Abstract
Somatodendritic Kv4.2 channels mediate transient A-type potassium currents (IA), and play critical roles in controlling neuronal excitability and modulating synaptic plasticity. Our studies have shown a NMDA receptor-dependent downregulation of Kv4.2 and IA. NMDA receptors are heteromeric complexes of NR1 combined with NR2A-NR2D, mainly NR2A and NR2B. Here, we investigate NR2B receptor-mediated modulation of Kv4.2 and IA in cultured hippocampal neurons. Application of glutamate caused a reduction in total Kv4.2 protein levels and Kv4.2 clusters, and produced a hyperpolarized shift in the inactivation curve of IA. The effects of glutamate on Kv4.2 and IA were inhibited by pretreatment of NR2B-selective antagonists. NR2B-containing NMDA receptors are believed to be located predominantly extrasynaptically. Like application of glutamate, selective activation of extrasynaptic NMDA receptors caused a reduction in total Kv4.2 protein levels and Kv4.2 clusters, which was also blocked by NR2B-selective antagonists. In contrast, specific stimulation of synaptic NMDA receptors had no effect on Kv4.2. In addition, the influx of Ca2+ was essential for extrasynaptic modulation of Kv4.2. Calpain inhibitors prevented the reduction of total Kv4.2 protein levels following activation of extrasynaptic NMDA receptors. These results demonstrate that the glutamate-induced downregulation of Kv4.2 and IA is mediated by NR2B-containing NMDA receptors and is linked to proteolysis by calpain, which might contribute to the development of neuronal hyperexcitability and neurodegenerative diseases.
Keywords: IA, synaptic, extrasynaptic, ischemia, epilepsy, calpain
Voltage-gated potassium channels (Kvs) play a critical role in regulating neuronal excitability and synaptic plasticity in the hippocampus. IA is a voltage-dependent, transient outward K+ current that activates rapidly upon depolarization (threshold ~ −50 mV), inactivates quickly and recovers fast from inactivation (Jerng et al., 2004). Electrophysiological studies have indicated that somadendritic IA modulates sub-threshold dendritic signal integration (Ramakers and Storm, 2002, Goldberg et al., 2003, Cai et al., 2004, Kim et al., 2005a). Kv channels are composed of four α subunits that come together as either homotetrameric or heterotetrameric channels. Among the Kv α subunits, Kv4 subunits (Kv4.1, Kv4.2 and Kv4.3) are the major subunits that give rise to somatodendritic IA in hippocampal neurons. Immunohistochemical studies have shown that Kv4.2 is robustly detected on the soma and dendrites of CA1–CA3 pyramidal neurons and granule cells in dentate gyrus (Sheng et al., 1992, Serodio et al., 1994, Serodio and Rudy, 1998, Rhodes et al., 2004). Whole-cell IA in CA1 pyramidal neurons is selectively eliminated by knockout of Kv4.2 gene (Chen et al., 2006, Andrasfalvy et al., 2008), demonstrating that Kv4.2 is most likely to encode somatodendritic IA in CA1 pyramidal neurons. The distribution of Kv4.2 on the neuronal surface is highly uneven. Kv4.2 locates in a cluster pattern on the surface of soma and dendrites of pyramidal neurons (Petrecca et al., 2000, Wong et al., 2002, Jinno et al., 2005). This clustering is proposed to be attributable to interaction with post-synaptic density (PSD-95) via C-terminal VSAL motif of Kv4.2 (Wong et al., 2002). Recently, synaptic and extrasynaptic localization of Kv4.2 have been carefully investigated by immunoelectron microscopic analysis (Jinno et al., 2005, Burkhalter et al., 2006). Kv4.2 is expressed synaptically in GABAergic synapses, but extrasynaptically in glutamatergic synapses. This extrasynaptic distribution of Kv4.2 is likely to be related to IA properties and neuronal excitability.
Recent evidence suggests that neuronal activity and glutamatergic neurotransmission regulate Kv4.2 expression and subsequently IA function. Induction of long-term potentiation (LTP) causes a hyperpolarized shift in the inactivation curve of IA, resulting in an increased excitability of hippocampal neurons in rat brain slices (Frick et al., 2004). Two recent studies have found an activity-dependent and PKA-mediated internalization of Kv4.2 in primary cultured hippocampal neurons (Kim et al., 2007, Hammond et al., 2008). Dysregulation of Kv4.2 and IA has also been described in many neurodegenerative diseases (Birnbaum et al., 2004). Our previous studies have shown that glutamate reduces total Kv4.2 levels, diminishes Kv4.2 clusters and shifts voltage-dependent inactivation of IA in the hyperpolarization direction (Lei et al., 2008). Activation of NMDA receptors and Ca2+ influx through open NMDA receptors contribute to these changes. NMDA receptors are assembled from the NR1 subunit and at least one type of NR2 subunit, mainly NR2A and/or NR2B. NR2A-containing NMDA receptors are believed to be located predominantly at synaptic sites, whereas NR2B-containing NMDA receptors are located predominantly extrasynaptically. Distinct roles of NR2A- versus NR2B-containing or synaptic versus extrasynaptic NMDA receptors have been extensively studied in synaptic plasticity and neurodegenerative diseases (Hardingham et al., 2002, Liu et al., 2004, Massey et al., 2004, Liu et al., 2007, Papadia et al., 2008).
In the present study, we examined the involvement of NR2B-containing NMDA receptors in the glutamate-induced Kv4.2 downregulation. We observed that specific NR2B-selective antagonists inhibited the effect of glutamate on Kv4.2 protein levels, Kv4.2 clusters and IA properties. Furthermore, selective activation of extrasynaptic, but not synaptic NMDA receptors caused the same effect as glutamate on Kv4.2. The extrasynaptic modulation of Kv4.2 was also blocked by NR2B-selective antagonists. Calpain inhibitors could prevent the reduction of total Kv4.2 protein levels following the activation of extrasynaptic NMDA receptors. Together, our findings suggest that activation of NR2B-containing NMDA receptors is essential for the downregulation of Kv4.2 induced by glutamate and that calpain-dependent proteolysis is involved in this process.
Experimental procedures
Primary hippocampal neuronal culture
Dissociated hippocampal neurons were prepared and maintained as previously described (Lei et al., 2006). Briefly, hippocampal tissues were collected from embryonic days 18 Wistar rat embryos. After digestion and centrifuge, cells were re-suspended in Neurobasal medium (Invitrogen, Carlsbad, CA, USA) containing 2% B27 (Invitrogen). Then cells from a single pregnant rat were seeded on glass coverslips (Fisher Scientific, Pittsburgh, PA, USA) or in 60 mm culture dishes (BD Biosciences, San Jose, CA, USA) coated with 0.01% (w/v) poly-L-lysine (Sigma, St Louis, MO, USA) at a density of 1.0 X 105 cells/cm2, and put into a standard incubator (Taibai Espec, Osaka, Japan) maintained at 37°C in 95% air, 5% CO2. A half medium was changed once a week. Experimental protocols were institutionally approved in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize both the suffering and number of animals used.
Treatments
Cultures were washed 3 times with pre-warmed (37 °C) Locke’s solution (154 mM NaCl, 5.6 mM KCl, 5 mM HEPES, 5 mM glucose, 2 mM CaCl2, 1 mM MgCl2, pH 7.4, osmolarity 300 mOsm/L) (Misonou et al., 2004). Cultures were exposed to 10 µM glutamate in Locke’s solution supplemented with 1 µM glycine for 10 min at 37 °C. Synaptic NMDA receptors were selectively stimulated by treatment with 50 µM bicuculline plus 2.5 mM 4-aminopyridine (4-AP) in Mg2+ free Locke’s solution for 10 min. To selectively activate extrasynaptic NMDA receptors, synaptic NMDA receptors were blocked by treatment with 50 µM bicuculline in the presence of 10 µM MK-801 for 10 min in Mg2+ free Locke’s solution, followed by thorough washes with Locke’s solution 3 times to remove any trace of MK-801. Then extrasynaptic NMDA receptors were selectively activated by treatment of 10 µM glutamate plus 1 µM glycine in Locke’s solution. NMDA receptor antagonist, MK-801 (10 µM); NR2B receptor antagonists, ifenprodil (10 µM), Ro 25–6981 (0.5 µM, Tocris, Ellisville, MO, USA) and Co 101244 (5 µM, Tocris); membrane-permeable Ca2+ chelator, BAPTA-AM (10 µM), voltage-dependent Ca2+ channel blocker, nimodipine (10 µM); irreversible inhibitor of the endoplasmic reticulum (ER) Ca2+-ATPase, thapsigargin (10 µM); and calpain inhibitors, MDL-28170 (20 µM) and ALLN (25 µM) were added to the bath medium 15 min before and throughout the treatments. For Ca2+-free solution, 2 mM CaCl2 was substituted with 5 mM EGTA. All treatments were performed at 37 °C in the culture incubator. All chemicals were purchased from Sigma, unless otherwise indicated.
Western blotting
After treatments, cultures were washed 3 times with cold PBS and lysed with ice-cold RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% Sodium deoxycholate, 0.1% SDS; Boston BioProducts, Worcester, MA, USA) supplemented with a protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Cell lysates were harvested with a cell scraper, and incubated an additional 15 min on ice. After brief sonication on ice, cell lysates were centrifuged at 12,000 g for 20 min at 4°C to pellet nuclei and debris, and the resulting supernatants were collected for analysis. Protein concentration was determined by BCA protein assay (Bio-Rad, Hercules, CA, USA). Protein samples were boiled in 2 × SDS gel-loading buffer (Invitrogen) prior to SDS-PAGE. Proteins (20 µg) were separated on 10% SDS-PAGE gels and transferred to nitrocellulose membranes (Millipore, Bedford, MA, USA). The membranes were rinsed with distilled water, blocked with 1% bovine serum albumin (BSA; Sigma) in TBS-0.1% Tween20 (TBST) for 1 h, and then incubated with primary antibodies overnight in a blocking buffer at 4°C. We used rabbit polyclonal anti-Kv4.2 (1:1,000; Chemicon, Temecula, CA, USA), anti-Kv4.3 (1:1,000; Chemicon), anti-Kv1.4 (1:1,000; Chemicon), or mouse monoclonal anti-β-actin antibodies (1:20,000; Sigma). The membranes were washed with TBST, and incubated at room temperature for 1 h with HRP-conjugated anti-rabbit (1:5,000; Chemicon) or anti-mouse secondary antibodies (1:20,000; Chemicon). Bands were detected by the enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ, USA) and visualized by exposing the membrane to X-ray films (Fuji, Tokyo, Japan). Band densitometry analysis of the membrane was performed using scanned images of non-saturated immunoblot films, using NIH ImageJ 1.37 analysis software.
Immunocytochemical staining
After treatments, cultures on glass coverslips were fixed with 4% paraformaldehyde in PBS for 15 min. Cultures were blocked and permeabilized with 10% goat serum, 0.1% Triton X-100 in PBS for 1 h at room temperature. Cultures were then incubated overnight at 4°C with rabbit polyclonal anti-Kv4.2 antibody (1:1,000; Chemicon). After being washed with PBS 3 times for 10 min each, cultures were incubated with a fluorescein-conjugated anti-rabbit secondary antibody (1:100; Vector Labs, Burlingame, CA, USA) for 1 h at room temperature. Finally, cultures were washed with PBS 3 times for 10 min each and mounted with Vectashield mounting medium (Vector Labs). Cells were imaged using a microscope (BX50; Olympus, Tokyo, Japan) equipped with a reflected light fluorescence attachment (BX-FLA; Olympus). Fluorescence images were acquired with a digital camera coupled to control software (DP70-BSW; Olympus) at 100 × magnification.
Electrophysiological Recording
Recording electrodes were prepared from borosilicate glass (Warner Instruments, Hamden, CT, USA) using a horizontal electrode puller (P-97; Sutter Instruments, Novato, CA, USA). To record IA, electrodes had resistance of 2–4 MΩ when filled with an intracellular solution containing: 120 mM KMeSO4, 12 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.2 mM CaCl2, 10 mM HEPES, and 2 mM Mg-ATP, pH 7.3, 290–295 mOsm/L. The extracellular solution was Locke’s solution described above. To record spontaneous excitatory postsynaptic currents (sEPSCs), electrodes were filled with an intracellular solution containing: 43 mM CsCl, 92 mM CsMeSO4, 5 mM TEA, 2 mM EGTA, 1 mM MgCl2, 10 mM HEPES, and 4 mM Mg-ATP, pH 7.3, 290–295 mOsm/L. The flow rate of extracellular solution was adjusted to 2–3 ml/min. Recordings were carried out at room temperature. Cells were visualized with an infrared-differential interference contrast microscope (BX50WI; Olympus) and a CCD camera. Whole-cell patch-clamp recordings were performed with an Axopatch 200B amplifier (Molecular Devices, Palo Alto, CA, USA). After tight-seal (> 1 GΩ ) formation, the electrode capacitance was compensated. The membrane capacitance, series resistance and input resistance of the recorded neurons were measured by applying a 5 mV (10 ms) hyperpolarizing voltage pulse from a holding potential of −70 mV. The series resistance was 8–15 MΩ. Cells with a series resistance > 10% of the input resistance were discarded. The membrane capacitance reading was used as the value for whole cell capacitance. During the experiment, the membrane capacitance and series resistance were periodically monitored. Cells with a series resistance change > 20% during the experiment were excluded from the analysis. Signals were filtered at 2 kHz and digitized at a sampling rate of 5 kHz using a data-acquisition program (Axograph 4.6; Molecular Devices).
At a holding potential of −70 mV, the voltage-dependent outward potassium currents were evoked by voltage steps (from −90 mV to +60 mV in 10 mV increments, 400 ms) following a 300 ms hyperpolarizing pulse of −130 mV in the presence of TTX (1 µM) and CdCl2 (300 µM) to block voltage-activated Na+ and Ca2+ currents, as well as Ca2+-activated potassium currents. Taking advantage of the rapid inactivation at depolarized membrane potential, IA was isolated by subtracting the currents evoked after depolarized pre-pulses (0 mV, 100 ms) from those evoked without depolarized pre-pulses.
The current density of IA for each neuron was obtained by dividing the membrane capacitance from current amplitude. The current amplitude of IA was measured at the peak of each current (~ 4 ms after the onset of the command pulses). The steady-state activation curves were established similarly to those previously reported (Deng et al., 2004). Briefly, the conductance (G) was calculated using the following equation: G = I/(Vm − Vk), where I was the current amplitude, Vm was the command potential and Vk was the reversal potential of potassium (Vk = −98 mV). The conductance was then normalized with respect to the maximum value and plotted as a function of the membrane potential during the test pulse. The resulting activation curves were fitted with a normalized Boltzmann distribution: G/Gmax = 1/[1 + exp(Vm - V1/2)/Vc], where Gmax was the maximum conductance at +60 mV, V1/2 was the membrane voltage at which the current amplitude was half-maximum and Vc was the slope factor. The steady-state inactivation properties of IA were determined by measuring the current availability with a testing pulse of +10 mV following 2 s pre-pulse between −130 mV and −10 mV in 10 mV increments. The plot of mean normalized peak currents as a function of pre-pulse voltage was fitted with a normalized Boltzmann distribution: I/Imax = 1/[1 +exp(V1/2 − Vm)/Vc], where Imax was the maximum current at +10 mV following the pre-pulse of −130 mV.
Data Analysis
The values were presented as mean ± SEM. The significance of the results was tested using a one-sample T-TEST or one-way ANOVA followed by a post hoc Scheffe’s test using a commercially available software (StatView 5.0; Abacus Concepts, Berkeley, CA, USA). Changes were considered significant if p < 0.05.
Results
NR2B-selective antagonists block the glutamate-induced reduction in total Kv4.2 protein levels
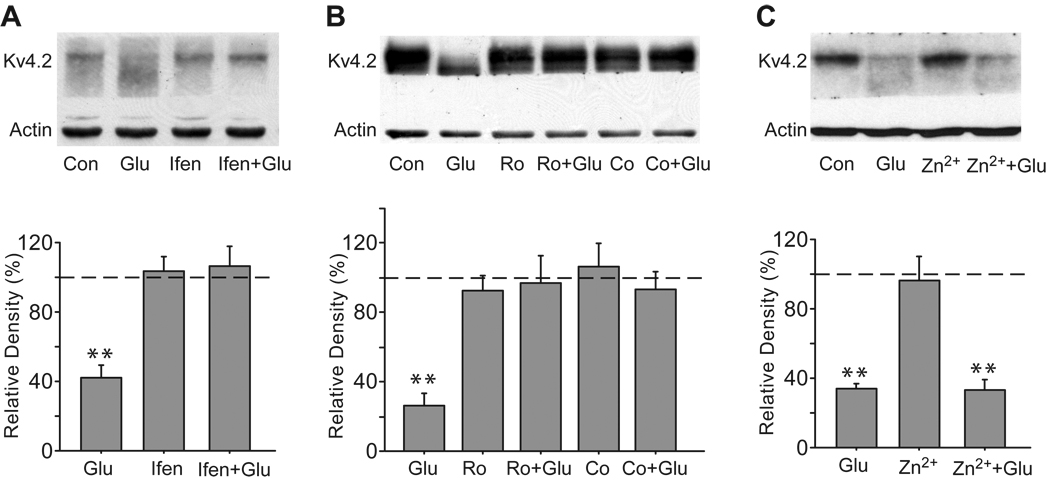
Consistent with our previous studies (Lei et al., 2008), at DIV 18, brief glutamate treatment (10 µM, 10 min) caused a profound reduction in total Kv4.2 levels in cultured hippocampal neurons (42.1 ± 6.3% of control, n = 6, p < 0.01 versus control, Fig. 1A). We first determined the effect of ifenprodil, a preferential antagonist for NR2B-containing NMDA receptors. Application of ifenprodil (10 µM, 15 min) completely blocked the glutamate-induced reduction in total Kv4.2 levels (106.2 ± 10.7% of control, n = 6, p > 0.05 versus control), while ifenprodil alone had no effect on total Kv4.2 levels (103.0 ± 8.2% of control, n = 6, p > 0.05 versus control, Fig. 1A). Due to possible interactions of ifenprodil with α-adrenergic receptors, serotonin receptors and especially calcium channels (Chenard et al., 1991, Church et al., 1994, McCool and Lovinger, 1995), we further used more potent and selective antagonists of NR2B receptors, Ro 25–6981 and Co 101244, to test the regulation of total Kv4.2 levels by NR2B-containing NMDA receptors. Ro 25–6981 is a widely used and well characterized NR2B-containing NMDA receptor antagonist. Co 101244 is a novel and selective NR2B-containing NMDA receptor antagonist. IC50 values of Co 101244 are 0.043 and >100 µM for NR2B- and NR2A-containing NMDA receptors respectively (Zhou et al., 1999). Like ifenprodil, both 0.5 µM Ro 25–6981 and 5 µM Co 101244 were able to abolish the glutamate-induced reduction in total Kv4.2 levels (Ro 25–6981: 96.8 ± 15.7% of control, n = 4, p > 0.05 versus control; Co 101244: 93.2 ± 10.0% of control, n = 4, p > 0.05 versus control, Fig. 1B). Zn2+ is highly potent at inhibiting NR2A-containing NMDA receptors (in the nanomolar range) and displays strong selectivity for NR2A-containing NMDA receptors over NR2B-containing NMDA receptors (> 100-fold) (Paoletti et al., 2000, Rachline et al., 2005). A concentration of 100 nM Zn2+ produces more than 70% inhibition of NR1/NR2A activity, whereas it only blocks NR1/NR2B activity by 10% (Rachline et al., 2005). The glutamate-induced reduction in Kv4.2 protein levels was not influenced by Zn2+ at a concentration of 100 nM (33.3 ± 5.2% of control, n = 4, p < 0.01 versus control, Fig. 1C). Together, the results suggest that the regulation of Kv4.2 by glutamate is selectively coupled to NR2B-containing NMDA receptors.
Fig. 1.
The glutamate-induced reduction in total Kv4.2 levels is mediated by NR2B-containing NMDA receptors. (A) Ifenprodil blocks the glutamate-induced reduction in total Kv4.2 levels. Cultured hippocampal neurons (DIV 18) were treated with ifenprodil (Ifen, 10 µM, 15 min) before and during glutamate exposure (Glu, 10 µM, 10 min). After treatment, cell lysates were Western blotted with anti-Kv4.2 and anti-β-actin antibodies. Β-actin works as a loading control. (B) Both Ro 25–6981 (Ro) and Co 101244 (Co) block the glutamate-induced reduction in total Kv4.2 levels. Neurons were treated with Ro 25–6981 (0.5 µM, 15 min) or Co 101244 (5 µM, 15 min) before and during glutamate exposure. (C) Zn2+ fails to block the glutamate-induced reduction in total Kv4.2 levels. Neurons were treated with Zn2+ (100 µM, 15 min) before and during glutamate exposure. Blots are representative of three to six independent experiments. The dashed line indicates the control value against which the other values are measured. Data are presented as mean ± SEM. Statistical analysis was performed by one-sample T-TEST. ** p < 0.01 versus control (Con).
NR2B-containing NMDA receptors are involved in the effect of glutamate on the cellular distribution of Kv4.2
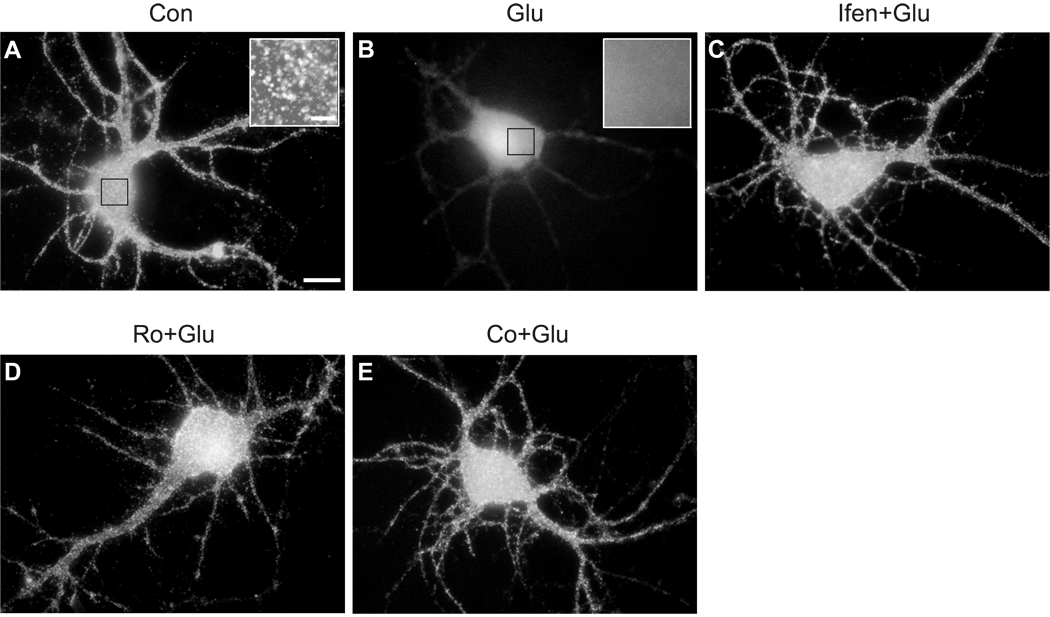
To provide further evidence to complement our Western blotting data, we tested whether NR2B-containing NMDA receptors are involved in the effect of glutamate on the cellular distribution of Kv4.2 in cultured hippocampal neurons by immunocytochemical staining. At DIV 18, hippocampal neurons showed strong immunoreactivity for Kv4.2 (Maletic-Savatic et al., 1995). Kv4.2 immunoreactivity displayed a dense cluster pattern throughout soma and dendrites in control neurons (Fig. 2A), in agreement with previous studies (Petrecca et al., 2000, Wong et al., 2002, Lei et al., 2008). Brief glutamate exposure dramatically reduced the abundance of Kv4.2 clusters in both neuronal soma and dendrites, as compared with control neurons (Fig. 2B). The glutamate effect on Kv4.2 clusters was completely attenuated in the presences of ifenprodil, Ro 25–6981 and Co 101244 (Fig. 2C–E).
Fig. 2.
Glutamate causes a reduction of Kv4.2 clusters on the soma and dendrites of cultured hippocampal neurons through NR2B-containing NMDA receptors. (A) A control neuron (DIV 18) was incubated in Locke’s solution for 10 min before fixation and immunostaining for Kv4.2. Insert is the high magnification image of indicated region, showing Kv4.2 clusters (Scale bar, 2 µm). (B) A neuron was treated with 10 µM glutamate (Glu) for 10 min. Insert is the high magnification image of indicated region. (C) A neuron was pretreated with 10 µM ifenprodil (Ifen) for 15 min, and then treated with 10 µM glutamate in the presence of 10 µM ifenprodil for 10 min. (D) A neuron was pretreated with 0.5 µM Ro 25–6981(Ro) for 15 min, and then treated with 10 µM glutamate in the presence of 0.5 µM Ro 25–6981 for 10 min. (E) A neuron was pretreated with 5 µM Co 101244 (Co) for 15 min, and then treated with 10 µM glutamate in the presence of 5 µM Co 101244 for 10 min. Kv4.2 clusters were dramatically decreased following glutamate treatment. This effect was attenuated in the presence of NR2B-selective NMDA antagonists ifenprodil, Ro 25–6981 or Co 101244. Scale bar, 10 µm.
Glutamate alters IA properties through NR2B-containing NMDA receptors
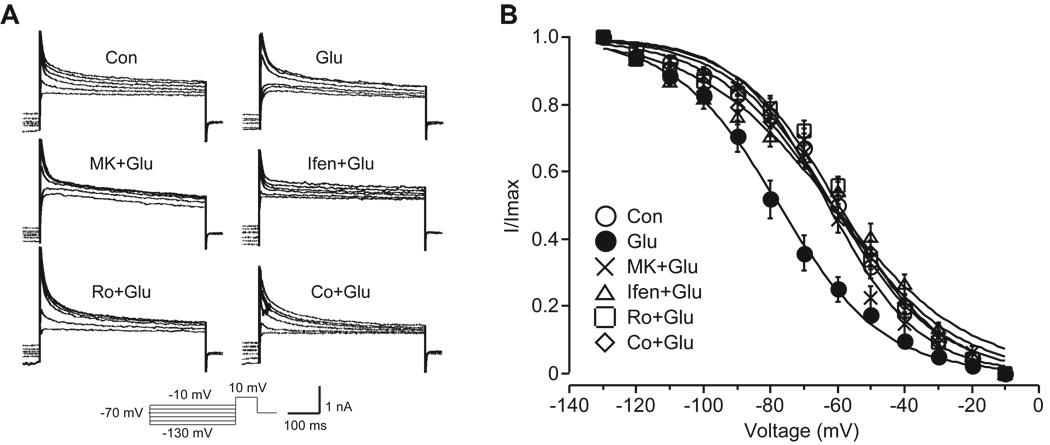
Since Kv4.2 underlies the majority of somatodendritic IA in hippocampal neurons, it is rational to hypothesize that IA should be changed with Kv4.2. To test this hypothesis, we performed whole-cell voltage-clamped recording to measure IA in cultured hippocampal neurons (DIV 18) after different treatments. Voltage steps from −90 mV to +60 mV evoked an outward current composed of two distinct components, a transient component (IA) and a sustained component (Ikd). IA was isolated by subtracting the sustained component from the total potassium currents. Subsequently, the voltage dependence of steady-state inactivation was recorded at +10 mV following 2 s conditioning voltage steps from −130 to −10 mV. As shown in Fig. 3, glutamate shifted the V1/2 of inactivation for IA by approximately 16 mV in the hyperpolarization direction (glutamate-treated neurons −77.8 ± 2.5 mV, n = 10, p < 0.05 versus control neurons −61.5 ± 2.3 mV, n = 16). Similar to the non-selective NMDA receptor blocker MK-801 (10 µM), all of the tested NR2B-selective antagonists prevented the shift in V1/2 of inactivation for IA after glutamate treatment (MK-801 + glutamate: −62.6 ± 1.9 mV, n = 8, p > 0.05 versus control; ifenprodil + glutamate: −61.3 ± 2.6 mV, n = 12, p > 0.05 versus control; Ro 25–6981 + glutamate: −60.6 ± 1.6 mV, n = 16, p > 0.05 versus control; Co 101244 + glutamate: −62.1 ± 2.5 mV, n = 10, p > 0.05 versus control). None of the tested chemicals alone changed the V1/2 of inactivation for IA (MK-801: −63.1 ± 3.8 mV, n = 6, p > 0.05 versus control; ifenprodil: −61.4 ± 1.9 mV, n = 6, p > 0.05 versus control; Ro 25–6981: −60.2 ± 2.1 mV, n = 12, p > 0.05 versus control; Co 101244: −59.3 ± 2.1 mV, n = 7, p > 0.05 versus control). In contrast to the V1/2 of inactivation, the V1/2 of activation for IA was unaltered after these treatments (data not shown).
Fig. 3.
Glutamate alters properties of IA currents in cultured hippocampal neurons through NR2B-contraining NMDA receptors. (A) Representative traces of IA for determining the voltage dependence of inactivation. The protocol is shown at the bottom. Neurons were pretreated with MK-801 (MK, 10 µM), ifenprodil (Ifen, 10 µM), Ro 25–6981 (Ro, 0.5 µM) or Co 101244 (Co, 5 µM) for 15 min, and then were treated with glutamate (Glu, 10 µM, 10 min) in the presence of MK-801, ifenprodil, Ro 25–6981 or Co 101244. (B) Group data showing inactivation curves for IA (n = 8 ~ 16). Glutamate shifted the V1/2 of inactivation for IA approximately 16 mV in the hyperpolarization direction. Like MK-801, NR2B-selective antagonists ifenprodil, Ro 25–6981 and Co 101244 prevented the shift in V1/2 of IA after glutamate treatment.
Activation of extrasynaptic NMDA receptors reduces total Kv4.2 levels and Kv4.2 clusters through NR2B-containing NMDA receptors
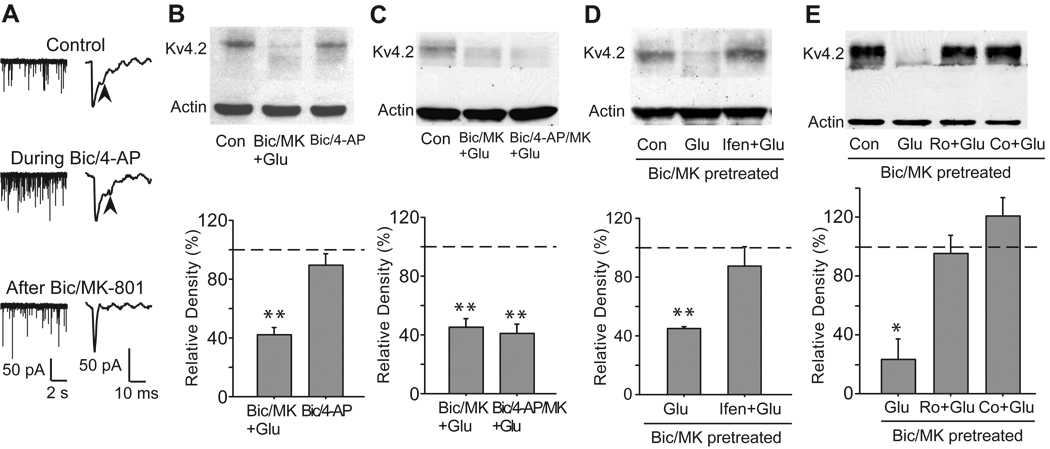
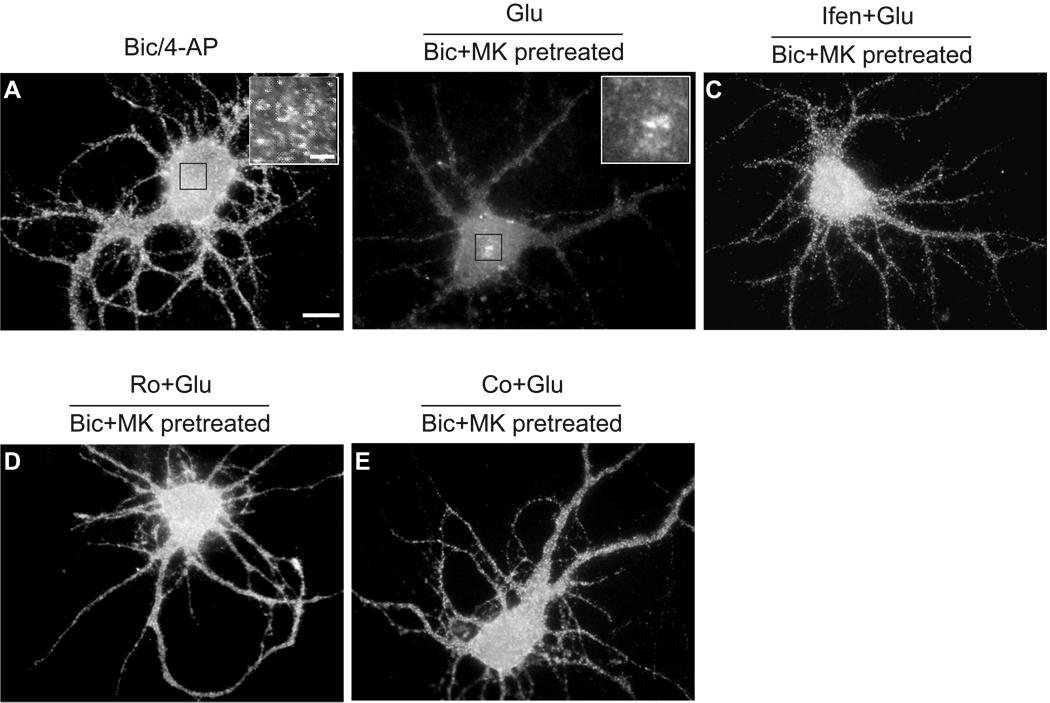
In contrast to the predominant expression of NR2A-containing NMDA receptors inside synapses, NR2B-containing NMDA receptors are widely believed to be the predominant NMDA receptors expressed at extrasynaptic sites in mature hippocampal neurons (Tovar and Westbrook, 1999). In our previous studies (Lei et al., 2008), we applied 50 µM bicuculline, a GABAA receptor inhibitor, to activate synaptic NMDA receptors. We found that bicuculline, unlike glutamate, had no significant effect on total Kv4.2 levels, which suggests that the glutamate-induced reduction of Kv4.2 expression might be mediated by extrasynaptic NMDA receptors. In the present study, we stimulated synaptic NMDA receptors with 50 µM bicuculline plus 2.5 mM 4-AP, a weak K+ channel blocker. It has been proved that this treatment increases synaptic glutamate release, activates synaptic NMDA receptors and elevates the Ca2+ plateau induced by synaptic NMDA receptors (Hardingham et al., 2002). The treatment with 50 µM bicuculline and 2.5 mM 4-AP dramatically increased the frequency of sEPSCs, reflecting an enhancement of synaptic activity (Fig. 4A). Application of bicuculline together with 4-AP failed to dramatically decrease total Kv4.2 levels (89.2 ± 7.9% of control, n = 6, p > 0.05 versus control, Fig. 4B). These results confirm that synaptic NMDA receptors are not involved in the glutamate-induced reduction of total Kv4.2 levels. Given the results demonstrating that total Kv4.2 levels were decreased by a bath application of glutamate but not by activation of synaptic NMDA receptors, we next examined the possibility that NMDA receptor modulation of Kv4.2 was associated with activation of extrasynaptic NMDA receptors. To test this hypothesis, we used a well-established method to selectively activate extrasynaptic NMDA receptors (Hardingham et al., 2002). First, synaptic NMDA receptors were blocked by co-application of 50 µM bicuculline and 10 µM MK-801 for 10 min. Bicuculline selectively activates synaptic NMDA receptors. Meanwhile, MK-801 is an irreversible blocker of open NMDA receptors and can only block the bicuculline-activated synaptic NMDA receptors. After the treatment with 50 µM bicuculline and 10 µM MK-801, synaptic NMDA components of sEPSCs was significant decreased, indicating an inhibition of synaptic NMDA receptors (Fig. 4A) (Ivanov et al., 2006). After thorough washout of bicuculline and MK-801, the residual extrasynaptic NMDA receptors were then selectively activated by the bath application of glutamate (10 µM, 10 min). Similar to the bath application of glutamate, selective activation of extrasynaptic NMDA receptors significantly deceased total Kv4.2 levels (40.2 ± 13.6% of control, n = 3, p > 0.05 versus control, Fig. 4B). Furthermore, we used 50 µM bicuculline and 2.5 mM 4-AP to activate synaptic NMDA receptors, and meanwhile used 10 µM MK-801 to block activated synaptic NMDA receptors. We found that this treatment caused the same degree of blockade of synaptic NMDA receptors as bicuculline/MK-801 treatment, because the following application of glutamate resulted in a similar reduction in Kv4.2 protein levels (41.2 ± 6.9% of control, n = 3, p < 0.01 versus control, Fig. 4C). As a result, we only used 50 µM bicuculline together with 10 µM MK-801 to block synaptic NMDA receptors in the following experiments. Ifenprodil (10 µM) could prevent the reduction in total Kv4.2 levels induced by activation of extrasynaptic NMDA receptors (91.5 ± 20% of control, n = 3, p > 0.05 versus control, Fig. 4D). Furthermore, both 0.5 µM Ro 25–6981 and 5 µM Co 101244 inhibited the decrease of total Kv4.2 levels after activation of extrasynaptic NMDA receptors (Ro 25–6981: 95.4 ± 12.3% of control, n = 4, p > 0.05 versus control; Co 101244: 120.9 ± 12.6% of control, n = 4, p > 0.05 versus control, Fig. 4E). In agreement with changes of total Kv4.2 levels, no obvious change in Kv4.2 clusters was observed after selective synaptic NMDA stimulation (Fig. 5A). However, selective extrasynaptic NMDA stimulation caused a dramatic decrease of Kv4.2 clusters (Fig. 5B), which could also be eliminated by ifenprodil, Ro 25–6981 and Co 101244 (Fig. 5C–E).
Fig. 4.
Activation of extrasynaptic NMDA receptors causes a reduction in total Kv4.2 levels in cultured hippocampal neurons, which is blocked by NR2B-selective antagonists. (A) Left panel: sample traces of continuous recording of sEPSCs show that the treatment with 50 µM bicuculline and 2.5 mM 4-AP increases the frequency of sEPSCs, reflecting an enhancement of synaptic activity. Right panels: sample traces of sEPSCs show that the treatment with 50 µM bicuculline and 10 µM MK-801 results in a reduction of synaptic NMDA components of sEPSCs, indicating an inhibition of synaptic NMDA receptors. Vh = −70 mV. Arrows indicate NMDA components of sEPSCs. (B) Selective activation of extrasynaptic, but not synaptic NMDA receptors causes a reduction in total Kv4.2 levels. Synaptic NMDA receptors were selectively stimulated by treatment with 50 µM bicuculline (Bic) plus 2.5 mM 4-AP in Mg2+ free Locke’s solution for 10 min. Extrasynaptic NMDA receptors were selectively activated by glutamate (Glu, 10 µM, 10 min) after the blockage of synaptic NMDA receptors with bicuculline (Bic, 50 µM) plus MK-801 (MK, 10 µM) for 10 min. (C) The blockage of synaptic NMDA receptors by 50 µM bicuculline/10 µM MK-801 is similar to that by 50 µM bicuculline/2.5 mM 4-AP/10 µM MK-801. (D) Ifenprodil (Ifen, 10 µM) blocks extrasynaptic NMDA receptor-induced reduction in total Kv4.2 levels. (E) Both Ro 25–6981 (Ro, 0.5 µM) and Co 101244 (Co, 5 µM) block extrasynaptic NMDA receptor-induced reduction in total Kv4.2 levels. Blots are representative of three or four independent experiments. The dashed line indicates the control value against which the other values are measured. Data are presented as mean ± SEM. Statistical analysis was performed by one-sample T-TEST. * p < 0.05, ** p < 0.01 versus control (Con).
Fig. 5.
NR2B-containing NMDA receptors are involved in the decrease of Kv4.2 clusters on the soma and dendrites of cultured hippocampal neurons after activation of extrasynaptic NMDA receptors. (A) A neuron was incubated with 50 µM bicuculline (Bic) plus 2.5 mM 4-AP for 10 min to selectively activate synaptic NMDA receptors. (B) A neuron was incubated with 10 µM glutamate (Glu) for 10 min to selectively activate extrasynaptic NMDA receptors after the blockage of synaptic NMDA receptors with 50 µM bicuculline (Bic) plus 10 µM MK-801 (MK) for 10 min. The inserts in (A) and (B) show the high magnification images of indicated region (Scale bar, 2 µm). (C) A neuron was pretreated with 10 µM ifenprodil (Ifen) for 15 min, and then extrasynaptic NMDA receptors were selectively stimulated in the presence of 10 µM ifenprodil. (D) A neuron was pretreated with 0.5 µM Ro 25–6981 (Ro) for 15 min, and then extrasynaptic NMDA receptors were selectively stimulated in the presence of 0.5 µM Ro 25–6981. (E) A neuron was pretreated with 5 µM Co 101244 (Co) for 15 min, and then extrasynaptic NMDA receptors were selectively stimulated in the presence of 5 µM Co 101244. Activation of extrasynaptic, but not synaptic, NMDA receptors decreased Kv4.2 clusters. This effect was attenuated in the presence of NR2B-selective NMDA antagonists ifenprodil, Ro 25–6981 or Co 101244. Scale bar, 10 µm.
The properties of extrasynaptic NMDA receptor-mediated Kv4.2 modulation
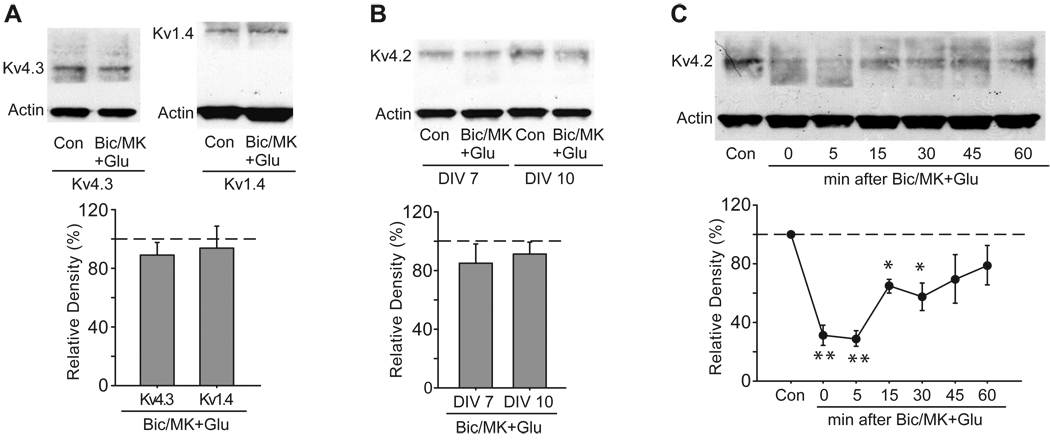
First, we examined the specificity of extrasynaptic NMDA receptor-mediated K+ channels modulation. Among the Kv4 members (Kv4.1, Kv4.2 and Kv4.3), Kv4.1 expression is quite low in the hippocampus. In CA1, Kv4.2 is abundantly expressed in pyramidal neurons, whereas Kv4.3 is only expressed in interneurons. Kv4.3 is colocalized with Kv4.2 in CA2–CA3 and the dentate gyrus. Although Kv4.3 shows 73% identity with Kv4.2 in their protein sequences (Serodio et al., 1996), total Kv4.3 levels were unchanged after activation of extrasynaptic NMDA receptors (89.0 ± 7.6% of control, n = 6, p > 0.05 versus control, Fig. 6A). We also examined whether activation of extrasynaptic NMDA receptors could reduce total Kv1.4 levels. Kv1.4 assembled with Kv β subunits encodes presynaptic IA in hippocampal neurons (Maletic-Savatic et al., 1995, Rhodes et al., 1995, Rhodes et al., 1997, Monaghan et al., 2001). No significant alteration was observed in total Kv1.4 levels after activation of extrasynaptic NMDA receptors (93.6 ± 14.8% of control, n = 3, p > 0.05 versus control, Fig. 6A). These data suggest that the reduction in total Kv4.2 levels after extrasynaptic NMDA stimulation is not a general effect on all IA channels. The composition of NMDA receptors changes during the development of cultured hippocampal neurons (Rao et al., 1998). Early in development, hippocampal neurons only express NR2B-containing NMDA receptors. Later, NR2A-containing synaptic NMDA receptors increase during a period of rapid synapse formation. Likewise, the sensitivity of Kv4.2 to activation of extrasynaptic NMDA receptors changed during the development of cultured hippocampal neurons. Total Kv4.2 levels showed no response to activation of extrasynaptic NMDA receptors before DIV 10 (DIV 7: 85.9 ± 12.9% of control, n = 3, p > 0.05 versus control; DIV 10: 91.3 ± 8.2% of control, n = 3, p > 0.05 versus control, Fig. 6B). In addition, the effect of extrasynaptic NMDA stimulation on Kv4.2 was reversible, in that total Kv4.2 levels partially recovered to control levels at 1 h after washout (79.1 ± 13.2% of control, n = 3, p > 0.05 versus control, Fig. 6C), and totally returned to control levels at 4 h after washout (106.6 ± 10.3% of control, n = 3, p > 0.05 versus control, data not shown).
Fig. 6.
Extrasynaptic modulation of Kv4.2 is reversible. (A) Specificity of extrasynaptic modulation of Kv4.2. Activation of extrasynaptic NMDA receptors had no influence on total Kv4.3 levels and total Kv1.4 levels. Extrasynaptic NMDA receptors were selectively activated by glutamate (Glu, 10 µM, 10 min) after the blockage of synaptic NMDA receptors with bicuculline (BiC, 50 µM) plus MK-801 (MK, 10 µM) for 10 min. (B) Age dependence of extrasynaptic modulation of Kv4.2 in cultured hippocampal neurons. Activation of extrasynaptic NMDA receptors did not cause a reduction of total Kv4.2 levels in 7- and 10-day-old neurons. (C) Time course of extrasynaptic modulation of Kv4.2. Neurons were treated with extrasynaptic NMDA stimulation, washed and then incubated for 5, 15, 30, 45 or 60 min in culture medium. Total Kv4.2 levels began to recover 15 min after treatment. Blots are representative of three to six independent experiments. The dashed line indicates the control value against which the other values are measured. Data are presented as mean ± SEM. Statistical analysis was performed by one-sample T-TEST. * p < 0.05, ** p < 0.01 versus control (Con).
The influx of Ca2+ is essential for the effect of extrasynaptic NMDA stimulation on Kv4.2
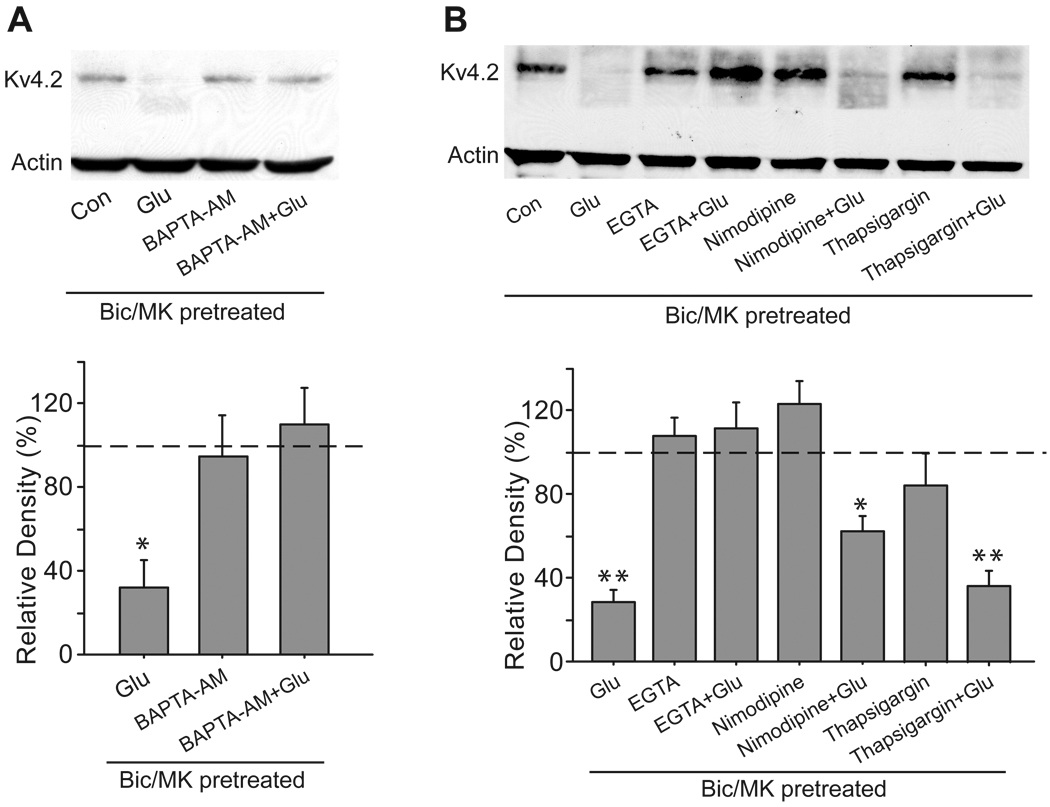
NMDA receptors are highly permeable to Ca2+, and Ca2+ influx through NMDA receptors is critical for long-lasting changes in synaptic efficacy and excitotoxicity. Therefore, we assessed the involvement of Ca2+ in the regulation of Kv4.2 by extrasynaptic NMDA stimulation. We pretreated neurons with 10 µM BAPTA-AM (a fast membrane-permeable Ca2+ chelator) for 15 min to remove Ca2+ from the neuronal cytosol, followed by extrasynaptic NMDA stimulation. BAPTA-AM completely inhibited the reduction in total Kv4.2 levels caused by extrasynaptic NMDA stimulation (110.2 ± 17.8% of control, n =3, p > 0.05 versus control, Fig 7A). These data suggest that cytosolic Ca2+ is required for the regulation of Kv4.2 by extrasynaptic NMDA receptors. Like other cells, neurons use both extracellular and intracellular sources to control cytosolic Ca2+ concentration (Berridge, 1998). These sources include Ca2+ influx through NMDA receptors and L-type voltage-dependent Ca2+ channels, and Ca2+ release from ER. We removed Ca2+ from the extracellular solution with 5 mM EGTA. Removal of extracellular Ca2+ resulted in a complete inhibition of the reduction of total Kv4.2 levels following extrasynaptic NMDA stimulation (117.1 ± 11.6% of control, n = 3, p > 0.05 versus control, Fig. 7B). We then pretreated neurons with 10 µM nimodipine for 15 min to block L-type voltage-dependent Ca2+ channels. In contrast to EGTA, nimodipine failed to fully block the reduction in total Kv4.2 levels after extrasynaptic NMDA stimulation (62.0 ± 7.5% of control, n = 3, p < 0.05 versus control, Fig. 7B). To test whether Ca2+ release from ER was involved in this process, we depleted intracellular Ca2+ stores of hippocampal neurons with 10 µM thapsigargin, an irreversible inhibitor of the ER Ca2+-ATPase. Extrasynaptic NMDA receptor modulation of Kv4.2 was not inhibited by thapsigargin (36.4 ± 7.5% of control, n = 3, p < 0.05 versus control, Fig. 7B). These results strongly suggest that Ca2+ entry through open extrasynaptic NMDA receptors is essential for extrasynaptic NMDA receptor modulation of Kv4.2.
Fig. 7.
Ca2+ influx is essential for extrasynaptic modulation of Kv4.2 in cultured hippocampal neurons. (A) Intracellular Ca2+ is required for extrasynaptic modulation of Kv4.2. Neurons were pretreated with BAPTA-AM (10 µM) for 15 min, and then extrasynaptic NMDA receptors were selectively activated for 10 min in the presence of BAPTA-AM (10 µM). Extrasynaptic NMDA receptors were selectively activated by glutamate (Glu, 10 µM, 10 min) after the blockage of synaptic NMDA receptors with bicuculline (Bic, 50 µM) plus MK-801 (MK, 10 µM) for 10 min. (B) Extrasynaptic modulation of Kv4.2 is dependent on Ca2+ influx. Neurons were incubated with EGTA (5 mM), nimodipine (10 µM) or thapsigargin (10 µM) for 15 min, and then extrasynaptic NMDA receptors were activated in the presence of EGTA (5 mM), nimodipine (10 µM) or thapsigargin (10 µM). Blots are representative of three independent experiments. The dashed line indicates the control value against which the other values are measured. Data are presented as mean ± SEM. Statistical analysis was performed by one-sample T-TEST. * p < 0.05, ** p < 0.01 versus control (Con).
The calpain-mediated proteolysis is involved in the effect of extrasynaptic NMDA stimulation on Kv4.2
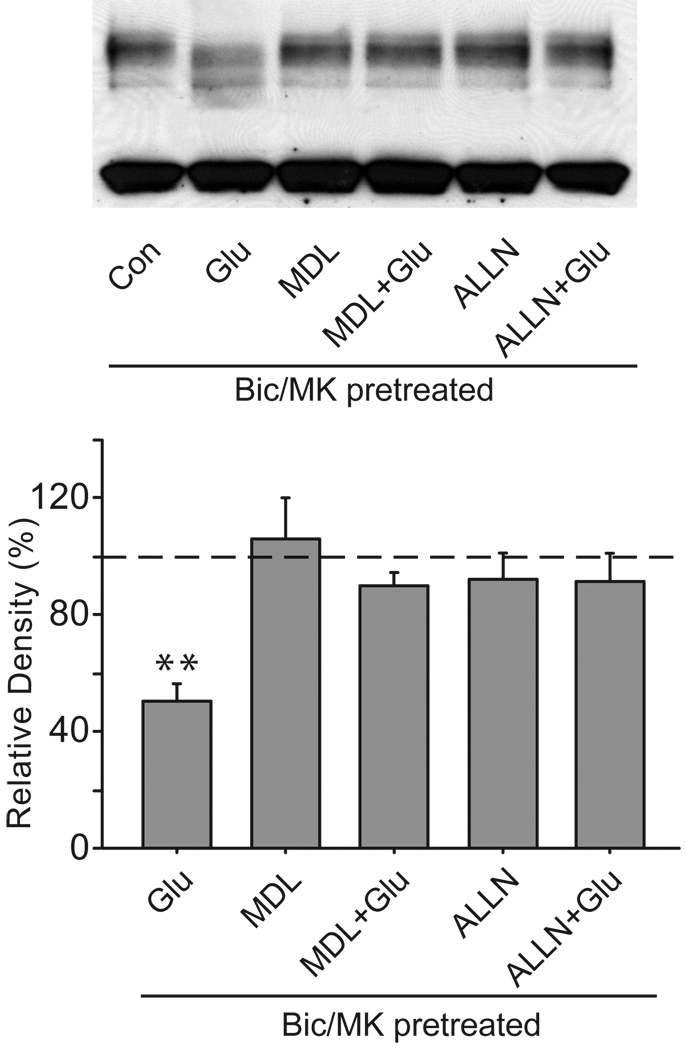
Our experiments demonstrate that Ca2+ influx contributes to the extrasynaptic NMDA receptor modulation of Kv4.2. The Ca2+ influx through open NMDA receptors is known to activate Ca2+-dependent proteases including calpain. The decrease in total Kv4.2 protein levels after extrasynaptic NMDA stimulation suggests that proteolytic activity may be responsible for this phenomenon. We therefore investigated whether calpain inhibitors, MDL-28170 and ALLN, could prevent the Kv4.2 protein’s alteration. We found that both MDL-28170 (20 µM) and ALLN (25 µM) significantly attenuated the decrease in Kv4.2 protein levels following extrasynaptic NMDA stimulation, as seen in Fig. 8 (MDL-2817: 90.0 ± 4.4% of control, n = 4, p > 0.05 versus control; ALLN: 91.3 ± 9.3% of control, n = 4, p > 0.05 versus control). These data indicate that the calpain-mediated proteolysis is involved in the effect of extrasynaptic NMDA stimulation on Kv4.2.
Fig. 8.
Calpain activity is required for extrasynaptic modulation of Kv4.2 in cultured hippocampal neurons. Neurons were pretreated with MDL-28170 (20 µM) or ALLN (25 µM) for 15 min, and then extrasynaptic NMDA receptors were selectively activated for 10 min in the presence of MDL-28170 (20 µM) or ALLN (25 µM). Extrasynaptic NMDA receptors were selectively activated by glutamate (Glu, 10 µM, 10 min) after the blockage of synaptic NMDA receptors with bicuculline (Bic, 50 µM) plus MK-801 (MK, 10 µM) for 10 min. Blots are representative of four independent experiments. The dashed line indicates the control value against which the other values are measured. Data are presented as mean ± SEM. Statistical analysis was performed by one-sample T-TEST. ** p < 0.01 versus control (Con).
Discussion
The present report provides the evidence showing NR2B-containing NMDA receptor-mediated modulation of Kv4.2 potassium channels. This conclusion is based on three major findings. First, brief glutamate treatment leads to a reduction of total Kv4.2 levels and Kv4.2 clusters, together with a hyperpolarized shift in the inactivation curve of IA. These effects are fully blocked by NR2B-selective antagonists. Second, like glutamate stimulation, selective activation of extrasynaptic NMDA receptors causes the reduction in total Kv4.2 levels and Kv4.2 clusters. Third, in contrast, selective activation of synaptic NMDA receptors has no effect on total Kv4.2 levels and Kv4.2 clusters.
The finding that selective inhibitors of NR2B-containing NMDA receptors prevent the glutamate-induced downregulation of Kv4.2 and IA demonstrates the involvement of this subtype of glutamate receptors in Kv4.2 modulation. It is well known that NR2B subunits are predominantly present at extrasynaptic sites, but they are also incorporated in synapses (Tovar and Westbrook, 1999). Furthermore, NMDA receptors are highly dynamic and can move bidirectionally between synaptic and extrasynaptic sites (Tovar and Westbrook, 2002, Bellone and Nicoll, 2007). NR2B-selective antagonists are unable to discriminate between synaptic and extrasynaptic pools of NR2B. As a result, we next addressed the role of extrasynaptic NR2B-containing NMDA receptors in Kv4.2 modulation. We found that selective activation of extrasynaptic NMDA receptors displays the same effect as glutamate on Kv4.2, which is also eliminated by NR2B-selective antagonists. Together, these results strongly suggest that extrasynaptic NR2B-containing NMDA receptors mediate the glutamate-induced downregulation of Kv4.2.
Why are NR2A- and NR2B-containing NMDA receptors different in Kv4.2 modulation? NR2A- and NR2B-containing NMDA receptors differ in a range of ways such as localizations, physiological properties, binding partners and downstream signaling molecules. Therefore, many mechanisms can be proposed, one of which is the differential Ca2+ influx. Our data indicate that only Ca2+ influx through activated extrasynaptic NMDA receptors is essential for Kv4.2 modulation. Although both NR2A-and NR2B-containing NMDA receptors are highly permeable to Ca2+, Ca2+ influx through NR2A- or NR2B-containing NMDA receptors determines the Ca2+ profile, leading to the activation of specific signaling pathways. Compared to NR2A-containing NMDA receptors, NR2B-containing NMDA receptors have slower decay kinetics and cause more sustained increase in intracellular Ca2+ concentration (McBain and Mayer, 1994, Hardingham and Bading, 2003, Vanhoutte and Bading, 2003). Calpain is a neutral, Ca2+-activated protease that regulates numerous downstream targets and participates in synaptic modification and excitotoxic neuronal death (Saido et al., 1994, Chan and Mattson, 1999). Only activation of NR2B- but not NR2A-containing NMDA receptors can result in calpain activation (Zhou and Baudry, 2006). Our results indicate that calpain-dependent proteolysis is involved in this process. Moreover, the intracellular domains of NR2A and NR2B subunits may differentially bind and regulate downstream signaling molecules. The calcium/calmodulin-dependent protein kinase II (CaMKII) is another signaling downstream of NMDA receptors and Ca2+. CaMKII is reported to bind preferentially to the cytoplasmic tail of NR2B rather than NR2A (Barria and Malinow, 2005). Besides CaMKII, the extracellular signal-regulated kinases (ERK) are also activated by Ca2+ influx through NMDA receptors and play an important role in neuronal plasticity and survival. In hippocampal neurons, NR2A-containing NMDA receptors promote, whereas NR2B-containing NMDA receptors inhibit ERK signaling cascades (Krapivinsky et al., 2003, Kim et al., 2005b, Ivanov et al., 2006). It is reported that Kv4.2 is directly phosphorylated and regulated by both CaMKII and ERK (Birnbaum et al., 2004). Phosphorylation of various proteins by kinases has been shown to control their proteolysis by calpain (Goll et al., 2003). Thus, the Kv4.2 phosphorylation state is likely to determine the susceptibility of Kv4.2 to calpain cleavage.
What are then functional implications of the modulation of Kv4.2 and IA mediated by NR2B-containing NMDA receptors? It has been proposed that activation of the postsynaptic NMDA receptors is required for both LTP and LTD, and that Ca2+ influx through activated NMDA receptors triggers a series of intracellular cascades that lead to persistent changes in the number and properties of postsynaptic AMPA receptors (Malenka and Bear, 2004). Whether NR2A- and NR2B-containing NMDA receptors have differential roles in synaptic plasticity is a hot ongoing debate (Liu et al., 2004, Massey et al., 2004, Berberich et al., 2005, Weitlauf et al., 2005, Fox et al., 2006, Bartlett et al., 2007, Morishita et al., 2007). Somatodendritic IA plays a central role in regulating membrane excitability of neurons, such as the back-propagation of dendritic action potentials and Ca2+ plateau potential, action potential initiation and half-width, and frequency-dependent action potential broadening (Hoffman et al., 1997, Goldberg et al., 2003, Cai et al., 2004, Kim et al., 2005a). Therefore, changes in somatodendritic IA are suggested to play an important role in synaptic plasticity (Ramakers and Storm, 2002, Watanabe et al., 2002, Frick et al., 2004, Chen et al., 2006, Losonczy et al., 2008). Since molecular biological and pharmacological studies have implicated Kv4.2 as a key subunit contributing to somatodendritic IA in the brain, knocking out Kv4.2 leads to a lower threshold for LTP induction (Chen et al., 2006), whereas augmenting Kv4.2 blocks LTP (Jung et al., 2008). A recent electron microscopic study shows that Kv4.2 is excluded from excitatory synapses in the hippocampus (Jinno et al., 2005). In agreement with this study, our results demonstrate a functional coupling of extrasynaptic NR2B-containing NMDA receptors and Kv4.2. Under the circumstances of LTP induction, high frequency stimulation of synaptic activity can elevate the extracellular concentration of glutamate sufficient to activate extrasynaptic NMDA receptors (Clark and Cull-Candy, 2002). Our observations demonstrate a downregulation of Kv4.2 and IA following activation of extrasynaptic NMDA receptors. It is proposed that the downregulation of Kv4.2 is likely to cause an enhanced amplitude of back-propagating action potential (BAP), provide a stronger postsynaptic depolarization to unblock NMDA receptors, and facilitate the induction of LTP. In addition, it has recently been shown that alterations of Kv4.2 are able to directly reorganize the NMDA receptor composition at synapses and influence the induction of LTP. Upregulation of Kv4.2 causes a decreased fraction of synaptic NR2B/NR2A and a reduced LTP, whereas downregulation of Kv4.2 results in an increased proportion of synaptic NR2B/NR2A and an increased LTP (Jung et al., 2008). It is also well known that a hyperpolarized shift in IA currents causes an increase in intrinsic neuronal excitability. It is reported that the induction of LTP is accompanied by a hyperpolarized shift in the inactivation curve of IA leading to an increase in dendritic excitability (Frick et al., 2004). The hyperpolarized shift in IA inactivation curve occurred under pathological conditions is also reported to be associated with neuronal hyperexcitability (Takeda et al., 2006, Xu et al., 2006).
The activation of extrasynaptic NMDA receptors is attributed mainly to excessive glutamate release occurring during pathological conditions, including ischemic and epileptic brain damage (Arundine and Tymianski, 2004, Sierra-Paredes and Sierra-Marcuno, 2007). Dysregulation of Kv4.2 and IA has also been described in epilepsy and ischemia (Chi and Xu, 2000, Bernard et al., 2004, Zou et al., 2005). Therefore, extrasynaptic NMDA receptors might initiate the cascade to reduce Kv4.2 levels and inhibit IA function, resulting in neuronal hyperexcitability and excitotoxic neuronal death. Although our data suggest that Ca2+ influx through activated extrasynaptic NMDA receptors and calpain-mediated proteolysis are involved in this process, a full understanding of how Kv4.2 and IA is down-regulated after activation of extrasynaptic NMDA receptors will need further study.
In summary, the present study indicates that glutamate modulation of Kv4.2 and IA is mediated by NR2B-containing NMDA receptors that are located outside of synapses. Ca2+ influx through open extrasynaptic NMDA receptors and subsequently activated calpain are critical to Kv4.2 modulation. The downregulation of Kv4.2 and IA mediated by NR2B-containing NMDA receptors is prone to increase neuronal intrinsic excitability, and might play a role in long-term synaptic plasticity and excitotoxic neuronal death.
Acknowledgments
This work was supported by NIH grant NS38053 and AHA grant 0655747Z to ZCX. ZL, PD, and YL are recipients of AHA fellowships (AHA0526007Z, AHA0425689Z, AHA0630172N, AHA0710027Z, AHA0825810G).
Abbreviations
- ALLN
Ac-LLnL-CHO
- AMPA
(±) α-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid hydrate
- 4-AP
4-aminopyridine
- BAP
back-propagating action potential
- BSA
bovine serum albumin
- CaMKII
Ca/calmodulin dependent kinase II
- Co 101244
1-[2-(4-Hydroxyphenoxy)ethyl]-4-piperidinol hydrochloride
- CREB
cAMP response element binding protein
- DIV
days in vitro
- ECL
enhanced chemiluminescence
- EGTA
ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid
- ER
endoplasmic reticulum
- ERK
extracellular-regulated kinases
- HRP
horseradish peroxidase
- IA
A-type K+ currents
- Kv
voltage-dependent K+ currents
- LTD
long-term depression
- LTP
long-term potentiation
- MAP-2
microtubule-associated protein-2
- MDL-28170
Z-Val-Phe-CHO
- MK-801
(5S, 10R)-(+)-5-methyl-10, 11-dihydro-5H-dibenzo-[a, d]-cyclo-hepten-5, 10- imine hydrogen maleate
- PBS
phosphate-buffered saline
- PKA
cAMP-dependent protein kinase
- PKC
protein kinase C
- PSD-95
post-synaptic density
- Ro 25-6981
(αR, βS)-α-(4-hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidinepropanol maleate
- SDS
sodium dodecyl sulfate
- sEPSCs
spontaneous excitatory postsynaptic currents
- TBS
tris-buffered saline
- TBST
TBS-0.1% Tween20
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests Statement
The authors declare that they have no competing financial interests.
References
- Andrasfalvy BK, Makara JK, Johnston D, Magee JC. Altered synaptic and non-synaptic properties of CA1 pyramidal neurons in Kv4.2 knockout mice. The Journal of physiology. 2008;586:3881–3892. doi: 10.1113/jphysiol.2008.154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science (New York, NY. 2004;305:532–535. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiological reviews. 2004;84:803–833. doi: 10.1152/physrev.00039.2003. [DOI] [PubMed] [Google Scholar]
- Burkhalter A, Gonchar Y, Mellor RL, Nerbonne JM. Differential expression of I(A) channel subunits Kv4.2 and Kv4.3 in mouse visual cortical neurons and synapses. J Neurosci. 2006;26:12274–12282. doi: 10.1523/JNEUROSCI.2599-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–364. doi: 10.1016/j.neuron.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Chan SL, Mattson MP. Caspase and calpain substrates: roles in synaptic plasticity and cell death. J Neurosci Res. 1999;58:167–190. [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenard BL, Shalaby IA, Koe BK, Ronau RT, Butler TW, Prochniak MA, Schmidt AW, Fox CB. Separation of alpha 1 adrenergic and N-methyl-D-aspartate antagonist activity in a series of ifenprodil compounds. J Med Chem. 1991;34:3085–3090. doi: 10.1021/jm00114a018. [DOI] [PubMed] [Google Scholar]
- Chi XX, Xu ZC. Differential changes of potassium currents in CA1 pyramidal neurons after transient forebrain ischemia. Journal of neurophysiology. 2000;84:2834–2843. doi: 10.1152/jn.2000.84.6.2834. [DOI] [PubMed] [Google Scholar]
- Church J, Fletcher EJ, Baxter K, MacDonald JF. Blockade by ifenprodil of high voltage-activated Ca2+ channels in rat and mouse cultured hippocampal pyramidal neurones: comparison with N-methyl-D-aspartate receptor antagonist actions. Br J Pharmacol. 1994;113:499–507. doi: 10.1111/j.1476-5381.1994.tb17017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BA, Cull-Candy SG. Activity-dependent recruitment of extrasynaptic NMDA receptor activation at an AMPA receptor-only synapse. J Neurosci. 2002;22:4428–4436. doi: 10.1523/JNEUROSCI.22-11-04428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P, Pang Z, Zhang Y, Xu ZC. Developmental changes of transient potassium currents in large aspiny neurons in the neostriatum. Brain Res Dev Brain Res. 2004;153:97–107. doi: 10.1016/j.devbrainres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Russell KI, Wang YT, Christie BR. Contribution of NR2A and NR2B NMDA subunits to bidirectional synaptic plasticity in the hippocampus in vivo. Hippocampus. 2006;16:907–915. doi: 10.1002/hipo.20230. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nature neuroscience. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Tamas G, Yuste R. Ca2+ imaging of mouse neocortical interneurone dendrites: Ia-type K+ channels control action potential backpropagation. The Journal of physiology. 2003;551:49–65. doi: 10.1113/jphysiol.2003.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiological reviews. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Lin L, Sidorov MS, Wikenheiser AM, Hoffman DA. Protein kinase a mediates activity-dependent Kv4.2 channel trafficking. J Neurosci. 2008;28:7513–7519. doi: 10.1523/JNEUROSCI.1951-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends in neurosciences. 2003;26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nature neuroscience. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. The Journal of physiology. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004;27:343–369. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Jinno S, Jeromin A, Kosaka T. Postsynaptic and extrasynaptic localization of Kv4.2 channels in the mouse hippocampal region, with special reference to targeted clustering at gabaergic synapses. Neuroscience. 2005;134:483–494. doi: 10.1016/j.neuroscience.2005.04.065. [DOI] [PubMed] [Google Scholar]
- Jung SC, Kim J, Hoffman DA. Rapid, bidirectional remodeling of synaptic NMDA receptor subunit composition by A-type K+ channel activity in hippocampal CA1 pyramidal neurons. Neuron. 2008;60:657–671. doi: 10.1016/j.neuron.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron. 2007;54:933–947. doi: 10.1016/j.neuron.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Wei DS, Hoffman DA. Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. The Journal of physiology. 2005a;569:41–57. doi: 10.1113/jphysiol.2005.095042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005b;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–784. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Lei Z, Deng P, Xu ZC. Regulation of Kv4.2 channels by glutamate in cultured hippocampal neurons. Journal of neurochemistry. 2008;106:182–192. doi: 10.1111/j.1471-4159.2008.05356.x. [DOI] [PubMed] [Google Scholar]
- Lei Z, Ruan Y, Yang AN, Xu ZC. NMDA receptor mediated dendritic plasticity in cortical cultures after oxygen-glucose deprivation. Neuroscience letters. 2006;407:224–229. doi: 10.1016/j.neulet.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science (New York, NY. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Lenn NJ, Trimmer JS. Differential spatiotemporal expression of K+ channel polypeptides in rat hippocampal neurons developing in situ and in vitro. J Neurosci. 1995;15:3840–3851. doi: 10.1523/JNEUROSCI.15-05-03840.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiological reviews. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- McCool BA, Lovinger DM. Ifenprodil inhibition of the 5-hydroxytryptamine3 receptor. Neuropharmacology. 1995;34:621–629. doi: 10.1016/0028-3908(95)00030-a. [DOI] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nature neuroscience. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- Monaghan MM, Trimmer JS, Rhodes KJ. Experimental localization of Kv1 family voltage-gated K+ channel alpha and beta subunits in rat hippocampal formation. J Neurosci. 2001;21:5973–5983. doi: 10.1523/JNEUROSCI.21-16-05973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology. 2007;52:71–76. doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Perin-Dureau F, Fayyazuddin A, Le Goff A, Callebaut I, Neyton J. Molecular organization of a zinc binding n-terminal modulatory domain in a NMDA receptor subunit. Neuron. 2000;28:911–925. doi: 10.1016/s0896-6273(00)00163-x. [DOI] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nature neuroscience. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrecca K, Miller DM, Shrier A. Localization and enhanced current density of the Kv4.2 potassium channel by interaction with the actin-binding protein filamin. J Neurosci. 2000;20:8736–8744. doi: 10.1523/JNEUROSCI.20-23-08736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci. 2005;25:308–317. doi: 10.1523/JNEUROSCI.3967-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers GM, Storm JF. A postsynaptic transient K(+) current modulated by arachidonic acid regulates synaptic integration and threshold for LTP induction in hippocampal pyramidal cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10144–10149. doi: 10.1073/pnas.152620399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Kim E, Sheng M, Craig AM. Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J Neurosci. 1998;18:1217–1229. doi: 10.1523/JNEUROSCI.18-04-01217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Carroll KI, Sung MA, Doliveira LC, Monaghan MM, Burke SL, Strassle BW, Buchwalder L, Menegola M, Cao J, An WF, Trimmer JS. KChIPs and Kv4 alpha subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci. 2004;24:7903–7915. doi: 10.1523/JNEUROSCI.0776-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Keilbaugh SA, Barrezueta NX, Lopez KL, Trimmer JS. Association and colocalization of K+ channel alpha- and beta-subunit polypeptides in rat brain. J Neurosci. 1995;15:5360–5371. doi: 10.1523/JNEUROSCI.15-07-05360.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. J Neurosci. 1997;17:8246–8258. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saido TC, Sorimachi H, Suzuki K. Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB J. 1994;8:814–822. [PubMed] [Google Scholar]
- Serodio P, Kentros C, Rudy B. Identification of molecular components of A-type channels activating at subthreshold potentials. Journal of neurophysiology. 1994;72:1516–1529. doi: 10.1152/jn.1994.72.4.1516. [DOI] [PubMed] [Google Scholar]
- Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. Journal of neurophysiology. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- Serodio P, Vega-Saenz de Miera E, Rudy B. Cloning of a novel component of A-type K+ channels operating at subthreshold potentials with unique expression in heart and brain. Journal of neurophysiology. 1996;75:2174–2179. doi: 10.1152/jn.1996.75.5.2174. [DOI] [PubMed] [Google Scholar]
- Sheng M, Tsaur ML, Jan YN, Jan LY. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992;9:271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- Sierra-Paredes G, Sierra-Marcuno G. Extrasynaptic GABA and glutamate receptors in epilepsy. CNS Neurol Disord Drug Targets. 2007;6:288–300. doi: 10.2174/187152707781387251. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Ikeda M, Nasu M, Kadoi J, Yoshida S, Matsumoto S. Enhanced excitability of rat trigeminal root ganglion neurons via decrease in A-type potassium currents following temporomandibular joint inflammation. Neuroscience. 2006;138:621–630. doi: 10.1016/j.neuroscience.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, Bading H. Opposing roles of synaptic and extrasynaptic NMDA receptors in neuronal calcium signalling and BDNF gene regulation. Curr Opin Neurobiol. 2003;13:366–371. doi: 10.1016/s0959-4388(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Hoffman DA, Migliore M, Johnston D. Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8366–8371. doi: 10.1073/pnas.122210599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W, Newell EW, Jugloff DG, Jones OT, Schlichter LC. Cell surface targeting and clustering interactions between heterologously expressed PSD-95 and the Shal voltage-gated potassium channel, Kv4.2. The Journal of biological chemistry. 2002;277:20423–20430. doi: 10.1074/jbc.M109412200. [DOI] [PubMed] [Google Scholar]
- Xu GY, Winston JH, Shenoy M, Yin H, Pasricha PJ. Enhanced excitability and suppression of A-type K+ current of pancreas-specific afferent neurons in a rat model of chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G424–G431. doi: 10.1152/ajpgi.00560.2005. [DOI] [PubMed] [Google Scholar]
- Zhou M, Baudry M. Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors. J Neurosci. 2006;26:2956–2963. doi: 10.1523/JNEUROSCI.4299-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZL, Cai SX, Whittemore ER, Konkoy CS, Espitia SA, Tran M, Rock DM, Coughenour LL, Hawkinson JE, Boxer PA, Bigge CF, Wise LD, Weber E, Woodward RM, Keana JF. 4-Hydroxy-1-[2-(4-hydroxyphenoxy)ethyl]-4- (4-methylbenzyl)piperidine: a novel, potent, and selective NR1/2B NMDA receptor antagonist. J Med Chem. 1999;42:2993–3000. doi: 10.1021/jm990246i. [DOI] [PubMed] [Google Scholar]
- Zou B, Li Y, Deng P, Xu ZC. Alterations of potassium currents in ischemia-vulnerable and ischemia-resistant neurons in the hippocampus after ischemia. Brain research. 2005;1033:78–89. doi: 10.1016/j.brainres.2004.11.023. [DOI] [PubMed] [Google Scholar]