Abstract
Purpose
We examined how an aerobic exercise intervention influenced circulating estradiol, estrone, sex hormone–binding globulin (SHBG), androstenedione, and testosterone levels, which may be involved in the association between physical activity and breast cancer risk.
Methods
A two-center, two-arm randomized controlled trial of exercise was conducted in 320 postmenopausal, sedentary women age 50 to 74 years. Participants were randomly assigned to a 1-year aerobic exercise intervention of 225 min/wk (n = 160) or to a control group who maintained their usual level of activity (n = 160). Baseline, 6-month, and 12-month assessments of estrone, estradiol, androstenedione, and testosterone were quantified by radioimmunoassay after extraction, and SHBG was quantified by an immunometric assay. Intent-to-treat analyses were performed using linear mixed models.
Results
Blood data were available on 309 women (96.6%) at 12 months. Women in the intervention group exercised an average of 3.6 d/wk for 178 min/wk. At 12 months, statistically significant reductions in estradiol (treatment effect ratio [TER] = 0.93; 95% CI, 0.88 to 0.98) and free estradiol (TER = 0.91; 95% CI, 0.87 to 0.96) and increases in SHBG (TER = 1.04; 95% CI, 1.02 to 1.07) were observed in the exercise group compared with the control group. No significant differences in estrone, androstenedione, and testosterone levels were observed between exercisers and controls at 12 months.
Conclusion
This trial found that previously sedentary postmenopausal women can adhere to a moderate- to vigorous-intensity exercise program that results in changes in estradiol and SHBG concentrations that are consistent with a lower risk for postmenopausal breast cancer.
INTRODUCTION
Consistent observational epidemiologic evidence suggests that physical activity is associated with reduced postmenopausal breast cancer risk.1–3 Several plausible biologic mechanisms have been hypothesized to underlie this association, but none have been established yet.4 Multiple inter-related mechanisms are likely operative,5 including changes in metabolic and sex hormones,6 growth factors,7 adiposity,8 and possibly immune function.9
One of the most plausible mechanisms is via the modification of sex hormone levels. High endogenous estrogen and androgen levels are fairly consistently associated with an increase in breast cancer risk,10–12 whereas increased sex hormone–binding globulin (SHBG) levels are associated with a decrease in risk.12 Previous studies in postmenopausal women, all but two of which were observational, have also observed that serum sex hormone concentrations decrease12–19 and SHBG concentrations increase13,20 with increasing physical activity; several mechanisms may be involved, including preventing weight gain after menopause.13–16,21,22
To provide more direct evidence regarding the biologic mechanisms than observational studies can provide, we conducted the Alberta Physical Activity and Breast Cancer Prevention Trial, a two-center, two-arm randomized controlled trial that examined how a 1-year aerobic exercise intervention, compared with a usual sedentary lifestyle, influences sex hormone concentrations, body composition, mammographic density, insulin-like growth factors, insulin resistance, and markers of obesity and inflammation. In this article, the first from this trial, we report the effects of the exercise intervention on sex hormone concentrations, which were the primary outcomes. Subsequent reports will present results for the other outcomes.
METHODS
Setting and Participants
This trial was conducted at the Westside Recreation Centre in Calgary and at the University of Alberta in Edmonton, Canada. The study and protocol were approved by the institutional review boards at the Alberta Cancer Board and the Universities of Calgary and Alberta, and all participants provided written informed consent. Women from the general population were recruited through targeted mailings to participants in the Alberta Breast Screening Program, via the Alberta Family Physicians Research Network, and through media campaigns. Eligibility criteria were as follows: English speaking; resident of Calgary or Edmonton; age 50 to 74 years; postmenopausal for at least 24 months; no previous cancer diagnosis besides nonmelanotic skin cancer; no major comorbidities; acceptable heart and lung function as assessed by a baseline fitness test; sedentary (< 90 min/wk recreational activity or, if between 90 and 120 min/wk of physical activity, having a maximal oxygen uptake < 34.5 mL/kg/min); able to undertake unrestricted physical activity as assessed by physician23; normal levels of fasting lipids, thyroid-stimulating hormone, and ALT; body mass index between 22 and 40 kg/m2; breast tissue density greater than a zero density level; nonsmoker; alcohol intake less than 14 drinks per week; no medications or exogenous hormones that might influence estrogen metabolism or breast tissue growth; not currently or planning to undertake a weight loss program; and not planning extended absences in the 18 months subsequent to enrollment. Eligibility was determined in several stages and included telephone screening, completion of questionnaires at an information session, a baseline mammogram, physician approval, blood screening, and a submaximal fitness test.
Random Assignment and Blinding
The random assignment sequence was created by the study biostatistician (R.F.B.) using a random number generator program in S-Plus (Version 3.3; Statistical Sciences, Seattle, WA). Random assignment was stratified by center (Calgary or Edmonton) and body mass index (< or ≥ 27.5 kg/m2), with blocks of random size between four and six. Numbered sealed envelopes were opened by the research coordinator in Calgary at the time of random assignment. All assessments of outcome measures were blinded.
Intervention
The exercise intervention was a monitored, structured program of at least 45 minutes of aerobic exercise performed 5 d/wk for 12 months at 70% to 80% heart rate reserve. At least three sessions per week were facility based with on-site exercise trainers, and the remainder of the sessions were home based. During the first 3 months of the intervention, the frequency, duration, and intensity of exercise were gradually increased from the starting level of three sessions a week of 15 to 20 minutes in duration at an intensity of 50% to 60% heart rate reserve to the final prescription achieved in week 12. Participants were instructed to perform at least half of their total workout time within their target heart rate zone. Any exercise could be performed to reach the required intensities.
Interim submaximal fitness tests were performed in the exercise group at weeks 12, 24, 36, and 48 to monitor progress, maintain motivation, and adjust individual exercise prescriptions to ensure continual training effects. Women in the control group were asked to maintain their regular sedentary lifestyle. Both exercise and control participants were also asked not to change their usual diet.
Several methods to increase adherence with the exercise intervention were implemented, including an individualized exercise program, with regularly scheduled sessions and automatic follow-up for missed sessions; plans for sessions missed because of vacations or illness; a comprehensive educational package; group sessions; social interaction; donated incentives awarded at different milestones; regular newsletters; and a study Web site. Control participants received newsletters and a 1-month pass to the fitness center at the end of the study, the educational materials, and time with fitness trainers to plan their own exercise program.
Exercise participants wore heart rate monitors (Polar A3; Polar, Lake Success, NY) and recorded their activities in weekly exercise logs that included type of activity, minutes of exercise completed, average heart rate, and Borg Rating of Perceived Exertion.24 Adherence was additionally monitored by the exercise trainers who kept separate logs of the same exercise variables. All situations of vacation, illness, or injury were recorded and tracked by the exercise trainers.
Baseline and End-of-Study Measures
Baseline data on demographics, medical and reproductive history, postmenopausal hormone use, and medication and nonprescription drug use were obtained from a self-administered questionnaire. At baseline and the 12-month follow-up, past year dietary intake was obtained using the US National Cancer Institute's 124-item Diet History Questionnaire adapted for use in Canadian populations,25 and physical activity was obtained using the Past Year Total Physical Activity Questionnaire.26 Physical fitness was assessed at baseline and at 12 months using a modified Balke treadmill protocol to estimate maximum oxygen consumption from submaximal exercise intensities. Oxygen consumption at the age-predicted maximum heart rate was estimated by extrapolating from the two last completed stages using the American College of Sports Medicine metabolic equations for estimating oxygen consumption at the workload of each stage.27
Blood Collection and Hormone Assays
Blood was collected after a minimum 10-hour fast at baseline (60 mL) and 6 and 12 months (40 mL each) after random assignment, and all medications taken in the past 24 hours were recorded. Blood collections occurred at the Calgary Laboratory Service clinic at the Tom Baker Cancer Centre and at the Cross Cancer Institute in Edmonton. All blood samples were collected, processed, and stored within 12 hours of collection. Blood samples were shipped and stored in our −86°C freezers until the time of the assays.
Laboratory assays were conducted at the Reproductive Endocrine Research Laboratory (University of Southern California, Los Angeles, CA). Each participant's samples from the three time points were included in a single batch, but the order was randomized. Each batch also had an equal number of samples from exercise and control participants with similar random assignment dates and two pooled quality control samples. Total estrone, estradiol, androstenedione, and testosterone levels were quantified by radioimmunoassay after organic solvent extraction and Celite (CAS 68855-54-9; Mallinckrodt Baker, Paris, KY) column partition chromatography.28–30 Chromatographic separation of the steroids was achieved by using different concentrations of toluene in iso-octane and ethyl acetate in iso-octane. We quantified SHBG via an immunometric assay using the Immulite Analyzer (Siemens Diagnostic Products, Los Angeles, CA). The SHBG concentration and an assumed albumin concentration of 43 g/L were then used in a validated algorithm with total testosterone or total estradiol to calculate free testosterone or free estradiol, respectively.31,32
For all analytes, the intrabatch coefficients of variation ranged between 4.0% and 7.5%, whereas the interbatch coefficients of variation ranged between 8.0% and 13%. The lower limits of detection for the assays for androstenedione, testosterone, estrone, estradiol, and SHBG were 0.03 ng/mL, 1.5 ng/dL, 5 pg/mL, 3 pg/mL, and 1 nmol/L, respectively. No woman had undetectable levels.
Statistical Analysis and Sample Size
The sample size calculations were based on the normal theory formula for comparing means of two independent samples.33 Taking a conservative approach, we considered the comparison of means of 12-month outcomes (log transformed, with no adjustment for baseline values) for the estrogen outcomes. Using preliminary results from the only previous trial14,34 for the standard deviations (SDs), a sample size of 150 participants per group was selected for an 80% power to detect anticipated changes of 10% to 20% in the three estrogen outcomes at α = .05. We enrolled 160 participants per group to allow for study dropouts.
The primary analysis assessed the intervention effect based on assigned group at random assignment regardless of adherence for all participants with complete data at baseline and end of study (intent to treat). All hormones were non-normally distributed, and therefore, analyses were performed on log-transformed values. The intervention effects were evaluated with linear mixed models considering the sex hormone measures at 6 and 12 months as repeated measures. The models included main effects of intervention and time, as well as their interaction term, and were adjusted for baseline values. The intervention effect was denoted by a geometric mean ratio for the exercise group over the control group from the mixed models.
As a secondary analysis, we examined whether or not the effect of exercise on hormone concentrations varied by adherence. We classified exercise adherence into the following three categories predefined by public health guidelines35,36: less than 150, 150 to 225, and more than 225 min/wk. To explore the possibility that the effect of exercise on changes in sex hormones was mediated by weight change, we further adjusted our models for weight change at 12 months. All statistical tests were two-sided, with a level of significance set at P = .05. Statistical analyses were performed using SAS software (Version 9.1; SAS Institute, Cary, NC).
RESULTS
Study Participants
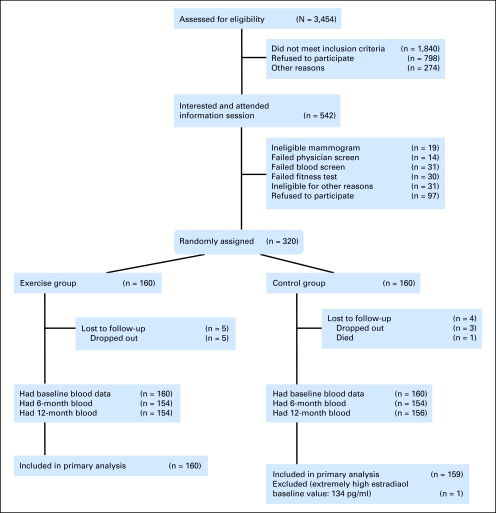
A total of 3,454 women were assessed for eligibility out of the 4,543 sent letters of invitation and the 2,170 women who responded after the media campaigns and other recruitment methods (Fig 1). Of these women, 1,840 did not meet the inclusion criteria, 798 refused participation, and 274 were excluded for other reasons including loss of contact. The main reasons for refusal to participate were lack of interest in the study (n = 574), unwillingness to be randomly assigned (n = 105), withdrawal before the information session (n = 48), and lack of time (n = 71). Of the 542 women who attended an information session, 106 were ineligible after additional screening tests, 97 decided against participating, and 320 women were randomly assigned. Nine women were lost to follow-up after random assignment, and no adverse effects related to the intervention occurred. The primary analysis included 160 exercisers and 159 control participants. Recruitment began in May 2003 and was completed in June 2006, and 12-month follow-up of the study participants ended in July 2007. The two groups were similar at baseline with respect to demographic characteristics, body composition, and sex hormone concentrations (Table 1).
Fig 1.
CONSORT diagram showing recruitment of participants into the Alberta Physical Activity and Breast Cancer Prevention Trial.
Table 1.
Baseline Demographics and Clinical Characteristics of Participants in the ALPHA Trial, Alberta, Canada, 2003-2007
| Characteristic | Exercisers (n = 160) | Controls (n = 160) |
|---|---|---|
| Age, years | ||
| Mean | 61.2 | 60.6 |
| SD | 5.4 | 5.7 |
| Body mass index, kg/m2 | ||
| Mean | 29.1 | 29.2 |
| SD | 4.5 | 4.3 |
| Alcohol intake, g/d | ||
| Mean | 4.4 | 5.0 |
| SD | 5.9 | 7.6 |
| Total energy intake, kcal/d | ||
| Mean | 1,551.2 | 1,527.3 |
| SD | 598.7 | 535.0 |
| Maximal oxygen consumption, mL/kg/min | ||
| Mean | 27.1 | 26.8 |
| SD | 6.2 | 6.0 |
| Past year total physical activity, MET-h/wk | ||
| Total physical activity | ||
| Mean | 114.2 | 129.1 |
| SD | 57.6 | 77.9 |
| Occupational activity | ||
| Mean | 50.4 | 52.2 |
| SD | 49.1 | 57.9 |
| Household activity | ||
| Mean | 52.9 | 63.9 |
| SD | 34.3 | 53.5 |
| Recreation activity | ||
| Mean | 10.2 | 12.1 |
| SD | 11.8 | 13.6 |
| Full-time employment | ||
| No. of patients | 82 | 79 |
| % | 55 | 51 |
| Education > high school | ||
| No. of patients | 112 | 102 |
| % | 70 | 64 |
| Married/common law | ||
| No. of patients | 113 | 125 |
| % | 71 | 78 |
| White race | ||
| No. of patients | 144 | 145 |
| % | 91 | 91 |
| First-degree family history of breast cancer | ||
| No. of patients | 31 | 35 |
| % | 19 | 22 |
| Ever used hormone therapy | ||
| No. of patients | 75 | 71 |
| % | 47 | 44 |
| Sex hormone–binding globulin, nmol/L | ||
| Median | 41.5 | 39.5 |
| Quartile 1-3 | 30.0-57.0 | 28.0-51.5 |
| Estrone, pg/mL | ||
| Median | 32.0 | 32.0 |
| Quartile 1-3 | 24.0-44.0 | 22.5-44.5 |
| Estradiol, pg/mL | ||
| Median | 9.0 | 10.0 |
| Quartile 1-3 | 7.0-12.0 | 7.0-14.0 |
| Free estradiol, pg/mL | ||
| Median | 0.23 | 0.24 |
| Quartile 1-3 | 0.17-0.31 | 0.18-0.36 |
| Androstenedione, pg/mL | ||
| Median | 576 | 547 |
| Quartile 1-3 | 434-779 | 417-736 |
| Testosterone, ng/dL | ||
| Median | 24.3 | 23.3 |
| Quartile 1-3 | 16.8-32.8 | 17.5-32.4 |
| Free testosterone, ng/dL | ||
| Median | 0.36 | 0.36 |
| Quartile 1-3 | 0.24-0.48 | 0.25-0.49 |
Abbreviations: ALPHA, Alberta Physical Activity and Breast Cancer Prevention Trial; SD, standard deviation; MET, metabolic equivalent.
Exercise Adherence
On their weekly logs, exercisers recorded a total of 29,872 individual exercise sessions noting 63 different activities (Table 2). At the fitness center, the most common activity was walking on the treadmill (51%), whereas for home-based activity, walking was the most frequent activity (59%). The exercisers recorded a mean of 3.6 sessions per week (SD = 1.3 sessions per week) for an average duration of 178.4 min/wk (SD = 76.1 min/wk). Objectively measured average heart rate was 80.1% (SD = 5.1%) of estimated heart rate maximum and 62.2% (SD = 9.8%) of estimated heart rate reserve. The average Borg Rating of Perceived Exertion reported was 12.7 (“somewhat hard” on the scale that ranges from 6 for “no exertion” to 20 for “maximal exertion”), and the average estimated energy expenditure was 5.7 metabolic equivalent-hours (MET-h) per session (SD = 0.7 MET-h per session).37,38
Table 2.
Type of Physical Activity Recorded by Exercisers in the Daily Exercise Activity Logs
| Recorded Activity | No. of Observations Recorded on the Exercise Log | % |
|---|---|---|
| At fitness facility | ||
| Walking on treadmill | 10,273 | 50.8 |
| Bicycling | 2,723 | 13.5 |
| Elliptical trainer | 2,480 | 12.3 |
| Cross trainer | 2,101 | 10.4 |
| Walking on the track | 2,093 | 10.3 |
| Swimming | 235 | 1.2 |
| Calisthenics | 62 | 0.3 |
| Stationary rowing machine | 71 | 0.4 |
| Running | 33 | 0.2 |
| Rowing | 32 | 0.2 |
| Other | 133 | 0.7 |
| Total | 20,237 | |
| At home | ||
| Walking | 5,689 | 59.0 |
| Walking on treadmill | 870 | 9.0 |
| Bicycling | 717 | 7.4 |
| Elliptical trainer | 640 | 6.6 |
| Cross trainer | 295 | 3.1 |
| Swimming | 274 | 2.8 |
| Running | 105 | 1.1 |
| Calisthenics | 107 | 1.1 |
| Stair climbing | 89 | 0.9 |
| Nordic skiing | 63 | 0.7 |
| Gardening | 55 | 0.6 |
| Curling | 38 | 0.4 |
| Golfing | 31 | 0.3 |
| Tennis | 27 | 0.3 |
| Rollerblading | 20 | 0.2 |
| Jogging | 18 | 0.2 |
| Skating | 17 | 0.2 |
| Other | 522 | 5.4 |
| Total | 9,635 |
On the Past Year Total Physical Activity Questionnaire, exercisers reported a larger increase in recreational activity than controls (20.2 v 3.2 MET-h/wk, respectively; P < .0001; Table 3). Among the control group, 16 participants (10%) reported increasing their level of recreational activity by ≥ 20 MET-h/wk (200 min/wk of activity at ≥ 6 MET). Increases in physical fitness, as measured by estimated maximal oxygen consumption, were also significantly greater in exercisers than controls (3.9 v 0.7 mL/kg/min, respectively; P < .001). Energy intake decreased among controls by an average 161 kcal/d, whereas in exercisers, it decreased an average of 45 kcal/d (P = .01). Exercisers also lost −1.8 kg (P < .001) more body weight than controls.
Table 3.
Physical Activity, Fitness, Dietary, and Weight Changes at 12 Months From Baseline by Group Assignment
| Measure | No. of Exercisers | No. of Controls | Exercisers |
Controls |
Difference |
P | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||||
| Total activity, h/wk | 152 | 153 | 5.3 | 2.3 to 8.3 | 0.6 | −3.6 to 4.8 | 4.7 | −0.5 to 9.8 | .075 |
| Total activity, MET-h/wk | 152 | 153 | 27.8 | 18.7 to 36.8 | −0.2 | −12.9 to 12.6 | 28.0 | 12.4 to 43.5 | .001 |
| Occupational activity, h/wk | 152 | 153 | 1.2 | −1.0 to 3.5 | −0.5 | −3.4 to 2.5 | 1.7 | −2.0 to 5.4 | .355 |
| Occupational activity, MET-h/wk | 152 | 153 | 4.6 | −1.6 to 10.9 | −1.5 | −10.3 to 7.4 | 6.1 | −4.7 to 16.9 | .268 |
| Household activity, h/wk | 152 | 153 | 0.5 | −1.4 to 2.3 | −0.2 | −2.7 to 2.4 | 0.7 | −2.5 to 3.8 | .679 |
| Household activity, MET-h/wk | 152 | 153 | 2.6 | −2.5 to 7.8 | −2.4 | −9.9 to 5.2 | 5.0 | −4.1 to 14.1 | .282 |
| Recreation activity, h/wk | 152 | 153 | 3.5 | 2.8 to 4.1 | 1.2 | 0.4 to 1.9 | 2.3 | 1.3 to 3.3 | < .001 |
| Recreation activity, MET-h/wk | 152 | 153 | 20.2 | 17.3 to 23.1 | 3.2 | 0.7 to 5.7 | 17.0 | 13.2 to 20.9 | < .001 |
| Maximal oxygen consumption, mL/kg/min | 147 | 151 | 3.9 | 2.8 to 4.9 | 0.7 | −0.2 to 1.6 | 3.2 | 1.7 to 4.6 | < .001 |
| Total energy intake, kcal/d* | 150 | 153 | −44.8 | −107.4 to 17.8 | −161.3 | −225.3 to −97.3 | 116.5 | 27.4 to 205.7 | .011 |
| Body weight, kg | 152 | 155 | −2.3 | −2.9 to −1.7 | −0.5 | −1.0 to 0.1 | −1.8 | −2.6 to −1.0 | < .001 |
Abbreviation: MET, metabolic equivalent.
Estimated from food frequency questionnaire.
Estrogen and Androgen Outcomes
The reduction in estradiol concentrations at 12 months was greater among exercisers than controls (P = .004; Table 4). Estrone changes were no different between exercisers and controls at 12 months (P = .57). An effect of exercise on SHBG levels (P = .001) and free estradiol levels (P = .001) was also observed.
Table 4.
Differences in Sex Hormone Concentrations Between Exercisers and Controls at Baseline and the 6- and 12-Month Follow-Ups
| Hormone | Baseline |
6 Months |
12 Months |
Treatment Effect |
Between-Group P | ||||
|---|---|---|---|---|---|---|---|---|---|
| Geometric Mean | 95% CI | Geometric Mean | 95% CI | Geometric Mean | (95% CI) | Ratio of Exercise/Control* | 95% CI | ||
| Estradiol, pg/mL | .004 | ||||||||
| Exercisers† | 10.1 | 9.3 to 10.9 | 9.4 | 8.7 to 10.1 | 8.7 | 8.2 to 9.3 | 0.93 | 0.88 to 0.98 | |
| Controls‡ | 10.2 | 9.4 to 11.1 | 10.0 | 9.3 to 10.8 | 9.9 | 9.1 to 10.7 | |||
| Estrone, pg/mL | .569 | ||||||||
| Exercisers | 31.4 | 29.2 to 33.7 | 31.3 | 29.3 to 33.5 | 29.4 | 27.6 to 31.4 | 0.99 | 0.94 to 1.03 | |
| Controls | 31.3 | 29.1 to 33.7 | 31.2 | 29.0 to 33.5 | 30.6 | 28.5 to 32.9 | |||
| SHBG, nmol/L | .001 | ||||||||
| Exercisers | 40.3 | 37.5 to 43.4 | 43.1 | 40.2 to 46.3 | 41.9 | 38.9 to 45.1 | 1.04 | 1.02 to 1.07 | |
| Controls | 38.1 | 35.7 to 40.8 | 39.2 | 36.7 to 41.9 | 38.4 | 35.9 to 41.1 | |||
| Free estradiol, pg/mL | .001 | ||||||||
| Exercisers | 0.24 | 0.22 to 0.26 | 0.22 | 0.20 to 0.24 | 0.21 | 0.19 to 0.22 | 0.91 | 0.87 to 0.96 | |
| Controls | 0.25 | 0.23 to 0.27 | 0.24 | 0.22 to 0.26 | 0.24 | 0.22 to 0.26 | |||
| Androstenedione, pg/mL | .334 | ||||||||
| Exercisers | 578 | 539 to 621 | 566 | 527 to 607 | 572.0 | 537 to 610 | 0.98 | 0.93 to 1.03 | |
| Controls | 553 | 514 to 595 | 560 | 520 to 604 | 577.3 | 534 to 624 | |||
| Testosterone, ng/dL | .620 | ||||||||
| Exercisers | 23.9 | 22.3 to 25.8 | 23.7 | 21.9 to 25.6 | 23.4 | 21.7 to 25.3 | 0.99 | 0.95 to 1.03 | |
| Controls | 23.1 | 21.3 to 25.1 | 23.1 | 21.2 to 25.1 | 23.7 | 21.8 to 25.7 | |||
| Free testosterone, ng/dL | .094 | ||||||||
| Exercisers | 0.35 | 0.32 to 0.38 | 0.33 | 0.30 to 0.36 | 0.33 | 0.31 to 0.36 | 0.96 | 0.92 to 1.01 | |
| Controls | 0.35 | 0.32 to 0.38 | 0.34 | 0.31 to 0.37 | 0.35 | 0.33 to 0.39 | |||
Abbreviation: SHBG, sex hormone–binding globulin.
The geometric mean ratios were estimated from least square means for the difference in treatment effect between exercisers and controls averaged across the entire study period adjusted for the baseline values and then back log-transformed.
Exercise group: baseline, n = 160; 6 months, n = 154; 12 months, n = 154.
Control group: baseline, n = 159; 6 months, n = 153; 12 months, n = 155.
Exercise adherence was not consistently associated with monotonic reductions in estradiol or estrone concentrations (Table 5). However, exercisers who adhered to 150 to 225 min/wk had an 18% reduction in estradiol concentrations compared with controls (P = .002), and a trend of percent change in estradiol levels with increasing exercise adherence was observed (P = .005). Similar results were found for free estradiol levels. A linear trend of increasing SHBG levels with decreasing exercise adherence was found (P = .01). Greater increases in SHBG levels were observed among exercisers who adhered to 150 to 225 min/wk (4.8%; P = .035) or more than 225 min/wk (5.3%; P = .030) compared with controls (−0.2%). After adjustment for weight change at 12 months, the intervention effect remained significant for estradiol (P = .01) and free estradiol (P = .006), whereas the SHBG results became nonsignificant (P = .29; Table 6).
Table 5.
Hormone Concentrations at Baseline and 12 Months in Controls and Exercisers by Three Adherence Levels
| Hormone | Baseline |
12 Months |
Ratio of 12 Months/Baseline* |
% Change† | P‡ | P for Trend§ | |||
|---|---|---|---|---|---|---|---|---|---|
| Geometric Mean | 95% CI | Geometric Mean | 95% CI | Mean | 95% CI | ||||
| Estradiol, pg/mL | |||||||||
| Controls | 10.3 | 9.5 to 11.2 | 9.9 | 9.1 to 10.7 | 0.96 | 0.91 to 1.01 | −4.4 | Reference | .005 |
| Exercisers (< 150 min/wk) | 10.1 | 8.7 to 11.7 | 9.6 | 8.4 to 10.9 | 0.95 | 0.87 to 1.03 | −5.3 | .778 | |
| Exercisers (150-225 min/wk) | 10.6 | 9.3 to 12.0 | 8.6 | 7.8 to 9.6 | 0.82 | 0.73 to 0.91 | −18.2 | .002 | |
| Exercisers (> 225 min/wk) | 8.9 | 7.9 to 10.0 | 8.2 | 7.3 to 9.2 | 0.92 | 0.85 to 1.00 | −8.1 | .128 | |
| Estrone, pg/mL | |||||||||
| Controls | 31.5 | 29.3 to 34.0 | 30.6 | 28.5 to 32.9 | 0.97 | 0.92 to 1.02 | −2.9 | Reference | .366 |
| Exercisers (< 150 min/wk) | 30.6 | 26.0 to 36.0 | 29.0 | 25.2 to 33.5 | 0.95 | 0.87 to 1.04 | −5.2 | .549 | |
| Exercisers (150-225 min/wk) | 31.2 | 28.2 to 34.6 | 29.9 | 27.2 to 32.9 | 0.96 | 0.87 to 1.05 | −4.2 | .721 | |
| Exercisers (> 225 min/wk) | 31.3 | 27.6 to 35.5 | 29.0 | 25.8 to 32.7 | 0.93 | 0.86 to 1.00 | −7.1 | .337 | |
| SHBG, nmol/L | |||||||||
| Controls | 38.5 | 36.0 to 41.2 | 38.4 | 35.9 to 41.1 | 1.00 | 0.97 to 1.02 | −0.2 | Reference | .010 |
| Exercisers (< 150 min/wk) | 38.6 | 33.3 to 44.8 | 37.9 | 32.7 to 44.0 | 0.98 | 0.93 to 1.04 | −1.9 | .574 | |
| Exercisers (150-225 min/wk) | 41.4 | 37.0 to 46.4 | 43.4 | 38.5 to 49.0 | 1.05 | 1.00 to 1.09 | 4.8 | .035 | |
| Exercisers (> 225 min/wk) | 41.1 | 36.0 to 46.9 | 43.3 | 38.3 to 48.9 | 1.05 | 1.00 to 1.11 | 5.3 | .030 | |
| Free estradiol, pg/mL | |||||||||
| Controls | 0.25 | 0.23 to 0.27 | 0.24 | 0.22 to 0.26 | 0.96 | 0.91 to 1.01 | −4.4 | Reference | .001 |
| Exercisers (< 150 min/wk) | 0.24 | 0.21 to 0.29 | 0.23 | 0.20 to 0.27 | 0.95 | 0.87 to 1.04 | −4.8 | .857 | |
| Exercisers (150-225 min/wk) | 0.25 | 0.22 to 0.29 | 0.20 | 0.18 to 0.23 | 0.80 | 0.72 to 0.89 | −19.7 | < .001 | |
| Exercisers (> 225 min/wk) | 0.21 | 0.18 to 0.24 | 0.19 | 0.17 to 0.22 | 0.91 | 0.83 to 0.99 | −9.4 | .078 | |
| Androstenedione, pg/mL | |||||||||
| Controls | 556 | 517 to 599 | 577 | 534 to 623 | 1.04 | 0.98 to 1.10 | 3.7 | Reference | .602 |
| Exercisers (< 150 min/wk) | 588 | 501 to 689 | 548 | 477 to 630 | 0.93 | 0.85 to 1.03 | −6.7 | .116 | |
| Exercisers (150-225 min/wk) | 583 | 525 to 648 | 599 | 546 to 656 | 1.03 | 0.94 to 1.12 | 2.7 | .927 | |
| Exercisers (> 225 min/wk) | 557 | 489 to 634 | 554 | 493 to 624 | 1.00 | 0.90 to 1.11 | −0.5 | .466 | |
| Testosterone, ng/dL | |||||||||
| Controls | 23.5 | 21.7 to 25.4 | 23.7 | 21.8 to 25.7 | 1.01 | 0.97 to 1.05 | 0.8 | Reference | .549 |
| Exercisers (< 150 min/wk) | 23.6 | 20.3 to 27.5 | 22.7 | 19.3 to 26.7 | 0.96 | 0.89 to 1.03 | −3.9 | .274 | |
| Exercisers (150-225 min/wk) | 24.5 | 21.9 to 27.4 | 24.7 | 21.9 to 27.8 | 1.01 | 0.93 to 1.09 | 1.0 | .876 | |
| Exercisers (> 225 min/wk) | 22.9 | 20.0 to 26.3 | 22.3 | 19.4 to 25.7 | 0.97 | 0.91 to 1.04 | −2.7 | .351 | |
| Free testosterone, ng/dL | |||||||||
| Controls | 0.35 | 0.32 to 0.38 | 0.35 | 0.33 to 0.39 | 1.01 | 0.97 to 1.05 | 0.9 | Reference | .112 |
| Exercisers (< 150 min/wk) | 0.35 | 0.30 to 0.42 | 0.34 | 0.29 to 0.40 | 0.97 | 0.90 to 1.05 | −2.9 | .379 | |
| Exercisers (150-225 min/wk) | 0.35 | 0.31 to 0.39 | 0.34 | 0.30 to 0.39 | 0.98 | 0.89 to 1.07 | −2.2 | .446 | |
| Exercisers (> 225 min/wk) | 0.33 | 0.28 to 0.38 | 0.31 | 0.27 to 0.36 | 0.95 | 0.88 to 1.01 | −5.4 | .088 | |
NOTE. Controls, n = 155; exercisers (< 150 min/wk), n = 40; exercisers (150-225 min/wk), n = 67; and exercisers (> 225 min/wk), n = 47.
Abbreviation: SHBG, sex hormone–binding globulin.
Ratio of geometric means at 12 months to geometric means at baseline.
Percent hormone change at 12 months from baseline for that group.
P values for hormone change at 12 months from baseline between controls and the level of exercise adherence group adjusted for the baseline value.
Trend analysis for hormone change at 12 months from baseline between controls and three adherence groups adjusted for the baseline value.
Table 6.
Treatment Effect Ratio of Exercisers to Controls on Sex Hormone Concentrations Between Baseline and 12 Months Before and After Adjustment for Change in Body Weight
| Hormone | Treatment Effect Ratio of Exercisers/Controls* | 95% CI | P |
|---|---|---|---|
| Estradiol | |||
| No adjustment for weight change | 0.93 | 0.88 to 0.98 | .004 |
| Adjustment for weight change | 0.94 | 0.89 to 0.99 | .014 |
| Estrone | |||
| No adjustment for weight change | 0.99 | 0.94 to 1.03 | .569 |
| Adjustment for weight change | 0.99 | 0.94 to 1.04 | .637 |
| SHBG | |||
| No adjustment for weight change | 1.04 | 1.02 to 1.07 | .001 |
| Adjustment for weight change | 1.01 | 0.99 to 1.04 | .288 |
| Free estradiol | |||
| No adjustment for weight change | 0.91 | 0.87 to 0.96 | .001 |
| Adjustment for weight change | 0.93 | 0.88 to 0.98 | .006 |
| Androstenedione | |||
| No adjustment for weight change | 0.98 | 0.93 to 1.03 | .334 |
| Adjustment for weight change | 0.98 | 0.93 to 1.03 | .384 |
| Testosterone | |||
| No adjustment for weight change | 0.99 | 0.95 to 1.03 | .620 |
| Adjustment for weight change | 1.00 | 0.95 to 1.04 | .889 |
| Free testosterone | |||
| No adjustment for weight change | 0.96 | 0.92 to 1.01 | .094 |
| Adjustment for weight change | 0.99 | 0.94 to 1.03 | .554 |
Abbreviation: SHBG, sex hormone–binding globulin.
The geometric mean ratios were estimated from least square means for the difference in treatment effect between exercisers and controls averaged across the entire study period adjusted for the baseline values and then back log-transformed.
No significant differences in the change in androstenedione or testosterone concentrations were observed between exercisers and controls at 6 and 12 months of follow-up (Table 4). Furthermore, changes in these hormone concentrations did not differ by exercise adherence (Table 5). There was a slight indication of a greater decrease in free testosterone among exercisers who exercised more than 225 min/wk (−5.4% change, P = .088); however, across the levels of adherence, there was no significant trend (P = .112).
DISCUSSION
This year-long aerobic exercise intervention program among postmenopausal sedentary women resulted in reductions in estradiol and free estradiol concentrations and increases in SHBG concentration that are consistent with a reduced risk of breast cancer. Although estrone concentrations also decreased in exercisers to a greater extent than in controls, the difference between groups did not achieve statistical significance. Likewise, changes in testosterone, androstenedione, and free testosterone levels were not significantly different between exercisers and controls. Greater adherence to the exercise intervention was also associated with greater decreases in estradiol concentration and increases in SHBG concentration. Finally, this study demonstrated that long-term adherence to a combination facility- and home-based exercise program is feasible and achievable for some women.
This study found stronger effects on endogenous estrogen levels than the two other published exercise trials.14,19 In the first study,14 a statistically significant effect of exercise on estrogen levels was observed at 3 months but not at 12 months. The second trial found no significant effects of exercise on estrogen levels.19 Our trial and the previous ones15,19 observed no impact of exercise on androgen levels overall, but in the second trial, androgen levels decreased significantly in the exercise group who lost more than 2% body fat.19 This study's results are consistent with some,12,13,18,22 but not all,21,39 observational studies that have found an association between physical activity and estrogen levels. The lack of effect of exercise on estrone levels found in our study may have occurred because estrone is rapidly converted to estrone sulfate and estrone levels in postmenopausal women are low, making changes in levels difficult to detect.
Physical activity may influence circulating estrogens by reducing adiposity, which decreases conversion of androgens to estrogens by aromatase.5 In the current study, additional adjustment for weight change during the intervention attenuated the effect of the exercise intervention on SHBG but not estradiol or free estradiol. Physical activity may have effects independent of a change in adiposity, including a reduction in insulin levels, which, in turn, increases SHBG levels and decreases estradiol bioavailability.5
Recent guidelines recommend that adults perform at least 150 min/wk of moderate-intensity or 75 min/wk of vigorous-intensity aerobic physical activity.35,36 This study provides evidence that circulating estrogens can be reduced and SHBG concentrations increased in women exercising 150 to 225 min/wk. The clinical significance of these findings can be evaluated by noting that the 1.2-pg/mL decrease in estradiol levels in this study was one fifth of the interquartile range (7 to 14 pg/mL) at baseline. Given that a large pooled analysis of prospective cohort studies12 found that a doubling of estradiol levels in postmenopausal women is associated with a 29% (95% CI, 15% to 44%) increase in breast cancer risk, the impact of our intervention on estradiol levels would translate into a small breast cancer risk reduction. Further decreases in estradiol may occur with continued exercise.
This trial's strengths include the large sample size, a full year of supervised exercise intervention, low dropout rate, excellent adherence to the intervention, and the valid measures of estrogens and androgens. One limitation is the lack of compliance among 10% of controls who increased their vigorous recreational activity levels by ≥ 200 min/wk. A second limitation is the select sample of women who participated in the trial. The Alberta Physical Activity and Breast Cancer Prevention Trial was conducted as an efficacy trial in a select population to add information about the mechanisms by which physical activity may lead to a reduction in the risk of breast cancer. It is unclear what percentage of women would be able to adopt and maintain such an exercise program over an extended time period. Subsequent pragmatic behavioral trials are needed to determine the best strategies for increasing physical activity in the general population of women at higher risk of breast cancer.
In conclusion, this trial provides new evidence that previously sedentary, mostly overweight, postmenopausal women can achieve and sustain high levels of aerobic exercise that result in statistically significant changes in estradiol and SHBG concentrations that are consistent with a reduced risk of postmenopausal breast cancer. Physical activity may offer an acceptable means of reducing breast cancer risk for postmenopausal women who are not at a high enough risk for the benefits to outweigh the adverse effects of chemoprevention options that are currently under investigation,40–42 particularly given the broad health benefits associated with regular exercise.35
Acknowledgment
We acknowledge the following people: study set-up: Kim van der Hoek and Marla Orenstein; study coordinators: Rosemary Crosby, Ame-Lia Tamburrini; fitness center managers: Ben Wilson, Lisa Workman, Diane Cook; exercise trainers: Shannon Hutchins, Kathy Traptow, Shannon Brown, Susan Daniel, Parissa Gillani, Stephanie Sanden, Karen Mackay, Sandra Olsen; data analysis: Sandra Blitz, Sony Brar.
Footnotes
See accompanying editorial on page 1445
Supported by Research Grant No. 017468 from the Canadian Breast Cancer Research Alliance. C.M.F. is supported by career awards from the Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research. K.S.C. is supported by the Canada Research Chairs Program.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00522262.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Christine M. Friedenreich, Anne McTiernan, Rachel Ballard-Barbash, Rollin F. Brant, Tim Terry, Norman F. Boyd, Martin J. Yaffe, Melinda L. Irwin, Charlotte A. Jones, Yutaka Yasui, Kristin L. Campbell, Margaret L. McNeely, Kristina H. Karvinen, Kerry S. Courneya
Financial support: Christine M. Friedenreich, Anne McTiernan, Rachel Ballard-Barbash, Rollin F. Brant, Tim Terry, Norman F. Boyd, Martin J. Yaffe, Melinda L. Irwin, Charlotte A. Jones, Yutaka Yasui, Kerry S. Courneya
Administrative support: Christine M. Friedenreich, Christy G. Woolcott, Anne McTiernan, Charlotte A. Jones, Kristin L. Campbell, Margaret L. McNeely, Kristina H. Karvinen, Qinggang Wang, Kerry S. Courneya
Provision of study materials or patients: Christine M. Friedenreich, Christy G. Woolcott, Kerry S. Courneya
Collection and assembly of data: Christine M. Friedenreich, Christy G. Woolcott, Frank Z. Stanczyk, Tim Terry, Kristin L. Campbell, Margaret L. McNeely, Kristina H. Karvinen, Kerry S. Courneya
Data analysis and interpretation: Christine M. Friedenreich, Christy G. Woolcott, Anne McTiernan, Rachel Ballard-Barbash, Rollin F. Brant, Frank Z. Stanczyk, Tim Terry, Norman F. Boyd, Martin J. Yaffe, Melinda L. Irwin, Charlotte A. Jones, Yutaka Yasui, Kristin L. Campbell, Margaret L. McNeely, Kristina H. Karvinen, Qinggang Wang, Kerry S. Courneya
Manuscript writing: Christine M. Friedenreich, Christy G. Woolcott, Frank Z. Stanczyk, Kerry S. Courneya
Final approval of manuscript: Christine M. Friedenreich, Christy G. Woolcott, Anne McTiernan, Rachel Ballard-Barbash, Rollin F. Brant, Frank Z. Stanczyk, Tim Terry, Norman F. Boyd, Martin J. Yaffe, Melinda L. Irwin, Charlotte A. Jones, Yutaka Yasui, Kristin L. Campbell, Margaret L. McNeely, Kristina H. Karvinen, Qinggang Wang, Kerry S. Courneya
REFERENCES
- 1.Friedenreich CM, Cust AE. Physical activity and breast cancer risk: Impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med. 2008;42:636–647. doi: 10.1136/bjsm.2006.029132. [DOI] [PubMed] [Google Scholar]
- 2.Monninkhof EM, Elias SG, Vlems FA, et al. Physical activity and breast cancer: A systematic review. Epidemiology. 2007;18:137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 3.Friedenreich CM. Physical activity and breast cancer risk: The effect of menopausal status. Exerc Sport Sci Rev. 2004;32:180–184. doi: 10.1097/00003677-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 4.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8:205–211. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 5.Neilson HK, Friedenreich CM, Brockton NT, et al. Physical activity and postmenopausal breast cancer: Proposed biologic mechanisms and areas for future research. Cancer Epidemiol Biomarkers Prev. 2009;18:11–27. doi: 10.1158/1055-9965.EPI-08-0756. [DOI] [PubMed] [Google Scholar]
- 6.Toniolo PG. Endogenous estrogens and breast cancer risk: The case for prospective cohort studies. Environ Health Perspect. 1997;105(suppl 3):587–592. doi: 10.1289/ehp.97105s3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 8.Friedenreich CM. Review of anthropometric factors and breast cancer risk. Eur J Cancer Prev. 2001;10:15–32. doi: 10.1097/00008469-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Shephard RJ, Rhind S, Shek PN. The impact of exercise on the immune system: NK cells, interleukins 1 and 2, and related responses. Exerc Sport Sci Rev. 1995;23:215–241. [PubMed] [Google Scholar]
- 10.Eliassen AH, Missmer SA, Tworoger SS, et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98:1406–1415. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 11.Kaaks R, Rinaldi S, Key TJ, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: The European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12:1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 12.Key T, Appleby P, Barnes I, et al. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 13.Chan MF, Dowsett M, Folkerd E, et al. Usual physical activity and endogenous sex hormones in postmenopausal women: The European Prospective Investigation into Cancer-Norfolk Population study. Cancer Epidemiol Biomarkers Prev. 2007;16:900–905. doi: 10.1158/1055-9965.EPI-06-0745. [DOI] [PubMed] [Google Scholar]
- 14.McTiernan A, Tworoger SS, Ulrich CM, et al. Effect of exercise on serum estrogens in postmenopausal women: A 12-month randomized clinical trial. Cancer Res. 2004;64:2923–2928. doi: 10.1158/0008-5472.can-03-3393. [DOI] [PubMed] [Google Scholar]
- 15.McTiernan A, Tworoger SS, Rajan KB, et al. Effect of exercise on serum androgens in postmenopausal women: A 12-month randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2004;13:1099–1105. [PubMed] [Google Scholar]
- 16.McTiernan A, Wu L, Chen C, et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring) 2006;14:1662–1677. doi: 10.1038/oby.2006.191. [DOI] [PubMed] [Google Scholar]
- 17.Verkasalo PK, Thomas HV, Appleby PN, et al. Circulating levels of sex hormones and their relation to risk factors for breast cancer: A cross-sectional study in 1092 pre- and postmenopausal women (United Kingdom) Cancer Causes Control. 2001;12:47–59. doi: 10.1023/a:1008929714862. [DOI] [PubMed] [Google Scholar]
- 18.van Gils CH, Peeters PH, Schoenmakers MC, et al. Physical activity and endogenous sex hormone levels in postmenopausal women: A cross-sectional study in the Prospect-EPIC Cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:377–383. doi: 10.1158/1055-9965.EPI-08-0823. [DOI] [PubMed] [Google Scholar]
- 19.Monninkhof EM, Velthuis MJ, Peeters PH, et al. Effect of exercise on postmenopausal sex hormone levels and role of body fat: A randomized controlled trial. J Clin Oncol. 2009;27:4492–4499. doi: 10.1200/JCO.2008.19.7459. [DOI] [PubMed] [Google Scholar]
- 20.Wu F, Ames R, Evans MC, et al. Determinants of sex hormone-binding globulin in normal postmenopausal women. Clin Endocrinol (Oxf) 2001;54:81–87. doi: 10.1046/j.1365-2265.2001.01183.x. [DOI] [PubMed] [Google Scholar]
- 21.Madigan MP, Troisi R, Potischman N, et al. Serum hormone levels in relation to reproductive and lifestyle factors in postmenopausal women (United States) Cancer Causes Control. 1998;9:199–207. doi: 10.1023/a:1008838412423. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz KH, Lin H, Sammel MD, et al. Association of physical activity with reproductive hormones: The Penn Ovarian Aging Study. Cancer Epidemiol Biomarkers Prev. 2007;16:2042–2047. doi: 10.1158/1055-9965.EPI-07-0061. [DOI] [PubMed] [Google Scholar]
- 23.British Columbia Ministry of Health, Canadian Society for Exercise Physiology. Physical activity readiness medical examination. http://www.precisionathletics.ca/docs/parmedx.pdf.
- 24.Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 25.Csizmadi I, Kahle L, Ullman R, et al. Adaptation and evaluation of the National Cancer Institute's Dietary History Questionnaire and nutrient database for use in Canadian populations. Public Health Nutr. 2007;10:88–96. doi: 10.1017/S1368980007184287. [DOI] [PubMed] [Google Scholar]
- 26.Friedenreich CM, Courneya KS, Neilson HK, et al. Reliability and validity of the Past Year Total Physical Activity Questionnaire. Am J Epidemiol. 2006;163:959–970. doi: 10.1093/aje/kwj112. [DOI] [PubMed] [Google Scholar]
- 27.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. ed 6. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 28.Goebelsmann U, Horton R, Mestman JH, et al. Male pseudohermaphroditism due to testicular 17 -hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab. 1973;36:867–879. doi: 10.1210/jcem-36-5-867. [DOI] [PubMed] [Google Scholar]
- 29.Goebelsmann U, Bernstein GS, Gale JA, et al. Serum gonadotropin, testosterone, estradiol and estrone levels prior to and following bilateral vasectomy. In: Lepow IH, Crozier R, editors. Vasectomy: Immunologic and Pathophysiologic Effects in Animals and Man. New York, NY: Academic Press; 1979. 165 pp. [Google Scholar]
- 30.Probst-Hensch NM, Ingles SA, Diep AT, et al. Aromatase and breast cancer susceptibility. Endocr Relat Cancer. 1999;6:165–173. doi: 10.1677/erc.0.0060165. [DOI] [PubMed] [Google Scholar]
- 31.Rinaldi S, Geay A, Dechaud H, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev. 2002;11:1065–1071. [PubMed] [Google Scholar]
- 32.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 33.Rosner B. Fundamentals of Biostatistics. ed 2. Boston, MA: Duxbury Press; 1986. [Google Scholar]
- 34.Irwin ML, Yasui Y, Ulrich CM, et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: A randomized controlled trial. JAMA. 2003;289:323–330. doi: 10.1001/jama.289.3.323. [DOI] [PubMed] [Google Scholar]
- 35.US Department of Health and Human Services. Physical activity guidelines advisory committee report, 2008. http://www.health.gov/paguidelines/Report/pdf/CommitteeReport.pdf. [DOI] [PubMed]
- 36.Warburton DE, Katzmarzyk PT, Rhodes RE, et al. Evidence-informed physical activity guidelines for Canadian adults. Can J Public Health. 2007;98(suppl 2):S16–S68. [PubMed] [Google Scholar]
- 37.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: Classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 39.Bjørnerem A, Straume B, Midtby M, et al. Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: The Tromso Study. J Clin Endocrinol Metab. 2004;89:6039–6047. doi: 10.1210/jc.2004-0735. [DOI] [PubMed] [Google Scholar]
- 40.Cuzick J. Aromatase inhibitors for breast cancer prevention. J Clin Oncol. 2005;23:1636–1643. doi: 10.1200/JCO.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 41.Dunn BK, Ryan A. Phase 3 trials of aromatase inhibitors for breast cancer prevention: Following in the path of the selective estrogen receptor modulators. Ann N Y Acad Sci. 2009;1155:141–161. doi: 10.1111/j.1749-6632.2009.03688.x. [DOI] [PubMed] [Google Scholar]
- 42.Visvanathan K, Chlebowski RT, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235–3258. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]