Abstract
Purpose
Abiraterone acetate is a prodrug of abiraterone, a selective inhibitor of CYP17, the enzyme catalyst for two essential steps in androgen biosynthesis. In castration-resistant prostate cancers (CRPCs), extragonadal androgen sources may sustain tumor growth despite a castrate environment. This phase I dose-escalation study of abiraterone acetate evaluated safety, pharmacokinetics, and effects on steroidogenesis and prostate-specific antigen (PSA) levels in men with CPRC with or without prior ketoconazole therapy.
Patients and Methods
Thirty-three men with chemotherapy-naïve progressive CRPC were enrolled. Nineteen patients (58%) had previously received ketoconazole for CRPC. Bone metastases were present in 70% of patients, and visceral involvement was present in 18%. Three patients (9%) had locally advanced disease without distant metastases. Fasted or fed cohorts received abiraterone acetate doses of 250, 500, 750, or 1,000 mg daily. Single-dose pharmacokinetic analyses were performed before continuous daily dosing.
Results
Adverse events were predominantly grade 1 or 2. No dose-limiting toxicities were observed. Hypertension (grade 3, 12%) and hypokalemia (grade 3, 6%; grade 4, 3%) were the most frequent serious toxicities and responded to medical management. Confirmed ≥ 50% PSA declines at week 12 were seen in 18 (55%) of 33 patients, including nine (47%) of 19 patients with prior ketoconazole therapy and nine (64%) of 14 patients without prior ketoconazole therapy. Substantial declines in circulating androgens and increases in mineralocorticoids were seen with all doses.
Conclusion
Abiraterone acetate was well tolerated and demonstrated activity in CRPC, including in patients previously treated with ketoconazole. Continued clinical study is warranted.
INTRODUCTION
Androgen deprivation therapy is the standard of care for patients with advanced prostate cancer. However, virtually all patients eventually develop castration-resistant prostate cancer (CRPC), the lethal form of prostate cancer where less than 20% of men survive beyond 3 years.1–3
Historically, castration-resistant tumors were thought to have no reliance on androgen receptor (AR) signaling for growth and survival, prompting characterization as androgen independent or hormone resistant. However, recent findings suggest that AR signaling persists in many of these tumors,4–7 the result of adaptive mechanisms that permit survival in the castrate-level androgen environment.8–10
Although medical or surgical androgen deprivation abrogates gonadal testosterone production, circulating testosterone of up to 10% of precastrate levels may persist as a result of androgen production from the adrenal glands or the tumor itself.10 Through its inhibitory action on the cholesterol side-chain cleavage enzyme as well as CYP17, ketoconazole has demonstrated activity as a secondary hormonal manipulation in CRPC. In a phase III clinical trial in metastatic CRPC, 28% of patients treated with ketoconazole experienced a ≥ 50% decline in prostatic-specific antigen (PSA), and the median survival time was approximately 16 months. Notably, progression of disease on this study was shown to be associated with an increase in adrenal androgen levels, indicating a failure of the drug to durably suppress hormone production.11
Abiraterone acetate and its metabolite, abiraterone, are potent and selective inhibitors of CYP17 α-hydroxylase and C17,20-lyase activities, both essential steps in androgen biosynthesis. In human microsomes, the concentration of abiraterone required to produce 50% inhibition of CYP17 is approximately 10% that of ketoconazole.12,13 The current report details findings from a phase I trial of abiraterone acetate in men with CRPC both with and without prior ketoconazole therapy and provides important insights into the endocrinologic and clinical effects of potent CYP17 inhibition.
PATIENTS AND METHODS
Major Eligibility Criteria
Men with histologically confirmed adenocarcinoma of the prostate and disease progression despite androgen deprivation therapy (either a luteinizing hormone–releasing hormone agonist or orchiectomy) were eligible. When appropriate, progression after antiandrogen withdrawal was required. Patients with metastatic disease or PSA-only progression by the PSA Working Group criteria14 were eligible. Prior chemotherapy for prostate cancer was not allowed. Use of other hormonal therapies, systemic corticosteroids, or any other product known to decrease PSA levels was not permitted within 4 weeks of treatment initiation. Eligibility required an Eastern Cooperative Oncology Group performance status of 0 or 1, serum creatinine ≤ 1.5× the institutional upper limit of normal [ULN], bilirubin ≤ 1. × ULN, AST and ALT ≤ 2.5 × ULN, serum potassium ≥ 3.5 mmol/L, and baseline adrenocorticotropic hormone (ACTH) stimulation test peak cortisol level of more than 18 μg/dL. Patients with uncontrolled hypertension, New York Heart Association Class III or IV congestive heart failure, autoimmune disease requiring corticosteroid therapy, or other illness interfering with study participation were ineligible. Prior ketoconazole therapy was not required for eligibility for the study.
Study Design and Treatment
The primary objective of this phase I, dose-escalation trial was determination of the maximum-tolerated dose (MTD) of abiraterone acetate administered orally on a continuous schedule in men with CRPC with and without prior ketoconazole therapy. Endocrine and pharmacokinetic effects were secondary objectives. The study was approved by the institutional review boards of the participating institutions and was conducted in accordance with the ethical principles of the World Medical Association Declaration of Helsinki. All patients provided written informed consent.
Medical maintenance of a castrate testosterone level was required for patients without prior orchiectomy. Abiraterone acetate was administered orally as a 250-mg tablet in escalating dose cohorts of 250, 500, 750, and 1,000 mg, with fed and fasted cohorts enrolled at each dose. On day –7, patients were administered a single dose of abiraterone acetate for pharmacokinetic analysis after an overnight fast or 30 minutes after starting a 800- to 1,000-calorie breakfast.15 After 7 days, drug was administered daily.
Dose-limiting toxicity (DLT) was defined as any drug-related grade ≥ 3 toxicity by the Common Terminology Criteria for Adverse Events (version 3) observed in the first 28 days. Neither fatigue responding to corticosteroid replacement nor grade 3 hypertension manageable with mineralocorticoid antagonists or corticosteroids was considered dose limiting. A single DLT event would expand a cohort to six patients, two DLT events would de-escalate to a lower dose cohort, and more than two DLTs among six patients would stop escalation and define the prior dose level as the MTD. Continuation of therapy beyond 28 days was allowed at the discretion of the physician.14,15
Use of a glucocorticoid was allowed for patients with clinical symptoms of adrenal insufficiency and fatigue, whereas mineralocorticoid excess could receive the aldosterone antagonists. Spironolactone was not permitted because of its potential to act as an AR agonist.16
Evaluations
Baseline evaluations included a history, examination, CBC counts and serum chemistries, PSA, serum testosterone, assessment of adrenal steroidogenesis (ACTH, luteinizing hormone, follicle-stimulating hormone, corticosterone, cortisol, 11-deoxycorticosterone, 11-deoxycortisol, aldosterone, and dehydroepiandrosterone sulfate [DHEA-S]), and imaging studies as clinically indicated. Testosterone levels were measured by a commercial assay method (liquid chromatography–tandem mass spectrometry, lower limit of quantitation of 1.0 ng/dL; Quest Diagnostics, Madison, NJ). Evaluations were repeated weekly throughout the first cycle of therapy and before day 1 of any subsequent cycles. Imaging studies were repeated at cycles 4, 7, and 10, as clinically warranted. Clinical activity was evaluated using PSA decline parameters according to PSA Working Group criteria.14
Pharmacokinetic Analysis
Blood samples for pharmacokinetic analysis were collected at hours 1, 2, 4, 8, 12, 24, and 48 after single-dose administration of abiraterone acetate on day −7, before dosing on days 1, 8, 15, and 22 of cycle 1, and before the first dose of any subsequent cycles. Abiraterone acetate and abiraterone plasma concentrations were analyzed by a liquid chromatography–tandem mass spectrometry assay developed and performed by the Institute of Cancer Research Drug Metabolism and Pharmacokinetics Team (Belmont, Sutton, United Kingdom). The assays were validated and linear within the range of 5 to 500 nmol/L. Noncompartmental pharmacokinetic analyses were performed using WINNonlin (Scientific Consultant, Apex, NC) software. Estimated parameters included maximum concentration (Cmax), time of maximum observed concentration, terminal half-life, total body apparent clearance, apparent volume of distribution (Vd), and area under the curve (AUC) from the time of dosing to the last measurable concentration (AUClast) and extrapolated to infinity (AUC0-∞).
RESULTS
Patient Characteristics
Between July 2006 and December 2007, 33 patients with CRPC were enrolled (Table 1). Seventy percent had bone metastasis, 18% had visceral involvement, and three patients (9%) had locally advanced disease without distant metastases. Nineteen patients (58%) had received prior ketoconazole therapy for CRPC. The median duration of ketoconazole therapy was 15 months (range, 1.6 to 42 months), and 16 (84%) of 19 patients had achieved a ≥ 50% decline in PSA on ketoconazole. The median interval from the date of discontinuation of ketoconazole until beginning therapy with abiraterone was 7.0 months (range, 1.8 to 31 months). Of the 19 patients, ketoconazole had been discontinued in 15 patients (79%) because of disease progression and in four patients (21%) because of toxicity.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | Total Patients (N = 33) | Abiraterone Acetate Dose |
|||
|---|---|---|---|---|---|
| 250 mg (n = 6) | 500 mg (n = 9) | 750 mg (n = 6) | 1,000 mg (n = 12) | ||
| Age, years | |||||
| Median | 72 | 65 | 71 | 74 | 76 |
| Range | 56-85 | 61-76 | 56-76 | 63-85 | 59-83 |
| ECOG performance status, No. of patients | |||||
| 0 | 30 | 5 | 9 | 6 | 10 |
| 1 | 3 | 1 | 0 | 0 | 2 |
| PSA, ng/mL | |||||
| Median | 33 | 35 | 45 | 29 | 28 |
| Range | 7-5,436 | 16-98 | 8-5,436 | 12-205 | 7-451 |
| Gleason score, No. of patients | |||||
| < 7 | 5 | 1 | 1 | 0 | 3 |
| 7 | 13 | 1 | 2 | 4 | 6 |
| > 7 | 15 | 4 | 6 | 2 | 3 |
| Disease involvement, No. of patients | |||||
| Elevated PSA | 33 | 6 | 9 | 6 | 12 |
| Prostate | 14 | 1 | 2 | 4 | 7 |
| Lymph node | 11 | 2 | 2 | 2 | 5 |
| Bone | 23 | 5 | 6 | 5 | 7 |
| Viscera | 6 | 0 | 3 | 1 | 2 |
| Prior ketoconazole therapy, No. of patients | 19 | 5 | 7 | 1 | 6 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PSA, prostate-specific antigen.
Dose Escalation
Tolerability was acceptable through 1,000 mg daily in both fasted and fed patients, and no DLTs were observed. The 500-mg dose cohort was expanded to include three additional patients (total of six patients in the fasted cohort) after one patient treated at this dose level experienced a syncopal event that was subsequently determined to be unrelated to study therapy. This type of event was not observed in subsequent patients. On the basis of evidence of clinical responses across several doses, maximization of the intended endocrinologic effects, and the favorable safety, dose escalation was ceased at 1,000 mg. These findings paralleled a concurrent study with abiraterone acetate.16
Toxicity
The most common adverse events were fatigue, hypertension, headache, nausea, and diarrhea (Table 2). Adverse events were predominantly grade 1 or 2. Grade 3 toxicities consisted of hypertension (n = 4), hypokalemia (n = 2), constipation (n = 1), diarrhea (n = 1), muscular weakness (n = 1), and arthralgia (n = 1). One patient treated at the 500-mg dose level developed grade 4 hypokalemia. The three episodes of grade 3 or 4 hypokalemia all occurred after cycle 1. Only one episode of grade 1 hypokalemia occurred in cycle 1. There was no observed increase in toxicity in patients who had previously been treated with ketoconazole. Grade 3 or 4 toxicities occurred in seven (37%) of 19 patients with prior ketoconazole exposure and in six (55%) of 11 patients without prior ketoconazole exposure.
Table 2.
Incidence of Most Common (> 10%) Adverse Events of Abiraterone Acetate in Patients With Castration-Resistant Prostate Cancer
| Adverse Event | All Grades (N = 33) |
Dose Cohort (No. of patients) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 250 mg (n = 6) |
500 mg (n = 9) |
750 (n = 6) |
1,000 mg (n = 12) |
|||||||
| No. of Patients | % | Grade 1/2 | Grade 3 | Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3 | Grade 1/2 | Grade 3 | |
| Abdominal pain | 6 | 18 | 1 | 2 | 2 | 1 | ||||
| Constipation | 7 | 21 | 2 | 1 | 2 | 2 | ||||
| Diarrhea | 10 | 30 | 3 | 2 | 1 | 4 | ||||
| Dry mouth | 7 | 21 | 2 | 3 | 2 | |||||
| Dyspepsia | 4 | 12 | 2 | 2 | ||||||
| Nausea | 11 | 33 | 1 | 3 | 1 | 6 | ||||
| Vomiting | 5 | 15 | 1 | 1 | 3 | |||||
| Asthenia | 8 | 24 | 1 | 3 | 2 | 2 | ||||
| Fatigue | 22 | 67 | 3 | 7 | 4 | 8 | ||||
| Edema | 5 | 15 | 1 | 3 | 1 | |||||
| Peripheral edema | 8 | 24 | 2 | 6 | ||||||
| Anorexia | 7 | 21 | 1 | 2 | 1 | 3 | ||||
| Hyperglycemia | 4 | 12 | 1 | 1 | 2 | |||||
| Hypokalemia | 8 | 24 | 1 | 3 | 1 | 2 | 1 | |||
| Arthralgia | 5 | 15 | 1 | 2 | 1 | 1 | ||||
| Back pain | 4 | 12 | 1 | 1 | 1 | 1 | ||||
| Muscular weakness | 5 | 15 | 2 | 1 | 1 | 1 | ||||
| Dizziness | 5 | 15 | 1 | 2 | 2 | |||||
| Headache | 11 | 33 | 3 | 2 | 6 | |||||
| Cough | 7 | 21 | 1 | 1 | 4 | 1 | ||||
| Hot flush | 7 | 21 | 2 | 2 | 3 | |||||
| Hypertension | 12 | 36 | 2 | 1 | 1 | 2 | 1 | 3 | 2 | |
| Hypotension | 4 | 12 | 1 | 3 | ||||||
Endocrine Effects
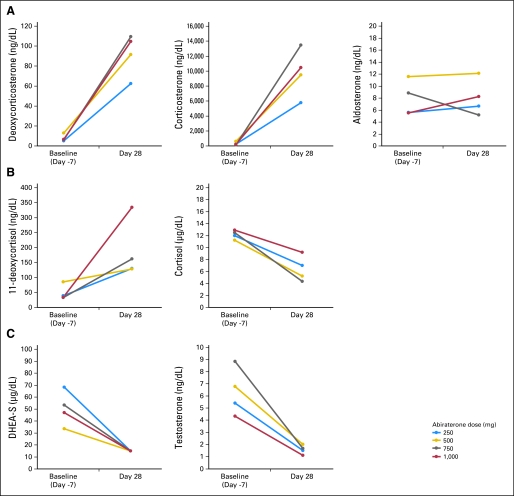
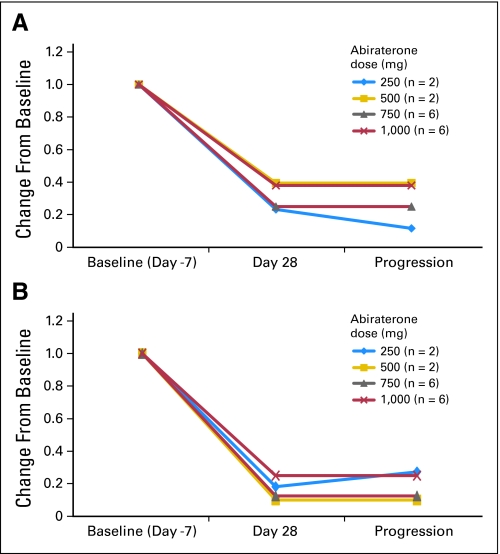
As predicted, therapy with abiraterone acetate resulted in a substantial reduction of circulating androgen levels and an increase in mineralocorticoids such as deoxycorticosterone, upstream of CYP17 (Fig A1, online only). A decrease in cortisol was observed (Fig 1). DHEA-S and testosterone levels decreased to undetectable or near undetectable levels. At the 1,000-mg dose level, the DHEA-S level decreased from baseline values (mean, 49 μg/dL; range, 15 to 99 μg/dL) to ≤ 15 μg/dL (assay lower limit of quantitation) at day 28 and remained at this level at time of progression. For testosterone, corresponding mean values were 4 ng/dL (range, 1 to 10 ng/dL) at baseline and ≤ 1 ng/dL (range, 1 to 2 ng/dL) at day 28; values remained at ≤ 1 ng/dL (range, 1 to 1.5 ng/dL) at time of progression (Fig 2). A possible trend in dose-response relationship was observed for deoxycorticosterone, corticosterone, and 11-deoxycortisol levels, although statistical comparison is limited by the small total number. No appreciable changes in follicle-stimulating hormone or luteinizing hormone levels were seen.
Fig 1.
Changes in mean levels of endocrine steroids from baseline to day 28 of therapy, by dose (A-C), in men with castration-resistant prostate cancer receiving abiraterone acetate. DHEA-S, dehydroepiandrosterone sulfate.
Fig 2.
Relative levels of (A) dehydroepiandrosterone sulfate and (B) testosterone (commercial assay) at baseline, at day 28, and at the time of disease progression in patients treated with abiraterone acetate, by dose.
Pharmacokinetics
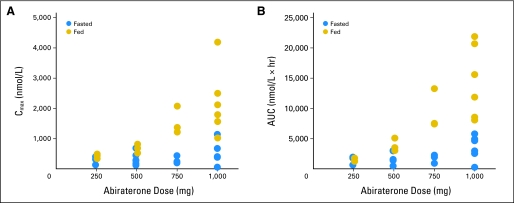
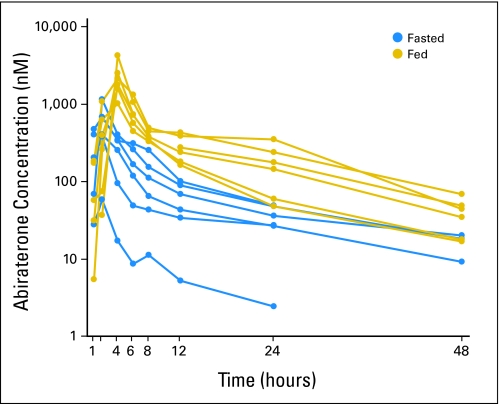
Pharmacokinetics were evaluated for all 33 patients. Abiraterone acetate was not detected in any sample, suggesting rapid conversion to abiraterone. Maximum drug concentrations (Cmax) were achieved within 1.5 to 4 hours (time of maximum observed concentration; Table 3). Less than proportional increases in both Cmax and AUC0-∞ were observed across dose levels in fed and fasted patients (Table 3; Fig A2, online only) but were less pronounced among fed patients. The small number of patients per cohort and the high degree of interpatient variability, often approaching 50%, limit further interpretation. Nonetheless, abiraterone exposures appeared higher in fed patients (Fig A3, online only), possibly suggesting that food may increase absorption. Terminal half-life ranged from 5 to 14 hours. At subsequent cycles, concentrations were lower (approximately 10% to 15%) than the highest concentration observed in cycle 1.
Table 3.
Pharmacokinetic Parameters of Abiraterone by Noncompartmental Analysis
| Parameter | Abiraterone Acetate Dose |
|||||||
|---|---|---|---|---|---|---|---|---|
| 250 mg |
500 mg |
750 mg |
1,000 mg |
|||||
| Fasted (n = 3) | Fed (n = 3) | Fasted (n = 6) | Fed (n = 3) | Fasted (n = 3) | Fed (n = 3) | Fasted (n = 6) | Fed (n = 6) | |
| Tmax, hours | ||||||||
| Mean | 2.0 | 2.0 | 1.5 | 2.7 | 2.0 | 2.0 | 1.8 | 4.0 |
| SD | 0 | 0.1 | 0.5 | 1.2 | 0.1 | 0 | 0.4 | 0 |
| % CV | 0 | 3.8 | 36.5 | 43.3 | 2.8 | 0 | 22.3 | 0 |
| Cmax, nM/L | ||||||||
| Mean | 283 | 421 | 331 | 676 | 290 | 1,552 | 510 | 2,194 |
| SD | 142.2 | 75.8 | 204.9 | 147.9 | 126.6 | 458.1 | 366.5 | 1,096.9 |
| % CV | 50.3 | 18.0 | 62.0 | 21.9 | 43.7 | 29.5 | 71.9 | 50.0 |
| AUC0-∞, nM/L · h | ||||||||
| Mean | 1,411 | 1,387 | 1,781 | 3,840 | 1,665 | 9,359 | 3,478 | 14,404 |
| SD | 697.2 | 290.2 | 986.8 | 1,080.9 | 704.6 | 3,338.6 | 2,012.2 | 5,971.0 |
| % CV | 49.4 | 20.9 | 55.4 | 28.2 | 42.3 | 35.7 | 57.9 | 41.5 |
| t1/2-λ, h | ||||||||
| Mean | 5.3 | 5.1 | 10.6 | 6.9 | 7.1 | 7.9 | 14.4 | 12.5 |
| SD | 1.7 | 1.0 | 6.3 | 5.7 | 3.4 | 2.6 | 7.7 | 1.2 |
| % CV | 31.7 | 20.3 | 59.8 | 82.7 | 48.6 | 33.2 | 53.3 | 9.8 |
| Apparent clearance, L/h | ||||||||
| Mean | 4,288 | 530 | 5,441 | 391 | 1,519 | 247 | 2,650 | 231 |
| SD | 1,319.3 | 99.5 | 4,844.5 | 99.1 | 822.7 | 73.0 | 4,617.0 | 97.7 |
| % CV | 30.8 | 18.8 | 89.0 | 25.4 | 54.2 | 29.6 | 174.2 | 42.2 |
| Apparent Vd, L | ||||||||
| Mean | 654 | 3,940 | 10,252 | 3,418 | 13,688 | 2,740 | 25,494 | 4,069 |
| SD | 447.3 | 1,275.4 | 12,268.8 | 1,934.4 | 4,265.4 | 1,084.7 | 18,670.2 | 1,462.6 |
| % CV | 68.4 | 32.4 | 119.7 | 56.6 | 31.2 | 39.6 | 73.2 | 36.0 |
NOTE. Clearance and volume of distribution are calculated as a function of bioavailability. However, the bioavailability of abiraterone is not known.
Abbreviations: Tmax, time of maximum observed concentration; SD, standard deviation; CV, coefficient of variation; Cmax, maximum concentration; AUC0-∞, area under the curve from the time of dosing extrapolated to infinity; t1/2-λ, terminal half-life; Vd, volume of distribution.
Efficacy
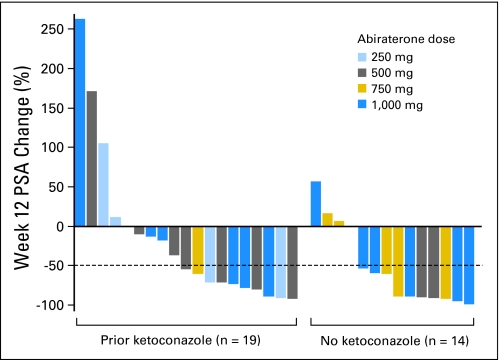
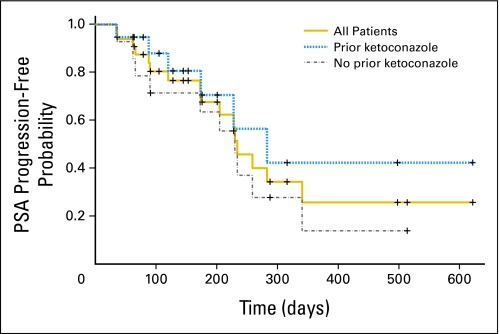
At week 12, confirmed decreases in PSA levels of ≥ 50% were seen in 18 (55%) of 33 patients overall, including nine (47%) of 19 patients with prior ketoconazole therapy and nine (64%) of 14 patients without prior ketoconazole therapy (Fig 3). A maximal PSA decrease of ≥ 50% at any time point was seen in 19 (58%) of 33 patients, including 10 patients (53%) with prior ketoconazole exposure and nine patients (64%) without. At 1,000 mg, seven (58%) of 12 patients had a ≥ 50% maximal decrease in PSA. Within the prior ketoconazole group, confirmed ≥ 50% decreases in PSA were seen in two (50%) of four patients who had discontinued ketoconazole as a result of toxicity and in seven (47%) of 15 patients who had discontinued ketoconazole as a result of disease progression. The median time to PSA progression for all patients was 234 days (95% CI, 174 to 341 days). Among patients with and without prior ketoconazole, median time to PSA progression was 283 days (95% CI, 174 days to not estimable) and 230 days (95% CI, 90 to 341 days), respectively (Fig A4, online only).
Fig 3.
Relative change in prostate-specific antigen (PSA) levels at week 12 of therapy in men with castration-resistant prostate cancer treated with abiraterone acetate. Patients who had received prior ketoconazole therapy appear on the left; those who had not received prior ketoconazole appear on the right.
Nine (56%) of 16 patients who previously responded to ketoconazole responded to abiraterone. Seven (37%) of 19 patients who responded to ketoconazole did not respond to abiraterone.
Of three patients who did not respond to ketoconazole, one has responded to abiraterone. Of four patients who discontinued ketoconazole as a result of toxicity, three responded to abiraterone. Of the 15 patients who developed ketoconazole-refractory disease (eg, experienced progression of disease on ketoconazole after experiencing a response), seven (46%) responded to abiraterone.
DISCUSSION
Abiraterone acetate has significant activity in patients with CRPC, as evidenced by a PSA response rate of 58% in this phase I trial and declines in PSA on all dose levels. Although the utility of using PSA reductions as a marker of clinical activity is debated, in general, most investigators agree that it is a reasonable tool to screen for activity. The activity of abiraterone acetate is attributed to the reduction of the total androgen pool, with a reduction in levels of both adrenal androgens and testosterone, thereby inhibiting persistent signaling through the AR. It is of considerable interest that abiraterone acetate has demonstrated activity in patients previously treated with ketoconazole.
Dose escalation was not discontinued as a result of the presence of DLTs. Abiraterone acetate was well tolerated up through the highest dose level evaluated (1,000 mg/d) with no MTD observed and no apparent toxicity differences among patients who had or had not received prior ketoconazole use. On the basis of safety, endocrinologic and pharmacokinetic parameters, and indications of activity, an abiraterone acetate dose of 1,000 mg/d is recommended for further study.
The adverse event and steroid endocrine profiles were consistent with anticipated outcomes of selective CYP17 inhibition (decrease in androgens and concomitant increase in upstream mineralocorticoid production). At the 1,000-mg dose level, hypertension and fatigue were the most commonly observed toxicities. The use of beta-blockers, diuretics, and eplerenone (often at doses > 25 mg daily) was modestly effective in managing abiraterone-induced hypertension. Administration of a corticosteroid was associated with normalization of mineralocorticoid levels and improvements in blood pressure. Of interest, two patients who received dexamethasone, which has little mineralocorticoid activity, experienced orthostatic hypotension, possibly related to a rapid decline in mineralocorticoid levels. This was not seen in patients who received hydrocortisone or prednisone. Therefore, we suggest that a low dose of a corticosteroid, such as prednisone, be administered with abiraterone acetate in future studies to optimize the safety profile.
The endocrine changes observed with abiraterone acetate were also consistent with its known inhibitory effects on 17α-hydroxylase and C17,20-lyase activities. Serum DHEA-S and testosterone declined substantially at all dose levels. At day 28, increased levels of adrenal steroids and upstream precursor molecules, including mineralocorticoids (corticosterone and deoxycorticosterone) were seen, consistent with a feedback mechanism resulting from increasing ACTH levels. Toxicities of mineralocorticoid excess (hypertension and potassium wasting) were mitigated with corticosteroid use as a result of suppression of the hypothalamic-pituitary adrenal axis.
No differences were observed in toxicity or PSA declines between fasted and fed patients. Interestingly, a high-fat meal substantially increased total drug exposure (AUC) in the fed patients, whereas an increased clearance and volume of distribution were observed in the fasted state. Although not directly observed, the potential for greater drug exposure to increase toxicities cannot be ruled out. To minimize diet-related variability in drug exposure, we suggest that future studies administer abiraterone acetate in a fasted state.
Confirmed PSA declines of ≥ 50% were seen in 58% of patients overall, including a 47% PSA response rate in patients with disease progression on prior ketoconazole therapy; in phase III trials of ketoconazole, the PSA response rate was approximately 30%,11 and phase II studies showed a 45% to 65% rate of response.17 Of note, at the time of disease progression on ketoconazole,11 adrenal androgen levels were increased relative to their nadir, suggesting that the suppressive effects of ketoconazole on adrenal androgen synthesis are not durable. Furthermore, prolonged disease suppression by ketoconazole may be complicated by induction of metabolism or drug-drug interactions. By contrast, the suppressive effects of abiraterone on adrenal androgen synthesis are durable because progression of disease on abiraterone is not accompanied by an increase in adrenal androgens (Fig 2), again potentially reflecting the more potent CYP17 inhibitory activity of abiraterone compared with ketoconazole. These observations may explain why a high proportion of patients were able to benefit from abiraterone after ketoconazole. Also of note, the patients with prior ketoconazole treatment had experienced longer than average responses to ketoconazole (15 months v reported median responses of approximately 5 months) and thus may represent a group of patients more likely to harbor disease that is dependent on CYP17-mediated androgen production. Although this may be reflective of an unintended selection bias, the high rate of response to abiraterone in ketoconazole-exposed patients is further evidence of the superior potency of abiraterone compared with ketoconazole. More analysis of this relationship in future studies is warranted.
From our data, it cannot be determined whether pretreatment adrenal androgen levels can identify patients more likely to respond to abiraterone, as has been suggested with ketoconazole,18,19 or whether changes in adrenal androgen levels correlate with clinical outcomes. However, such correlations are of interest and should be pursued. The exclusion of patients with prior chemotherapy for CRPC in this study was intended to allow for preliminary evaluation of abiraterone acetate in a patient population typically treated with secondary hormonal manipulation, chiefly as a means of delaying use of traditional cytotoxics. Ongoing studies are evaluating abiraterone in patients with both chemotherapy-refractory and chemotherapy-naïve CRPC.
Despite these findings, several unaddressed issues remain. First, although abiraterone did not cause adrenal insufficiency in any patients, the impact of concurrent prednisone on response proportion and durability or long-term toxicity is not known. Second, despite the high PSA decline rate, the relationship between PSA decline and survival in the prechemotherapy setting is undefined. Finally, the observation of abiraterone efficacy in a ketoconazole-refractory CRPC population requires prospective validation. Such studies are ongoing.
In summary, these data suggest that abiraterone is an active agent for CRPC and is associated with acceptable toxicity. Mineralocorticoid-induced hypertension is a unique toxicity but is controlled by corticosteroids. Preliminary observations of responses to abiraterone in patients with prior ketoconazole therapy suggest that cross-resistance between these therapies may not exist. Further definitive studies of this agent are warranted.
Acknowledgment
We thank Vivian Huey and Janina Valiente for data coordination and Christine Gutheil for editorial assistance. Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL; the 2008 Genitourinary Cancers Symposium, February 14-16, 2008, San Francisco, CA; the American Association for Cancer Research–National Cancer Institute–European Organisation for Research and Treatment of Cancer meeting, October 22-26, 2007, San Francisco, CA; and the 42nd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Appendix
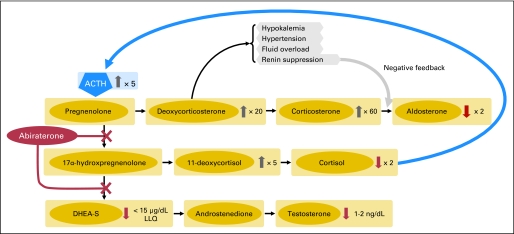
Fig A1.
Effects of abiraterone acetate on adrenal steroid biosynthesis. Inhibition of 17α-hydroxylase and and C17,20-lyase resulted in substantial decreases in circulating androgen levels (dehydroepiandrosterone sulfate [DHEA-S] and testosterone), with increases in upstream mineralocorticoids (deoxycorticosterone and corticosterone). Adrenocorticotropic hormone (ACTH) levels increased in response to decreases in circulating cortisol levels. LLQ, lower limit of quantitation.
Fig A2.
Abiraterone (A) maximum concentrations (Cmax) and (B) area under the curve (AUC) by dose level in fed and fasting patients.
Fig A3.
Abiraterone concentration-time profiles at the 1,000-mg dose level in fed and fasting patients.
Fig A4.
Kaplan-Meier plot of the probability of prostate-specific antigen (PSA) progression over time in all patients (N = 33) and subgroups of patients with prior ketoconazole therapy (n = 19) and without prior ketoconazole therapy (n = 14).
Footnotes
Supported by Cougar Biotechnology, the Department of Defense Prostate Cancer Clinical Trials Consortium, and Grant No. K23CA115775 (C.J.R.) from the National Institutes of Health. V.M. and F.R. are employees of The Institute of Cancer Research, which has a commercial interest in the development of abiraterone and operates a rewards to inventors scheme. The Institute of Cancer Research has been involved in a commercial collaboration with Cougar Biotechnology.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00473746.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Thian Kheoh, Cougar Biotechnology (C); Arturo Molina, Cougar Biotechnology (C) Consultant or Advisory Role: Charles J. Ryan, Cougar Biotechnology (U); Matthew R. Smith, Cougar Biotechnology (U); Philip Kantoff, Cougar Biotechnology (C); Eric J. Small, Cougar Biotechnology (C) Stock Ownership: Thian Kheoh, Cougar Biotechnology; Arturo Molina, Cougar Biotechnology Honoraria: None Research Funding: Philip Kantoff, Cougar Biotechnology; Florence Raynaud, Cougar Biotechnology; Vanessa Martins, Cougar Biotechnology Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Charles J. Ryan, Vanessa Martins, Arturo Molina
Administrative support: Jennifer Kim
Provision of study materials or patients: Charles J. Ryan, Lawrence Fong, Philip Kantoff, Gloria Lee, Arturo Molina
Collection and assembly of data: Charles J. Ryan, Matthew R. Smith, Jonathan E. Rosenberg, Philip Kantoff, Florence Raynaud, Vanessa Martins, Thian Kheoh, Jennifer Kim, Arturo Molina, Eric J. Small
Data analysis and interpretation: Charles J. Ryan, Matthew R. Smith, Lawrence Fong, Jonathan E. Rosenberg, Philip Kantoff, Florence Raynaud, Vanessa Martins, Gloria Lee, Thian Kheoh, Jennifer Kim, Arturo Molina, Eric J. Small
Manuscript writing: Charles J. Ryan, Matthew R. Smith, Lawrence Fong, Jonathan E. Rosenberg, Philip Kantoff, Arturo Molina, Eric J. Small
Final approval of manuscript: Charles J. Ryan, Matthew R. Smith, Lawrence Fong, Jonathan E. Rosenberg, Philip Kantoff, Florence Raynaud, Vanessa Martins, Gloria Lee, Thian Kheoh, Jennifer Kim, Arturo Molina, Eric J. Small
REFERENCES
- 1.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 2.Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 3.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 4.Slovin SF. Neuroendocrine differentiation in prostate cancer: A sheep in wolf's clothing? Nat Clin Pract Urol. 2006;3:138–144. doi: 10.1038/ncpuro0435. [DOI] [PubMed] [Google Scholar]
- 5.Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1665–1671. doi: 10.1158/1078-0432.CCR-06-0067. [DOI] [PubMed] [Google Scholar]
- 6.Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–1490. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 8.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 9.Linja MJ, Savinainen KJ, Saramäki OR, et al. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–3555. [PubMed] [Google Scholar]
- 10.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 11.Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: A phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–1033. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 12.Haidar S, Ehmer PB, Barassin S, et al. Effects of novel 17alpha-hydroxylase/C17, 20-lyase (P450 17, CYP 17) inhibitors on androgen biosynthesis in vitro and in vivo. J Steroid Biochem Mol Biol. 2003;84:555–562. doi: 10.1016/s0960-0760(03)00070-0. [DOI] [PubMed] [Google Scholar]
- 13.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 14.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: Recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration, Center for Evaluation and Research. Guidance for industry: Food-effect bioavailability and fed bioequivalence studies. http://www.fda.gov/ohrms/dockets/ac/02/briefing/3860b1_01_GFI-Food-effect.pdf.
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Luthy IA, Begin DJ, Labrie F. Androgenic activity of synthetic progestins and spironolactone in androgen-sensitive mouse mammary carcinoma (Shionogi) cells in culture. J Steroid Biochem. 1988;31:845–852. doi: 10.1016/0022-4731(88)90295-6. [DOI] [PubMed] [Google Scholar]
- 18.Small EJ, Ryan CJ. The case for secondary hormonal therapies in the chemotherapy age. J Urol. 2006;176:S66–S71. doi: 10.1016/j.juro.2006.06.071. [DOI] [PubMed] [Google Scholar]
- 19.Ryan CJ, Halabi S, Ou SS, et al. Adrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: Results from a Cancer and Leukemia Group B study. Clin Cancer Res. 2007;13:2030–2037. doi: 10.1158/1078-0432.CCR-06-2344. [DOI] [PubMed] [Google Scholar]