Abstract
Neural responses to a repeated stimulus typically diminish, an effect known as repetition suppression. We here demonstrate what appear to be parallel effects of repetition on subjective duration, even when stimuli are presented too rapidly for explicit temporal judgments. When a brief visual stimulus (e.g., a letter, word, object, or face) was serially flashed in different locations, several stimuli appeared to be present simultaneously due to persistence of vision—we term this the Proliferation Effect. Critically, fewer stimuli were perceived to be simultaneously present when the same stimulus was flashed repeatedly than when a different stimulus was used for each flash, indicating that persistence of vision (and hence subjective duration) shrinks for predictable stimuli. These short-timescale experiments demonstrate that subjective durations are computed at a preconscious and implicit level of processing, thereby changing the temporal interpretation of visual scenes. Further, these findings suggest a new, instant diagnostic test for deficits in repetition suppression, such as those found in schizophrenia.
Keywords: time perception, duration, repetition suppression, novelty, predictability, schizophrenia, proliferation effect
Introduction
How the brain represents duration remains an unsolved problem (Eagleman et al., 2005). It is clear that physical time does not have a direct mapping onto perceived time—instead, subjective duration judgments are surprisingly prone to distortions (Eagleman, 2008; Kanai, Paffen, Hogendoorn, & Verstraten, 2006; Morrone, Ross, & Burr, 2005; Nakajima, ten Hoopen, Hilkhuysen, & Sasaki, 1992; Yarrow, Haggard, Heal, Brown, & Rothwell, 2001), such that two stimuli of identical duration can be perceived to last different amounts of time. For example, observers watching a repeated stimulus erroneously report that the first presentation (Kanai & Watanabe, 2006; Pariyadath & Eagleman, 2007; Rose & Summers, 1995) and any ‘oddball’ presentation (Pariyadath & Eagleman, 2007; Tse, Intriligator, Rivest, & Cavanagh, 2004; Ulrich, Nitschke, & Rammsayer, 2006) appear longer in duration than the other presentations.
Although these duration illusions were originally suggested to be caused by increases in attention (Rose & Summers, 1995; Tse et al., 2004), we have previously shown that the emotional salience of the oddball has no effect on the illusion (Pariyadath & Eagleman, 2007), suggesting that the effect has more to do with the stimulus predictability than the amount of attentional deployment. We suggested that the perceived duration of novel and repeated stimuli may map on to measured neural responses to the same (Eagleman, 2008).
While earlier studies have used stimuli lasting hundreds of milliseconds, we here explored duration judgments of stimuli briefer than 100 ms, presented too quickly for explicit duration judgments. To achieve this, we developed a novel modification to the traditional flicker fusion paradigm. In flicker fusion experiments, a light is rapidly turned on and off: at a low frequency, flicker is perceived, while at a high frequency, the light appears to be steady. The frequency at which perception switches from flicker to a steady light is called the critical flicker fusion threshold (CFFT). In light of repetition suppression, we took note of the fact that CFFT experiments always consist of a single stimulus (the light) presented repeatedly. Because there are subjective duration differences when viewing familiar versus novel stimuli, we hypothesized that the CFFT would change if the rapid stimulus could somehow be made novel each time it appeared. To this end, we derived a variation of the flicker fusion paradigm to study the differences in duration judgments for novel and familiar stimuli.
Methods
Apparatus and stimuli
Participants consisted of graduate students and staff between the age of 18 and 45 at the Texas Medical Center with normal or corrected-to-normal vision; all were compensated for taking part in the experiments. Participants sat 59 cm from a CRT computer monitor (refresh rate 100 Hz) and fixated a cross at the center of the screen.
On each trial, stimuli were flashed one at a time in a randomized location within 19.5° of fixation. In 72 randomly interleaved trials, stimulus durations were 10, 20, or 30 ms (fixed within a trial), and the inter-stimulus interval was always equal to the stimulus duration; this yielded presentation rates of 50, 25, and 17 Hz. To ensure that stimuli were not presented in close proximity on successive frames, each stimulus was presented in a different quadrant from the previous presentation.
Trials lasted 1320 ms and ended with a mask of white noise. Participants then used a numberpad to report the number of stimuli perceived to have been present on screen at any one moment of time.
Letters (Figure 1) subtended 2° × 2° of visual angle and were presented at 18.9 cd/m2 on a black background. Photographs (Figure 2) subtended 1.98° × 1.98°. All stimuli appeared within 19.5° × 19.5° centered on fixation. Stimuli were generated using Matlab and the psychophysical toolbox (psychtoolbox.org). Demonstrations and sample code are provided at http://eaglemanlab.net/proliferation.
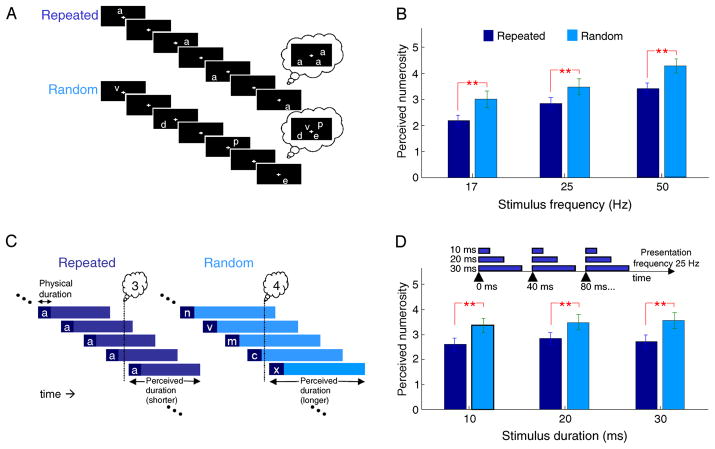
Figure 1.
Repeated stimuli subjectively proliferate less than random stimuli. (A) Example sequences of stimulus presentation and perceived numerosity for repeated and random stimuli. (B) Number of characters perceived to be present for repeated and random stimuli. Participants report more characters present on screen when the stimuli are different than when they are repeated. n = 31. Error bars SEM. Demonstration videos: http://eaglemanlab.net/proliferation. (C) To explain these results, we hypothesize that the visual persistence of a repeated stimulus is contracted compared to a novel stimulus. Even while participants are not asked to make an explicit temporal judgment, the duration distortion changes their interpretation of the visual scene. (D) Duty cycle does not change numerosity estimates. Stimuli were presented for different physical durations while keeping the presentation frequency constant. No significant difference was found in perceived numerosity for different stimulus durations when maintaining a constant stimulus presentation frequency (** indicates p < 0.01; * indicates p < 0.05, paired t-tests). n = 31. Error bars SEM.
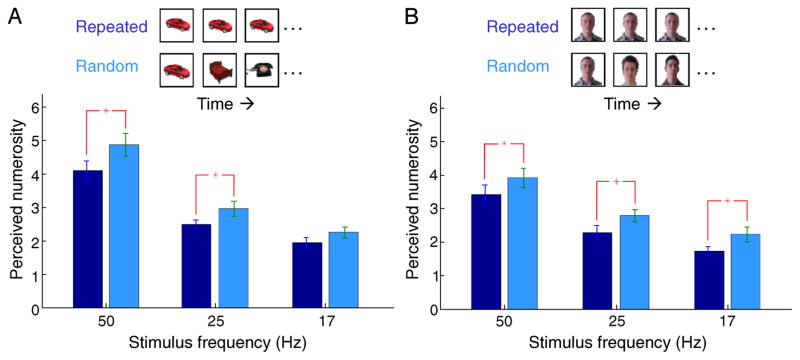
Figure 2.
Decreased numerosity for repeated stimuli generalizes to objects and faces. (A) Participants report that more objects were simultaneously present on screen when the objects were different (random) as compared to repeated (n = 16). (B) More faces were reported to be simultaneously present when the faces were different as compared to repeated (n = 15). * indicates p < 0.05, paired t-test. Error bars SEM. Conditions were identical to the first experiment (Figure 1).
Results
The proliferation effect differs for novel and repeated stimuli
As described in the Methods section, stimuli were rapidly flashed one at a time in different positions on the screen. Perceptually, there appear to be several stimuli simultaneously present because of visual persistence. Visual persistence refers to the phenomenon that a briefly presented stimulus appears to last longer than the time it was physically presented: in general, stimuli < 100 ms in physical duration seem to last for ~100 ms (Bowen, Pola, & Matin, 1974; Di Lollo, 1977; Efron, 1970). Beyond this threshold, stimuli are perceived approximately accurately, i.e., close to their true physical duration. Because of visual persistence, the physically present stimulus is accompanied by the ‘ghosts’ of stimuli that were presented recently. Thus more than one character appears to temporally overlap on screen. We refer to the perceived multiplicity of stimuli as the proliferation effect. We employed two conditions: in the first, the same character was presented (‘Repeated’); in the second, different characters were presented (‘Random’, Figure 1A). Participants reported perceived numerosity, i.e., how many characters appeared to be present on screen at any instant.
Participants’ estimates of how many characters they perceived simultaneously on screen differed significantly between the repeated and random conditions (Figure 1B). At a 50-Hz presentation rate, for example, observers reported an average of 3.4 characters on screen in the ‘repeated’ condition and 4.2 in the ‘random’ condition (p < 10−5, paired t-test; average within-subject standard deviation 0.91 [repeated] and 1.00 [random]). We summarize the different numerosities in the two conditions by calculating a repeat-to-random ratio, which in this case was 0.81. The difference between the two conditions holds across different stimulus frequencies, even while the absolute numerosity declines with lower frequencies.
We interpret these results to mean that repetition contracts the duration of visual persistence, and therefore there is less temporal overlap and a reduced number of stimuli perceived to be simultaneously present (Figure 1C).
To further address whether the proliferation effect is predicated on visual persistence, we turned to the fact that brief stimuli (<100 ms) will be perceptually expanded to ~100 ms, irrespective of their physical durations (e.g., a 10-ms stimulus and a 30-ms stimulus will appear to last the same duration). Thus, in our next experiment, we fixed the presentation frequency at 25 Hz but changed the stimulus duration to 10, 20, or 30 ms (Figure 1D, inset). Changing the duty cycle in this way had no effect on numerosity (Figure 1D), consistent with the hypothesis that the effect results from visual persistence of the stimuli, which makes the 10, 20, and 30 ms presentations perceptually equivalent.
We next calculated the values of visual persistence that could account for the reported numerosities. Since we have no way of knowing whether participants chose their numerosities based on the average number of perceived stimuli, or instead on the maximum number of perceived stimuli, we calculated visual persistence using both methods. The resulting estimates of visual persistence range from 84 to 180 ms (average method) or 61to 121 ms (maximum method) for repeated stimuli and 68 to 132 ms (average method) or 41 to 81 ms (maximum method) for repeated stimuli. A single value for the window of visual persistence does not account for the perceived numerosities at all 3 frequencies in Figure 1B; however, the data translate into visual persistence values consistent with their typically reported range of visual persistence (Di Lollo, 1977). The variability suggests the possibility of additional mechanisms at play in the different presentation frequencies.
The repetition effect generalizes to pictures of objects and faces
To determine whether the differential proliferation effect for repeated and random stimuli generalized beyond characters, we next presented participants with variations of the experiment in which the stimuli consisted of photographs of everyday objects (Figure 2A) or faces (Figure 2B). In the ‘repeated’ conditions, the same image was serially presented; in the ‘random’ conditions, different images were randomly selected (from a bank of 75 objects or 106 faces). Trial types were randomly interleaved. As in the first experiment, participants perceived fewer stimuli on screen when the stimulus was presented repeatedly as compared with random stimuli (Figure 2) with an average repeat-to-random ratio of 0.88 in both cases. Therefore, repetition related duration distortions generalize beyond letters.
As a final control, we considered the possibility that the perception of numerosity is sensitive to similarity or differences among stimulus elements and that our results may have nothing to do with duration. To test this, we presented 7 participants with brief static displays of the same or different stimuli (analogous to the repeated and random conditions). Specifically, 3, 4, or 5 letters were presented simultaneously at random locations of the screen for three possible durations (10, 20, or 30 ms). With such static displays, participants perceived no difference in numerosity between the same and different conditions (p > 0.66, data not shown). Therefore, our results cannot be explained by differences in numerosity perception based on the low-level properties of the stimulus elements.
Discussion
We have shown that the perceived durations of repeated stimuli are briefer than those of novel stimuli, even when participants are not asked to make explicit temporal judgments. These subjective durations have direct impact on the interpretation of the visual scene: participants perceive a different number of stimuli in repeated and random conditions. These perceptual repercussions suggest that distorted durations are assigned on the fly instead of labeled retrospectively.
We had originally considered the possibility that the data in Figure 1 might be explained by timing differences between normal apparent motion and transformational apparent motion (in which an object is perceived to change shape as it moves), but previous studies have found no difference in the strength of apparent motion when using the same or different shapes (Burt & Sperling, 1981; Kolers & von Grünau, 1976; Navon, 1976), thus weighing against such an explanation. Attentional theories posit that an increased allocation of attentional resources results in increased perceived duration. These theories rest on the purported >120 ms required for allocating attention to a stimulus [see Tse et al., 2004]; such an explanation is untenable for the present duration distortions. A third possibility could be that neural fatigue induced by repetitive presentation of a stimulus rendered some of the repeated stimuli invisible; however, this appears unlikely, since repetition only increases detection thresholds from about 2% to 3–4% (Hammett & Snowden, 1995), not nearly enough to push our high-contrast stimuli under the threshold of visibility.
We hypothesize that the perceived duration of a stimulus is dependent on the amplitude of the neural response it engenders (Eagleman, 2008; Pariyadath & Eagleman, 2007). In non-human primates, neuronal firing rates in higher cortical areas quickly diminish in response to repeated presentations of a stimulus (Fahy, Riches, & Brown, 1993; Miller & Desimone, 1994), an effect known as repetition suppression. In humans, these differential responses to familiar and novel stimuli are seen using electroencephalography (Grill-Spector et al., 2006), functional magnetic resonance imaging (Grill-Spector et al., 2006; Henson & Rugg, 2001), positron emission tomography (Buckner et al., 1998), and magnetoencephalography (Ishai, Bikle, & Ungerleider, 2006).
Our findings on the visual persistence of repeated stimuli provide an alternative explanation for previous reports. For example, the smallest interval required for two flashes to be perceived as separate is greater than the smallest interval needed between successive flashes in a train (Herrick, 1974). In other words, when measured in frequency, the two-flash flicker threshold is smaller than the critical flicker fusion threshold. Herrick (1974) appealed to probability summation to explain these results (Herrick, 1974); we make the simpler alternative suggestion that when two flashes are presented one after the other, the visual persistence of the first causes it to overlap temporally with the second (provided that the ISI is less than 100 ms). But when a train of such flashes is presented, the visual persistence of the flashes contracts with repetition and one perceives the train as a series of events. Our hypothesis that the visual persistence of brief stimuli is contracted by repetition also offers a new framework for understanding ‘change-related persistence’: when a moving object undergoes a sudden change it is momentarily perceived as two separate objects (Moore, Mordkoff, & Enns, 2007), presumably because of the increased visual persistence of the novel presentation.
Novelty and efficiency of encoding
The increased numerosity with random stimuli may also account for another observation: random dot kinematograms with lower coherence appear to have higher dot density (A. Tolias, personal communication). We hypothesize that in conditions of high coherence, repetition suppression caused by neural fatigue or by a more efficient encoding (Grill-Spector et al., 2006; Summerfield, Trittschuh, Monti, Mesulam, & Egner, 2008) decreases the visual persistence of the dots. Thus, fewer dots are perceived on screen simultaneously.
The current findings suggest that future electrophysiology experiments might profitably compare neural responses to repeated and random stimuli with concurrent duration (or numerosity) judgments. This would be an inroad to potentially reveal direct correlations between features of the neural response (e.g., onset latency, magnitude, decay characteristics, etc.) and subjective duration.
Finally, these findings suggest a novel visual method to rapidly and non-invasively appraise disorders of repetition suppression in human subjects, such as schizophrenia.
Deficits in repetition suppression in schizophrenia are evidenced by an impaired pre-pulse inhibition of the startle response (Hong et al., 2007; Swerdlow et al., 2006), impaired mismatch negativity (Javitt, Grochowski, Shelley, & Ritter, 1998; Light & Braff, 2005), and abnormal processing of oddball stimuli (Kiehl & Liddle, 2001). Relatedly, schizophrenic patients have a lowered CFFT (Black, Franklin, de Silva, & Wijewickrama, 1975; Saucer & Sweetbaum, 1958) and generally a lower sensitivity for detecting flicker (Slaghuis & Bishop, 2001), presumably because of a non-diminishing visual persistence. Collectively, these findings paint a picture of reduced or absent repetition suppression in schizophrenic patients, presumably resulting from a deficit in cortical inhibition (Daskalakis et al., 2002). Roughly speaking, to a schizophrenic brain, certain types of repeated stimuli will continue to appear novel (Guillem et al., 2001). Consistent with these observations, our preliminary results indicate that schizophrenic patients fail to perceive a differential numerosity for repeated and random stimuli (Gandhi, Pariyadath, Wassef, & Eagleman, 2007). Thus, it is possible that in the future the proliferation effect may be useful as a non-invasive, rapid screening tool for early diagnosis.
Acknowledgments
We thank Andreas Tolias and Chess Stetson for feedback. This work was supported by NIH R01 grant NS053960 (DME).
Footnotes
Commercial relationships: none.
Contributor Information
Vani Pariyadath, Department of Neuroscience, Baylor College of Medicine, Houston, TX, USA.
David M. Eagleman, Department of Neuroscience and Department of Psychiatry, Baylor College of Medicine, Houston, TX, USA
References
- Black S, Franklin LM, de Silva FP, Wijewickrama HS. The flicker-fusion threshold in schizophrenia and depression. The New Zealand Medical Journal. 1975;81:244–246. [PubMed] [Google Scholar]
- Bowen RW, Pola J, Matin L. Visual persistence: Effects of flash luminance, duration and energy. Vision Research. 1974;14:295–303. doi: 10.1016/0042-6989(74)90079-0. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, et al. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Burt P, Sperling G. Time, distance, and feature trade-offs in visual apparent motion. Psychological Review. 1981;88:171–195. [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Archives of General Psychiatry. 2002;59:347–354. doi: 10.1001/archpsyc.59.4.347. [DOI] [PubMed] [Google Scholar]
- Di Lollo V. Temporal characteristics of iconic memory. Nature. 1977;267:241–243. doi: 10.1038/267241a0. [DOI] [PubMed] [Google Scholar]
- Eagleman DM. Human time perception and its illusions. Current Opinion in Neurobiology. 2008;18:131–136. doi: 10.1016/j.conb.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleman DM, Tse PU, Buonomano D, Janssen P, Nobre AC, Holcombe AO. Time and the brain: How subjective time relates to neural time. Journal of Neuroscience. 2005;25:10369–10371. doi: 10.1523/JNEUROSCI.3487-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron R. The minimum duration of a perception. Neurophysiologia. 1970;8:57–63. doi: 10.1016/0028-3932(70)90025-4. [DOI] [PubMed] [Google Scholar]
- Fahy FL, Riches IP, Brown MW. Neuronal activity related to visual recognition memory: Long-term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Experimental Brain Research. 1993;96:457–472. doi: 10.1007/BF00234113. [DOI] [PubMed] [Google Scholar]
- Gandhi SK, Pariyadath V, Wassef AA, Eagleman DM. Timing judgments in schizophrenia. Paper presented at the Society for Neuroscience.2007. [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: Neural models of stimulus-specific effects. Trends in Cognitive Sciences. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Guillem F, Bicu M, Hooper R, Bloom D, Wolf MA, Messier J, et al. Memory impairment in schizophrenia: A study using event-related potentials in implicit and explicit tasks. Psychiatry Research. 2001;104:157–173. doi: 10.1016/s0165-1781(01)00305-5. [DOI] [PubMed] [Google Scholar]
- Hammett ST, Snowden RJ. The effect of contrast adaptation on briefly presented stimuli. Vision Research. 1995;35:1721–1725. doi: 10.1016/0042-6989(94)00283-r. [DOI] [PubMed] [Google Scholar]
- Henson R, Rugg M. Effects of stimulus repetition on latency of the BOLD impulse response. Neuroimage. 2001;13:683. [Google Scholar]
- Herrick RM. Frequency thresholds for two-flash flicker and critical flicker: Why they differ. Perception & Psychophysics. 1974;15:79–82. [Google Scholar]
- Hong LE, Summerfelt A, Wonodi I, Adami H, Buchanan RW, Thaker GK. Independent domains of inhibitory gating in schizophrenia and the effect of stimulus interval. American Journal of Psychiatry. 2007;164:61–65. doi: 10.1176/ajp.2007.164.1.61. [DOI] [PubMed] [Google Scholar]
- Ishai A, Bikle PC, Ungerleider LG. Temporal dynamics of face repetition suppression. Brain Research Bulletin. 2006;70:289–295. doi: 10.1016/j.brainresbull.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Grochowski S, Shelley AM, Ritter W. Impaired mismatch negativity (MMN) generation in schizophrenia as a function of stimulus deviance, probability, and interstimulus/interdeviant interval. Electroencephalography and Clinical Neurophysiology. 1998;108:143–153. doi: 10.1016/s0168-5597(97)00073-7. [DOI] [PubMed] [Google Scholar]
- Kanai R, Paffen CL, Hogendoorn H, Verstraten FA. Time dilation in dynamic visual display. Journal of Vision. 2006;6(12):8, 1421–1430. doi: 10.1167/6.12.8. http://journalofvision.org/6/12/8/ [DOI] [PubMed]
- Kanai R, Watanabe M. Visual onset expands subjective time. Perception & Psychophysics. 2006;68:1113–1123. doi: 10.3758/bf03193714. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF. An event-related functional magnetic resonance imaging study of an auditory oddball task in schizophrenia. Schizophrenia Research. 2001;48:159–171. doi: 10.1016/s0920-9964(00)00117-1. [DOI] [PubMed] [Google Scholar]
- Kolers PA, von Grünau M. Shape and color in apparent motion. Vision Research. 1976;16:329–335. doi: 10.1016/0042-6989(76)90192-9. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Archives of General Psychiatry. 2005;62:127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263:520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- Moore CM, Mordkoff JT, Enns JT. The path of least persistence: Object status mediates visual updating. Vision Research. 2007;47:1624–1630. doi: 10.1016/j.visres.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Ross J, Burr D. Saccadic eye movements cause compression of time as well as space. Nature Neuroscience. 2005;8:950–954. doi: 10.1038/nn1488. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, ten Hoopen G, Hilkhuysen G, Sasaki T. Time-shrinking: A discontinuity in the perception of auditory temporal patterns. Perception & Psychophysics. 1992;51:504–507. doi: 10.3758/bf03211646. [DOI] [PubMed] [Google Scholar]
- Navon D. Irrelevance of figural identity for resolving ambiguities in apparent motion. Journal of Experimental Psychology: Human Perception and Performance. 1976;2:130–138. doi: 10.1037//0096-1523.2.1.130. [DOI] [PubMed] [Google Scholar]
- Pariyadath V, Eagleman DM. The effect of predictability on subjective duration. PLoS ONE. 2007;2:e1264. doi: 10.1371/journal.pone.0001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D, Summers J. Duration illusions in a train of visual stimuli. Perception. 1995;24:1177–1187. doi: 10.1068/p241177. [DOI] [PubMed] [Google Scholar]
- Saucer RT, Sweetbaum H. Perception of the shortest noticeable dark time by schizophrenics. Science. 1958;127:698–699. doi: 10.1126/science.127.3300.698. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL, Bishop AM. Luminance flicker sensitivity in positive- and negative-symptom schizophrenia. Experimental Brain Research. 2001;138:88–99. doi: 10.1007/s002210100683. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Trittschuh EH, Monti JM, Mesulam MM, Egner T. Neural repetition suppression reflects fulfilled perceptual expectations. Nature Neuroscience. 2008;11:1004–1006. doi: 10.1038/nn.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: Relationship to medications, symptoms, neurocognition, and level of function. Archives of General Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Tse PU, Intriligator J, Rivest J, Cavanagh P. Attention and the subjective expansion of time. Perception & Psychophysics. 2004;66:1171–1189. doi: 10.3758/bf03196844. [DOI] [PubMed] [Google Scholar]
- Ulrich R, Nitschke J, Rammsayer T. Perceived duration of expected and unexpected stimuli. Psychological Research. 2006;70:77–87. doi: 10.1007/s00426-004-0195-4. [DOI] [PubMed] [Google Scholar]
- Yarrow K, Haggard P, Heal R, Brown P, Rothwell JC. Illusory perceptions of space and time preserve cross-saccadic perceptual continuity. Nature. 2001;414:302–305. doi: 10.1038/35104551. [DOI] [PubMed] [Google Scholar]