Abstract
Purpose
The 21-gene OncotypeDX recurrence score (RS) assay quantifies the risk of distant recurrence in tamoxifen-treated patients with node-negative, estrogen receptor (ER)–positive breast cancer. We investigated the association between RS and risk for locoregional recurrence (LRR) in patients with node-negative, ER-positive breast cancer from two National Surgical Adjuvant Breast and Bowel Project (NSABP) trials (NSABP B-14 and B-20).
Patients and Methods
RS was available for 895 tamoxifen-treated patients (from both trials), 355 placebo-treated patients (from B-14), and 424 chemotherapy plus tamoxifen-treated patients (from B-20). The primary end point was time to first LRR. Distant metastases, second primary cancers, and deaths before LRR were censored.
Results
In tamoxifen-treated patients, LRR was significantly associated with RS risk groups (P < .001). The 10-year Kaplan-Meier estimate of LRR was 4.% (95% CI, 2.3% to 6.3%) for patients with a low RS (< 18), 7.2% (95% CI, 3.4% to 11.0%) for those with intermediate RS (18-30), and 15.8% (95% CI, 10.4% to 21.2%) for those with a high RS (> 30). There were also significant associations between RS and LRR in placebo-treated patients from B-14 (P = .022) and in chemotherapy plus tamoxifen–treated patients from B-20 (P = .028). In multivariate analysis, RS was an independent significant predictor of LRR along with age and type of initial treatment.
Conclusion
Similar to the association between RS and risk for distant recurrence, a significant association exists between RS and risk for LRR. This information has biologic consequences and potential clinical implications relative to locoregional therapy decisions for patients with node-negative and ER-positive breast cancer.
INTRODUCTION
Gene expression profiling has emerged as a useful tool for assessing risk of distant recurrence in patients with early-stage breast cancer and has provided additional information to that obtained from traditional histopathologic factors and biomarkers.1–5 Several gene expression signatures have been reported to predict risk of distant recurrence in both untreated patients and those treated with hormone therapy and/or chemotherapy.1–6
The 21-gene recurrence score (RS) assay (OncotypeDX; Genomic Health Inc, Redwood City, CA) quantifies risk of distant recurrence in patients with node-negative, estrogen receptor (ER)–positive, tamoxifen-treated breast cancer and has been validated in two independent data sets.5,7 Recently, the RS was also shown to significantly predict benefit from adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy in node-negative, ER-positive patients in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-20 trial and benefit from adjuvant cyclophosphamide, doxorubicin, and fluorouracil chemotherapy in node-positive, hormone receptor–positive patients in the Southwest Oncology Group 8814 trial.8,9 Both American Society of Clinical Oncology and National Comprehensive Cancer Network guidelines have included the RS in the management of node-negative, ER-positive breast cancer.10,11
Locoregional recurrence (LRR) is a significant predictor of distant recurrence.12,13 All types of LRR (ipsilateral breast tumor recurrence, chest wall recurrence, and regional nodal recurrence) have been associated with a significant increase in risk for subsequent distant recurrence, although the magnitude of risk varies depending on the type of LRR.12,13
Despite significant progress in identifying genomic profiles associated with risk of distant recurrence, risk assessment for LRR is still primarily based on traditional anatomic and histopathologic factors (such as tumor size, grade, pathologic nodal status, and lymphovascular invasion). Given the strong association between LRR and distant recurrence, we and others have hypothesized that genomic profiles that predict risk for distant recurrence will also predict risk for LRR.14–16 The primary objective of this study was to examine the relationship between the RS and risk of LRR in tamoxifen-treated patients (as evidenced in patients from the NSABP B-14 and B-20 trials). As secondary objectives, we examined the relationship in placebo-treated patients (from NSABP B-14) and in chemotherapy plus tamoxifen-treated patients (from NSABP B-20).
PATIENTS AND METHODS
Patient Population
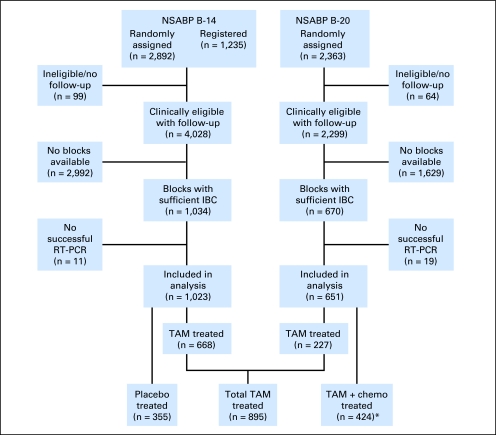
Detailed information on the design, patient eligibility, treatment regimens, and outcome results from the two trials has been published.17–20 In short, between January 1982 and January 1988, 2,892 patients who were node negative and ER positive were randomly assigned in NSABP trial B-14 to 5 years of placebo or 5 years of tamoxifen. Between January 1988 and October 1988, 1,235 additional patients were registered to 5 years of tamoxifen to later address a tamoxifen duration question (10 v 5 years). Of 4,127 patients in the B-14 trial, 4,028 were clinically eligible with follow-up. Blocks containing sufficient invasive breast cancer were available for 1,034 patients. In the remaining patients, blocks were either never obtained by the NSABP or were exhausted from use in prior studies. Reverse transcription polymerase chain reaction (RT-PCR) was successful in 1,023 (99%) of the blocks, providing RS information on 668 tamoxifen-treated patients (290 randomly assigned and 378 registered) and 355 placebo-treated patients (CONSORT Figure 1).
Fig 1.
CONSORT diagram for patients from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 and B-20 trials. (*) Methotrexate and fluorouracil with leucovorin rescue (MFT): 203 patients; tamoxifen plus cyclophosphamide, methotrexate, and fluorouracil (CMFT): 221 patients. IBC, invasive breast cancer; RT-PCR, reverse transcription polymerase chain reaction; TAM, tamoxifen; chemo, chemotherapy.
Between October 1988 and March 1993, 2,363 node-negative, ER-positive patients were randomly assigned in B-20 to tamoxifen alone or tamoxifen plus either cyclophosphamide, methotrexate, and fluorouracil or sequential methotrexate and fluorouracil with leucovorin rescue. Of 2,363 patients in trial B-20, 2,299 were clinically eligible with follow-up. Blocks containing sufficient invasive breast cancer were available for 670 patients. RT-PCR was successful in 651 of the blocks (97%), providing RS information on 227 tamoxifen-treated and 424 chemotherapy plus tamoxifen–treated patients (methotrexate and fluorouracil with leucovorin rescue: 203 patients; tamoxifen plus cyclophosphamide, methotrexate, and fluorouracil: 221 patients; Fig 1).
Because there was no significant difference in clinical outcomes between tamoxifen-treated patients in B-14 and B-20, we prespecified that the tamoxifen-only arms from these trials would be combined for the primary analyses. Similarly, since there were no outcome differences between the two chemotherapy plus tamoxifen arms in B-20, we prespecified that these two arms would be combined for the primary analyses. As a result, the tamoxifen-treated group included 895 patients (668 from B-14 and 227 from B-20) and the chemotherapy plus tamoxifen–treated group included 424 patients from B-20 (Fig 1).
Study Design and End Points
Patients were eligible for the present analysis if they were eligible with follow-up, if a tumor block was available in the NSABP tumor bank, and if successful assessment of RS had been previously performed. Exclusion criteria included insufficient tumor (< 5% of the overall tissue) as assessed by histopathology, insufficient RNA (< 0.5 μg), or weak RT-PCR signal (average cycling threshold for the reference genes > 35).
Patients in both trials had lumpectomy plus axillary node dissection or modified radical mastectomy as their surgical procedure. All lumpectomy-treated patients were required per protocol to receive standard breast irradiation. However, chest wall irradiation after mastectomy was not allowed per protocol. Similarly, regional nodal irradiation was not allowed, irrespective of surgical procedure. As a result, there were two types of initial locoregional (LR) treatment in the two trials: lumpectomy plus breast irradiation (L + XRT) or mastectomy.
In both trials, ER/progesterone receptor were measured by ligand-binding assay. For the 668 tamoxifen-treated B-14 patients in the RS validation study, tumor grade was independently determined by an NSABP pathologist and two board-certified pathologists (from Stanford University and University of California, San Francisco) using the Elston modification of the Bloom-Richardson grading criteria. Since all three tumor grade ratings were highly associated with risk of distant recurrence with only modest concordance between readings, the tumor grade rating used in the analyses was the one whose association with risk of distant recurrence was neither the strongest nor the weakest of the three. Tumor grades for the 355 B-14 placebo patients and the 424 chemotherapy plus tamoxifen B-20 patients were also determined by the same pathologist.
The primary prespecified end point for this analysis of LRR was defined as time from study entry to ipsilateral breast tumor recurrence (after L+XRT), local chest wall recurrence (after mastectomy), and regional nodal recurrence (after either initial LR treatment). Occurrence of contralateral breast cancer, other second primary cancers, distant recurrence, and death before LRR were considered censoring events. The cutoff points for the RS were those that were prespecified before the performance of the validation study of the RS,5 which categorized patients into low RS (< 18), intermediate RS (18-30), and high RS (≥ 31) groups.
The study was approved by the Essex institutional review board (IRB; NJ), the Allegheny General Hospital IRB (PA), and the University of Pittsburgh IRB (PA).
Sample Preparation, Genes, RS Algorithm
Information on patient eligibility for inclusion in the development and validation of the RS, methods of RNA extraction, the RT-PCR methodology used and the genes included in the RS have been previously published in detail5 and are summarized in short in the online-only Appendix.
Statistical Analysis
The log-rank test was used as the primary analysis to assess the association between the RS categories (low, intermediate, and high) and time to LRR. Kaplan-Meier estimates were used to determine proportions of LRR in patient subgroups. Cox proportional hazards models were utilized to examine the association between RS and risk of LRR, adjusting for other clinical variables, such as age, clinical tumor size, tumor grade, and type of initial LR treatment (mastectomy v L + XRT). The likelihood ratio test was used for testing the significance of such association by comparing the reduced model that excluded the RS to the full model that included the RS, in addition to clinical variables, such as age, clinical tumor size, tumor grade, and type of initial LR treatment. A P value less than .05 for the likelihood ratio test was considered significant.
RESULTS
Patient Demographics
In trial B-14, the distributions of demographic, clinical, and treatment characteristics were similar between the 1,023 evaluable patients with RS information and the 4,028 clinically eligible patients with follow-up (Appendix Table A1, online only). In B-20, the distributions of demographic, clinical, and tumor characteristics were also similar between the 651 evaluable patients with RS information and the 2,299 clinically eligible patients with follow-up (Appendix Table A2, online only).
Among the 1,023 evaluable patients from B-14, 509 patients (49.8%) were in the low RS group, 234 patients (22.9%) in the intermediate RS group, and 280 (27.4%) in the high RS group. Among the 651 evaluable patients from B-20, 353 (54.2%) were in the low RS group, 134 (20.6%) in the intermediate RS group, and 164 (25.1%) in the high RS group. Median follow-up time for LRR was 14.8 years for the 355 B-14 placebo-treated patients, 13.9 years for the 668 B-14 tamoxifen-treated patients, 10.6 years for the 227 B-20 tamoxifen-treated patients (12.5 years for all 895 tamoxifen-treated patients), and 10.3 years for the 424 B-20 chemotherapy plus tamoxifen–treated patients.
Among the 1,023 evaluable patients from B-14, the 10-year Kaplan-Meier estimate of the proportion of patients with LRR was 14.9% (95% CI, 10.7% to 19.1%) for patients treated with placebo and 7.7% (95% CI, 5.7% to 10.2%) for those treated with tamoxifen. Among the 651 evaluable patients from B-20, the respective rates were 7.8% (95% CI, 4.7% to 12.6%) for patients treated with tamoxifen alone and 3.5% (95% CI, 0.2% to 5.3%) for those treated with chemotherapy plus tamoxifen. The tamoxifen groups from B-14 (668 patients) and B-20 (227 patients) were combined in subsequent analyses.
Type of LRR in Tamoxifen-Treated Patients
A total of 73 LRRs were observed as first treatment failure among the 895 tamoxifen-treated patients. Forty-two (10.8%) of 390 L + XRT patients and 31 (6.1%) of 505 mastectomy patients experienced LRR. In L + XRT patients, 88.1% of the LRRs were local (mostly ipsilateral breast tumor recurrences). In mastectomy patients, 58.1% of the LRRs were local (chest wall and scar). The most common sites of regional recurrences were the axilla and the supraclavicular area. Two patients had simultaneously detected local and regional recurrence. Table 1 provides details on the nature of LRR according to type of initial treatment.
Table 1.
Sites of the First Locoregional Recurrence Among Tamoxifen- Treated Patients From NSABP Trials B-14 and B-20 According to Type of Initial Treatment (N = 895)
| Type of Initial Treatment | Group Total (No.) | Local Site |
Regional Site |
||||
|---|---|---|---|---|---|---|---|
| IBTR | Chest Wall | Scar | Axilla | Supraclavicular | Local and Regional | ||
| Lumpectomy + XRT | 390 | 34 | 3 | 0 | 1 | 3 | 1 |
| Mastectomy | 505 | 0 | 17 | 1 | 9 | 3 | 1 |
Abbreviations: NSABP, National Surgical Adjuvant Breast and Bowel Project; IBTR, ipsilateral breast tumor recurrence; XRT, radiation therapy.
Association Between RS and LRR in Tamoxifen-Treated Patients
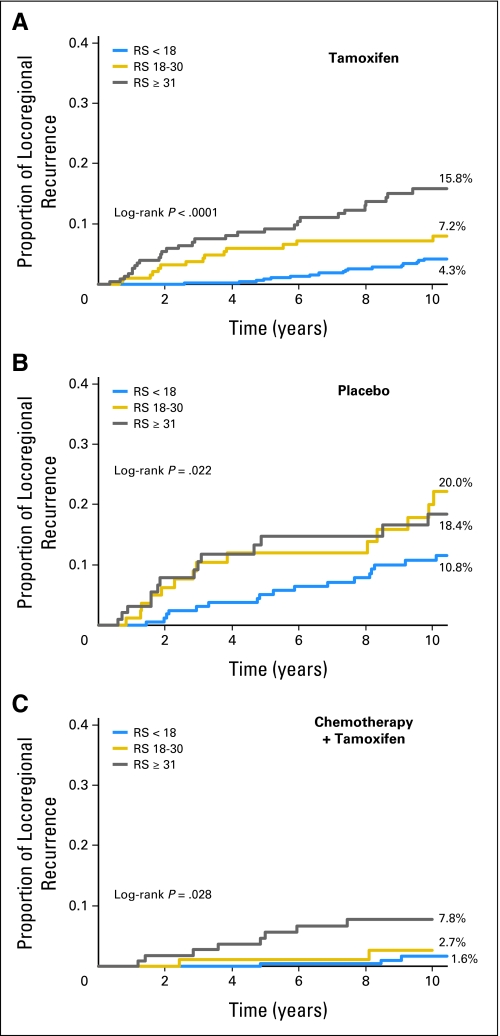
In the 895 evaluable tamoxifen-treated patients, RS was significantly associated with risk of LRR (log-rank test P < .001). The 10-year Kaplan-Meier estimates of the proportion of patients with LRR were 4.3% (95% CI, 2.3% to 6.3%) for patients with low RS, 7.2% (95% CI, 3.4% to 11%) for those with intermediate RS, and 15.8% (95% CI, 10.4% to 21.2%) for those with high RS (Fig 2A and Table 2).
Fig 2.
(A) Kaplan-Meier estimates of the proportion of B-14 and B-20 tamoxifen-treated patients with locoregional recurrence at 10 years according to the three recurrence score (RS) categories. (B) Kaplan-Meier estimates of the proportion of B-14 placebo-treated patients with locoregional recurrence at 10 years according to the three RS categories. (C) Kaplan-Meier estimates of the proportion of B-20 chemotherapy plus tamoxifen-treated patients with locoregional recurrence at 10 years according to the three RS categories.
Table 2.
Kaplan-Meier Estimates and 95% CIs of the Proportion of Patients With Locoregional Recurrence at 10 Years for 355 Placebo-Treated Patients (NSABP B-14), 895 Tamoxifen-Treated Patients (NSABP B-14 andB-20) and 424 Tamoxifen Plus Chemotherapy–Treated Patients (NSABP B-20)
| Treatment Group and Recurrence Score Group | 10-Year Kaplan-Meier Estimate (%) | 95% CI | Log-Rank P | No. of Events/No. at Risk |
|---|---|---|---|---|
| Placebo | ||||
| Low (< 18) | 10.8 | 5.8% to 15.8% | .022 | 19/171 |
| Intermediate (18-30) | 20.0 | 9.9% to 30.0% | 15/85 | |
| High (≥ 31) | 18.4 | 9.5% to 27.4% | 19/99 | |
| Tamoxifen | ||||
| Low (< 18) | 4.3 | 2.3% to 6.3% | < .001 | 24/473 |
| Intermediate (18-30) | 7.2 | 3.4% to 11.0% | 16/194 | |
| High (≥ 31) | 15.8 | 10.4% to 21.2% | 33/228 | |
| Chemotherapy + tamoxifen | ||||
| Low (< 18) | 1.6 | 0.0% to 3.5% | .028 | 4/218 |
| Intermediate (18-30) | 2.7 | 0.0% to 6.4% | 2/89 | |
| High (≥ 31) | 7.8 | 2.6% to 13.0% | 8/117 |
NOTE. Results are given for all patients and for the pre-specified recurrence score risk categories.
Abbreviation: NSABP, National Surgical Adjuvant Breast and Bowel Project.
Association Between RS and LRR in Placebo Patients
In the 355 evaluable placebo-treated patients from B-14, RS was also significantly associated with risk of LRR (log-rank test P = .022). The 10-year Kaplan-Meier estimates of the proportion of patients with LRR were 10.8% (95% CI, 5.8% to 15.8%) for patients with low RS, 20.0% (95% CI, 9.9% to 30.0%) for those with intermediate RS, and 18.4% (95% CI, 9.5% to 27.4%) for those with high RS (Fig 2B and Table 2).
Association Between RS and LRR in Chemotherapy Plus Tamoxifen–Treated Patients
Similarly, in the 424 evaluable chemotherapy plus tamoxifen–treated patients from B-20, RS was significantly associated with LRR (log-rank P = .028). The 10-year Kaplan-Meier estimates of the proportion of patients with LRR were 1.6% (95% CI, 0% to 3.5%) for patients with low RS, 2.7% (95% CI, 0% to 6.4%) for those with intermediate RS, and 7.8% (95% CI, 2.6% to 13%) for those with high RS (Fig 2C and Table 2).
Multivariate Cox Regression Analysis in Tamoxifen-Treated Patients
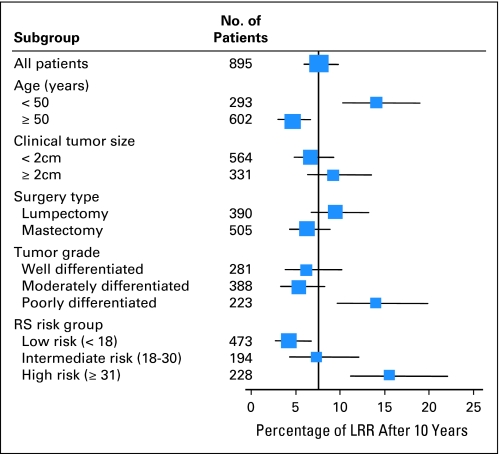
Based on data from the 895 tamoxifen-treated patients, multivariate Cox regression analysis demonstrated that RS (as a continuous variable) was significantly associated with risk of LRR. The results of analysis when including RS as a continuous variable are presented in Table 3. After adjusting for age, clinical tumor size, type of initial treatment, and tumor grade, a hazard ratio of 2.16 was associated with an increment of 50 units in RS (95% CI, 1.26 to 3.68; P = .007). Other statistically significant variables associated with LRR included age (≥ 50 v < 50: hazard ratio, 0.4; P < .001) and type of initial LR treatment (mastectomy v L+XRT: hazard ratio, 0.62; P = .047). Interestingly, after the inclusion of RS in the multivariate model, clinical tumor size and tumor grade did not have a statistically significant association with LRR. The 10-year Kaplan-Meier estimates of the proportion of LRR for various patient subgroups according to RS and other clinical variables (age, clinical tumor size, tumor grade, type of initial treatment) are presented in Figure 3 for the 895 tamoxifen-treated patients.
Table 3.
Multivariate Cox Regression Analysis of Predictors of Locoregional Recurrence in the Cohort of 895 Tamoxifen-Treated Patients From NSABP Trials B-14 and B-20
| Variable | Hazard Ratio | 95% CI | Wald Test P |
|---|---|---|---|
| Age (≥ 50 v < 50) | 0.40 | 0.25 to 0.65 | .0002 |
| Mastectomy v L + XRT | 0.62 | 0.39 to 0.99 | .047 |
| Clinical tumor size (> 2 v ≤ 2 cm) | 0.98 | 0.61 to 1.59 | .933 |
| Tumor grade (moderate v well) | 1.10 | 0.54 to 1.92 | .113 |
| Tumor grade (poor v well) | 1.76 | 0.89 to 3.48 | |
| Recurrence score* | 2.16 | 1.26 to 3.68 | .005 |
Abbreviations: L, lumpectomy; XRT, radiation therapy; LRR, locoregional recurrence; NSABP, National Surgical Adjuvant Breast and Bowel Project.
Recurrence score was a continuous variable, with the hazard ratio for LRR calculated relative to an increment of 50 units (chosen to dichotomize the recurrence score and thus improve comparability of the hazard ratio with the hazard ratios based on the clinical covariates). The P value for the likelihood ratio test on RS is .007.
Fig 3.
Percentage of patients with locoregional recurrence (LRR) at 10 years according to various subgroups in the 895 tamoxifen-treated patients in National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14/B-20 trials. RS, recurrence score.
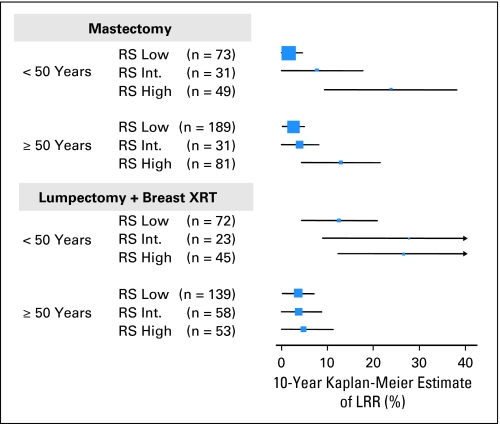
Among the 895 tamoxifen-treated patients, 390 underwent L + XRT, and the 10-year Kaplan-Meier estimates of the proportion with LRR for the RS low, intermediate, and high groups were: 6.8%, 10.8%, and 14.6%, respectively (log-rank test, P = .043). Five hundred five underwent mastectomy, and the 10-year Kaplan-Meier estimates of the proportion with LRR for the RS low, intermediate, and high groups were: 2.3%, 4.7%, and 16.8%, respectively (log-rank test, P < .001). To further explore the risk of LRR by subgroups, Figure 4 shows the 10-year Kaplan-Meier estimates (± 95% CI) of the proportion of patients with LRR by RS, type of initial LR treatment, and age. For patients treated with L + XRT the rates of LRR appeared considerably higher in younger women than in older women compared with the findings in women treated with mastectomy. In addition, for patients treated with mastectomy there was a consistent association between RS and risk of LRR but for those treated with L + XRT, that association was less straightforward. In the multivariate Cox model that included age, clinical tumor size, tumor grade, type of initial LR treatment, RS, and their interaction (Appendix Table A3), the interaction between RS risk group and type of initial LR treatment was statistically significant (P = .036). However, the interaction between RS, as a continuous variable, and type of initial LR treatment was not statistically significant (P = .166).
Fig 4.
Ten-year Kaplan-Meier estimates of the proportions of locoregional recurrence (LRR) according to recurrence score (RS), initial locoregional treatment, and age in the 895 tamoxifen-treated patients in National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14/B-20 trials. XRT, radiation therapy.
DISCUSSION
Our study demonstrates that in node-negative, ER-positive, tamoxifen-treated patients there is a significant association between the 21-gene RS and risk for LRR, similar to that demonstrated between RS and distant recurrence in the same group of patients. Given the strong association between LRR and distant recurrence, our findings are not surprising. Recognizing that our study is exploratory and hypothesis generating, and requires independent confirmation by other datasets (preferably with a prospective design), this is the first demonstration of such an association based on a sizeable population of patients from randomized clinical trials of adjuvant therapy.
Similar associations have been reported in smaller studies with other genomic profiles. Cheng et al15 explored the association between gene expression profiles and LRR in 94 patients with breast cancer who underwent mastectomy without radiotherapy. They identified two sets of gene expression profiles that were independent predictors of LRR (one with 258 and another with 34 genes). They concluded that these gene expression-based predictive indexes can be used to select patients for postmastectomy radiotherapy. In contrast, Nuyten et al16 evaluated microarray-based gene expression profiles with proven value in predicting metastasis-free and overall survival (wound-response signature, 70-gene prognosis profile and hypoxia-induced profile) as predictors of LRR in patients treated with breast conserving surgery and RT. Only the wound-response signature (after gene set enrichment analysis) independently separated patients at high (29%) versus low (5%) risk of LRR at 10 years.
It is interesting that, similarly to the previously shown significant association between RS and distant recurrence in placebo-treated patients,21 we also observed a significant, but less robust, association between RS and LRR in placebo-treated patients. This is not surprising given that the RS contains genes (such as ER) that are only weakly prognostic but highly predictive of tamoxifen benefit. In fact, a significant reduction in distant recurrence was observed with tamoxifen in B-14 for patients with low and intermediate RS but not in those with high RS.22 Our data also indicate that the RS was significantly associated with LRR in chemotherapy plus tamoxifen–treated patients, but again, this association was less robust than that observed in tamoxifen-treated patients, possibly reflecting a differential effect of chemotherapy in reducing LRR by RS categories, similar to that shown with distant recurrence.8
Our exploratory data on LRR according to RS, age, and initial LR treatment reveal a complex relationship. Results from multivariate Cox models that include interaction terms suggest that there are significant interactions between type of initial LR treatment and RS group and type of initial LR treatment and patient age after adjusting for tumor size and tumor grade (Fig 4 and Appendix Table A3). Interestingly, an interaction between age and type of initial LR treatment is also observed in the entire population of B-14 and B-20 tamoxifen-treated patients (Appendix Tables A4 and A5, online only).
Our results further suggest that in tamoxifen-treated patients who undergo mastectomy, the association between RS and LRR is straightforward and independent of age. Patients with low RS had very low 10-year rates of LRR whether they were younger than 50 years of age (1.5%) or ≥ 50 years (2.6%). In contrast, the association was less straightforward in patients treated with L + XRT, where patients younger than 50 years with a low RS had still a 12.5% 10-year rate of LRR (mostly in-breast recurrences) versus a rate of 3.6% in those ≥ 50 years with low RS. Whether this difference is the result of chance alone, undetected synchronous multicentric foci in the premenopausal breast, lower efficacy of radiation in the younger group, higher rates of second primary cancers in the ipsilateral breast, or an enhanced ability of residual cells to grow and proliferate in premenopausal breast tissue cannot be determined from our data set. One possible explanation for the apparent different patterns of association between RS and LRR in mastectomy versus L + XRT-treated patients is that the effect of radiation may not be uniform across RS categories but that radiation may be more effective as RS increases. This is also suggested by the two previously mentioned studies in which genomic profiling appeared to discriminate better in mastectomy-treated patients (without RT) than in patients treated with L + XRT.
In summary, similar to the association between RS and risk for distant recurrence, a significant association also exists between RS and risk for LRR. Although these observations may or may not apply to other endocrine therapy or chemotherapy regimens, these results have biologic and potential clinical implications for LR therapy decisions for patients with node-negative, ER-positive breast cancer. Although we identified high-risk subgroups in which regional RT after lumpectomy or chest wall/regional RT after mastectomy may be entertained (eg, for patients < 50 with high RS), such patients would currently receive chemotherapy plus hormone therapy, which reduces their risk for LRR. However, as the evaluation of RS expands to node-positive patients,9 the association between RS and LRR, if also shown in this group, could become important in identifying subgroups with one to three or ≥ four positive nodes at low versus high risk for LRR who may or may not need chest wall and/or regional radiotherapy.
Acknowledgment
Bernard Fisher, MD, was responsible for the concept, design, and initial published findings from the B-14 and B-20 trials, which provided the opportunity for this study. We thank Barbara C. Good, PhD, for editorial assistance with this manuscript.
Appendix
This section is the summary of patient eligibility for inclusion in the development and validation of the 21-gene Oncotype DX recurrence score (RS) assay, methods of RNA extraction, reverse transcription polymerase chain reaction (RT-PCR) methodology used, and genes included in the RS.
Gene expression in fixed paraffin-embedded tumor tissue was performed by Genomic Health, Inc, using the previously described Oncotype DX assay.5,23 After RNA extraction and DNase I treatment, total RNA content was measured, and the absence of DNA contamination was verified. Gene-specific reverse transcription was performed, followed by quantitative TaqMan RT-PCR reactions in 384 well plates using Applied Biosystems PRISM 7900HT (Applied Biosystems, Foster City, CA) instruments.
Expression of each gene was measured in triplicate and normalized relative to a set of five reference genes (beta-actin, GAPDH, GUS, RPLPO, and TFRC). Reference-normalized expression measurements range from 0 to 15; a one unit increase reflects approximately a two-fold increase in RNA. The RS is calculated on a scale from 0 to 100 and is derived from the reference-normalized expression measurements for the 16 cancer-related genes (Ki67, STK15, Survivin or BIRC5, CCNB1 or cyclin B1, MYBL2, GRB7, HER2, ER, PGR, BCL2, SCUBE2, MMP11 or stromelysin 3, CTSL2 or cathepsin L2, GSTM1, CD68, and BAG1) and the five reference genes.
The cutoff points were prespecified before the performance of the validation study of the RS,5 which categorized patients into low RS (RS < 18), intermediate RS (18 to 30), and high RS (≥ 31) groups, which were also prespecified in this study.
Table A1.
Distribution of Demographic, Clinical, and Treatment Characteristics of Patients in NSABP Trial B-14 Who Had Assessment of Recurrence Score and Those Who Did Not
| Characteristic | Recurrence Score Population in B-14 |
Remaining B-14 Population |
All NSABP B-14 Population |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| No. of patients | 1,023 | 3,005 | 4,028 | |||
| Treatment enrollment | ||||||
| Placebo | 355 | 35 | 1,058 | 35 | 1,413 | 35 |
| TAM randomly assigned | 290 | 28 | 1,114 | 37 | 1,404 | 35 |
| TAM registered | 378 | 37 | 833 | 28 | 1,213 | 30 |
| Age at enrollment, years | ||||||
| < 50 | 298 | 29 | 947 | 32 | 1,245 | 31 |
| 50 ≤ <60 | 292 | 29 | 912 | 30 | 1,204 | 30 |
| ≥ 60 | 433 | 42 | 1,146 | 38 | 1,579 | 39 |
| Race | ||||||
| White | 933 | 91 | 2,711 | 90 | 3,644 | 90 |
| Black | 48 | 5 | 151 | 5 | 199 | 5 |
| Other | 42 | 4 | 143 | 5 | 185 | 5 |
| Clinical tumor size, cm | ||||||
| 0-1.0 | 153 | 15 | 536 | 18 | 689 | 17 |
| 1.1-2.0 | 448 | 44 | 1,347 | 45 | 1,795 | 45 |
| 2.1-4.0 | 369 | 36 | 996 | 33 | 1,365 | 34 |
| 4.1+ | 53 | 5 | 126 | 4 | 179 | 4 |
| Initial LR treatment | ||||||
| L + XRT | 393 | 38 | 1,186 | 39 | 1,579 | 39 |
| Mastectomy | 630 | 62 | 1,819 | 61 | 2,449 | 61 |
Abbreviations: NSABP, National Surgical Adjuvant Breast and Bowel Project; TAM, tamoxifen; L, lumpectomy; XRT, radiation therapy; LR, locoregional.
Table A2.
Distribution of Demographic, Clinical, and Treatment Characteristics of Patients in NSABP Trial B-20 Who Had Assessment of Recurrence Score and Those Who Did Not
| Characteristic | Recurrence Score Population in B-20 |
Remaining B-20 Population |
NSABP B-20 Population |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| No. of patients | 651 | 1,648 | 2,299 | |||
| Treatment enrollment | ||||||
| TAM only | 227 | 35 | 543 | 33 | 770 | 33 |
| TAM + chemotherapy | 290 | 65 | 1,239 | 67 | 1,529 | 67 |
| Age at enrollment, years | ||||||
| < 50 | 289 | 44 | 752 | 46 | 1,041 | 45 |
| 50 ≤ < 60 | 166 | 26 | 478 | 29 | 644 | 28 |
| ≥ 60 | 196 | 30 | 418 | 25 | 614 | 27 |
| Race | ||||||
| White | 593 | 91 | 1,430 | 87 | 2,023 | 88 |
| Black | 33 | 5 | 102 | 6 | 135 | 6 |
| Other | 25 | 4 | 116 | 7 | 141 | 6 |
| Clinical tumor size, cm | ||||||
| 0-1.0 | 110 | 17 | 341 | 21 | 451 | 20 |
| 1.1-2.0 | 318 | 49 | 801 | 49 | 1,119 | 49 |
| 2.1-4.0 | 196 | 30 | 423 | 26 | 619 | 27 |
| 4.1+ | 24 | 4 | 55 | 3 | 79 | 3 |
| Unknown | 3 | 0 | 28 | 2 | 31 | 1 |
| Initial LR treatment | ||||||
| L + XRT | 277 | 43 | 750 | 46 | 1,027 | 45 |
| Mastectomy | 374 | 57 | 898 | 54 | 1,272 | 55 |
Abbreviations: NSABP, National Surgical Adjuvant Breast and Bowel Project; TAM, tamoxifen; L, lumpectomy; XRT, radiation therapy.
Table A3.
Multivariate Cox Regression Analysis of Predictors of LRR in the Cohort of Tamoxifen-Treated Patients in NSABP Trials B-14 and B-20 (n = 895)
| Variable | Hazard Ratio | 95% CI | Wald Test P |
|---|---|---|---|
| Age (≥ 50 v < 50) | 0.24 | 0.12 to 0.46 | < .0001 |
| Mastectomy v L + XRT | 0.26 | 0.1 to 0.69 | .007 |
| Clinical tumor size (> 2 v ≤ 2 cm) | 1.05 | 0.65 to 1.69 | .845 |
| Tumor grade | |||
| Moderate v well | 0.91 | 0.48 to 1.75 | .159 |
| Poor v well | 1.54 | 0.78 to 3.06 | |
| RS | |||
| Intermediate risk (v low risk) | 2.16 | 0.98 to 4.75 | .143 |
| High risk (v low risk) | 1.67 | 0.78 to 3.58 | |
| Interaction | |||
| Initial LR treatment and RS intermediate risk* | 0.73 | 0.19 to 2.83 | .039 |
| Initial LR treatment and RS high risk* | 3 | 1.01 to 8.98 | |
| Initial LR treatment and age | 2.75 | 1.03 to 7.29 | .043 |
Abbreviations: LRR, locoregional recurrence; NSABP, National Surgical Adjuvant Breast and Bowel Project; L, lumpectomy; XRT, radiation therapy; RS, recurrence score.
P value for the likelihood ratio test for the interaction between type of initial LR treatment and RS risk groups is .036.
Table A4.
Multivariate Cox Regression Analysis of Predictors of Locoregional Recurrence in the Cohort of Tamoxifen-Treated Patients in the NSABP B-14 Trial (n = 2,615)
| Variable | Hazard Ratio | 95% CI | Wald Test P |
|---|---|---|---|
| Age (≥ 50 v < 50) | 0.34 | 0.23 to 0.51 | < .001 |
| Mastectomy v L + XRT | 0.45 | 0.29 to 0.69 | < .001 |
| Clinical tumor size (> 2 v ≤ 2 cm) | 1.43 | 1.06 to 1.92 | .018 |
| Interaction: initial LR treatment and age* | 2.01 | 1.12 to 3.63 | .02 |
Abbreviations: NSABP, National Surgical Adjuvant Breast and Bowel Project; L, lumpectomy; XRT, radiation therapy.
The P value for the likelihood ratio test for the interaction between type of initial treatment and age is .019.
Table A5.
Multivariate Cox Regression Analysis of Predictors of Locoregional Recurrence in the Cohort of Tamoxifen-Treated Patients in the NSABP B-20 Trial (n = 655)
| Variable | Hazard Ratio | 95% CI | Wald Test P* |
|---|---|---|---|
| Age (≥ 50 v < 50 years) | 0.26 | 0.12 to 0.55 | < .001 |
| Mastectomy v L + XRT | 0.23 | 0.1 to 0.5 | < .001 |
| Clinical tumor size (> 2 v ≤ 2 cm) | 1.58 | 0.89 to 2.8 | .004 |
| Tumor grade | |||
| Moderate v well | 0.91 | 0.39 to 2.14 | |
| Poor v well | 2.34 | 1 to 5.49 | .05 |
| Interaction: initial LR treatment and age* | 4.03 | 1.21 to 13.41 | .023 |
Abbreviations: NSABP, National Surgical Adjuvant Breast and Bowel Project; L, lumpectomy; XRT, radiation therapy; LR, locoregional recurrence.
The P value for the likelihood ratio test for the interaction between type of initial treatment and age is .021.
Footnotes
Supported in part by Public Health Service Grants No. U10CA-12027, U10CA-69974, U10CA-37377, and U10CA-69651 from the National Cancer Institute, Department of Health and Human Services.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: CT# PDQ: NSABP-14, PDQ: NSABP-20.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Steven Shak, Genomic Health, Inc (C); Drew Watson, Genomic Health, Inc (C) Consultant or Advisory Role: Eleftherios P. Mamounas, Genomic Health, Inc (C), Agendia (C) Stock Ownership: Steven Shak, Genomic Health, Inc; Drew Watson, Genomic Health, Inc Honoraria: Eleftherios P. Mamounas, Genomic Health, Inc; D. Lawrence Wickerham, AstraZeneca, Eli Lilly Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Eleftherios P. Mamounas, Gong Tang, Steven Shak, Soonmyung Paik, Norman Wolmark
Financial support: Steven Shak
Administrative support: Joseph P. Costantino, Norman Wolmark
Provision of study materials or patients: Eleftherios P. Mamounas, Soonmyung Paik, D. Lawrence Wickerham
Collection and assembly of data: Eleftherios P. Mamounas, Gong Tang, Soonmyung Paik, Steven Shak, Joseph P. Costantino, Drew Watson, D. Lawrence Wickerham
Data analysis and interpretation: Eleftherios P. Mamounas, Gong Tang, Soonmyung Paik, Steven Shak, Drew Watson, Charles E. Geyer Jr
Manuscript writing: Eleftherios P. Mamounas, Gong Tang, Steven Shak, Soonmyung Paik, Joseph P. Costantino, Charles E. Geyer Jr, D. Lawrence Wickerham
Final approval of manuscript: Eleftherios P. Mamounas, Gong Tang, Bernard Fisher, Steven Shak, Soonmyung Paik, Joseph P. Costantino, Drew Watson, Charles E. Geyer Jr, D. Lawrence Wickerham, Norman Wolmark
REFERENCES
- 1.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van De Vijver M, He YD, Van t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 3.van 't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 4.Foekens JA, Atkins D, Zhang Y, et al. Multicenter validation of a gene expression-based prognostic signature in lymph node-negative primary breast cancer. J Clin Oncol. 2006;24:1665–1671. doi: 10.1200/JCO.2005.03.9115. [DOI] [PubMed] [Google Scholar]
- 5.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 6.Dowsett M, Cuzick J, Wales C, et al. Risk of distant recurrence using Oncotype DX in postmenopausal primary breast cancer patients treated with anastrozole or tamoxifen: A TransATAC study. Cancer Res. 2009;69(suppl):75s. abstract 53. [Google Scholar]
- 7.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8:R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 9.Albain KS, Barlow W, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal, node-positive, ER-positive breast cancer (S8814, INT0100) Br Cancer Res Treat. 2007;106(suppl 1) abstr 10. [Google Scholar]
- 10.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology Breast Cancer, (version 2.2008) http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 12.Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028–2037. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 13.Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: Results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22:4247–4254. doi: 10.1200/JCO.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 14.Mamounas E, Tang G, Bryant J, et al. Association between the 21-gene recurrence score assay (RS) and risk of locoregional failure in node-negative, ER-positive breast cancer: Results from NSABP B-14 and NSABP B-20. Br Ca Res Treat. 2005;94:S18. abstr 29. [Google Scholar]
- 15.Cheng SH, Horng C-F, West M, et al. Genomic prediction of locoregional recurrence after mastectomy in breast cancer. J Clin Oncol. 2006;24:4594–4602. doi: 10.1200/JCO.2005.02.5676. [DOI] [PubMed] [Google Scholar]
- 16.Nuyten DS, Kreike B, Hart AA, et al. Predicting a local recurrence after breast-conserving therapy by gene expression profiling. Breast Cancer Res. 2006;8:R62. doi: 10.1186/bcr1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: Updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684–690. doi: 10.1093/jnci/93.9.684. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89:1673–1682. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: Long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet. 2004;364:858–868. doi: 10.1016/S0140-6736(04)16981-X. [DOI] [PubMed] [Google Scholar]
- 21.Paik S, Shak S, Tang G, et al. Expression of the 21 genes in the recurrence score assay and tamoxifen clinical benefit in the NSABP study B-14 of node negative, estrogen receptor positive breast cancer. J Clin Oncol. 2005;23:6s. abstr 510. [Google Scholar]
- 22.Paik S, Shak S, Tang G, et al. Expression of the 21 genes in the recurrence score assay and prediction of the clinical benefit from tamoxifen in NSABP study B-14 and chemotherapy in NSABP study B-20. Br Ca Res Treat. 2004;88:S15. abstr 24. [Google Scholar]
- 23.Cronin M, Pho M, Dutta D, et al. Measurement of gene expression in archival paraffin-embedded tissues: Development and performance of a 92-gene reverse transcriptase-polymerase chain reaction assay. Am J Pathol. 2004;164:35–42. doi: 10.1016/S0002-9440(10)63093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]