Abstract
Purpose
The principal objective of this trial was to evaluate the antitumor activity of abiraterone acetate, an oral, specific, irreversible inhibitor of CYP17 in docetaxel-treated patients with castration-resistant prostate cancer (CRPC).
Patients and Methods
In this multicenter, two-stage, phase II study, abiraterone acetate 1,000 mg was administered once daily continuously. The primary end point was achievement of a prostate-specific antigen (PSA) decline of ≥ 50% in at least seven of 35 patients. Per an attained phase II design, more than 35 patients could be enrolled if the primary end point was met. Secondary objectives included: PSA declines of ≥ 30% and ≥ 90%; rate of RECIST (Response Evaluation Criteria in Solid Tumors) responses and duration on study; time to PSA progression; safety and tolerability; and circulating tumor cell (CTC) enumeration.
Results
Docetaxel-treated patients with CRPC (N = 47) were enrolled. PSA declines of ≥ 30%, ≥ 50% and ≥ 90% were seen in 68% (32 of 47), 51% (24 of 47), and 15% (seven of 47) of patients, respectively. Partial responses (by RECIST) were reported in eight (27%) of 30 patients with measurable disease. Median time to PSA progression was 169 days (95% CI, 113 to 281 days). The median number of weeks on study was 24, and 12 (25.5%) of 47 patients remained on study ≥ 48 weeks. CTCs were enumerated in 34 patients; 27 (79%) of 34 patients had at least five CTCs at baseline. Eleven (41%) of 27 patients had a decline from at least five to less than 5 CTCs, and 18 (67%) of 27 had a ≥ 30% decline in CTCs after starting treatment with abiraterone acetate. Abiraterone acetate was well tolerated.
Conclusion
Abiraterone acetate has significant antitumor activity in post-docetaxel patients with CRPC. Randomized, phase III trials of abiraterone acetate are underway to define the future role of this agent.
INTRODUCTION
Castration-resistant prostate cancer (CRPC) is the second-most common cause of cancer-related death in men in the developed world.1,2 Docetaxel is the only currently approved drug to have shown a survival advantage in CRPC, with a median survival advantage of 2 to 3 months and improved quality of life reported.3 The continued importance of androgen receptor (AR) activation and signaling in CRPC is increasingly recognized.4–6 AR-activating ligands originating in the adrenal glands or occurring by de novo synthesis may be activating CRPC.7–10 The successful development of aromatase (CYP19) inhibitors in breast cancer raises the question of whether CYP targeting may be similarly successful in prostate cancer (PCa).11
The key enzyme in steroid biosynthesis leading to production of androgenic and estrogenic steroids is CYP17.12 Inhibition of CYP17 results in decreased levels of downstream androgens and reduced peripheral conversion to the more potent androgens testosterone and dihydrotestosterone capable of AR activation. Estrogens also are decreased, which may be important, as there is increasing evidence that they may increase the expression of hormone-regulated oncogenic ETS fusion genes.13
Abiraterone (CB7598) is an oral, potent, selective, and irreversible inhibitor of CYP17 that is 10- to 30-fold more potent than the nonselective inhibitor, ketoconazole. The parent drug has poor bioavailability; therefore, a prodrug was generated.14,15 The 3-β-O-acetate prodrug (ie, abiraterone acetate, CB7630) is rapidly deacetylated to the active metabolite in vivo.14,15 In our recent, first-in-man, phase I evaluation of continuous daily abiraterone in chemotherapy-naïve men, no dose-limiting toxicities were observed, and—significantly—abiraterone acetate was well tolerated; moreover, abiraterone was active at all doses tested, as evidenced by prostate-specific antigen (PSA) declines, disease regression in both soft tissue and bone, and symptomatic improvements.5 This study reported that castrate, but detectable, testosterone levels at baseline declined rapidly to less than 1 ng/dL after treatment with abiraterone acetate.5 Compatible with complete CYP17 inhibition, treatment with abiraterone acetate also decreases estradiol, dehydroepiandrostenedione (DHEA), and androstenedione. Increases in steroids upstream of CYP17, corticosterone and deoxycorticosterone, reached a plateau at doses greater than 750 mg. A 1,000-mg dose, therefore, was selected for phase II evaluation. Pharmacokinetic testing confirmed a once-daily dosing schedule. Our phase II study in men who have not yet received chemotherapy has confirmed that abiraterone is well tolerated and active in this setting.4
As AR activation and signalling remain key targets in later stages of the disease, we hypothesized that postdocetaxel patients would derive benefit from abiraterone acetate. Therefore, we conducted a phase II study to evaluate the antitumor activity of abiraterone acetate 1,000 mg administered daily, continuously, to castrate men with CRPC who had previously received docetaxel.
PATIENTS AND METHODS
Patient Population
This was a multicenter, phase II study (COU-AA-003) conducted at the Royal Marsden NHS Foundation Trust, Memorial Sloan-Kettering Cancer Center, and the University of California, San Francisco, Comprehensive Cancer Center. Castrate (ie, serum testosterone < 50 ng/dL, < 2.0 nmol/L) patients with Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 to 2 who had a histologic diagnosis of prostate adenocarcinoma, a PSA greater than 5 ng/mL, and progressive disease as defined by PSA Working Group (PSAWG)16 criteria were eligible. Patients were permitted to be on stable low doses of steroids if the steroids were required to maintain fitness for the study. Patients were required to have a minimum washout period of 4 weeks after the use of PCa therapy (except for luteinizing hormone-releasing hormone [LHRH] agonists) and were required to have 6 weeks of washout after antiandrogens were stopped. Patients were required to have received prior docetaxel chemotherapy for PCa. Other eligibility criteria included normal serum potassium and adequate bone marrow, renal, and hepatic functions. Patients were excluded if they had brain metastases or spinal cord compression, active autoimmune disease that required corticosteroid therapy, uncontrolled hypertension, a history of cardiac failure class III or IV, or a serious concurrent medical illness. The study was approved by the institutional ethics review committees of all participating centers and was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation/WHO Good Clinical Practice standards. Written informed consent was obtained from all patients before any study procedures were performed.
Study Design and Response Assessment
This was a single-arm, open-label, two-stage, phase II study. The primary objective of the study was to evaluate the antitumor activity of abiraterone acetate in patients with CRPC who had received prior docetaxel chemotherapy, and the primary end point was measured by the proportion of patients achieving a PSA decline of ≥ 50% from baseline, as recommended by PSAWG criteria.16 A confirmatory PSA value decline was obtained ≥ 4 weeks later. For patients with a ≥ 50% PSA decline from baseline, PSA progression was defined as an increase in PSA of 50% (minimum, 5 ng/mL) greater than nadir value and was confirmed by a second reading ≥ 4 weeks later. For patients without a ≥ 50% PSA decline from baseline, PSA progression was defined as an increase in PSA of 25% (minimum, 5 ng/mL) greater than nadir and was confirmed by a second reading a minimum of 4 weeks later (according to PSAWG). After this study was written, revised criteria for assessing response in prostate clinical trials has been published (ie, Prostate Cancer Clinical Trials Working Group) that recommends the reporting of the maximal PSA decline from baseline at 12 weeks and at any point on study.17 Measurable disease response rate, at least 3 months after the start of treatment per investigator's assessment using Response Evaluation Criteria in Solid Tumors (RECIST), was reported. As conducted in our previous chemotherapy-naïve studies, ≥ 30% and ≥ 90% declines in PSA were also reported. The median time to PSA progression was defined according to the PSAWG criteria. Patients with PSA progression but stable measurable disease by RECIST and no symptoms of clinical progression were allowed to continue abiraterone acetate therapy and duration on study is therefore also reported.
Treatment and Procedures
Four capsules (250 mg each) of abiraterone acetate powder were administered once daily, continuously, to patients in a fasted state in 28-day cycles. All patients underwent a history, physical exam, and safety evaluations that included hematology, coagulation, biochemistry, liver and renal function studies at baseline after the first week and at 4-week intervals. PSA, alkaline phosphatase, and albumin levels were also measured. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3.0. Expected toxicities of hypertension, hypokalemia, and fluid retention arising from a syndrome of secondary mineralocorticoid excess were managed with either a mineralocorticoid receptor antagonist (eplerenone 50 to 200 mg) or low-dose glucocorticoids. Baseline high-resolution computed tomography (CT) scans and bone scans were performed and repeated at 3-month and 6-month intervals, respectively. Patients were offered to sign an optional secondary protocol to have blood samples (7.5 mL) collected into CellSave tubes (Veridex LLC, Raritan, NJ) at baseline and every 4 weeks thereafter for the enumeration of CTC by using the CellSearch system (Veridex), as described previously.18 Fluorescent in situ hybridization to investigate ERG gene status was performed on CTCs, as described previously.19,20
Statistical Analyses
The primary objective was to determine the rate of patients that demonstrated a ≥ 50% decline in PSA. A two-stage, Green and Dahlberg, attained-phase II trial design was utilized that permitted additional patients to be recruited if sufficient activity was demonstrated in the second stage.21 By using a response rate of ≤ 10% for the null hypothesis versus an alternative hypothesis response rate of ≥ 30%, 20 patients were to be recruited to the first stage. If at least three of 20 patients had a PSA decline by ≥ 50%, the study would proceed to the second stage, with an additional 13 patients recruited. The null hypothesis would be rejected if there were at least seven patients who had a PSA decline by ≥ 50% (α = .05; β = .01). As defined a priori in the trial protocol, all patients treated were included in the assessment of antitumor activity. Patients with at least five CTCs per 7.5mL at baseline were reported as to whether they had a decrease in CTC to less than five CTCs; a decrease by 50%; or a decrease by 30%.22–24
RESULTS
Patient Characteristics
Forty-seven patients were enrolled from December 2006 to August 2007. Table 1 lists the patient demographic and clinical characteristics. Median age was 67 years (range, 48 to 87 years). All patients had experienced progression on LHRH agonists, and 46 patients had received prior antiandrogens. The median numbers of prior hormonal therapies and prior chemotherapy agents were four and one, respectively. All patients had previously received steroid therapy with docetaxel; 27 (57%) of 47 patients also had received single-agent steroid treatment (ie, prednisone, dexamethasone, or hydrocortisone), and 17 (36%) of 47 patients had received prior estrogens as well. Eight (17%) of 47 patients had received prior ketoconazole. Eighteen of 47 patients started the study on a stable dose of steroids to maintain PS. The median baseline PSA was 403 ng/mL (range, 9.9 to 10,325 ng/mL). Sixty-two percent of patients had an albumin of ≤ 35 or 3.5 g/dL. Thirty patients had measurable disease on baseline CT scan. Overall, 45 of 47 patients had evidence of bony metastases at baseline. Twenty-seven (79%) of 34 patients (who were in a seperate CTC acquisition protocol) had at least five CTCs at baseline; the median CTC count in the 27 patients with at least five CTCs at baseline was 36 (range, five to 1,712). Among patients with a CTC count of at least five at baseline, 14 (52%) of 27 patients had a count of five to 50 cells, and 13 (48%) of 27 patients had a CTC count of greater than 50.
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | No. of Patients |
|---|---|
| Age, years | |
| Median | 67 |
| Range | 48-87 |
| Gleason score | |
| < 6 | 4 |
| 6-7 | 20 |
| 8-9 | 17 |
| 10 | 1 |
| Unknown | 5 |
| Baseline PSA, ng/mL | |
| Median | 403 |
| Range | 9.9-10,325 |
| ECOG performance status | |
| 0 | 16 |
| 1 | 27 |
| 2 | 4 |
| Hemoglobin, g/dL | |
| Median | 11.8 |
| Range | 9.4-14.6 |
| Albumin, g/L | |
| Median | 36 |
| Range | 23-46 |
| Alkaline phosphatase, μ/L | |
| Median | 185 |
| Range | 34-110 |
| Predominant metastases | |
| Bone only | 4 |
| Visceral only | 0 |
| Soft tissue only | 1 |
| Bone and soft tissue | 33 |
| Bone and visceral | 1 |
| Soft tissue and visceral | 1 |
| Bone, soft tissue, and visceral | 7 |
| Prior hormone therapies | |
| LHRH analogues | 47 |
| Antiandrogens | 46 |
| Steroids | 27 |
| Estrogens | 17 |
| Ketoconazole | 8 |
| Prior chemotherapy | |
| Docetaxel | 47 |
| Vinorelbine | 3 |
| ECarboF | 1 |
| Mitoxantrone | 8 |
| Cyclophosphamide | 2 |
| Carboplatin | 2 |
| Estramustine | 2 |
| Paclitaxel | 2 |
Abbreviations: PSA, prostate specific antigen; ECOG, Eastern Cooperative Oncology Group; LHRH, luteinizing hormone-releasing hormone; ECarboF, carboplatin plus fluorouracil plus epirubicin.
Antitumor Activity: PSA Changes and Measurable Disease Responses
A PSA decline of ≥ 50% from the start of treatment was observed in 24 (51%) of 47 patients at least once on study. Thirty-two (68%) of 47 patients and seven (15%) of 47 patients had ≥ 30% and ≥ 90% declines in PSA, respectively. These responses were confirmed at least 4 weeks later with a second PSA except for 3 and 4 patients with PSA declines of ≥ 50% and ≥ 30% respectively for whom a confirmatory PSA four weeks later was not available. The percentage of change in PSA from baseline to 12 weeks and the maximum decline in PSA at any point on study are depicted for each patient in waterfall plots (Fig 1).17 Thirty patients had measurable disease at baseline, and eight (27%) of 30 patients met the criteria for partial response (PR) by RECIST per investigator's assessment (Fig 2).
Fig 1.
Waterfall plots of prostate-specific antigen (PSA) changes. (A) Waterfall plot of greatest percentage change in PSA of individual patients on abiraterone acetate. (B) Waterfall plot of PSA change from baseline at 12 weeks for individual patients on abiraterone acetate. Brown, gold and gray lines indicate a decline in PSA of 30%, 50% and 90%, respectively. Some patients had a PSA decline on study but this was short-lived; PSA then increased again, which explains why the week-12 and maximal PSA declines are different.
Fig 2.
Radiologic responses. (A) Patient 010 had previously experienced progression on an luteinizing hormone-releasing hormone (LHRH) agonist, flutamide, bicalutamide, docetaxel, the monoclonal antibody to IGF-1R, CP-751, 871, and the survivin inhibitor YM155. Before starting treatment with abiraterone acetate, the prostate-specific antigen (PSA) was 789, and there was evidence of nodal and bony metastatic disease. Circulating tumor cells (CTCs) were not detected. Red oval, retroperitoneal nodal disease on a baseline computed tomography (CT) scan (A). After 3 months on abiraterone acetate, PSA decreased to a nadir of 1.4 (ie, > 90% decline), and (B) the red oval shows that the nodal disease has largely disappeared. Patient 010 continues to take study drug, now in the third year of treatment. (C) Patient 025 had previously experienced progression on an LHRH agonist, antiandrogen, stilboestrol, and docetaxel. Baseline PSA was 10,325, and it decreased to a PSA nadir of 46 (ie, > 90% decline) after 4 months on abiraterone acetate. Baseline CTC count was 20, which decreased to a CTC count nadir of 0 after 4 weeks. Green oval, CT scan at baseline demonstrated an axillary nodal metastasis (C). Reduction in size is seen in a follow-up scan performed at 6 months (green oval; D). (E) Whole-body, 99mTc-MDP bone scintigraphy at baseline (again for patient 025). A response in the bony disease can be seen in (F) after 6 months on abiraterone acetate. This patient remained on study in excess of a year (ie, 482 days).
Symptomatic Improvements
At baseline, 16 patients had an ECOG PS of 0; 27 patients had an ECOG PS of 1; and four patients had an ECOG PS of 2. An improvement in performance status was observed during treatment in 11 patients: 10 patients shifted from ECOG 1 to ECOG 0, and one patient shifted from ECOG 2 to ECOG 1. ECOG PS did not change from baseline after treatment in 35 patients, as ECOG PS scores of 0, 1, and 2 remained unchanged in 15 of 16, 17 of 27, and three of four patients, respectively.
Time to PSA Progression and Duration on Study
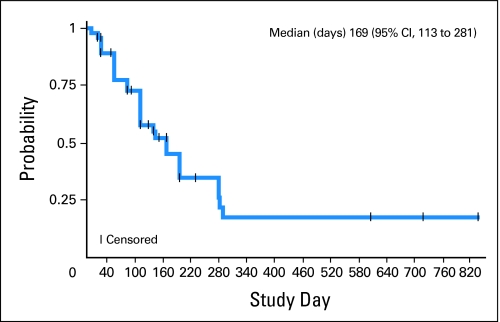
The median time to PSA progression was 169 days (ie, 24 weeks; 95% CI, 113 to 281 days; Fig 3). Five (10.6%) of 47 patients were on study fewer than 12 weeks; 18 (38.3%) of 47 patients were on study for 12 to 23 weeks; 12 (25.5%) of 47 patients were on study 24 to 47 weeks; and 12 (25.5%) of 47 patients remained on study at least 48 weeks. At the time of data cutoff (ie, May 2009), five patients continued on abiraterone acetate (+913, +886, +795, +726, +698 days, respectively).
Fig 3.
Time to prostate-specific antigen (PSA) progression. Median time to PSA progression is 169 days (95% CI, 113 to 281 days).
Declines in CTC Count
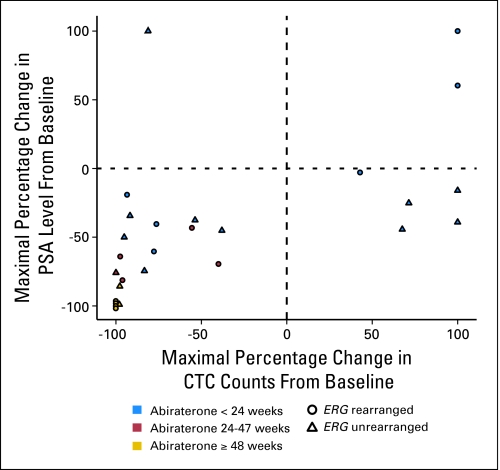
After treatment with abiraterone acetate was started, 11 (41%) of 27 patients had a decline in CTC count from five or greater to less than five. Seventeen (63%) of 27 patients had a decline in CTC count by ≥ 50%, and 18 (67%) of 27 patients had a decline in CTC count by ≥ 30% after starting treatment with abiraterone acetate. CTC count declines from five or greater to less than 5 and declines of 50% and 30% have been associated previously with improved overall survival.22–24 One of 27 patients with a CTC count of ≥ 5 only had a baseline reading. A waterfall plot of maximal percentage CTC count decline on abiraterone acetate is shown in Figure 4. Maximal change in CTC count did not correlate with maximal PSA change (r = 0.093; P = .762 by Pearson Rho test) in the population as a whole. However, for patients with ERG gene–rearranged tumors, PSA and CTC count declines were significantly correlated (r = 0.762; P = .001); conversely, PSA and CTC count declines were not correlated for patients with ERG gene–unrearranged disease (r = 0.493; P = .010; Appendix Fig A1, online only).
Fig 4.
Waterfall plot of maximal circulating tumor cell (CTC) count declines in individual patients on abiraterone acetate. Twenty-six patients are featured in the plot as 1/27 patients with ≥ 5 CTCs only had a baseline measurement. Brown, gold and gray lines indicate a decline in CTC count of 30%, 50% and 90% respectively. Red dots indicate clipped CTC count values.
Safety
All 47 patients were evaluable for adverse events. Abiraterone acetate was, in general, well tolerated. The expected and related toxicities of hypokalemia, hypertension, and fluid retention occurred in 26 (55%), eight (17%), and seven (15%) patients, respectively, and were easily managed (eplerenone/low-dose glucocorticoids). Three deaths occurred on study. One patient developed pneumonia, and another patient developed progressive disease and deteriorated rapidly. Neither of these deaths was considered related to study drug. The third patient, who had diabetes and a cardiac history, was admitted to hospital with groin pain at the site of extensive bony metastases. During the admission, he experienced chest discomfort and was treated for a possible upper respiratory tract infection. He had an asystolic cardiac arrest attributed to a myocardial infarct or pulmonary embolus. A postmortem was not conducted. Table 2 lists adverse events.
Table 2.
Incidence of Most Frequent Treatment-Related Adverse Events
| Event | Patients by Event Grade (N = 47) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Nausea | 3 | 6 | 2 | 4 | 2 | 4 | 0 | |
| Constipation | 1 | 2 | 4 | 9 | 0 | 0 | ||
| Fatigue | 2 | 4 | 10 | 21 | 3 | 6 | 0 | |
| Peripheral edema | 2 | 4 | 5 | 11 | 0 | 0 | ||
| Hypokalaemia | 25 | 53 | 0 | 1 | 2 | 0 | ||
| Anorexia | 4 | 9 | 4 | 9 | 1 | 2 | 0 | |
| Hyperglycemia | 1 | 2 | 5 | 11 | 0 | 0 | ||
| Headache | 3 | 6 | 2 | 4 | 0 | 0 | ||
| Hypertension | 3 | 6 | 5 | 11 | 0 | 0 | ||
NOTE. Treatment-related effects from abiraterone acetate or steroid treatment. Only events occurring in ≥ 10% of patients are reported in this Table. One patient reported grade 4 increase in transaminases not shown in the Table.
DISCUSSION
This study in patients with late-stage metastatic CRPC who have received prior docetaxel treatment demonstrates that selective, irreversible CYP17 inhibition with abiraterone acetate results in significant antitumor activity. The antitumor activity is in keeping with levels reported in our chemotherapy-naïve studies4,5 and by Ryan et al25 in this issue of Journal of Clinical Oncology (JCO). Declines in PSA of ≥ 50% were seen in 51% of patients. PSA declines were supported by radiologic responses, improvement in symptoms, and CTC declines. Similar activity in this patient population is reported by Danila et al26 in this issue of JCO. Overall, these results suggest that a significant proportion of CRPC remains hormone driven after docetaxel treatment, which supports therapeutic targeting of the AR and AR signaling in this population. These data led to the development of an 1,158 patient registration phase III trial of prednisone with either abiraterone acetate or placebo in a 2:1 random assignment in docetaxel-treated patients, for which overall survival was the primary end point (ie, NCT00638690). The CTC data in this phase II study led to the incorporation of CTC count evaluation into the phase III trial to establish whether the impact of treatment on CTC count can serve as an intermediate end point of overall survival in CRPC. Concordance was seen between PSA and CTC for patients with ERG gene–rearranged disease but not for patients without an ERG gene rearrangement. As ERG gene rearrangements and PSA production are hormone regulated, one might expect that PSA should more accurately reflect a therapeutic effect and be concordant with CTC counts in the patients with ERG gene–rearranged disease. These data, albeit preliminary and in small numbers, are in keeping with the mechanism of action of abiraterone acetate.
The patients recruited to this study had many poor prognostic features, including the following: albumin ≤ 35 or 3.5 g/dL (62%); PS of ≥ 1 (66%); and CTC count of at least five (79%).22–24,27 All of these patients had disease that was resistant to most standard and some experimental lines of therapy. Specifically, all patients had received docetaxel, the only agent to have shown a survival benefit in this group.3
Importantly, despite the more advanced stage of the disease and the poorer patient PS, the adverse events seen in these post-docetaxel patients were similar to those in our recently reported phase I and II pre-docetaxel studies (N = 54 patients) of abiraterone acetate.4,5 As reported in our previous phase I experience, no hypoadrenalism was seen. Hypokalemia, hypertension, and fluid retention—all expected toxicities that occur because of increased adrenocorticotrophic hormone and a resultant excess of mineralocorticoid steroids upstream of CYP17—were effectively managed with a mineralocorticoid receptor antagonist or a low-dose of glucocorticoid.
This study provides additional evidence that selective CYP17 inhibition with abiraterone acetate in postdocetaxel patients with CRPC is well tolerated and has significant antitumor activity. These results highlight the continued importance of the AR axis, even in the most advanced stages of the disease, and warrant phase III confirmation.
Acknowledgment
We thank research nurse Emilda Thompson for her care and organization of patients and Gal Maier for data management and collection in this study. Supported by Cougar Biotechnology. A.H.M.R., G.A., N.B.O., D.O., P.C.F., L.R.M., J.H., C.M., C.P., D.D., and J.S.d.B are in the Section of Medicine, which is supported by a Cancer Research UK program grant and an Experimental Cancer Medicines Centre grant from Cancer Research UK and the Department of Health (Ref: C51/A7401) and a Medical Research Council (UK) Biomarkers Grant. Also supported by the Royal Marsden Hospital Research Fund (A.H.M.R. and G.A.); the Prostate Cancer Foundation, Santa Monica, CA (G.A.); by Cancer Research UK and the National Cancer Research Institute Prostate Cancer Collaborative (C.P.). We also acknowledge National Health Service funding to the National Institute for Health Research Biomedical Research Centre. Abiraterone acetate was developed at The Institute of Cancer Research, which therefore has a commercial interest in the development of this agent. D.D., C. M., D.O., L.R.M., C.P., A.H.M.R., G.A., and J.S.d.B. are employees of The Institute of Cancer Research.
Appendix
Fig A1.
Correlation of the maximal percentage change in prostate-specific antigen (PSA) with the maximal percentage change in circulating tumor cells (CTCs). Twenty-seven patients had five or more CTCs at baseline. This plot shows 26 patients, as one patient had only a baseline CTC measurement. Of the 10 patients with five or greater CTCs who remained on study for ≥ 24 weeks (indicated in red and gold), seven patients (70%) had an ERG gene rearrangement. However, of the 16 patients who remained on study for less than 24 weeks (indicated in blue), six of these (37.5%) had an ERG gene rearrangement.
Footnotes
Presented at the 98th Annual Meeting of the American Association for Cancer Research, April 14-18, 2007, Los Angeles, CA; Annual European Society for Medical Oncology Conference, July 5-8, 2007, Lugano, Switzerland; Annual National Cancer Research Institute Conference, September 30-October 3, 2007, Birmingham, United Kingdom; Annual Genitourinary Meeting of the American Society of Clinical Oncology, February 14-16, 2008, San Francisco, CA; 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL; and the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00474383.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Thian Kheoh, Cougar Biotechnology (C); Arturo Molina, Cougar Biotechnology (C) Consultant or Advisory Role: Alison H.M. Reid, Cougar Biotechnology (U); Gerhardt Attard, Cougar Biotechnology (U); Daniel C. Danila, Cougar Biotechnology (U); Leon W.M.M. Terstappen, Veridex (C); Eric Small, Cougar Biotechnology (C); Howard I. Scher, Cougar Biotechnology (C), Veridex LLC (U); Johann S. de Bono, Cougar Biotecnology (U) Stock Ownership: Thian Kheoh, Cougar Biotechnology; Arturo Molina, Cougar Biotechnology Honoraria: None Research Funding: Howard I. Scher, Veridex, Cougar Biotechnology Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Alison H.M. Reid, Johann S. de Bono
Financial support: Arturo Molina
Administrative support: Gloria Lee, Thian Kheoh, Arturo Molina
Provision of study materials or patients: Alison H.M. Reid, Gerhardt Attard, Daniel C. Danila, Nikhil Babu Oommen, David Olmos, Peter C. Fong, L. Rhoda Molife, Joanne Hunt, Christopher Parker, David Dearnaley, Charles J. Ryan, Eric Small, Howard I. Scher, Johann S. de Bono
Collection and assembly of data: Alison H.M. Reid, Gerhardt Attard, Daniel C. Danila, Nikhil Babu Oommen, David Olmos, Peter C. Fong, L. Rhoda Molife, Joanne Hunt, Christina Messiou, Joost F. Swennenhuis, Leon W.M.M. Terstappen, Thian Kheoh, Charles J. Ryan, Johann S. de Bono
Data analysis and interpretation: Alison H.M. Reid, David Olmos, Thian Kheoh, Johann S. de Bono
Manuscript writing: Alison H.M. Reid, Johann S. de Bono
Final approval of manuscript: Alison H.M. Reid, Gerhardt Attard, Daniel C. Danila, Nikhil Babu Oommen, David Olmos, Peter C. Fong, L. Rhoda Molife, Joanne Hunt, Christina Messiou, Christopher Parker, David Dearnaley, Joost F. Swennenhuis, Leon W.M.M. Terstappen, Gloria Lee, Thian Kheoh, Arturo Molina, Charles J. Ryan, Eric Small, Howard I. Scher, Johann S. de Bono
REFERENCES
- 1.National Cancer Institute. Surveillance, Epidemiology and End Results. www.seer.cancer.gov.
- 2.Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Attard G, Reid AH, A'Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 6.Attard G, Sarker D, Reid A, et al. Improving the outcome of patients with castration-resistant prostate cancer through rational drug development. Br J Cancer. 2006;95:767–774. doi: 10.1038/sj.bjc.6603223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 9.Mohler JL, Gregory CW, Ford OH, 3rd, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geisler J, Lonning PE. Aromatase inhibition: Translation into a successful therapeutic approach. Clin Cancer Res. 2005;11:2809–2821. doi: 10.1158/1078-0432.CCR-04-2187. [DOI] [PubMed] [Google Scholar]
- 12.Hakki T, Bernhardt R. CYP17- and CYP11B-dependent steroid hydroxylases as drug development targets. Pharmacol Ther. 2006;111:27–52. doi: 10.1016/j.pharmthera.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Setlur SR, Mertz KD, Hoshida Y, et al. Estrogen-dependent signaling in a molecularly distinct subclass of aggressive prostate cancer. J Natl Cancer Inst. 2008;100:815–825. doi: 10.1093/jnci/djn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan FC, Potter GA, Barrie SE, et al. 3- and 4-pyridylalkyl adamantanecarboxylates: Inhibitors of human cytochrome P450(17 alpha) (17 alpha-hydroxylase/C17,20-lyase)—Potential nonsteroidal agents for the treatment of prostatic cancer. J Med Chem. 1996;39:3319–3323. doi: 10.1021/jm950749y. [DOI] [PubMed] [Google Scholar]
- 15.Barrie SE, Potter GA, Goddard PM, et al. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase) J Steroid Biochem Mol Biol. 1994;50:267–273. doi: 10.1016/0960-0760(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 16.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: Recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 17.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 19.Attard G, Swennenhuis JF, Olmos D, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69:2912–2918. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 20.Swennenhuis JF, Tibbe AG, Levink R, et al. Characterization of circulating tumor cells by fluorescence in situ hybridization. Cytometry A. 2009;75:520–527. doi: 10.1002/cyto.a.20718. [DOI] [PubMed] [Google Scholar]
- 21.Green SDS. Planned versus attained design in phase II clinical trials. Stat Med. 1992;11:853–862. doi: 10.1002/sim.4780110703. [DOI] [PubMed] [Google Scholar]
- 22.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 23.Olmos D, Arkenau HT, Ang JE, et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): A single-centre experience. Ann Oncol. 2009;20:27–33. doi: 10.1093/annonc/mdn544. [DOI] [PubMed] [Google Scholar]
- 24.Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–239. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP 17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danila DC, Morris MJ, De-Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in docetaxel-treated, castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smaletz O, Scher HI, Small EJ, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;20:3972–3982. doi: 10.1200/JCO.2002.11.021. [DOI] [PubMed] [Google Scholar]