Inclusion and exclusion criteria for participation in clinical trials are meant to select patients who are appropriate for the disease under study and to protect clinical research subject-participants from undue risk related to the research. The selection criteria should not arbitrarily exclude subjects. In 1994, the National Institutes of Health (NIH) published guidelines on the inclusion of women and minorities in clinical trials. The guidelines were intended to ensure that women and members of minorities are included in all human subject research and specified that concerns for cost could not be viewed as an acceptable reason for excluding these groups. It is thus incumbent on researchers to ensure that undue barriers are not placed on groups of individuals that would cause their underrepresentation in publicly funded clinical research. Toward meeting these goals, the guidelines called for the initiation of programs and support for outreach efforts to recruit underrepresented groups into clinical studies. Although these efforts have led to the increased participation in some instances of previously excluded groups onto clinical trials, there may be circumstances where eligibility criteria can exclude participation of certain groups disproportionately.1–4
Rigid requirement for minimal WBC numbers may be one such criterion deserving of further consideration in this regard. Benign ethnic neutropenia (BEN) is a condition used to describe individuals of African descent with neutrophil counts less than 1.5 × 109 cells/L in the absence of other causes5 and constitutes one such group for whom minimal WBC numbers may arbitrarily exclude from participation in clinical trials. The risk of febrile neutropenia is determined by the duration and depth of neutropenia, with the risk greatest when the neutrophils are below 0.5 × 109 cells/L.6–8 There are currently insufficient data to indicate that individuals with BEN would face a higher risk of febrile neutropenia greater than those without BEN. Therefore, BEN may unduly exclude clinical trials participation in a greater proportion of individuals from certain ethnic groups compared with others. It follows that exclusion of individuals with BEN may be at odds with NIH guidelines aimed toward enhancing minority accrual onto clinical trials. Interpretation of the NIH guidelines would suggest that efforts to implement reasonable measures to ameliorate theoretical risks would be preferable to categoric exclusion of patients who are excluded on the basis of BEN. Consideration of the neutrophil physiology, the definition of neutropenia, and BEN (including the epidemiology, clinical correlates, and neutrophil response to various stimulating agents) may be useful toward developing rational and flexible evaluation of individuals with BEN with a view toward including them in clinical trials where there is no excess risk.
NEUTROPHIL PHYSIOLOGY
The absolute number of circulating neutrophils as measured in routine blood tests reflects a small number of the available pool of biologically active cells. Approximately half of the biologic pool of neutrophils outside the marrow are circulating; the other half marginate to vascular endothelium.9 Additionally, there is a large reserve of mature neutrophils in marrow before exiting into blood circulation. Because circulating neutrophils comprise approximately 4.5% of mature marrow neutrophils and 1.7% total marrow granulocytes, minor reduction in peripheral-blood neutrophil counts should constitute no increased risk of infection.10–12
HISTORY AND DEFINITION OF NEUTROPENIA
The definition of neutropenia is categorically based and represents a range that is arbitrarily considered subnormal. It is typically defined by an absolute neutrophil count (ANC) less than 1.5 × 109 cells/L. This cutoff value was popularized by the use of the National Cancer Institute (NCI) Common Toxicology Criteria, which was first developed in 1982. It was standardized by clinical trial investigators in Canada, Europe, Japan, and the United States in 1999 and revised to the current version of Common Terminology Criteria for Adverse Events (version 3.0) in 2006 by the NCI Cancer Therapy Evaluation Program. A further revision to Common Terminology Criteria for Adverse Events, which will make the new version consistent with the recommendations of the International Conference on Harmonization, is currently underway. An ANC of less than 1.5 × 109 cells/L defines the upper limit of grade 2 toxicity and has become the minimum threshold at which to begin therapy in many clinical trials. This is based in part on the risk of inducing a prolonged state of neutropenia after administering myelosuppressive cytotoxic chemotherapy in this setting. It does not take into account neutrophil biology and may not be relevant to BEN.
BEN
To consider whether an individual with low neutrophils has BEN, it is important to be able to distinguish it from several distinct clinical forms of neutropenia. BEN is clinically different from congenital neutropenia (also known as severe congenital neutropenia or Kostmann syndrome), cyclic neutropenia, and chronic idiopathic neutropenia. All these disorders are diagnosed mostly in whites, are exhibited by very low neutrophil count (less than 0.5 × 109 cells/L), and lead to frequent oral, cutaneous, or systemic infections, which are serious and potentially life-threatening. These latter disorders should not be confused with BEN. Other concomitant inciting events causing secondary neutropenia include pharmacologically induced suppression, comorbid infections, neoplastic disease, autoimmune diseases, metabolic disturbances, or hematologic disorders.
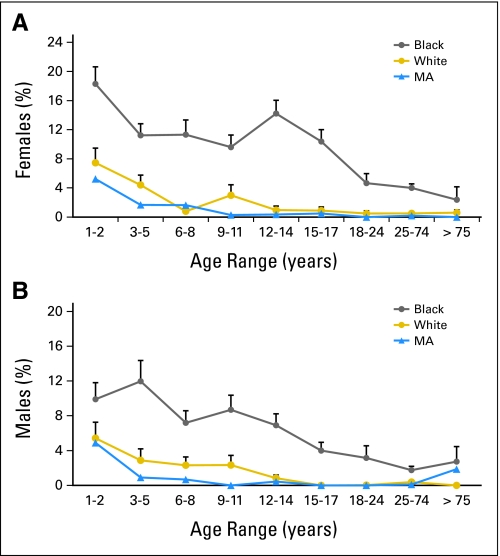
BEN, in contrast, is more commonly observed in individuals of African descent (Fig 1), although there are case reports of benign neutropenia in whites.13–16 Outside of the United States, BEN has been described in up to 25% to 40% of those of African descent.17–19 In the United States, prevalence decreases with age and is much lower: 4% in adult African American men and 2% to 3% in adult African American women, compared with less than 1% in whites.5 BEN is typically diagnosed by repeated ANC values less than 1.5 × 109 cells/L over many months, in the absence of other secondary neutropenia. Recent population-based analysis showed that most BEN-related neutrophil measurements are at least 1.0 × 109 cells/L5.
Fig 1.
Percent of male and female participants with absolute neutrophil count (ANC) less than 1.5 × 109 cells/L in NHANES 1999 to 2004.5 Reprinted with permission. MA, Mexican-Americans.
BEN is not associated with an increased risk of oral or systemic infections,10,20,21 as seen in other forms of congenital neutropenia. There are no compensatory increases in lymphocyte or monocytes counts.20,22 Large US studies have shown that African American adults have slightly higher lymphocyte counts (Table 1) and similar percentages but slightly reduced absolute number of monocytes (Table 1).23,24 Patients with BEN are logically presumed to be normal, and no additional treatment is recommended for the usual non–chemotherapy-related infections.
Table 1.
Mean Weighted Leukocyte and WBC Differential in National Health and Nutritional Examination Survey Database Between Whites and Blacks
| Age (years) | Leukocytes |
Neutrophils |
Lymphocytes |
Monocytes |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Afr Am | Whites | Mex Am | Afr Am | Whites | Mex Am | Afr Am | Whites | Mex Am | Afr Am | Whites | Mex Am | |
| 1-5 | 7.81 | 8.69 | 9.47 | 3.04 | 3.61 | 4.25 | 4.01 | 4.27 | 4.39 | 0.48 | 0.49 | 0.52 |
| 6-11 | 7.06 | 7.89 | 8.32 | 3.32 | 3.99 | 4.31 | 2.97 | 3.1 | 3.17 | 0.41 | 0.45 | 0.47 |
| 12-18 | 6.78 | 8.05 | 8.26 | 3.53 | 4.66 | 4.79 | 2.55 | 2.65 | 2.68 | 0.39 | 0.45 | 0.46 |
| 19-65 | 6.85 | 8.05 | 8.3 | 3.67 | 4.92 | 4.97 | 2.56 | 2.47 | 2.66 | 0.30 | 0.44 | 0.43 |
| ≥ 66 | 6.56 | 8.22 | 7.96 | 3.70 | 5.04 | 4.99 | 2.23 | 2.42 | 2.21 | 0.39 | 0.51 | 0.47 |
NOTE. Data adapted.24 Mean cell counts ×109/L are shown.
Abbreviations: Whites, non-Hispanic whites; Afr Am, non-Hispanic blacks; Mex Am, Mexican-Americans.
ETIOLOGY OF BEN
The etiology of BEN is not well understood, but the mechanism for it does not appear to represent a biologic abnormality. Computational modeling of gene marking or telomere length shortening over time suggests that stem-cell numbers were estimated to be 11,000 to 22,000 in mice, cats, nonhuman primates, or humans. Because the life span of the mammals are different, the number of cell divisions per stem cell varied to sustain the life of each mammal.25 If the total stem-cell number was similar between lower and higher ordered mammals, then stem-cell number is likely similar between individuals of different ethnic backgrounds.
Recently single-nucleotide polymorphism at the Duffy antigen receptor for chemokine on chromosome 1 (rs2814778)26–28 has been linked to leukocyte counts. This finding and earlier reports describing familial clustering of BEN29,30 suggest that there are potential genetic influences for this condition. How the Duffy antigen receptor for chemokine and potential other genetic factors cause neutropenia is unknown.
Direct bone marrow examinations in 12 individuals with BEN (ANC < 2.0 × 109 cells/L) reported earlier showed normal cellularity and leukocyte maturation.20,31 More recently, 27 Africans or Caribbeans of African descent were shown to have lower numbers of myeloid marrow progenitors compared with white controls.21 This minor reduction is further supported by our retrospective analysis of the database from the Center for International Blood and Marrow Transplant Research, where bone marrow collections from 240 African American donors contained lower nucleated cell and CD34+ cell number per recipient weight (Table 2). Additionally, when cord blood units as an alternative source of hematopoietic stem cells were studied, African Americans had lower total nucleated cells.32,33 These data can be summarized as African Americans having normal stem-cell number, normal myeloid maturation, and a minor reduction in hematopoietic myeloid progenitors at steady-state.
Table 2.
Bone Marrow Collections in African- and White-Descent Donors for Hematopoietic Stem-Cell Transplantation
| Characteristic | African Descent Bone Marrow | White Descent Bone Marrow | P |
|---|---|---|---|
| No. of patients | 244 | 2,594 | |
| Recipient age, years | < .001 | ||
| Median | 17 | 25 | |
| Range | < 1-62 | < 1-69 | |
| Donor age, years | < .001 | ||
| Median | 20 | 27 | |
| Range | 1-68 | 1-92 | |
| Recipient BMI before transplant, kg/m2* | |||
| No. of patients evaluable | 242 | 2,565 | |
| Median | 20.9 | 22.6 | .012† |
| 5th-95th percentile | 14.3-39.1 | 14.1-33.7 | |
| Nucleated cells after processing, ×108 cells/weight of recipient (kg) | |||
| No. of patients evaluable | 232 | 2,490 | |
| Median | 2.15 | 2.62 | < .001† |
| 5th-95th percentile | 0.03-10.3 | 0.06-7.32 | |
| C34+ cells after processing, ×106 cells/weight of recipient (kg) | |||
| Median | 2.17 | 2.67 | |
| 5th-95th percentile | 0.02-13.7 | 0.04-14.1 | < .001† |
NOTE. Data provided by Center for International Blood and Marrow Transplant Research from 1995 to 2007. Related donor transplants were selected for this analysis. Donor ethnic backgrounds were assumed to be the same as recipients and grouped based on the recipient ethnicities.
Abbreviation: BMI, body mass index.
Donor weight and BMI not available.
0-5th percentile and 95-100th percentile observations in each group were deemed as outliers and were excluded in the Wilcoxon test.
NEUTROPHIL RESPONSE TO PHYSIOLOGIC STIMULI
The lower number of marrow myeloid progenitors is further reflected in the lower physiologic release of neutrophils from vascular endothelium and bone marrow stores. The neutrophil increments after corticosteroids were the lowest in individuals with BEN; even nonneutropenic ethnic controls had lower neutrophil increases after these stimulating agents when compared with whites.20,34,35 This lack of robust response has effectively disproved neutrophil margination to vascular endothelium as a cause for the observed BEN. Smoking is also a well-known inducer of leukocyte counts. African American smokers have lower leukocyte and neutrophil counts than white smokers in the United States.5,36 This is consistent with the observation that Nigerian smokers have a nonstatistically significant increase in WBCs than Nigerian nonsmokers.37 Additionally, African healthy volunteers undergoing 10-minute exercises38 and African marathon runners39 have lower leukocyte and neutrophil counts compared with white controls. These studies together indicate that individuals with BEN at baseline may have a lower “set point” of committed hematopoietic progenitors and a lower “reserve” of mature neutrophils in the bone marrow, resulting in fewer total neutrophils circulating systemically and marginating in vascular endothelium and leading to a diminished neutrophil release.
BETTER HEMATOPOIETIC STEM-CELL MOBILIZATION IN AFRICAN AMERICANS
When granulocyte colony-stimulating factor (G-CSF) was administered to African Americans with and without sickle cell trait to ascertain the safety of stem-cell mobilization in preparation for transplantation,40 there was a trend toward higher average number of myeloid colony–forming units and CD34+ cells/L of apheresis product in sickle cell trait African Americans. A larger subsequent study of G-CSF mobilization of peripheral-blood stem cells among different ethnicities demonstrated that African Americans mobilized peripheral-blood stem cells better than whites.41 Our recent query to the Center for International Blood and Marrow Transplant Research showed that African Americans have comparable numbers of peripheral-blood CD34+ cells after G-CSF mobilization, despite having slightly lower steady-state bone marrow CD34+ cells (Table 3). Thus available evidence indicates that enhanced stem-cell proliferative and mobilization response to G-CSF are observed in individuals of African descent. These findings reinforce that there was no defect in stem-cell or myeloid maturation and that rigid neutrophil cutoffs may not be relevant to clinical trials inclusions when there is BEN.
Table 3.
G-CSF–Mobilized Peripheral-Blood Stem-Cell Grafts in African and White Descent for Hematopoietic Stem-Cell Transplantation
| Characteristic | African Descent Peripheral Blood | White Descent Peripheral Blood | P |
|---|---|---|---|
| No. of patients | 273 | 5,265 | |
| Recipient age, years | < .001 | ||
| Median | 40 | 45 | |
| Range | 1-68 | < 1-77 | |
| Donor age, years | < .001 | ||
| Median | 39 | 44 | |
| Range | 2-93 | <1-85 | |
| Recipient BMI before transplant, kg/m2* | |||
| Median | 25.8 | 25.1 | .778† |
| 5th-95th percentile | 16.2-39.6 | 17.8-36.6 | |
| C34+ cells after processing, ×108 cells/weight of recipient (kg) | |||
| Median | 5.48 | 5.32 | |
| 5th-95th percentile | 0.05-29.1 | 0.15-20.6 | .441† |
NOTE. Data provided by the Center for International Blood and Marrow Transplant Research from 1995 to 2007. Related donor transplants were selected for this analysis. Donor ethnic backgrounds were assumed to be the same as recipients and grouped based on the recipient ethnicities.
Abbreviations: G-CSF, granulocyte colony-stimulating factor; BMI, body mass index.
Donor weight and BMI not available.
0-5th percentile and 95-100th percentile observations in each group were deemed as outliers and were excluded in the Wilcoxon test.
IMPLICATION OF BEN IN ELIGIBILITY CRITERIA FOR ONCOLOGY TRIALS
Access of African Americans to cancer clinical trials is estimated at 10% or less.42,43 Although many factors limit their access, a rigid neutrophil count cutoff represents another barrier. To estimate the potential impact of BEN on access disparity to clinical trials, we identified all of the active NCI-sponsored phase III clinical trials in cancers of the breast, prostate, lung, colorectal, kidney, bladder, urinary tract, head and neck, female reproductive system, myeloma, and non-Hodgkin's lymphoma. Fifty percent (23 of the 46 active trials) required an ANC of at least 1.5 × 109 cells/L. There are substantially more phase I and II trials, which represent many more clinical trials that likely exclude those with ANC counts less than 1.5 × 109 cells/L. Thus a substantial proportion of publically funded clinical trials may have the unintended consequence of limiting access to individuals with BEN.
Several investigators have observed that African Americans have lower prechemotherapy and postchemotherapy leukocyte and neutrophil counts when compared with white patients.44,45 This reduction has been proposed to be one of many reasons for the frequent dose modification, treatment delay, and possibly higher cancer-related mortality in African Americans.44–46 Smith et al45 showed that, although the counts were lower before and after chemotherapy, the nadir leukocyte and neutrophil counts and incidence of febrile neutropenia were similar between 86 white and 19 African American patients with early-stage breast cancer. This observation should be further confirmed using currently available databases, as such an analysis could potentially inform what may represent a critical treatment and outcomes issue. The benign nature of lower leukocyte and neutrophil counts, although not explicitly stated, can also be inferred from no reported increase in febrile neutropenia incidence among African Americans in large community oncology clinics.47,48
Silber et al49 used mathematic models and validated in approximately 100 patients with early-stage breast cancer that the nadir neutrophil count after first cycle chemotherapy was a better predictor for subsequent treatment delays than pretreatment leukocyte or neutrophil count. Furthermore, two other groups reported that when African Americans were enrolled onto clinical trials, they receive similar relative dose-intensity of chemotherapy1,50 and experience similar rates of chemotherapy-related toxicities50 as their white counterparts. These reports indicate that when access is not a barrier, African Americans tolerate standard chemotherapy dosing and benefit from clinical trials as much as their white counterparts.
FINAL REMARKS
In conclusion, our current understanding about BEN is that this condition has normal hematopoietic stem-cell number and myeloid maturation and results from a lower set point in the number of marrow-nucleated cells, leading to slightly fewer mature neutrophils in the periphery. This minor reduction has not been reported to cause increased infections in otherwise healthy individuals. If an individual has an ANC count of at least 1.0 × 109 cells/L or higher over several months, and other causes of neutropenia have been excluded, then BEN is likely to be the cause.
How do these considerations inform decisions regarding clinical research eligibility? The current evidences indicate that there is not an increased risk of febrile neutropenia secondary to myelosuppressive therapy in a patient with BEN. Moreover, there seems to be an enhanced response to G-CSFs among those with BEN. These factors suggest that support with prophylactic G-CSF (5 μg/kg/d) and monitoring of blood counts as usual would provide sufficient safety for individuals with BEN to participate in most clinical research trials through explicit changes in eligibility criteria. There may be more justifiable concern, however, in cases where the baseline ANC count is less than 1.0 × 109 cells/L. Although BEN may fully explain the findings, common causes of neutropenia such as intercurrent viral illness, autoimmune disorders, medications, nutritional deficiencies, or the effect of the tumor itself should be assessed. This may include examination of the bone marrow for metastatic disease, marrow failure syndromes, or myelodysplastic syndromes. The clinical findings will guide the appropriate decision making.
In patients in whom BEN is the most likely cause of the neutropenia and have enrolled onto clinical trials, available evidence suggests they can receive effective doses of chemotherapy. Chemotherapy may be administered safely when the neutrophils range from 0.5 to 1.5 × 109 cells/L, and prophylactic G-CSF could be given if chemotherapy is expected to lead to neutropenia (below 0.5 × 109 cells/L). If cycles of therapy have already been administered, the subsequent dose adjustment depends on nadir duration of the ANC, signs or symptoms for infection, and other comorbidities. Awareness of higher cancer- and treatment-related mortalities in African Americans should prompt clinicians to weigh the risks and benefits carefully. However, reflexive exclusion of those with low WBCs on the basis of BEN is not medically justified and can have the unwanted effect of unduly excluding minority participation in cancer clinical trials. The impact of current ANC barrier at 1.5 × 109 cells/L is substantially larger than previously realized.
Acknowledgment
The data presented in Tables 2 and 3 are preliminary and were obtained from the Statistical Center of the Center for International Blood and Marrow Transplant Research (CIBMTR). The analysis has not been reviewed or approved by the Advisory or Scientific Committee of the CIBMTR. We thank Jerry Wang from CIBMTR for data analysis. This work is supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute and the National Institute of Diabetes, Digestive, and Kidney Diseases at the National Institutes of Health.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Data analysis and interpretation: Matthew M. Hsieh, Richard F. Little
Manuscript writing: Matthew M. Hsieh, Edward L. Trimble, John F. Tisdale, Neal S. Young, Griffin P. Rodgers, Richard F. Little
Final approval of manuscript: Matthew M. Hsieh, Edward L. Trimble, John F. Tisdale, Neal S. Young, Griffin P. Rodgers, Richard F. Little
REFERENCES
- 1.Griggs JJ, Sorbero ME, Stark AT, et al. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81:21–31. doi: 10.1023/A:1025481505537. [DOI] [PubMed] [Google Scholar]
- 2.Adams-Campbell LL, Ahaghotu C, Gaskins M, et al. Enrollment of African Americans onto clinical treatment trials: Study design barriers. J Clin Oncol. 2004;22:730–734. doi: 10.1200/JCO.2004.03.160. [DOI] [PubMed] [Google Scholar]
- 3.Unger JM, Coltman CA, Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol. 2006;24:141–144. doi: 10.1200/JCO.2005.02.8928. [DOI] [PubMed] [Google Scholar]
- 4.Albrecht TL, Eggly SS, Gleason ME, et al. Influence of clinical communication on patients' decision making on participation in clinical trials. J Clin Oncol. 2008;26:2666–2673. doi: 10.1200/JCO.2007.14.8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh MM, Everhart JE, Byrd-Holt DD, et al. Prevalence of neutropenia in the U.S. population: Age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146:486–492. doi: 10.7326/0003-4819-146-7-200704030-00004. [DOI] [PubMed] [Google Scholar]
- 6.Bodey GP, Buckley M, Sathe YS, et al. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 7.Bodey GP, Rodriguez V, Chang HY, et al. Fever and infection in leukemic patients: A study of 494 consecutive patients. Cancer. 1978;41:1610–1622. doi: 10.1002/1097-0142(197804)41:4<1610::aid-cncr2820410452>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: The major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345–351. doi: 10.7326/0003-4819-100-3-345. [DOI] [PubMed] [Google Scholar]
- 9.Athens JW, Haab OP, Raab SO, et al. Leukokinetic studies: IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest. 1961;40:989–995. doi: 10.1172/JCI104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddy TB, Rana SR, Castro O. Benign ethnic neutropenia: What is a normal absolute neutrophil count? J Lab Clin Med. 1999;133:15–22. doi: 10.1053/lc.1999.v133.a94931. [DOI] [PubMed] [Google Scholar]
- 11.Cartwright GE, Athens JW, Wintrobe MM. The kinetics of granulopoiesis in normal man. Blood. 1964;24:780–803. [PubMed] [Google Scholar]
- 12.Crosby WH. How many “polys” are enough? Arch Intern Med. 1969;123:722–723. [PubMed] [Google Scholar]
- 13.Kyle RA, Linman JW. Chronic idiopathic neutropenia: A newly recognized entity? N Engl J Med. 1968;279:1015–1019. doi: 10.1056/NEJM196811072791902. [DOI] [PubMed] [Google Scholar]
- 14.Dancey JT, Brubaker LH. Neutrophil marrow in chronic benign idiopathic neutropenia. Am J Med. 1980;68:251–254. doi: 10.1016/0002-9343(80)90362-9. [DOI] [PubMed] [Google Scholar]
- 15.Cutting HO, Lang JE. Familial benign chronic neutropenia. Ann Intern Med. 1964;61:876–887. doi: 10.7326/0003-4819-61-5-876. [DOI] [PubMed] [Google Scholar]
- 16.Mant MJ, Gordon PA, Akabutu JJ. Bone marrow granulocyte reserve in chronic benign idiopathic neutropenia. Clin Lab Haematol. 1987;9:281–288. doi: 10.1111/j.1365-2257.1987.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 17.Rippey JJ. Leucopenia in West Indians and Africans. Lancet. 1967;290:44. [Google Scholar]
- 18.Weingarten MA, Pottick-Schwartz EA, Brauner A. The epidemiology of benign leukopenia in Yemenite Jews. Isr J Med Sci. 1993;29:297–299. [PubMed] [Google Scholar]
- 19.Shoenfeld Y, Alkan ML, Asaly A, et al. Benign familial leukopenia and neutropenia in different ethnic groups. Eur J Haematol. 1988;41:273–277. doi: 10.1111/j.1600-0609.1988.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 20.Mason BA, Lessin L, Schechter GP. Marrow granulocyte reserves in black Americans: Hydrocortisone-induced granulocytosis in the “benign” neutropenia of the black. Am J Med. 1979;67:201–205. doi: 10.1016/0002-9343(79)90391-7. [DOI] [PubMed] [Google Scholar]
- 21.Rezvani K, Flanagan AM, Sarma U, et al. Investigation of ethnic neutropenia by assessment of bone marrow colony-forming cells. Acta Haematol. 2001;105:32–37. doi: 10.1159/000046530. [DOI] [PubMed] [Google Scholar]
- 22.Reed WW, Diehl LF. Leukopenia, neutropenia, and reduced hemoglobin levels in healthy American blacks. Arch Intern Med. 1991;151:501–505. [PubMed] [Google Scholar]
- 23.Beutler E, West C. Hematologic differences between African-Americans and whites: The roles of iron deficiency and alpha-thalassemia on hemoglobin levels and mean corpuscular volume. Blood. 2005;106:740–745. doi: 10.1182/blood-2005-02-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollowell JG, Van Assendelft OW, Gunter EW, et al. Hematological and iron-related analytes: Reference data for persons aged 1 year and over—United States 1988-94. National Center for Health Statistics. Vital Health Stat. 2005;11:1–156. [PubMed] [Google Scholar]
- 25.Abkowitz JL, Catlin SN, McCallie MT, et al. Evidence that the number of hematopoietic stem cells per animal is conserved in mammals. Blood. 2002;100:2665–2667. doi: 10.1182/blood-2002-03-0822. [DOI] [PubMed] [Google Scholar]
- 26.Nalls MA, Wilson JG, Patterson NJ, et al. Admixture mapping of white cell count: Genetic locus responsible for lower white blood cell count in the Health ABC and Jackson Heart studies. Am J Hum Genet. 2008;82:81–87. doi: 10.1016/j.ajhg.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grann VR, Ziv E, Joseph CK, et al. Duffy (Fy), DARC, and neutropenia among women from the United States, Europe and the Caribbean. Br J Haematol. 2008;143:288–293. doi: 10.1111/j.1365-2141.2008.07335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reich D, Nalls MA, Kao WH, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet. 2009;5:e1000360. doi: 10.1371/journal.pgen.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jumean HG, Sudah FI. Chronic benign idiopathic neutropenia in Jordanians. Acta Haematol. 1983;69:59–60. doi: 10.1159/000206841. [DOI] [PubMed] [Google Scholar]
- 30.Shoenfeld Y, Shapiro Y, Portugeeze D, et al. Maximal backpack load for long distance hiking. J Sports Med Phys Fitness. 1977;17:147–151. [PubMed] [Google Scholar]
- 31.Mintz U, Sachs L. Normal granulocyte colony-forming cells in the bone marrow of Yemenite Jews with genetic neutropenia. Blood. 1973;41:745–751. [PubMed] [Google Scholar]
- 32.Ballen KK, Kurtzberg J, Lane TA, et al. Racial diversity with high nucleated cell counts and CD34 counts achieved in a national network of cord blood banks. Biol Blood Marrow Transplant. 2004;10:269–275. doi: 10.1016/j.bbmt.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Kurtzberg J, Cairo MS, Fraser JK, et al. Results of the cord blood transplantation (COBLT) study unrelated donor banking program. Transfusion. 2005;45:842–855. doi: 10.1111/j.1537-2995.2005.04428.x. [DOI] [PubMed] [Google Scholar]
- 34.Haddy TB, Rana SR. Leukocyte response to administration of corticosteroid in healthy black children with neutropenia. J Pediatr. 1994;124:739–741. doi: 10.1016/s0022-3476(05)81366-6. [DOI] [PubMed] [Google Scholar]
- 35.Shoenfeld Y, Modan M, Berliner S, et al. The mechanism of benign hereditary neutropenia. Arch Intern Med. 1982;142:797–799. [PubMed] [Google Scholar]
- 36.McGrath CR. Total white blood cell count for persons ages 1-74 years with differential leukocyte counts for adults ages 25-74 years: United States 1971-75—National Center for Health Statistics. Vital Health Stat. 1982;11:1–36. [PubMed] [Google Scholar]
- 37.Aghaji M, Nnabuko R, Uzuegbunam C, et al. The relationship of white blood cell and platelet counts to cigarette smoking in adult Nigerians. Cent Afr J Med. 1990;36:273–278. [PubMed] [Google Scholar]
- 38.Phillips D, Rezvani K, Bain BJ. Exercise induced mobilisation of the marginated granulocyte pool in the investigation of ethnic neutropenia. J Clin Pathol. 2000;53:481–483. doi: 10.1136/jcp.53.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bain BJ, Phillips D, Thomson K, et al. Investigation of the effect of marathon running on leucocyte counts of subjects of different ethnic origins: Relevance to the aetiology of ethnic neutropenia. Br J Haematol. 2000;108:483–487. doi: 10.1046/j.1365-2141.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- 40.Kang EM, Areman EM, David-Ocampo V, et al. Mobilization, collection, and processing of peripheral blood stem cells in individuals with sickle cell trait. Blood. 2002;99:850–855. doi: 10.1182/blood.v99.3.850. [DOI] [PubMed] [Google Scholar]
- 41.Vasu S, Leitman SF, Tisdale JF, et al. Donor demographic and laboratory predictors of allogeneic peripheral blood stem cell mobilization in an ethnically diverse population. Blood. 2008;112:2092–2100. doi: 10.1182/blood-2008-03-143677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swanson GM, Bailar JC., 3rd Selection and description of cancer clinical trials participants: Science or happenstance? Cancer. 2002;95:950–959. doi: 10.1002/cncr.10785. [DOI] [PubMed] [Google Scholar]
- 43.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 44.Hershman D, Weinberg M, Rosner Z, et al. Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. J Natl Cancer Inst. 2003;95:1545–1548. doi: 10.1093/jnci/djg073. [DOI] [PubMed] [Google Scholar]
- 45.Smith K, Wray L, Klein-Cabral M, et al. Ethnic disparities in adjuvant chemotherapy for breast cancer are not caused by excess toxicity in black patients. Clin Breast Cancer. 2005;6:260–266. doi: 10.3816/CBC.2005.n.029. discussion 267-269. [DOI] [PubMed] [Google Scholar]
- 46.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23:6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 47.Crawford J, Dale DC, Kuderer NM, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: The results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 2008;6:109–118. doi: 10.6004/jnccn.2008.0012. [DOI] [PubMed] [Google Scholar]
- 48.Morrison VA, Wong M, Hershman D, et al. Observational study of the prevalence of febrile neutropenia in patients who received filgrastim or pegfilgrastim associated with 3-4 week chemotherapy regimens in community oncology practices. J Manag Care Pharm. 2007;13:337–348. doi: 10.18553/jmcp.2007.13.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silber JH, Fridman M, DiPaola RS, et al. First-cycle blood counts and subsequent neutropenia, dose reduction, or delay in early-stage breast cancer therapy. J Clin Oncol. 1998;16:2392–2400. doi: 10.1200/JCO.1998.16.7.2392. [DOI] [PubMed] [Google Scholar]
- 50.Hershman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: Retrospective analysis of Southwest Oncology studies S8814/S8897. J Clin Oncol. 2009;27:2157–2162. doi: 10.1200/JCO.2008.19.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]