Abstract
Purpose
Most of the esophageal squamous cell carcinomas (ESCCs) and cancers of the head and neck (H&N) region are diagnosed at later stages. To achieve better survival, early detection is necessary. We compared the real-time diagnostic yield of superficial cancer in these regions between conventional white light imaging (WLI) and narrow band imaging (NBI) in high-risk patients.
Patients and Methods
In a multicenter, prospective, randomized controlled trial, 320 patients with ESCC were randomly assigned to primary WLI followed by NBI (n = 162) or primary NBI followed by WLI (n = 158) in a back-to-back fashion. The primary aim was to compare the real-time detection rates of superficial cancer in the H&N region and the esophagus between WLI and NBI. The secondary aim was to evaluate the diagnostic accuracy of these techniques.
Results
NBI detected superficial cancer more frequently than did WLI in both the H&N region and the esophagus (100% v 8%, P < .001; 97% v 55%, P < .001, respectively). The sensitivity of NBI for diagnosis of superficial cancer was 100% and 97.2% in the H&N region and the esophagus, respectively. The accuracy of NBI for diagnosis of superficial cancer was 86.7% and 88.9% in these regions, respectively. The sensitivity and accuracy were significantly higher using NBI than WLI in both regions (P < .001 and P = .02 for the H&N region; P < .001 for both measures for the esophagus, respectively).
Conclusion
NBI could be the standard examination for the early detection of superficial cancer in the H&N region and the esophagus.
INTRODUCTION
Esophageal cancer is the eighth most common cancer worldwide, accounting for 462,000 new cases in 2002, and is the sixth most common cause of cancer-related death (386,000 deaths).1 Squamous cell carcinoma (SCC) is the most common histologic type worldwide.1 Head and neck (H&N) cancer accounted for 607,000 new cases and 261,000 deaths in 2002.1 The most common histologic type of H&N cancer is also SCC.
The early detection of cancer offers the best prognosis. Currently, however, esophageal SCC (ESCC) and H&N SCC (HNSCC) are detected at a late stage and then have poor prognoses.1 Early detection of these cancers is difficult by conventional endoscopic white light imaging (WLI). Lugol chromoendoscopy can be used to detect superficial ESCC, but it causes unpleasant adverse effects such as severe chest pain and chest discomfort,2–4 and it cannot be used for HNSCC screening because of the risk of aspiration.
The narrow band imaging (NBI) system is an innovative optical image-enhanced technology that uses narrow bandwidth NBI filters.5,6 The central wavelengths of the NBI filters are 415 and 540 nm and each has a bandwidth of 30 nm. This system is easily activated by pushing a button on the endoscope. NBI combined with magnifying endoscopy can clearly visualize the microvascular structure of the organ surface,6,7 because the 415-nm light is well absorbed by hemoglobin. Surface microvascular irregularities provide useful landmarks for identifying an early neoplasm in the H&N region, bronchus, and the GI tract.7–15 We previously reported that NBI was useful for identifying HNSCC at an early stage.8 Watanabe et al16,17 also reported the usefulness of NBI rhinolaryngovideoscopy for the diagnosis of HNSCC. Yoshida et al18 reported that NBI improves the accuracy of magnifying WLI in the assessment of ESCC.
However, the diagnostic yield of NBI in the early detection of superficial SCC has not been investigated. We conducted a prospective randomized study to directly compare WLI and NBI in the early diagnosis of SCC in the H&N region and the esophagus among high-risk patients.
PATIENTS AND METHODS
Study Rationale
Because ESCC patients frequently develop multiple intraesophageal SCC and second primary HNSCC synchronously and metachronously,4,19–22 they provide a good cancer screening model. Whereas massively invasive SCC is easy to detect by endoscope, superficial cancer has been difficult. Furthermore, detection of high-grade intraepithelial neoplasia (HGIN) is clinically important because HGINs have the potential to become malignant invasive cancers.23,24 Therefore, in this study, we targeted only macroscopic superficial cancer including HGIN that appeared as slightly elevated lesions lower than 5 mm, flat lesions, and lesions with a shallow depression. Lesions with an apparent elevation greater than 5 mm or those with apparent deeper ulceration were not evaluated.
The primary analysis of this study was a comparison of the detection rates of superficial cancer (HGIN, carcinoma in situ, and microinvasive SCC) using WLI and NBI. The secondary analysis was a comparison of the diagnostic accuracy (sensitivity and specificity) of the two imaging methods, size of the lesion detected, and the examination time. To evaluate diagnostic accuracy, we used the histologic diagnosis from a biopsy specimen as the gold standard diagnosis.
Study Populations
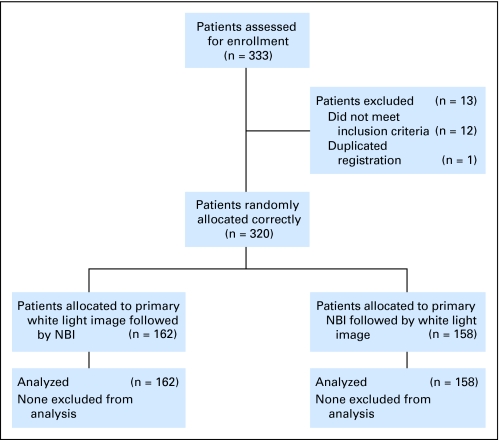
The protocol and consent form for this study were approved by the institutional review board at each participating institution, and written informed consent was obtained from all patients. The inclusion criteria were histologically confirmed present or previous ESCC and an age of 20 years or older. Although this study included patients with advanced ESCC, we evaluated only concomitant superficial cancer but not primary advanced cancer. Patients who had been previously treated for ESCC by endoscopic mucosal resection were included, because their esophagus was preserved with minimal damage. Patients with prior chemotherapy, radiotherapy, chemoradiotherapy, or surgical resection for ESCC or HNSCC were excluded, because their esophagus or pharynx was removed or too damaged to evaluate. Patients referred from another hospital with newly diagnosed ESCC were also included because they required more detailed examination (Fig 1). The endoscopists were blinded to the endoscopic information. Patients with esophageal stricture, esophageal varices, or allergy to lugol dye solution were excluded.
Fig 1.
CONSORT diagram; overview of the study design. NBI, narrow band imaging.
Study Design
Patients were randomly assigned to receive primary WLI or primary NBI. To investigate whether a lesion detected by primary imaging could be identified subsequently by the other type of imaging, or whether a lesion missed by primary imaging could be identified subsequently by the other type of imaging, we performed both imaging methods in a back-to-back fashion so that primary WLI was followed by NBI and primary NBI was followed by WLI. To avoid affecting the first imaging results, the report of the first examination was completed before the second imaging was started.
To improve the quality of the reporting in the diagnostic accuracy study, we complied with the Standards for Reporting of Diagnostic Accuracy (STARD) initiative.25 We set WLI as reference standard and NBI as index test.
Random assignment was performed in each case by an investigator using a computer-aided system on Medical Research Support Web site (Kyoto, Japan). This Web site was available only to the study participants. Using a minimization algorithm, the selection of the primary examination was balanced with respect to five stratification variables: institution, age (< 60 and ≥ 60 years), sex, alcohol consumption, and smoking habit.
Calculation of the Sample Size
For the purposes of this study, we set the probability for error (α) to .05 with a power of 0.80 (reflecting a β error of .2). Because there are no published comparative studies of NBI in ESCC patients, we estimated that the NBI system would increase the detection yield for superficial cancer by at least threefold compared with conventional WLI. This resulted in a calculated sample size of 250 patients (125 per group). Finally, we recruited an additional 50 patients in anticipation of instances of ineligibility or withdrawal during the examination because of discomfort (25 per group).
Endoscopic Examination
We used the same magnifying endoscope, with the capability for 80 times optical magnification (GIF-Q240Z, Olympus Medical Systems, Tokyo, Japan) for both WLI and NBI. The two imaging methods can be performed in a same video-endoscopy system (EVIS LUCERA system, Olympus Medical Systems, Tokyo, Japan). The details of the NBI system have been published elsewhere.1,2,26,27 To maintain the quality of the endoscopic images, we used the same liquid-crystal color display for both imaging methods. Before the study started, all the participating endoscopists were trained using a central review of demonstrable NBI images of superficial squamous lesions (13 neoplasias and seven non-neoplastic lesions).
All endoscopic observations were made according to the protocol. During the first imaging, all parts of the oropharynx and hypopharynx were evaluated. The nasopharynx was not included the examination. After the first imaging was completed, an assistant physician immediately recorded the results on the case record form (CRF). After completion of the first imaging CRF, the second imaging of the oropharynx and hypopharynx was performed and the results were recorded on the CRF.
Next, all parts of the esophagus were evaluated using the same imaging as used for the H&N region. The endoscope was inserted to gain a view from the cervical esophagus to the esophagogastric junction, and the results were recorded on the CRF. The second imaging was performed on withdrawal of the endoscope, and the results were recorded on the CRF. During the procedure, we measured the examination time from start to finish of each imaging at each site. These procedure times included the evaluation of the lesion but not the biopsy procedure. The findings obtained by lugol chromoendoscopy are not included in this study.
Endoscopic Evaluation of Superficial Cancers
In this study, the real-time on-site diagnosis was evaluated because making an accurate diagnosis during an examination is clinically more important than a retrospective evaluation using a stored database. On WLI, if the lesion showed both a reddish color with uneven surface and disappearance of the vascular network pattern (Fig 2A), we diagnosed it as endoscopically suspected “superficial cancer.” On NBI, if the lesion exhibited a well-demarcated brownish area as well as irregular microvascular patterns (Fig 2B), we diagnosed it as endoscopically suspected “superficial cancer.” Details of these findings have been described previously.7,8 If the lesion did not show these characteristics, the lesion was diagnosed as “non-cancer.” Mucosal abnormalities were recorded with regard to endoscopic diagnosis, location, and size of the lesion.
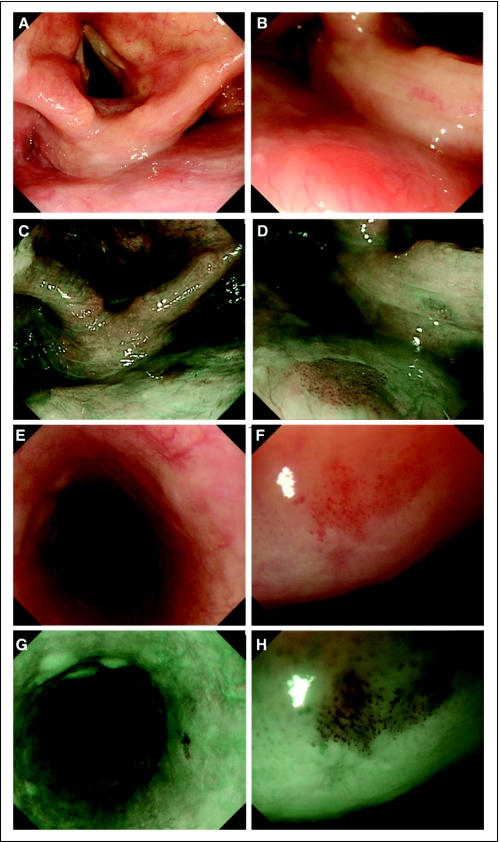
Fig 2.
Superficial cancer in the head and neck region and esophagus. (A) White light imaging (WLI) shows a small reddish area (arrows) in the posterior wall of the hypopharynx. (B) Magnifying WLI shows a slightly reddish area with tiny microdots. (C) Narrow band imaging (NBI) shows a well-demarcated brownish area (arrows) in the posterior wall of the hypopharynx. (D) Magnifying NBI shows many tiny dots in the brownish area. This lesion was diagnosed histologically as squamous cell carcinoma in situ. (E) WLI shows a slightly reddish and depressed lesion (arrows) in the esophagus, although it is difficult to detect by WLI alone. (F) Magnifying WLI shows a slightly reddish area with an irregular microvascular pattern. (G) NBI shows a well-demarcated brownish area (arrows). (H) Magnifying NBI shows many tiny dots in the brownish area. This lesion was diagnosed histologically as high-grade intraepithelial cancer.
Pathologic Evaluation
Biopsy specimens were taken from each lesion after the completion of both types of imaging. Histologic evaluation was performed by central review by four experienced pathologists (H.S., A.O., T.S., and H.W.) who were blinded to the recorded endoscopic assessment. Histologic diagnoses were made according to WHO criteria23 and were classified into two groups. One group included superficial cancers and the other group included non-cancers such as parakeratosis and inflammation. Microinvasion was estimated by the subepithelial invasion. The final pathologic diagnosis was made by the agreement of three of the four pathologists.
Statistical Analysis
The absolute and relative frequencies for qualitative variables were calculated for each group. Statistical analysis was performed using SPSS version 17 software (SPSS, Chicago, IL). The continuous variables are expressed as medians and ranges. Continuous data were compared using the Mann-Whitney U test. Pearson's χ2 test or Fisher's exact test was used to analyze categoric data to compare proportions. All P values were two-tailed, and a P value of < .05 was considered significant.
RESULTS
Between March 2005 and December 2005, 333 patients were enrolled onto this study (Fig 1). Twelve patients did not meet the inclusion criteria, and one was registered twice, so the remaining 320 patients were randomly assigned correctly into two groups: (1) 162 patients who underwent primary WLI followed by NBI, and (2) 158 patients who were examined by primary NBI followed by WLI.
The characteristics of the two groups are listed in Table 1. The two groups did not differ significantly in age, sex, alcohol consumption, smoking habits, or history of esophageal cancer treatment. In both groups, approximately 70% of the patients had newly diagnosed ESCC. Sixty-three (39%) patients in the primary WLI group and 71 (45%) patients in the primary NBI group had advanced ESCC deeper than the submucosal layer.
Table 1.
Characteristics of Patients
| Characteristic | Primary WLI (n = 162) |
Primary NBI (n = 158) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | |||||
| Median | 64 | 64 | |||
| Range | 39-84 | 46-84 | .99 | ||
| Male sex | 143 | 88 | 141 | 89 | .86 |
| Alcohol habit | |||||
| Drinking duration, years | 157 | 97 | 148 | 94 | .19 |
| Median | 41 | 40 | .17 | ||
| Range | 10-63 | 5-60 | |||
| Favorite beverage | |||||
| Beer | 61 | 38 | 59 | 37 | 1.00 |
| Shochu | 66 | 41 | 55 | 35 | .30 |
| Sake | 43 | 27 | 48 | 30 | .71 |
| Whisky | 22 | 14 | 24 | 15 | .75 |
| Wine | 8 | 5 | 7 | 4 | 1.00 |
| Others | 1 | 0.6 | 0 | 0 | 1.00 |
| Hot flashes | |||||
| Formerly had hot flashes | 117 | 72 | 109 | 69 | .62 |
| Currently has hot flashes | 75 | 46 | 70 | 44 | .91 |
| Smoking habit | |||||
| No. of smokers | 145 | 90 | 142 | 90 | 1.00 |
| Smoking duration, years | |||||
| Median | 37 | 40 | |||
| Range | 1-61 | 5-61 | .41 | ||
| No. of packs per day | |||||
| Median | 1 | 1 | |||
| Range | 0.05-4 | 0.125-4 | .64 | ||
| No. of packs per year | |||||
| Median | 41 | 42 | |||
| Range | 0.5-180 | 1.3-160 | .89 | ||
| Esophageal cancer | |||||
| No. of patients newly diagnosed | 110 | 68 | 115 | 73 | .39 |
| Previously treated EMR | 52 | 32 | 43 | 27 | .39 |
| Duration from previous EMR, years | |||||
| > 1 | 17 | 10 | 20 | 13 | .60 |
| 1 | 45 | 28 | 33 | 21 | .16 |
| Depth of invasion | |||||
| Tis-T1a | 74 | 46 | 67 | 42 | .57 |
| T1b | 25 | 15 | 20 | 13 | .27 |
| T2 | 12 | 7 | 22 | 14 | .07 |
| T3 | 49 | 30 | 46 | 29 | .90 |
| T4 | 2 | 1 | 3 | 2 | .68 |
Abbreviations: WLI, white light imaging; NBI, narrow band imaging; EMR, endoscopic mucosal resection.
Table 2 provides the distribution of histologically confirmed superficial cancers. The total numbers of superficial cancer in the H&N region and the esophagus were 28 and 212, respectively. Total numbers of histologically confirmed non-cancer were 36 and 38 in each region. In all patients, superficial cancers were detected in 8% (26 of 320) in the H&N region and in 38% (121 of 320) in the esophagus. Multiple cancers were found in 0.6% of the patients in the H&N region and in 12% in the esophagus. The number of patients with superficial cancer, total number of superficial cancers, and their sizes and distribution did not differ between the two groups.
Table 2.
Distribution of Histologically Confirmed Superficial Cancer According to Lesion in the Head and Neck Region and the Esophagus
| Variable | Primary WLI (n = 162) |
Primary NBI (n = 158) |
P | ||||
|---|---|---|---|---|---|---|---|
| No. | % | 95% CI | No. | % | 95% CI | ||
| Head and neck region | |||||||
| No. of patients | 12 | 7 | 3.3 to 11.4 | 14 | 9 | 4.4 to 13.3 | .66 |
| No. of lesions per patient | |||||||
| 1 | 12 | 7 | 3.3 to 11.4 | 14 | 9 | 4.4 to 13.3 | > .999 |
| ≥ 2 | 1 | 0.6 | −0.6 to 1.8 | 1 | 0.6 | −0.5 to 1.9 | |
| Total No. of superficial neoplasias | 13 | 15 | |||||
| Size threshold, mm | |||||||
| < 10 | 7 | 10 | .50 | ||||
| 11-20 | 5 | 5 | |||||
| ≥ 21 | 1 | 0 | |||||
| Histologic diagnosis | |||||||
| High-grade intraepithelial neoplasia or carcinoma in situ | 10 | 15 | .09 | ||||
| Microinvasive cancer | 3 | 0 | |||||
| Esophagus | |||||||
| No. of patients | 58 | 36 | 28.4 to 43.2 | 63 | 40 | 32.2 to 47.6 | .49 |
| No. of lesions per patient | |||||||
| 1 | 39 | 24 | 17.4 to 30.7 | 43 | 27 | 20.3 to 34.2 | > .999 |
| ≥ 2 | 19 | 12 | 6.7 to 16.7 | 20 | 13 | 7.4 to 17.9 | |
| Total No. of superficial cancers | 105 | 107 | |||||
| Size threshold, mm | |||||||
| < 10 | 18 | 18 | .91 | ||||
| 11-20 | 21 | 19 | |||||
| ≥ 21 | 66 | 70 | |||||
| Histologic diagnosis | |||||||
| High-grade intraepithelial neoplasia or carcinoma in situ | 73 | 84 | .16 | ||||
| Microinvasive cancer | 32 | 23 | |||||
Abbreviations: WLI, white light imaging; NBI, narrow band imaging.
The diagnostic yields for superficial cancer using primary WLI and primary NBI detection are summarized in Table 3. The total numbers of superficial cancers detected by primary imaging differed between the two groups. In the H&N region, primary NBI detected all (100%; 15 of 15) of the superficial cancers, but primary WLI detected only one lesion (8%; 1 of 13). In the esophagus, only 58 (55%) lesions were detected by primary WLI, whereas 104 (97%) lesions were detected by primary NBI. All these differences were statistically significant (P < .001). The detection rate was significantly higher with primary NBI than with primary WLI, even for small lesions (< 10 mm in diameter) in both the H&N region (P < .001) and the esophagus (P = .03).
Table 3.
Diagnostic Yield of Primary WLI and Primary NBI for Detection of Superficial Cancer in the Head and Neck Region and the Esophagus
| Variable | Primary WLI (n = 162) |
Primary NBI (n = 158) |
P | ||||
|---|---|---|---|---|---|---|---|
| No. | % | 95% CI | No. | % | 95% CI | ||
| Head and neck region | |||||||
| No. of superficial cancers | 1/13 | 8 | 0.2 to 36.0 | 15/15 | 100 | 78.2 to 100 | < .001 |
| Size of superficial cancer, mm | |||||||
| < 10 | 0/7 | 0 | 0 to 41.0 | 10/10 | 100 | 69.2 to 100 | < .001 |
| 11-20 | 1/5 | 20 | 0.5 to 71.6 | 5/5 | 100 | 48.7 to 100 | .12 |
| ≥ 21 | 0/1 | 0 | 0.0 to 0.0 | to | — | ||
| Esophagus | |||||||
| No. of superficial cancers | 58/105 | 55 | 45.2 to 65.0 | 104/107 | 97 | 92.0 to 99.4 | < .001 |
| Size of superficial cancer, mm | |||||||
| < 10 | 7/18 | 39 | 17.3 to 64.3 | 17/18 | 94 | 72.7 to 99.9 | .03 |
| 11-20 | 7/21 | 33 | 14.6 to 57.0 | 18/19 | 95 | 74.0 to 99.9 | .02 |
| ≥ 21 | 44/66 | 67 | 54.0 to 77.8 | 69/70 | 99 | 92.3 to 100 | < .005 |
Abbreviations: WLI, white light imaging; NBI, narrow band imaging.
In the back-to-back analysis, secondary NBI after primary WLI significantly increased the detection rate in both the H&N region (8% v 77%; P < .001) and esophagus (55% v 95%; P < .001; Appendix Table A1, online only). In contrast, secondary WLI after NBI significantly decreased the detection rate (Appendix Table A1). Moreover, 16 (57%) superficial cancers in the H&N region and 48 (23%) superficial cancers in the esophagus were detected only by NBI (Appendix Table A2, online only). In contrast, no lesion was detected only by WLI, except one lesion of > 20 mm in the esophagus. No lesions were undetected by both WLI and NBI in either region.
Table 4 summarizes the diagnostic performance of primary WLI and primary NBI for detecting superficial cancer. The sensitivity of primary NBI was significantly higher than that of primary WLI in both the H&N region (100% v 7.7%; P < .001) and the esophagus (97.2% v 55.2%; P < .001). Accuracy was also significantly higher for primary NBI than for primary WLI in both regions (85.7% v 62.9%, P = .02 and 88.9% v 56.5%, P < .001, respectively). Specificity was not significantly different in the two regions (P = .28 and P = .33, respectively). The positive predictive value did not differ between the two imaging techniques, but the negative predictive value was significantly higher for primary NBI than for primary WLI in both the H&N region (P = .02) and the esophagus (P < .002).
Table 4.
Diagnostic Performance of Primary WLI and Primary NBI Observation for Detection of Superficial Cancer in the Head and Neck Region and the Esophagus
| Variable | Primary WLI |
Primary NBI |
P | ||||
|---|---|---|---|---|---|---|---|
| No. | % | 95% CI | No. | % | 95% CI | ||
| Head and neck | |||||||
| Sensitivity | 1/13 | 7.7 | 0.2 to 36.0 | 15/15 | 100 | 100 | < .001 |
| Specificity | 21/22 | 95.5 | 77.2 to 99.9 | 11/14 | 78.6 | 54.6 to 98.1 | .28 |
| Accuracy | 22/35 | 62.9 | 47.6 to 76.4 | 26/29 | 86.7 | 72.6 to 97.8 | .02 |
| PPV | 1/2 | 50 | 1.3 to 98.7 | 15/18 | 83.3 | 58.6 to 96.4 | .37 |
| NPV | 21/33 | 63.6 | 54.1 to 79.6 | 11/11 | 100 | 100 | .02 |
| Esophagus | |||||||
| Sensitivity | 58/105 | 55.2 | 45.2 to 65.0 | 104/107 | 97.2 | 92.0 to 99.4 | < .001 |
| Specificity | 12/19 | 63.2 | 38.4 to 83.7 | 8/19 | 42.1 | 20.3 to 66.5 | .33 |
| Accuracy | 70/124 | 56.5 | 47.3 to 65.3 | 112/126 | 88.9 | 82.1 to 93.8 | < .001 |
| PPV | 58/65 | 89.2 | 79.1 to 95.6 | 104/115 | 90.4 | 85.3 to 95.1 | .80 |
| NPV | 12/59 | 20.3 | 11.0 to 32.8 | 8/11 | 72.8 | 39 to 94 | < .002 |
Abbreviations: WLI, white light imaging; NBI, narrow band imaging; PPV, positive predictive value; NPV, negative predictive value.
The median procedure times of primary WLI and primary NBI for the H&N region were 120 seconds (range, 34 to 275 seconds) and 162 seconds (range, 30 to 525 seconds), respectively. Those for the esophagus were 95 seconds (range, 30 to 360 seconds) and 135 seconds (range, 30 to 616 seconds), respectively. These differences were statistically significant (P < .001). The procedure times in the secondary imaging in the back-to-back experiments also differed significantly between WLI and NBI in both regions (Appendix Table A3, online only). There were no serious adverse events related to examination with either procedure. All patients tolerated both procedures well.
DISCUSSION
This study clearly demonstrates that NBI is a more sensitive method for detecting and diagnosing superficial SCC in the H&N region and the esophagus. According to the concept of “field cancerization,”28 patients with ESCC or HNSCC are at high risk for the development of multiple SCCs. In the clinical context, the early detection strategy for superficial SCC is the same between patients at high risk and those at risk because of heavy drinking, smoking, or aldehyde dehydrogenase 2 deficiency.20–35 In addition, detection technique should not only be sensitive but should also be easily applicable. From this perspective, NBI is easily applied with a modicum of experience and will have a rapid learning curve compared with WLI. Thus, NBI is the ideal method for effectively detecting superficial SCC.
Detecting cancer at an early stage is an optimal strategy for preventing the development of advanced cancer and improving survival. Furthermore, early detection uses a minimally invasive treatment (eg, endoscopic resection) with curative intent.8,36–38 In fact, in our study, 75% (21 of 28) of the superficial HNSCCs were completely removed by endoscopic resection or biopsy alone, while early detection of HNSCC had been quite difficult. These results provide us with new diagnostic and treatment strategies for ESCC patients, because the risk of development of HNSCC after esophagectomy is quite high.21
As the criteria for diagnosing superficial SCC by NBI, we used two endoscopic findings: a well-demarcated brownish area and an irregular microvascular pattern.7–9 Using only these two findings, the sensitivity of primary NBI for the diagnosis of superficial SCC was 100% in the H&N region and 97.2% in the esophagus. The diagnostic accuracy was nearly 90%. These results indicate that these NBI findings are quite useful for the accurate diagnosis of superficial SCC.
Lugol chromoendoscopy is useful for the detection of superficial ESCC.2–3 However, the administration of lugol solution is time-consuming, and accurate diagnosis by lugol chromoendoscopy is difficult4 because the staining pattern shows wide variations.2 This increases the incidence of false-positive lesions and leads to unnecessary biopsies. In contrast, NBI is easily manipulated and shows high sensitivity. Thus, NBI could reduce the number of unnecessary biopsies and shorten examination time. Furthermore, lugol chromoendoscopy is more invasive than both WLI and NBI, and WLI is still the gold standard for cancer screening. Therefore, we did not compare the diagnostic yield of NBI and lugol chromoendoscopy, and we used WLI as the standard reference to compared the diagnostic yield of WLI and NBI.
NBI required a significantly longer examination time than WLI. This might be related to the high detection rate and more frequent time spent in magnification during NBI, because if the lesions were not seen by WLI, no magnification was performed. The actual time difference between NBI and WLI was only 20 to 42 seconds. This is clinically acceptable, because the important time issue is not that NBI takes slightly longer than WLI, but rather that endoscopists spend more time in the careful observation of high-risk patients.
In this study, ESCC patients referred from another hospital were included. Even if the biopsies were previously done, the earlier biopsy sites were healed by the time of this study and were not generally detectable by either imaging method. Therefore, we thought that it was not a confounding factor.
The same endoscopists performed both imaging procedures in this study, whereas the endoscopists ideally should be separated and blinded to each imaging procedure. However, it was clinically impossible to change and blind the endoscopists during this series of examinations. Furthermore, the result produced with NBI first followed by WLI might underestimate the benefit of NBI because NBI is more sensitive than WLI. However, the detection and diagnosis of superficial SCC by NBI was significantly better than that using WLI in both the H&N region and the esophagus, regardless of whether NBI was primary or secondary. These results indicate that NBI should be the standard examination.
Significant detection results seen in this study were all achieved without the newest generation high-definition endoscope. If we use the newest high-definition endoscope with NBI, the rates of detection might increase compared with those found in this study. Furthermore, the endoscopy system used in this study and in most Asian countries was different from those used in North America and Europe.26,27 However, we previously reported that even the nonmagnifying laryngoscope based on same system as that used in North America and Europe could dramatically improve the visualization of both the brownish area and irregular microvascular patterns.39 Therefore, we believe that differences in the system are no longer as important as careful observation by NBI.
In conclusion, NBI combined with magnifying endoscopy significantly improved the detection rates for SCC with quite high sensitivity, and this new image-enhanced technology can be applied easily in clinical practice. Furthermore, early detection facilitates the potential of minimally invasive treatment, such as endoscopic resection or partial surgical resection.
Acknowledgment
We are grateful to the following collaborative members of this study: Chikatoshi Katada, MD, Takashi Kojima, MD, Keisei Taku, MD, at National Cancer Center Hospital East, Kashiwa, and Haruhisa Suzuki, MD, Kozu Takahiro, MD, at National Cancer Center Hospital, Tokyo, for technical support, Kenichi Yoshimura, PhD, Translational Research Center, Kyoto University, Kyoto, for statistical analysis support, and Ayako Nasu, Medical Research Support, Osaka, for data collection and management.
Appendix
Results
Among the 28 superficial head and neck squamous cell carcinomas (HNSCCs) detected, 16 lesions were treated with endoscopic resection, five lesions disappeared after only biopsy, and one lesion was treated with radiotherapy. The remaining six lesions were not treated, because the concomitant esophageal cancers had distant metastasis. Among 16 lesions removed by endoscopic resection, seven lesions were carcinoma in situ and the remaining nine lesions were microinvasive SCC. With a median follow-up of 33 months (range, 6 to 59 months), no patients developed lymph node metastasis from HNSCC.
Among the 212 superficial esophageal squamous cell carcinomas, those with accompanying advanced cancers and those with submucosal invasive cancers were treated with surgery or chemotherapy with or without radiotherapy. The remaining superficial cancers within the mucosal layer were removed by endoscopic resection.
Discussion
Most research on the endoscopic detection and diagnosis of GI disease has been performed by retrospectively reviewing static images in a database and selecting only the best of the stored images (Singh R: Endoscopy 40:457-463, 2008; Sharma P: Gastroenterology 133:454-464, 2007; Chiu HM: Gut 56:373-379, 2007). Evaluating selected stored images by retrospective review does not exclude the possibility of selection bias. From the viewpoint of clinical practice, real-time detection and diagnosis are important. To avoid selection bias and to evaluate the actual diagnostic yield, we recorded the data during the procedure and completely separated the evaluation of white light imaging and narrow band imaging. We also conducted this study according to the Standards for Reporting of Diagnostic Accuracy checklist29 to obtain high-quality data and to assess the generalizability and applicability of the results. Our data are therefore relevant to daily clinical practice.
Table A1.
Rate of Superficial Cancer by Method of Detection in Back-to-Back Fashion
| Region | No. of Patients | Primary Examination WLI |
Secondary Examination NBI |
P | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % | 95% CI | No. | % | 95% CI | |||
| Head and neck | 13 | 1 | 8 | 0.2 to 36.0 | 10 | 77 | 46.2 to 95 | < .001 |
| Esophagus | 105 | 58 | 55 | 45.2 to 65.0 | 100 | 95 | 89.2 to 98.4 | < .001 |
| NBI | WLI | |||||||
| Head and neck | 15 | 15 | 100 | 78.2 to 100 | 5 | 33 | 11.8 to 61.6 | < .001 |
| Esophagus | 107 | 104 | 97 | 92 to 99.4 | 85 | 79 | 70.5 to 86.6 | < .001 |
Abbreviations: WLI, white light imaging; NBI, narrow band imaging.
Table A2.
Size of Superficial Cancer by Method of Detection
| Tumor Size (mm) | WLI Positive |
NBI Positive |
NBI and WLI Positive |
Both Negative (false negative) |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Head and neck region | ||||||||
| < 10 | 0 | 0 | 12 | 70 | 5 | 30 | 0 | 0 |
| 11-20 | 0 | 0 | 4 | 40 | 6 | 60 | 0 | 0 |
| ≥ 21 | 0 | 0 | 0 | 0 | 1 | 100 | 0 | 0 |
| Total | 0 | 0 | 16 | 57 | 12 | 43 | 0 | 0 |
| Esophagus | ||||||||
| < 10 | 0 | 0 | 14 | 39 | 22 | 58 | 0 | 0 |
| 11-20 | 0 | 0 | 12 | 30 | 28 | 70 | 0 | 0 |
| ≥ 21 | 1 | 0.7 | 22 | 16 | 113 | 83 | 0 | 0 |
| Total | 1 | 0.5 | 48 | 23 | 163 | 77 | 0 | 0 |
Abbreviations: WLI, white light imaging; NBI, narrow band imaging.
Table A3.
Median Procedure Time
| Region | Primary WLI (seconds) |
Primary NBI (seconds) |
P | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Head and neck | 120 | 34-275 | 162 | 30-525 | < .001 |
| Esophagus | 95 | 30-360 | 135 | 30-616 | < .001 |
| Secondary WLI | Secondary NBI | ||||
| Head and neck | 90 | 10-300 | 135 | 30-540 | < .001 |
| Esophagus | 80 | 19-776 | 45 | 18-700 | < .005 |
Abbreviations: WLI, white light imaging; NBI, narrow band imaging.
Footnotes
Supported in part by Grant No. H15-008 from the Ministry of Health, Labor, and Welfare of Japan.
Presented in part at Digestive Disease Week, May 20-23, 2007, Washington, DC.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: UMIN 000000242.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Manabu Muto, Hideki Ishikawa
Financial support: Daizo Saito
Administrative support: Manabu Muto, Hisao Tajiri, Daizo Saito
Provision of study materials or patients: Manabu Muto, Keiko Minashi, Tomonori Yano, Yutaka Saito, Ichiro Oda, Satoru Nonaka, Tai Omori, Hitoshi Sugiura, Kenichi Goda, Mitsuru Kaise, Haruhiro Inoue, Atsushi Ochiai, Tadakazu Shimoda, Hidenobu Watanabe
Collection and assembly of data: Manabu Muto, Hitoshi Sugiura, Hideki Ishikawa, Atsushi Ochiai, Tadakazu Shimoda, Hidenobu Watanabe
Data analysis and interpretation: Manabu Muto, Hideki Ishikawa
Manuscript writing: Manabu Muto
Final approval of manuscript: Manabu Muto, Keiko Minashi, Tomonori Yano, Yutaka Saito, Ichiro Oda, Satoru Nonaka, Tai Omori, Hitoshi Sugiura, Kenichi Goda, Mitsuru Kaise, Haruhiro Inoue, Atsushi Ochiai, Tadakazu Shimoda, Hidenobu Watanabe, Hisao Tajiri, Daizo Saito
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Mori M, Adachi Y, Matsushima T, et al. Lugol staining pattern and histology of esophageal lesions. Am J Gastroenterol. 1993;88:701–705. [PubMed] [Google Scholar]
- 3.Inoue H, Rey JF, Lightdale C. Lugol chromoendoscopy for esophageal squamous cell cancer. Endoscopy. 2001;33:75–79. [PubMed] [Google Scholar]
- 4.Muto M, Hironaka S, Nakane M, et al. Association of multiple Lugol-voiding lesions with synchronous and metachronous esophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest Endosc. 2002;56:517–521. doi: 10.1067/mge.2002.128104. [DOI] [PubMed] [Google Scholar]
- 5.Gono K, Yamazaki K, Doguchi N, et al. Endoscopic observation of tissue by narrow band illumination. Opt Rev. 2003;10:211–215. [Google Scholar]
- 6.Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue feature in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568–577. doi: 10.1117/1.1695563. [DOI] [PubMed] [Google Scholar]
- 7.Muto M, Katada C, Sano Y, et al. Narrow band imaging: A new diagnostic approach to visualize angiogenesis in the superficial neoplasia. Clin Gastroenterol Hepatol. 2005;3(suppl 1):S16–S20. doi: 10.1016/s1542-3565(05)00262-4. [DOI] [PubMed] [Google Scholar]
- 8.Muto M, Nakane M, Katada C, et al. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004;101:1375–1381. doi: 10.1002/cncr.20482. [DOI] [PubMed] [Google Scholar]
- 9.Muto M, Ugumori T, Sano Y, et al. Narrow-band imaging combined with magnified endoscopy for the cancer at the head and neck region. Dig Endoscopy. 2005;17:S23–S24. [Google Scholar]
- 10.Shibuya K, Hoshino H, Chiyo M, et al. High magnification bronchovideoscopy combined with narrow band imaging could detect capillary loops of angiogenic squamous dysplasia in heavy smokers at high risk for lung cancer. Thorax. 2003;58:989–995. doi: 10.1136/thorax.58.11.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamamoto Y, Endo T, Nosho K, et al. Usefulness of narrow-band imaging endoscopy for diagnosis of Barrett's esophagus. J Gastroenterol. 2004;39:14–20. doi: 10.1007/s00535-003-1239-z. [DOI] [PubMed] [Google Scholar]
- 12.Sharma P, Bansal A, Mathur S, et al. The utility of a novel narrow band imaging endoscopy system in patients with Barrett's esophagus. Gastrointest Endosc. 2006;64:167–175. doi: 10.1016/j.gie.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 13.Nakayoshi T, Tajiri H, Matsuda K, et al. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: Correlation of vascular pattern with histopathology. Endoscopy. 2004;36:1080–1084. doi: 10.1055/s-2004-825961. [DOI] [PubMed] [Google Scholar]
- 14.Sumiyama K, Kaise M, Nakayoshi T, et al. Combined use of a magnifying endoscope with a narrow band imaging system and a multibending endoscope for en bloc EMR of early stage gastric cancer. Gastrointest Endosc. 2004;60:79–84. doi: 10.1016/s0016-5107(04)01285-4. [DOI] [PubMed] [Google Scholar]
- 15.Machida H, Sano Y, Hamamoto Y, et al. Narrow-band imaging in the diagnosis of colorectal lesions: A pilot study. Endoscopy. 2004;36:1094–1098. doi: 10.1055/s-2004-826040. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe A, Tsujie H, Taniguchi M, et al. Laryngoscopic detection of pharyngeal carcinoma in situ with narrowband imaging. Laryngoscope. 2006;116:650–654. doi: 10.1097/01.mlg.0000204304.38797.34. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe A, Taniguchi M, Tsujie H, et al. The value of narrow band imaging endoscope for early head and neck cancers. Otolaryngol Head Neck Surg. 2008;138:446–451. doi: 10.1016/j.otohns.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida T, Inoue H, Usui S, et al. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004;59:288–295. doi: 10.1016/s0016-5107(03)02532-x. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu Y, Tukagoshi H, Fujita M, et al. Metachronous squamous cell carcinoma of the esophagus arising after endoscopic mucosal resection. Gastrointest Endosc. 2001;54:190–194. doi: 10.1067/mge.2001.116877. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu Y, Tsukagoshi H, Fujita M, et al. Head and neck cancer arising after endoscopic mucosal resection for squamous cell carcinoma of the esophagus. Endoscopy. 2003;35:322–326. doi: 10.1055/s-2003-38151. [DOI] [PubMed] [Google Scholar]
- 21.Matsubara T, Yamada K, Kakegawa A. Risk of second primary malignancy after esophagectomy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:4336–4341. doi: 10.1200/JCO.2003.12.074. [DOI] [PubMed] [Google Scholar]
- 22.Kumagai Y, Kawano T, Nakajima Y, et al. Multiple primary cancers associated with esophageal carcinoma. Surg Today. 2001;31:872–876. doi: 10.1007/s005950170025. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton R, Aaltonen LA, editors. WHO Classification of Tumors of the Digestive System. Lyon, France: IARC Press; 2000. Tumors of the esophagus; pp. 11–19. [Google Scholar]
- 24.Schlemper RJ, Dawsey SM, Itabashi M, et al. Differences in diagnostic criteria for esophageal squamous cell carcinoma between Japanese and Western pathologists. Cancer. 2000;88:996–1006. doi: 10.1002/(sici)1097-0142(20000301)88:5<996::aid-cncr8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Schulz KF, Altman D. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel group randomized trials. JAMA. 2001;285:1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- 26.Muto M, Horimatsu T, Ezoe Y, et al. Narrow-band imaging of the gastrointestinal tract. J Gastroenterol. 2009;44:13–25. doi: 10.1007/s00535-008-2291-5. [DOI] [PubMed] [Google Scholar]
- 27.Muto M, Horimastu T, Ezoe Y, et al. Improving visualization techniques by narrow band imaging and magnification endoscopy. J Gastroenterol Hepatol. 2009;24:1333–1346. doi: 10.1111/j.1440-1746.2009.05925.x. [DOI] [PubMed] [Google Scholar]
- 28.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium: Clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann Intern Med. 2003;138:40–44. doi: 10.7326/0003-4819-138-1-200301070-00010. [DOI] [PubMed] [Google Scholar]
- 30.Franco EL, Kowalski LP, Kanda JL. Risk factors for second cancers of the upper respiratory and digestive systems: A case-control study. J Clin Epidemiol. 1991;44:615–625. doi: 10.1016/0895-4356(91)90021-z. [DOI] [PubMed] [Google Scholar]
- 31.Hsairi M, Luce D, Point D, et al. Risk factors for simultaneous carcinoma of the head and neck. Head Neck. 1989;11:426–430. doi: 10.1002/hed.2880110508. [DOI] [PubMed] [Google Scholar]
- 32.Morita M, Kuwano H, Ohno S, et al. Multiple occurrence of carcinoma in the upper aerodigestive tract associated with esophageal cancer: Reference to smoking, drinking, and family history. Int J Cancer. 1994;58:207–210. doi: 10.1002/ijc.2910580211. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama A, Kato H, Yokoyama T, et al. Genetic polymorphisms of alcohol and aldehyde dehydrogenases and glutathione S-transferase M1 and drinking, smoking, and diet in Japanese men with esophageal squamous cell carcinoma. Carcinogenesis. 2002;23:1851–1859. doi: 10.1093/carcin/23.11.1851. [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama A, Watanabe H, Fukuda H, et al. Multiple cancers associated with esophageal and oropharyngolaryngeal squamous cell carcinoma and the aldehyde dehydrogenase-2 genotype in male Japanese drinkers. Cancer Epidemiol Biomarkers Prev. 2002;11:895–900. [PubMed] [Google Scholar]
- 35.Muto M, Takahashi M, Ohtsu A, et al. Risk of multiple squamous cell carcinomas both in the esophagus and the head and neck region. Carcinogenesis. 2005;26:1008–1012. doi: 10.1093/carcin/bgi035. [DOI] [PubMed] [Google Scholar]
- 36.Katada C, Muto M, Momma K, et al. Clinical outcome after endoscopic mucosal resection for esophageal squamous cell carcinoma invading the muscularis mucosae—a multicenter retrospective cohort study. Endoscopy. 2007;39:779–783. doi: 10.1055/s-2007-966761. [DOI] [PubMed] [Google Scholar]
- 37.Katada C, Muto M, Manabe T, et al. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc. 2005;61:219–225. doi: 10.1016/s0016-5107(04)02756-7. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu Y, Yamamoto J, Kato M, et al. Endoscopic submucosal dissection for treatment of early stage hypopharyngeal carcinoma. Gastrointest Endosc. 2006;64:255–259. doi: 10.1016/j.gie.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 39.Ugumori T, Muto M, Hayashi R, et al. Prospective study of early detection of pharyngeal superficial carcinoma with the narrowband imaging laryngoscope. Head Neck. 2009;31:189–194. doi: 10.1002/hed.20943. [DOI] [PubMed] [Google Scholar]