2/M progression and degradation after mitosis. J. Cell Sci. 2002;115:3403–3414. doi: 10.1242/jcs.115.17.3403. [DOI] [PubMed] [Google Scholar]
- Ishiki M, Klip A. Minireview: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology. 2005;146:5071–5078. doi: 10.1210/en.2005-0850. [DOI] [PubMed] [Google Scholar]
- Ishiki M, Randhawa VK, Poon V, Jebailey L, Klip A. Insulin regulates the membrane arrival, fusion, and C-terminal unmasking of glucose transporter-4 via distinct phosphoinositides. J. Biol. Chem. 2005;280:28792–28802. doi: 10.1074/jbc.M500501200. [DOI] [PubMed] [Google Scholar]
- Jagadish MN, Fernandez CS, Hewish DR, Macaulay SL, Gough KH, Grusovin J, Verkuylen A, Cosgrove L, Alafaci A, Frenkel MJ, Ward CW. Insulin-responsive tissues contain the core complex protein SNAP-25 (synaptosomal-associated protein 25) A and B isoforms in addition to syntaxin 4 and synaptobrevins 1 and 2. Biochem. J. 1996;317:945–954. doi: 10.1042/bj3170945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu. Rev. Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- Kawanishi M, Tamori Y, Okazawa H, Araki S, Shinoda H, Kasuga M. Role of SNAP23 in insulin-induced translocation of GLUT4 in 3T3-L1 adipocytes. Mediation of complex formation between syntaxin4 and VAMP2. J. Biol. Chem. 2000;275:8240–8247. doi: 10.1074/jbc.275.11.8240. [DOI] [PubMed] [Google Scholar]
- Kockx M, Guo DL, Huby T, Lesnik P, Kay J, Sabaretnam T, Jary E, Hill M, Gaus K, Chapman J, et al. Secretion of apolipoprotein E from macrophages occurs via a protein kinase A and calcium-dependent pathway along the microtubule network. Circ. Res. 2007;101:607–616. doi: 10.1161/CIRCRESAHA.107.157198. [DOI] [PubMed] [Google Scholar]
- Kondratova AA, Neznanov N, Kondratov RV, Gudkov AV. Poliovirus protein 3A binds and inactivates LIS1, causing block of membrane protein trafficking and deregulation of cell division. Cell Cycle. 2005;4:1403–1410. doi: 10.4161/cc.4.10.2041. [DOI] [PubMed] [Google Scholar]
- Konstantinidou AE, Korkolopoulou P, Kavantzas N, Mahera H, Thymara I, Kotsiakis X, Perdiki M, Patsouris E, Davaris P. Mitosin, a novel marker of cell proliferation and early recurrence in intracranial meningiomas. Histol. Histopathol. 2003;18:67–74. doi: 10.14670/HH-18.67. [DOI] [PubMed] [Google Scholar]
- Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc. Natl. Acad. Sci. USA. 2002;99:10446–10451. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Yu W, Li Y, Yang Z, Yan X, Huang Q, Zhu X. Nudel functions in membrane traffic mainly through association with Lis1 and cytoplasmic dynein. J. Cell Biol. 2004;164:557–566. doi: 10.1083/jcb.200308058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Winkfein RJ, Mack G, Rattner JB, Yen TJ. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J. Cell Biol. 1995;130:507–518. doi: 10.1083/jcb.130.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhao X, Zhu X. Mitosin/CENP-F in mitosis, transcriptional control, and differentiation. J. Biomed. Sci. 2006;13:205–213. doi: 10.1007/s11373-005-9057-3. [DOI] [PubMed] [Google Scholar]
- Mallard F, Tang BL, Galli T, Tenza D, Saint-Pol A, Yue X, Antony C, Hong W, Goud B, Johannes L. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 2002;156:653–664. doi: 10.1083/jcb.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Shewan A, Millar CA, Gould GW, James DE. Vesicle-associated membrane protein 2 plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter GLUT4 in 3T3-L1 adipocytes. J. Biol. Chem. 1998;273:1444–1452. doi: 10.1074/jbc.273.3.1444. [DOI] [PubMed] [Google Scholar]
- Martin S, Tellam J, Livingstone C, Slot JW, Gould GW, James DE. The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J. Cell Biol. 1996;134:625–635. doi: 10.1083/jcb.134.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Ushkaryov YA, Edelmann L, Link E, Binz T, Niemann H, Jahn R, Sudhof TC. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993;364:346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Tanoue A, Wu C, Mobley WC. TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc. Natl. Acad. Sci. USA. 2006;103:10444–10449. doi: 10.1073/pnas.0603914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalewski S, Popowicz P, Thyberg J. Functions of the Golgi complex in cell division: formation of cell-matrix contacts and cell-cell communication channels in the terminal phase of cytokinesis. J. Submicrosc. Cytol. Pathol. 1994;26:9–20. [PubMed] [Google Scholar]
- Pabon-Pena LM, Goodwin RL, Cise LJ, Bader D. Analysis of CMF1 reveals a bone morphogenetic protein-independent component of the cardiomyogenic pathway. J. Biol. Chem. 2000;275:21453–21459. doi: 10.1074/jbc.M000518200. [DOI] [PubMed] [Google Scholar]
- Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J. Biol. Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- Pevsner J, Hsu SC, Braun JE, Calakos N, Ting AE, Bennett MK, Scheller RH. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Pooley RD, Reddy S, Soukoulis V, Roland JT, Goldenring JR, Bader DM. CytLEK1 is a regulator of plasma membrane recycling through its interaction with SNAP-25. Mol. Biol. Cell. 2006;17:3176–3186. doi: 10.1091/mbc.E05-12-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones B, Riento K, Olkkonen VM, Hardy S, Bennett MK. Syntaxin 2 splice variants exhibit differential expression patterns, biochemical properties and subcellular localizations. J. Cell Sci. 1999;112:4291–4304. doi: 10.1242/jcs.112.23.4291. [DOI] [PubMed] [Google Scholar]
- Rattner JB, Rao A, Fritzler MJ, Valencia DW, Yen TJ. CENP-F is a.ca 400 kDa kinetochore protein that exhibits a cell-cycle dependent localization. Cell Motil. Cytoskeleton. 1993;26:214–226. doi: 10.1002/cm.970260305. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Salmon ED. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 1998;8:310–318. doi: 10.1016/s0962-8924(98)01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Corradi N, Malosio ML, Taverna E, Halban P, Meldolesi J, Rosa P. Blockade of membrane transport and disassembly of the Golgi complex by expression of syntaxin 1A in neurosecretion-incompetent cells: prevention by rbSEC1. J. Cell Sci. 1999;112(Pt 12):1865–1877. doi: 10.1242/jcs.112.12.1865. [DOI] [PubMed] [Google Scholar]
- Salaun C, James DJ, Greaves J, Chamberlain LH. Plasma membrane targeting of exocytic SNARE proteins. Biochim. Biophys. Acta. 2004;1693:81–89. doi: 10.1016/j.bbamcr.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Shionoya A, Ishida M, Gambello MJ, Yingling J, Wynshaw-Boris A, Hirotsune S. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–696. doi: 10.1016/s0896-6273(00)00146-x. [DOI] [PubMed] [Google Scholar]
- Schnapp BJ. Trafficking of signaling modules by kinesin motors. J. Cell Sci. 2003;116:2125–2135. doi: 10.1242/jcs.00488. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Rogers GC, Scholey JM. Microtubule motors in mitosis. Nature. 2000;407:41–47. doi: 10.1038/35024000. [DOI] [PubMed] [Google Scholar]
- Smith DS, Niethammer M, Ayala R, Zhou Y, Gambello MJ, Wynshaw-Boris A, Tsai LH. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat. Cell Biol. 2000;2:767–775. doi: 10.1038/35041000. [DOI] [PubMed] [Google Scholar]
- Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat. Rev. Mol. Cell. Biol. 2006;7:897–908. doi: 10.1038/nrm2060. [DOI] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Soukoulis V, Reddy S, Pooley RD, Feng Y, Walsh CA, Bader DM. Cytoplasmic LEK1 is a regulator of microtubule function through its interaction with the LIS1 pathway. Proc. Natl. Acad. Sci. USA. 2005;102:8549–8554. doi: 10.1073/pnas.0502303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma T, Arakawa T, Okayama M, Mizoguchi I, Tanimura A, Tajima Y. Trafficking of green fluorescent protein-tagged SNARE proteins in HSY cells. J. Biochem. 2002;132:729–735. doi: 10.1093/oxfordjournals.jbchem.a003280. [DOI] [PubMed] [Google Scholar]
- Terada S, Nakata T, Peterson AC, Hirokawa N. Visualization of slow axonal transport in vivo. Science. 1996;273:784–788. doi: 10.1126/science.273.5276.784. [DOI] [PubMed] [Google Scholar]
- Thurmond DC, Ceresa BP, Okada S, Elmendorf JS, Coker K, Pessin JE. Regulation of insulin-stimulated GLUT4 translocation by Munc18c in 3T3L1 adipocytes. J. Biol. Chem. 1998;273:33876–33883. doi: 10.1074/jbc.273.50.33876. [DOI] [PubMed] [Google Scholar]
- Tortorella LL, Pilch PF. C2C12 myocytes lack an insulin-responsive vesicular compartment despite dexamethasone-induced GLUT4 expression. Am. J. Physiol. Endocrinol. Metab. 2002;283:E514–E524. doi: 10.1152/ajpendo.00092.2002. [DOI] [PubMed] [Google Scholar]
- Vedrenne C, Hauri HP. Morphogenesis of the endoplasmic reticulum: beyond active membrane expansion. Traffic. 2006;7:639–646. doi: 10.1111/j.1600-0854.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- Vergnolle MA, Taylor SS. Cenp-F links kinetochores to Ndel1/Nde1/Lis1/dynein microtubule motor complexes. Curr. Biol. 2007;17:1173–1179. doi: 10.1016/j.cub.2007.05.077. [DOI] [PubMed] [Google Scholar]
- Volchuk A, Wang Q, Ewart HS, Liu Z, He L, Bennett MK, Klip A. Syntaxin 4 in 3T3-L1 adipocytes: regulation by insulin and participation in insulin-dependent glucose transport. Mol. Biol. Cell. 1996;7:1075–1082. doi: 10.1091/mbc.7.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Cansino V, Mathews JR, Graham M, Burgoyne RD, Wilson MC. Cysteine residues of SNAP-25 are required for SNARE disassembly and exocytosis, but not for membrane targeting. Biochem. J. 2001;357:625–634. doi: 10.1042/0264-6021:3570625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RT, Pessin JE. Transmembrane domain length determines intracellular membrane compartment localization of syntaxins 3, 4, and 5. Am. J. Physiol., Cell Physiol. 2001;281:C215–C223. doi: 10.1152/ajpcell.2001.281.1.C215. [DOI] [PubMed] [Google Scholar]
- Wei CJ, Xu X, Lo CW. Connexins and cell signaling in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:811–838. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- Wilcke M, Johannes L, Galli T, Mayau V, Goud B, Salamero J. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-golgi network. J. Cell Biol. 2000;151:1207–1220. doi: 10.1083/jcb.151.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Ramirez A, Cui B, Ding J, Delcroix JD, Valletta JS, Liu JJ, Yang Y, Chu S, Mobley WC. A functional dynein-microtubule network is required for NGF signaling through the Rap1/MAPK pathway. Traffic. 2007;8:1503–1520. doi: 10.1111/j.1600-0854.2007.00636.x. [DOI] [PubMed] [Google Scholar]
- Xiang X, Zuo W, Efimov VP, Morris NR. Isolation of a new set of Aspergillus nidulans mutants defective in nuclear migration. Curr. Genet. 1999;35:626–630. doi: 10.1007/s002940050461. [DOI] [PubMed] [Google Scholar]
- Yang Z, Guo J, Chen Q, Ding C, Du J, Zhu X. Silencing mitosin induces misaligned chromosomes, premature chromosome decondensation before anaphase onset, and mitotic cell death. Mol. Cell. Biol. 2005;25:4062–4074. doi: 10.1128/MCB.25.10.4062-4074.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, McKay J, Buss JE. H-Ras does not need COP I- or COP II-dependent vesicular transport to reach the plasma membrane. J. Biol. Chem. 2007;282:25760–25768. doi: 10.1074/jbc.M700437200. [DOI] [PubMed] [Google Scholar]
- Zhou X, Wang R, Fan L, Li Y, Ma L, Yang Z, Yu W, Jing N, Zhu X. Mitosin/CENP-F as a negative regulator of activating transcription factor-4. J. Biol. Chem. 2005;280:13973–13977. doi: 10.1074/jbc.M414310200. [DOI] [PubMed] [Google Scholar]
- Zhu X, Chang KH, He D, Mancini MA, Brinkley WR, Lee WH. The C terminus of mitosin is essential for its nuclear localization, centromere/kinetochore targeting, and dimerization. J. Biol. Chem. 1995a;270:19545–19550. doi: 10.1074/jbc.270.33.19545. [DOI] [PubMed] [Google Scholar]
- Zhu X, Mancini MA, Chang KH, Liu CY, Chen CF, Shan B, Jones D, Yang-Feng TL, Lee WH. Characterization of a novel 350-kilodalton nuclear phosphoprotein that is specifically involved in mitotic-phase progression. Mol. Cell. Biol. 1995b;15:5017–5029. doi: 10.1128/mcb.15.9.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Ding L, Pei G. Carboxyl terminus of mitosin is sufficient to confer spindle pole localization. J. Cell Biochem. 1997;66:441–449. [PubMed] [Google Scholar]