Abstract
Bves was discovered through subtractive screens designed to identify heart-enriched transcripts. Bves is a transmembrane protein that possesses a highly conserved structure among species of the animal kingdom. Various approaches have been employed to elucidate the expression pattern of Bves mRNA and protein as well as its function in developing and mature organisms. Emerging evidence indicates that this protein is present in muscle and epithelia of developing embryos and the adult. In vitro functional studies predict a role in cell-cell interaction and/or adhesion. In vivo analysis of protein function is very limited at present, but recent work in Xenopus supports the importance of Bves in epithelial integrity. Presented in this review is a compilation of published findings concerning Bves gene and protein characteristics, expression patterns in embryos and cells, and functional significance as determined thus far. Presently, the literature supports a hypothesis that Bves is essential to the junctional architecture of muscle and epithelial cell types. Although there remain aspects of Bves structure, expression and function that are not completely resolved, now is an appropriate time to summarize current knowledge about this protein, the remaining questions, and what its potential role in development might be. This review will serve as a departure point for others who become interested in the study of this highly conserved protein.
Keywords: Bves, Popeye, Popdc, muscle, epithelia, cell adhesion, tight junction
Discovery
To identify novel genes expressed during heart development, a subtractive hybridization screen of HH stage 18 chick heart was performed by David Bader and colleagues (Reese et al., 1999). Bves (blood vessel epicardial substance) was a novel message identified through this screen, as reported by Reese et al. (1999). The name Bves was assigned because the first antiserum produced (D033) recognized Bves-positive cells in the epicardium and developing coronary vascular system (discussed below). Using a similar screen, Thomas Brand and colleagues identified a chicken cDNA identical to Bves, termed “Pop1a” (Andree et al., 2000). In the same study, Andree et al. also described a related family of genes in chick and several other species (Andree et al., 2000; Andree et al., 2002b; Hitz et al., 2002; Breher et al., 2004). Because transcripts were identified primarily in heart and skeletal muscle, the name ‘Popeye’ was assigned to the gene family to reflect the robust expression pattern in these muscle types. The Duncan laboratory also independently identified Bves/Pop1a in a screen for genes transcriptionally-regulated during eye development (M. Duncan, personal communication). In order to unify the nomenclature, the gene in mouse and human was subsequently called Bves, and accepted synonyms are Popeye 1 and Popdc1 (Popeye domain-containing). The accepted names for the other two mammalian family members are Popdc2 and Popdc3, respectively (Mouse Genome Informatics, Jackson Labs; HUGO Gene Nomenclature Committee). The gene family is known as the Popdc family.
Gene Structure
Since the initial identification of Bves in chicken, mouse and human, cDNAs representing this gene have been reported in all classes of vertebrates. Homologous Bves sequences have been identified in several invertebrates, including insects Drosophila and Anopheles, through a search for EST clones with internet-based search engines (NCBI, Ensembl). Additionally, related transcripts have been reported in the cephalochordate Amphioxus floridae (Andree et al., 2000), as well as Ciona intestinalis (Davidson and Levine, 2003) and the ascidian Boltenia villosa (Davidson et al., 2003). To date, Bves has not been reported to be in the C. elegans genome, in any single cell organism, or in plant species. The evolution and conservation patterns of the Popdc gene family have been presented (Brand, 2005).
The genomic location and structure of Bves is known for several species (Table 1). Mouse Bves lies on chromosome 10, human Bves has been mapped to chromosome 6q21, and the chicken gene is located on chromosome 3. The cloning of Bves in many organisms has allowed a cross-species comparison of gene structure, which varies considerably. The mouse gene consists of 11 exons, while human Bves has only five exons. The chicken and Drosophila Bves genes have eight and seven exons, respectively (Table 1). Chicken Bves generates a message of approximately 1.7kb, while mouse Bves is 1.8kb (Reese et al., 1999; Andree et al., 2000). A high sequence similarity exists at the nucleotide level (>70%). In addition, the presence of at least four individual transcripts from the chicken Bves gene has been demonstrated, and originally reported as Pop1A-Pop1D (Andree et al., 2000). The splice variants appear to be generated principally in the extreme 5′ and 3′ ends of the coding region (Andree et al., 2000). To date, no links concerning function have been derived from the genomic structure. Currently, none of the promoter or enhancer elements that drive cell-specific expression have been described for Bves or any other Popdc genes, although Bves has been identified as a target of Pax3, a key developmental transcription factor (Barber et al., 2002).
Table 1.
Characteristics of the Popdc family genes and proteins
| Species | Gene | mRNAs | accession # | Gene position |
protein | size (aa) |
Isolated/reported by |
|---|---|---|---|---|---|---|---|
| Bves | Bves | NM_007073 | 6q21 | Bves | 357 | Reese et al. (1999) | |
| H. sapiens | Popdc2 | Popdc2 | NM_022135 | 3q13 | Popdc2 | 364 | Andree et al. (2000) |
| Popdc3 | Popdc3 | NM_022361 | 6q21 | Popdc3 | 291 | " | |
|
| |||||||
| Bves | Bves | AF204174 | 10 | Bves | 358 | Reese et al. (1999) | |
| M. musculus | Popdc2 | Popdc2 | AF204175 | 16 | Popdc2 | 367 | Andree et al. (2000) |
| Popdc3 | Popdc3 | AF204176 | 10 | Popdc3 | 291 | " | |
|
| |||||||
| Bves | Bves | AF208398 | 3 | Bves | 360 | Reese et al. (1999) | |
| " | Pop1b | AF208399 | " | Popdc1b | 288 | Andree et al. (2000) | |
| " | Pop1c | AF208400 | " | Popdc1c | 305 | " | |
| " | Pop1d | AF208401 | " | Popdc1d | 695 | " | |
| Popdc2 | Popdc2a | AY388621 | 1 | Popdc2a | 356 | " | |
| G. gallus | " | Popdc2b | AY388622 | " | Popdc2b | 353 | " |
| " | Popdc2a/b | AY427076 | " | Popdc2a/b | 378 | " | |
| " | Popdc2c | AY388624 | " | Popdc2c | 276 | " | |
| " | Popdc2d | AY388623 | " | Popdc2d | 276 | " | |
| Popdc3 | Popdc3 | AF204170 | 3 | Popdc3 | 305 | " | |
|
| |||||||
| D. melanogaster | Bves | Bves | AF247183 | X | (dm)Bves | 415 | S. Lin et al. (abstract) |
|
| |||||||
| X. laevis | XBves | Bves | AF527799 | ? | XBves | 338 | Ripley et al. (in press) |
|
| |||||||
| D. rerio | Popdc1 | Popdc1 | AY293117 | 13 | Popdc1 | 316 | Brand et al. (abstract) |
| Popdc3 | Popdc3 | AY293116 | 25 | Popdc3 | 298 | " | |
This review focuses primarily on the Bves gene and its product, Bves. However, it is necessary and appropriate to point out similarities and differences between the other family members, Popdc2 and Popdc3, both of which have been identified in mouse, human, and chicken (Andree et al., 2000; Breher et al., 2004). The gene structure, size, and chromosomal location of the Popdc2 and Popdc3 genes have been determined in many species (Table 1). Interestingly, the Popdc3 gene is located on the same chromosome, in close proximity to Bves in chicken, mouse and human, while Popdc2 is found on a different chromosome in all three species. This indicates that the genomic region spanning Bves and Popdc3 is evolutionary conserved from chicken to human. Furthermore, close proximity of Bves and Popdc3 suggests that these two genes may have evolved from gene duplication. However, not all species have multiple Popdc genes. To date, only one transcript has identified in Xenopus (Hitz et al., 2002; Ripley et al., in press), and in insects through EST databases and cDNA cloning (NCBI).
In sum, Popdc genes appear in a broad spectrum of invertebrate and vertebrate species. Due to potential redundancy of Popdc gene family members, genetic analysis of function may proceed more rapidly in organisms that have only one gene, whereas studies in mice may require disruption of two or more of the genes (Andree et al., 2000).
Protein Size and Structure
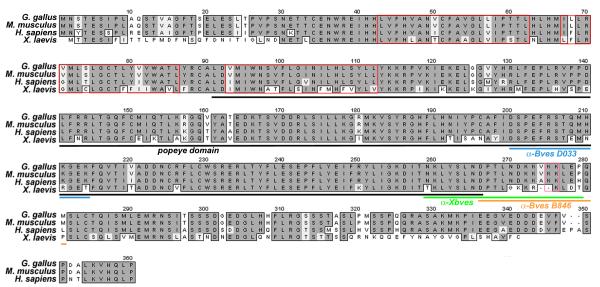
The full-length protein sequence of Bves has been determined in chicken (357 a.a.), mouse (358 a.a.), human (360 a.a.), and frog (338 a.a.) (Figure 1,Table 1). Conservation of Bves protein sequence exists across a wide variety of vertebrate species (~80%; Figure 1). At present, the Bves protein is thought to consist of three transmembrane domains, an extracellular N-terminus (~43 a.a.) with two N-glycosylation sites, and an intracellular C-terminus (~248 a.a.) (Andree et al., 2002b; Knight et al., 2003). A closer examination of sequence similarity reveals that certain regions of the protein exhibit higher degrees of homology across species. For example, while the amino acid sequence of chick and mouse are 75% homologous, the highest degree of homology (92%) lies within the C-terminus. Outside of the regions that encode glycosylation sites, the N-terminal sequence is rather diverse across species (Andree et al., 2000; Figure 1). For vertebrate species, the Popdc2 gene encodes a ~360 a.a. (41kD) protein, and Popdc3 encodes a ~290 a.a. protein, which has a shorter C-terminus (37kD) (Table 1). Like Bves, the other proteins in the Popdc family are highly conserved across species (~80%). However, within a given species, Bves is ~25% homologous to Popdc2 and Popdc3, while Popdc2 and Popdc3 have ~50% identity at the amino acid level. This indicates that Popdc2 and Popdc3 are more closely related to each other than to Bves, which appears to be the outlier of this family. This finding is surprising in light of the arrangement of the three family members in the genome (see Table 1).
Figure 1. Alignment of vertebrate Bves sequences.
Bves is highly conserved across species. Transmembrane/hydrophobic sequences are contained by red boxes. The Popeye domain is denoted (black line). Sequences to which antibodies were generated are indicated by blue (D033), green (XBves) and orange (B846) lines. An additional monoclonal antibody generated by DiAngelo et al. (2001) was raised against amino acids 91-358 of the chick sequence. A pair of lysine residues within the C-terminus, denoted by a pink box, has been shown to be critical for epithelial integrity.
Immunochemical detection of Bves in heart tissue and transfected cells yields one band ranging between ~42 and 58kD under reducing and denaturing conditions (Reese et al., 1999; Andree et al., 2000; DiAngelo et al., 2001; Knight et al., 2003; Osler and Bader, 2004; Vasavada et al., 2004). The size variation likely results from posttranslational modifications of the protein, including N-linked glycosylation (Knight et al., 2003). Early computer-based modeling predicted that Bves contains three hydrophobic domains. Presence of these hydrophobic domains was confirmed and shown to be essential for membrane insertion/retention, as demonstrated by in vitro transcription/translation reactions in the presence of microsomes (Wada et al., 2001). While Wada et al. (2001) originally reported that the C-terminus of Bves was extracellular, subsequent immunocytochemical and biochemical data suggest that the short N-terminus (36-39 a.a.) is extracellular and the longer C-terminus is intracellular (Knight et al., 2003).
The C-terminal intracellular portion of the protein is a likely candidate region for interactions with other proteins and, thus, its functional dissection has been a primary focus. However, the C-terminus of Bves lacks known motifs, such as a PDZ, SH3, leucine zippers, or other protein-protein interaction domains that would suggest a function. Interestingly, the highly conserved Popeye domain (a.a. 172-266) within the C-terminus has brought about the renaming of the Popeye family to Popdc (Popeye-domain containing), although no function has been assigned to this domain (NCBI, pfam04831). Since recognizable domains are seemingly absent, identification of binding partners and the eventual definition of their interaction domains within Bves may lead us to an understanding of molecular function and the involvement in known signaling pathways. A search for interacting proteins has been initiated in our laboratory using immunoprecipitation, protein pull-downs, and yeast two-hybrid analyses. A recent study identifies the tight junction (TJ) component ZO-1 (discussed below) as an interacting protein, and is the first work to tie Bves to known molecular regulators (Osler et al., 2005). However, this is certainly only the beginning of such experiments.
Expression of Bves during development
Bves expression has been analyzed at the RNA and protein level in the mouse, chick, and frog during various developmental stages (Reese et al., 1999; Andree et al., 2000; DiAngelo et al., 2001; Wada et al., 2001; Andree et al., 2002b; Hitz et al., 2002; Osler and Bader, 2004; Ripley et al., 2004; Vasavada et al., 2004; Ripley et al., in press). Interestingly, expression analysis using in situ hybridization or antibody detection did not always lead to congruent results. Instead, some of the Bves expression data appear difficult to reconcile. One major point of contention has been the question whether Bves is expressed in the epicardium of the heart, and more generally, in epithelial cells. In this review, we aim to provide a comprehensive overview on Bves expression in embryonic and postnatal tissues according to published reports. We will report consistent expression results but also point out conflicting findings.
Published literature agrees that Bves is clearly expressed in muscle cell types. Reese et al. (1999) and Andree et al. (2000) originally identified the Bves transcript in a screen for gene expression in the developing chick heart. Without doubt, in situ hybridization, Northern blot, RTPCR, and lacZ knock-in experiments have demonstrated that Bves is highly expressed in muscle cells of the embryonic heart in all vertebrates examined thus far (Reese et al., 1999; Andree et al., 2000; Andree et al., 2002a; Andree et al., 2002b; Hitz et al., 2002; Ripley et al., in press ; Table 2). Immunocytochemical studies have confirmed this robust cardiac muscle expression. Using the B846 polyclonal antiserum (Wada et al., 2001; Osler and Bader, 2004; Ripley et al., 2004; Figure 2A), the XBves polyclonal antiserum (Ripley et al., in press; Figure 2B), the 3F11 monoclonal antiserum (DiAngelo et al., 2001; Vasavada et al., 2004), and the SB1 monoclonal antiserum (Smith and Bader, in review), Bves has been detected in the developing myocardium of embryos and in the adult heart. Expanded analysis of both mRNA and protein expression have detected Bves in skeletal muscle of chick, mouse and frog (Andree et al., 2000; Andree et al., 2002a; Ripley et al., in press). It should be noted that Hitz et al. (2002) report no Bves expression in the skeletal muscle of Xenopus embryos by in situ hybridization. The reason for this conflicting result is presently not known. Interestingly, Swalla and colleagues found an ascidian Popdc gene to be expressed in cells of the primordial heart and tail muscle lineages, suggesting a conserved function for Bves in striated muscle across species (Davidson et al., 2003). In toto, these published studies permit the conclusion that Bves is expressed in all striated muscle continuously from development through adulthood.
Table 2. Bves expression in muscle cell types in vertebrates.
Expression data are reported as interpreted by published literature. Sources are numbered in order of publication date. Corresponding numbers are used to indicate expression findings in chart form based on technique. A consensus of expression is presented as positive, negative or both, in the case where conflicting reports exist. Reference numbers with a strikethrough indicate that expression was analyzed in the noted report and not observed. A question mark indicates that the expression has not been tested and/or determined. Species are abbreviated as C (chick), M (mouse), H (human), X (frog). Not applicable (n/a).
| Muscle type | cardiac | skeletal | vascular smooth |
extra-cardiac smooth |
primary myocytes |
Species, Reported by |
|---|---|---|---|---|---|---|
| mRNA detection | + | + | − | + | + |
|
| in situ | 1-3,5,13 | 1-3,5,13 | ? | 5 | n/a | |
| RT-PCR | 1-3,5,9 | 2,3,5,7,11,12 | ? | 3 | ? | |
| Northern blot | 1-3,11 | 1-3,11 | ? | 3 | ? | |
| lacZ embryos | 5,6 | 5,6 | 5,6 | 5,6 | ||
| Protein detection | + | + | +/− | + | + | |
| D033 pAb | 1 | 9 | 1,4 | ? | n/a | |
| B846 pAb | 9,14 | 15 | 4,14 | 14 | 15 | |
| XBves pAb | 13 | 13 | ? | 14 | n/a | |
| 3F11-D9-E8 mAb | 8,10 | 10,12 | ? | 10 | ||
| SB1 mAb | 15 | 15 | 15 | ? | 15 |
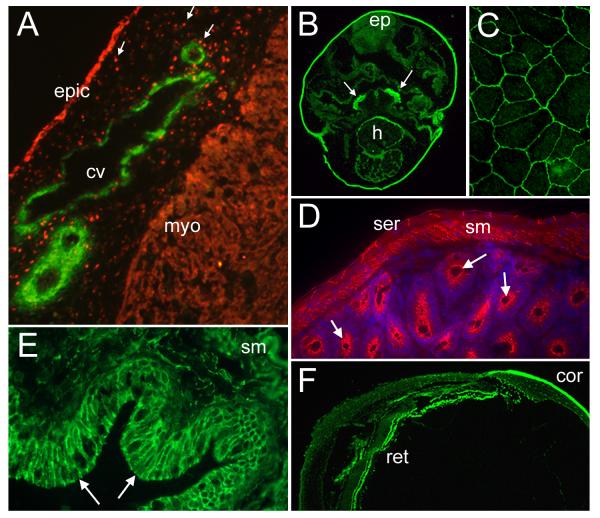
Figure 2. Bves expression in muscle and epithelia.
A. B846 polyclonal immunoreagent detects Bves (red) in the epithelial epicardium (epic), delaminated migratory cells (arrows), and myocardium (myo) in a section through a developing chick heart. Smooth muscle actin (green) labels forming coronary vessels (cv) in the subepicardial space. B. Five-day frog embryo labeled with α-XBves (green). Positive cells are found in the epidermis (epi), the developing heart (h), and the velar plate (arrows). C. Bves is distributed at the cell circumference in cultured human corneal epithelial cells, as labeled by B846 polyclonal antisera. D. Bves is detected by α-Bves B846 (red) in the serosa (ser), the smooth muscle (sm), and the gastric epithelium (arrows) of the adult mouse intestine. DAPI labels the cell nuclei (blue). E. α-XBves detects epithelium (arrows) and the smooth muscle (sm) cells of the adult Xenopus gut (green). F. Xenopus Bves is recognized by α-XBves in the epithelial layers of the adult frog eye, including the cornea (cor) and retina (ret).
EST database searches suggest that the message also exists in non-striated muscle sources. Using Northern blot and RT-PCR analyses, the Bves transcript was identified in other organs such as the brain, kidney, stomach, lung, spleen, and the uterus of a pregnant mouse, (Andree et al., 2000; Table 2). Based on these data and the results described above, Brand and co-workers hypothesized that Bves is expressed within the smooth muscle of these organs. However, Northern blot and RT-PCR analyses of organs or tissues do not allow the identification of the specific cell types expressing a certain transcript, presenting a disadvantage of these techniques for detailed expression studies at the cellular level. Using immunocytochemistry, Bves protein has been detected in the smooth muscle surrounding the coronary vessels and in gastric visceral smooth muscle (Reese and Bader, 1999; Wada et al., 2001; Figure 2D,E), but also in epithelia, as discussed below. Analysis of Bves lacZ knock-in mice further suggested Bves expression exists in the smooth muscle of the gut tube, notochord, and neural tube (Andree et al., 2002b; Table 2). While absolute resolution has not been reached concerning vascular smooth muscle expression, literature agrees that Bves is present in several smooth muscle cell types throughout the embryo.
Furthermore, primary and immortal myocyte cell lines have been employed to analyze Bves expression and localization in a cell culture system. Both Brand and Bader laboratories have demonstrated that Bves protein is expressed in and localizes to the cell contacts of adjoining muscle cells (C2C12 myoblasts and primary cardiomyocytes) (Brand, 2005; Smith and Bader, in review). Overall, the published work pertaining to Bves expression in muscle indicates that Bves mRNA and protein are clearly observed in cardiac, skeletal, and smooth muscle types. Current findings are summarized in Table 2.
A strong line of evidence now exists that Bves is expressed in epithelial cell types, in addition to expression in various striated and smooth muscle cells. Since Bves had originally been isolated from a screen for heart-specific genes (Reese et al., 1999; Andree et al., 2000), the expression pattern was consequently expected to be restricted to the cardiac progenitors and the myocardium. Thus, the initial investigations concentrated only on heart development, with a broadened focus on muscle development upon detection of the gene/protein in skeletal and smooth muscle. Consequently, studies of Bves expression were extended to embryogenesis as a whole. Following comprehensive mRNA and protein analysis in many species, Bves was found to be expressed in a large variety of epithelial tissues in addition to muscle (Wada et al., 2001; Osler and Bader, 2004; Ripley et al., 2004; Vasavada et al., 2004; Osler et al., 2005; Ripley et al., in press; Smith and Bader, in review; Table 3).
Table 3. Bves expression in epithelial cell types in vertebrates.
Expression data are reported as interpreted by published literature. Sources are numbered in order of publication date. Corresponding numbers are used to indicate expression findings in chart form based on technique. A consensus of expression is presented as positive, negative or both, in the case where conflicting reports exist. Reference numbers with a strikethrough indicate that expression was analyzed in the noted report and not observed. A question mark indicates that the expression has not been tested and/or determined. Species are abbreviated as C (chick), M (mouse), H (human), X (frog). Not applicable (n/a).
| Epithelial type | epicardium | gut | gastrula | eye | epidermis | early somite |
cell lines |
Species, Reported by: |
|---|---|---|---|---|---|---|---|---|
| mRNA detection | − | + | + | + | + | + | + |
|
| in situ | ? | 13 | 13 | 13 | 13 | n/a | ||
| RT-PCR | 8 | 8 | 7 | 8 | ? | 8 | ||
| lacZ embryos | ? | ? | ? | ? | ? | n/a | ||
| Protein detection | +/− | + | + | + | + | + | + | |
| D033 pAb | 1,3,8 | ? | 8 | ? | 8 | 8 | ? | |
| B846 pAb | 3,8,14 | 8 | 8 | 7 | 8 | 8 | 3,7,8,11 | |
| XBves pAb | ? | 14 | 13 | 13 | 13 | ? | 13 | |
| 3F11-D9-E8 mAb | 9 | ? | ? | ? | ? | ? | ? | |
| SB1 mAb | ? | 15 | ? | ? | 15 | 15 | 15 |
While existing data does not suggest whether Bves is restricted to a particular subset of epithelia, the protein is expressed in all three germ layers of the developing embryo, and in many epithelial structures during morphogenesis and in the adult (Osler and Bader, 2004; Ripley et al., 2004; Osler et al., 2005; Ripley et al., in press; Smith and Bader, in review). The first clue that Bves could be a significant epithelial component arose with the identification of expression in the chick proepicardium and its derivative, the epicardium. (Figure 2A; Table 3). Thus far, Bves expression in the epicardium has been detected using several monoclonal and polyclonal immunoreagents, in chick and mouse embryos (Reese et al., 1999; Wada et al., 2001; Osler and Bader, 2004; Vasavada et al., 2004). However, Bves expression was reported to be of transient nature in one particular analysis using a monoclonal α-Bves antibody (Vasavada et al., 2004). These data point out that the persistence/continuity of Bves expression in the epicardium is not resolved at present. Furthermore, all reports of epicardial expression come from studies in the chick embryo and it remains unclear whether this observation could be species-specific.
In addition to the epicardial epithelium, Bves is detected in a variety of other epithelia, including the gut epithelium and the serosa, epithelia of the respiratory system, the epidermis, the eye and the ependyma (Osler and Bader, 2004; Ripley et al., 2004; Ripley et al., in press; Smith and Bader, in review; Figure 2). Of these organs, the eye expression pattern is of particular interest since Duncan and colleagues identified the Bves transcript from a screen for genes essential for eye development (Duncan, personal communication). Importantly, proper eye development results from appropriate orchestration of signals between the primordial retina, lens and cornea, all of which are epithelial in nature. Unpublished work in Xenopus underscores the importance of Bves in epithelial morphogenesis of the eye, as development is impaired following XBves depletion (Osler, unpublished data). Notably, the Bves transcript has also been detected by RT-PCR in early embryonic stages in the chick and frog, prior to differentiation of the heart and skeletal muscle (Osler and Bader, 2004; Ripley et al., 2004; Ripley et al., in press). This supported the idea that Bves must be an epithelial component, since the cell layers of early, gastrulating embryos are epithelial in nature, and lack muscle gene-expressing cells. Finally, new monoclonal antibodies (SB1) (Smith and Bader, in review) directed against mouse Bves protein have been recently generated. These antibodies avidly recognize protein expression in epithelia, thus confirming previously published studies.
Epithelial cell lines have permitted the analysis of Bves expression in a controlled culture system. Our group demonstrated a conserved presence in clonal epithelial cell lines of endodermal, mesodermal and ectodermal origin, as predicted by expression in embryos (Wada et al., 2001; Osler and Bader, 2004; Ripley et al., in press). Antibodies (B846, D033, XBves, SB1) recognize Bves in various epithelia and leave little doubt that this protein is present in epithelia (Figure 2; Table 3). Although a consensus has not been reached regarding the epicardial expression of Bves, published literature undoubtedly supports an epithelial identity for Bves.
Function
Currently, the most important issue facing groups interested in Bves is ascertaining its function in the developing and adult organism. This task has been complicated by the lack of identifiable protein motifs and a potential redundancy of function between members of the Popdc gene family in coelomates. By necessity, initial experiments conducted to determine function were broad in nature. Thomas Brand and colleagues published the first report of a Bves-null mouse (Andree et al., 2000). These animals displayed no overt embryonic phenotype (Andree et al., 2002a), presumably due to redundant functions of Bves with Popdc2 and 3. However, the adult mice showed a delay in skeletal muscle regeneration in vivo following cardiotoxin injection. Attempts to determine the developmental function using genetics in the mouse await generation of an animal where all Popdc genes are inactivated. Genetic analyses in Drosophila and zebrafish are also attractive, as only one Popdc family gene is present in Drosophila, while the number is currently unresolved in zebrafish. Recently, Bader and colleagues addressed function in vivo by depleting the X. laevis Bves homolog in developing frog embryos (Ripley et al., in press). In this study, Ripley and Osler et al. show that global depletion of XBves by α-XBves morpholino oligonucleotides (Gene Tools, Inc.) causes a gastrulation block, while clonal depletion of XBves results in rogue movements by the progeny of the injected cell. These findings are the first to identify an in vivo function in embryos and underscore the essential nature of this protein in large-scale epithelial rearrangements that occur during early development (Ripley et al., in press).
In the absence of genetic systems, significant advances in examination of Bves function have developed from in vitro studies using cell culture models (Wada et al., 2001; Ripley et al., 2004; Osler et al., 2005). Early findings support a role in cell-cell adhesion/cell-cell interaction. Experiments using fibroblastic L-cell hanging-drop aggregation assays support an adhesive function for Bves. These standard adhesion assays demonstrated that transfection of chicken, Xenopus, or Drosophila Bves resulted in increases in cell-cell adhesion in these normally non-adherent cells (Wada et al., 2001; Ripley et al., in press; Wada and Bader, unpublished data). Whether Bves heterophilically interacts with related Popdc family members is currently unknown.
Published work from the Brand and Bader groups points to Bves-Bves homophilic interaction in both N- and C-termini (Wada et al., 2001; Andree et al., 2002b) and supports the results of L-cell adhesion assays. Vasavada et al. have also demonstrated that Bves in its native conformation forms dimers (2004). Additional work from the Backstrom laboratory has indicated that intermolecular disulphide bonding plays a role in Bves-Bves interactions (Knight et al., 2003). Thus, it is plausible that oligomeric forms of Bves participate in cell-cell adhesion at some level. Furthermore, Bader and colleagues have recently identified lysine residues within the C-terminus of Bves that are critical to the function of epithelial cells (Kawaguchi et al., in review; Figure 1). Clearly, evidence exists from a variety of experimental methods that Bves is important for cell-cell interaction/adhesion.
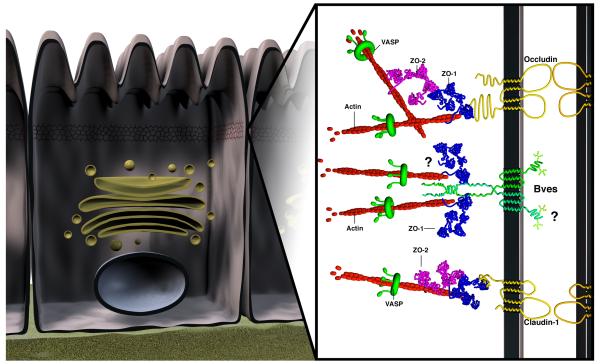
Cultured cardiac myocytes and epithelial cells express Bves at points of cell-cell contact, reminiscent of proteins involved in cell adhesion (Wada et al., 2001; Brand, 2005; Osler et al., 2005; Smith and Bader, in review). In confluent epithelial cells, Bves surrounds the cell border and significantly colocalizes with TJ proteins Occludin and ZO-1 (Osler et al., 2005). Clearly, investigation of Bves interaction with other proteins is a critical avenue of exploration for determination of Bves function. Thus, with this finding, GST pull-down analysis, an interaction was demonstrated between the intracellular C-terminus and ZO-1 (Osler et al., 2005). ZO-1 is a scaffolding protein that interacts with a multitude of tight, adherens and gap junction proteins (Itoh et al., 1993; Fanning et al., 1998; Itoh et al., 1999; Barker et al., 2001). The nature of this interaction nor the precise domain of Bves that interacts with ZO-1 has been determined. While studies have determined that a pool of extra-junctional Bves does exist (Smith, unpublished data), a model depicting Bves at the TJ has been proposed (Figure 3).
Figure 3. Bves interaction at the tight junction.
A schematic representation depicts Bves at the intercellular tight junction (TJ) of polarized epithelial cells. The TJ acts as a paracellular diffusion barrier but also functions regulating processes such as proliferation and differentiation. The TJ consists of several integral membrane proteins including occludin and claudins that bind the actin network via peripheral membrane components, such as ZO proteins. It remains possible that, through ZO-1, Bves also interacts with the actin cytoskeleton to maintain TJ integrity. The proteins and protein interactions shown here do not encompass all that exist at the TJ. The adherens junction, desmosome, and gap junction (not shown) lie subadjacent to the TJ within the lateral membrane.
Consequently, the localization and interaction at the TJ led to a functional assessment of TJ integrity in Bves-depleted human corneal epithelial cells. Knockdown of Bves in epithelial cells leads to a disruption of epithelial sheet integrity, a concomitant loss of transepithelial resistance, and displacement of ZO-1 from the TJ domain, further suggesting an important interaction between Bves and the TJ (Osler et al., 2005). Furthermore, similar treatment of these cells induces increased rates of migration in a wound-healing assay (Ripley et al., 2004), suggesting cells with reduced Bves function are more motile due to a decrease in the adhesive/interactive nature of the cells. It is presently unclear how Bves interaction at the TJ relates to Bves homophilic binding and further studies will be required to unravel this relationship. Together, published studies in epithelial cells support a putative function for Bves in cell interaction/epithelial integrity and regulation of the TJ.
In support of the above findings on cell-cell adhesion/interaction, Andree et al. (2002) postulated that retardation of skeletal muscle regeneration in Bves-null mice may be the result of a decrease in myocyte cell-cell interaction. Obviously, resolution of the role of Bves in the muscle is a major question for ongoing and future research. Likely, parallel functions exist in muscle and epithelial cells and thus, examining Bves in both systems will almost certainly be the most resourceful approach to elucidating the ultimate importance of Bves in development and adult organisms.
Conclusions
We present a summary of the current state of the field and possible future directions. At present, the distribution of Bves in developing and adult tissue is nearly resolved. The techniques and tools available to study Bves have indeed given a clear picture of expression such that the field can now progress to aspects of more critical importance, such as the determination of embryonic function. Together, studies from the literature suggest an important developmental role in both muscle and epithelia during critical phases of morphogenesis.
Still, some divergence of RNA and protein detection persists, namely in embryonic epithelial structures such as the epicardium. This may arise from the variable sensitivity of the methods used to detect message and protein. Because adhesion in epithelia is a defining characteristic, it is possible that Bves is most essential during the developmental stages of heart development when EMT is occurring. Bves may regulate proper cell-cell interaction of epicardium during this window of development, which may account for the transient expression observed by Vasavada et al. (2003). If epicardial expression is downregulated and/or lost after this critical stage, the message and protein may be undetectable, which could contribute to the discrepancies observed in the literature (see Table 3). Meticulous analysis of lacZ animals and further studies with new reagents (Smith, in review) will likely resolve this issue.
Even with residual expression differences, investigation of Bves function commenced. Thus far, studies in genetic mouse models and cell lines demonstrate a functional significance for Bves in muscle and epithelia and support our general hypothesis that this protein family plays a regulative role in cell/cell adhesion and/or interaction. This work has generated a foundation for future investigation, which will be geared toward unraveling the molecular mechanism of Bves during development and in the adult. Although a potential redundancy of Popdc family members has complicated strategies to generate knockout mice, the Brand group continues their efforts to identify roles for these proteins (T. Brand, personal communication). Knockout and transgenic strategies will be essential in targeting Bves function during development. Organisms with only one Popdc gene, such as Drosophila, will undoubtedly be instrumental in determining the role of Bves in development. Still, in vitro cell culture models will continue to be important to more easily ask and/or verify questions concerning function.
Furthermore, while functional data suggest that the Bves/ZO-1 interaction is significant, our knowledge of the nature of this interaction is in its infancy. Discovery of additional Bves interaction partners may not only help us to understand the Bves/TJ relationship but also link Bves to other known molecular pathways. Future studies are necessary to unravel the precise role of Bves and family members at epithelial junctional complexes and in muscle. However, only one activity for Bves has been proposed and it is highly probable that other functions for Bves exist, potentially in intracellular signaling or even in transcription. Contributions from all laboratories interested in Bves have begun to piece the molecular puzzle of this novel protein family together, but clearly, a complete understanding of the regulation of these proteins is far from complete.
Acknowledgments
Grant information: NIH PO1 HL-76105, AHA 0415184B, AHA 0415252B
References
- Andree B, Fleige A, Arnold HH, Brand T. Mouse Pop1 is required for muscle regeneration in adult skeletal muscle. Mol Cell Biol. 2002a;22:1504–1512. doi: 10.1128/mcb.22.5.1504-1512.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andree B, Fleige A, Hillemann T, Arnold HH, Kessler-Icekson G, Brand T. Molecular and functional analysis of Popeye genes: A novel family of transmembrane proteins preferentially expressed in heart and skeletal muscle. Exp Clin Cardiol. 2002b;7:99–103. [PMC free article] [PubMed] [Google Scholar]
- Andree B, Hillemann T, Kessler-Icekson G, Schmitt-John T, Jockusch H, Arnold HH, Brand T. Isolation and characterization of the novel popeye gene family expressed in skeletal muscle and heart. Dev Biol. 2000;223:371–382. doi: 10.1006/dbio.2000.9751. [DOI] [PubMed] [Google Scholar]
- Barber TD, Barber MC, Tomescu O, Barr FG, Ruben S, Friedman TB. Identification of target genes regulated by PAX3 and PAX3-FKHR in embryogenesis and alveolar rhabdomyosarcoma. Genomics. 2002;79:278–284. doi: 10.1006/geno.2002.6703. [DOI] [PubMed] [Google Scholar]
- Barker RJ, Price RL, Gourdie RG. Increased co-localization of connexin43 and ZO-1 in dissociated adult myocytes. Cell Commun Adhes. 2001;8:205–208. doi: 10.3109/15419060109080724. [DOI] [PubMed] [Google Scholar]
- Brand T. The Popeye domain-containing gene family. Cell Biochem Biophys. 2005;43:95–103. doi: 10.1385/CBB:43:1:095. [DOI] [PubMed] [Google Scholar]
- Breher SS, Mavridou E, Brenneis C, Froese A, Arnold HH, Brand T. Popeye domain containing gene 2 (Popdc2) is a myocyte-specific differentiation marker during chick heart development. Dev Dyn. 2004;229:695–702. doi: 10.1002/dvdy.20015. [DOI] [PubMed] [Google Scholar]
- Davidson B, Levine M. Evolutionary origins of the vertebrate heart: Specification of the cardiac lineage in Ciona intestinalis. Proc Natl Acad Sci U S A. 2003;100:11469–11473. doi: 10.1073/pnas.1634991100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B, Wallace SE Smith, Howsmon RA, Swalla BJ. A morphological and genetic characterization of metamorphosis in the ascidian Boltenia villosa. Dev Genes Evol. 2003;213:601–611. doi: 10.1007/s00427-003-0363-3. [DOI] [PubMed] [Google Scholar]
- DiAngelo JR, Vasavada TK, Cain W, Duncan MK. Production of monoclonal antibodies against chicken Pop1 (BVES) Hybrid Hybridomics. 2001;20:377–381. doi: 10.1089/15368590152740789. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Hitz MP, Pandur P, Brand T, Kuhl M. Cardiac specific expression of Xenopus Popeye-1. Mech Dev. 2002;115:123–126. doi: 10.1016/s0925-4773(02)00085-0. [DOI] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M, Wada AM, Presley S, Chang MS, Koyama T, Bader DM. Identification of a domain important for Bves homophillic interaction. in review.
- Knight RF, Bader DM, Backstrom JR. Membrane topology of Bves/Pop1A, a cell adhesion molecule that displays dynamic changes in cellular distribution during development. J Biol Chem. 2003;278:32872–32879. doi: 10.1074/jbc.M301961200. [DOI] [PubMed] [Google Scholar]
- Osler ME, Bader DM. Bves expression during avian embryogenesis. Dev Dyn. 2004;229:658–667. doi: 10.1002/dvdy.10490. [DOI] [PubMed] [Google Scholar]
- Osler ME, Chang MS, Bader DM. Bves modulates epithelial integrity through an interaction at the tight junction. J Cell Sci. 2005 doi: 10.1242/jcs.02588. (In press) [DOI] [PubMed] [Google Scholar]
- Reese DE, Bader DM. Cloning and expression of hbves, a novel and highly conserved mRNA expressed in the developing and adult heart and skeletal muscle in the human. Mamm Genome. 1999;10:913–915. doi: 10.1007/s003359901113. [DOI] [PubMed] [Google Scholar]
- Reese DE, Zavaljevski M, Streiff NL, Bader D. bves: A novel gene expressed during coronary blood vessel development. Dev Biol. 1999;209:159–171. doi: 10.1006/dbio.1999.9246. [DOI] [PubMed] [Google Scholar]
- Ripley AN, Chang MS, Bader DM. Bves is expressed in the epithelial components of the retina, lens, and cornea. Invest Ophthalmol Vis Sci. 2004;45:2475–2483. doi: 10.1167/iovs.04-0013. [DOI] [PubMed] [Google Scholar]
- Ripley AN, Osler ME, Wright CVE, Bader DM. Xbves is a regulator of epithelial movement during early Xenopus laevis development. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.0506095103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Bader DM. Bves expression during mouse embryogenesis. in review.
- Vasavada TK, DiAngelo JR, Duncan MK. Developmental expression of Pop1/Bves. J Histochem Cytochem. 2004;52:371–377. doi: 10.1177/002215540405200308. [DOI] [PubMed] [Google Scholar]
- Wada AM, Reese DE, Bader DM. Bves: prototype of a new class of cell adhesion molecules expressed during coronary artery development. Development. 2001;128:2085–2093. doi: 10.1242/dev.128.11.2085. [DOI] [PubMed] [Google Scholar]