Abstract
Study Objectives:
To describe sex differences in the associations between severity of obstructive sleep apnea (OSA) and measures of obesity in body regions defined using both dual-energy absorptiometry and traditional anthropometric measures in a sleep-clinic sample.
Design:
A prospective case-series observational study.
Setting:
The Western Australian Sleep Health Study operating out of the Sir Charles Gairdner Hospital Sleep Clinic, Perth, Western Australia.
Participants:
Newly referred clinic patients (60 men, 36 women) suspected of having OSA.
Interventions:
N/A
Measurements and Results:
Obstructive sleep apnea severity was defined by apnea-hypopnoea index from laboratory-based overnight polysomnography. Body mass index, neck, waist and hip circumference, neck-to-waist ratio, and waist-to-hip ratio were measured. Dual energy absorptiometry measurements included percentage fat and lean tissue. Multivariate regression models for each sex were developed. In women, percentage of fat in the neck region and body mass index together explained 33% of the variance in apnea-hypopnea index. In men, percentage of fat in the abdominal region and neck-to-waist ratio together accounted for 37% of the variance in apnea-hypopnea index.
Conclusions:
Regional obesity is associated with obstructive sleep apnea severity, although differently in men and women. In women, a direct influence of neck fat on the upper airway patency is implicated. In men, abdominal obesity appears to be the predominant influence. The apnea-hypopnea index was best predicted by a combination of Dual Energy Absorptiometry-measured mass and traditional anthropometric measurements.
Citation:
Simpson L; Mukherjee S; Cooper MN; Ward KL; Lee JD; Fedson AC; Potter J; Hillman Fanzca DR; Eastwood P; Palmer LJ; Kirkness J. Sex differences in the association of regional fat distribution with the severity of obstructive sleep apnea. SLEEP 2010;33(4):467-474
Keywords: Obesity, body mass index, abdominal fat, absorptiometry
OBSTRUCTIVE SLEEP APNEA (OSA) IS CHARACTERIZED BY REPETITIVE UPPER AIRWAY OBSTRUCTION DURING SLEEP.1 IT RESULTS FROM A COMBINATION of anatomic features that narrow the upper airway and the permissive effect of insufficient neuromuscular compensation during sleep.2 OSA is prevalent to a clinically significant degree in 2% of women and 4% of men,2 and obesity is the most common known risk factor.3–5
Excessive fat deposition may play a mechanistic role in OSA severity. Fat in the peripharyngeal area of the neck is thought to directly compress the upper airway.6 Chest-wall fat compresses the rib cage, reducing lung volume.7 Abdominal fat is thought to result in cranial displacement of the diaphragm, decreasing longitudinal tracheal traction on the upper airway and leading to increased propensity for upper airway collapse.8 Reduced prevalence and severity of OSA in women is likely to be a consequence of a more favorable pattern of distribution of excess fat. Specifically, women tend to distribute fat peripherally around the hips, buttocks, and thighs, whereas men tend to distribute excess fat more centrally on the abdomen and neck.9 As a result, although women have proportionally greater fat mass than men, they have less mechanical loading on their upper airway.9 However it is notable that most clinical studies examining the influence of obesity have been conducted in male populations10,11 or have included only small numbers of women12; therefore, the potentially important explanatory role of differences in pattern of obesity in determining differences in severity of OSA remains inadequately defined. Furthermore, traditional anthropometric measures used in studies of OSA include body mass index (BMI), waist and neck circumferences, neck-to-waist ratio (NWR), waist-to-hip ratio (WHR), and skin-fold anthropometry, which are of limited accuracy in determining fat distribution.5,13–15
More sophisticated methods are available to measure fat mass, such as magnetic resonance imaging (MRI) and computed tomography (CT), although they are unsuitable for studies requiring large populations. Dual energy absorptiometry (DXA) scanning is an accurate alternative measure of fat mass that is well suited for studies in clinical settings due to its relatively lower associated costs, training expertise, and radiation exposure.16 To date, it remains unknown whether DXA-measured regional fat can predict severity of OSA better than traditional anthropometric measures.
In this study, we investigated whether traditional anthropometric measures, DXA-measured fat, or a combination of both best predicted OSA severity. We also investigated whether associations between OSA severity and regional fat distribution differed in men and women.
METHODS
The study population comprised a case series (Table 1) recruited as part of the Western Australian Sleep Health Study (WASHS) between October 2007 and March 2008. The WASHS case series is an epidemiologic, genetic, and biospecimen resource created to enable research into sleep disorders, which recruits approximately 90% of all patients presenting at the Sir Charles Gairdner Hospital sleep clinic, Western Australia's largest facility for the diagnosis and treatment of OSA and other sleep disorders. In March 2008, when formal recruitment of participants for the DXA substudy ceased, the full WASHS case series comprised 1759 mainly Caucasian participants with questionnaire, biospecimen, and polysomnography data, of which 96 were further characterized for this study. In compliance with national guidelines for medical research involving humans, patients were posted an information pack outlining the study protocol prior to their first presentation at the sleep clinic. Informed written consent for the DXA study was obtained by clinic physicians who recruited patients consecutively. Exclusion criteria included suspected pregnancy, prior exceptional radiation exposure, concurrent use of continuous positive airway pressure, and inability to attend the appointment for the scanning and body-surface measurements. The height and weight limits of the DXA system further excluded patients who weighed more than 130 kg or were taller than 1.8m. This study was approved by the Sir Charles Gairdner Hospital Human Research Ethics Committee.
Table 1.
Characteristics of the study population
| Men (n = 60) | Women (n = 36) | P Value | |
|---|---|---|---|
| Age, y | 50 ± 14 | 51 ± 14 | NS |
| AHI, events/h | 33 (41) | 15 (21) | < 0.001 |
| Mean SaO2 | 93 (4) | 95 (2) | 0.002 |
| Lowest SaO2 | 86 (14) | 89 (5) | 0.004 |
| Number of times SaO2 90% | 163 (1103) | 8 (135) | 0.004 |
| Weight, kg | 98 ± 17) | 89 (22) | 0.03 |
| Height, cm | 175 ± 7 | 161 ± 7 | < 0.0001 |
| BMI, kg/m2 | 32 ± 5 | 34 ± 8 | NS |
| ESS, score | 10 ± 5 | 10 ± 5 | NS |
| Neck circumference, cm | 44 ± 4 | 38 ± 4 | < 0.001 |
| Waist circumference, cm | 111 ± 13 | 105 ± 18 | NS |
| Hip circumference, cm | 112 ± 10 | 120 ± 16 | 0.002 |
| WHR | 1.0 ± 0.1 | 0.9 ± 0.1 | < 0.0001 |
| NWR | 0.37 ± 0.03 | 0.40 ± 0.04 | < 0.0001 |
Data are presented as mean ± SD or median (interquartile range); NS refers to not significant at a P value of > 0.05; AHI, apnea-hypopnea index; BMI, body mass index; ESS, Epworth Sleepiness Scale; WHR, waist-to-hip ratio; NWR, neck-to-waist ratio.
OSA severity was defined by apnea-hypopnea index (AHI)17 from laboratory-based overnight polysomnography (Profusion 2, Compumedics, Victoria, Australia) and scored using American Academy of Sleep Medicine criteria.18 Factors with an established relationship with OSA severity—age (years), sex, Epworth sleepiness score (ESS), and BMI (kg/m2)—were recorded. Body surface, upper airway, and DXA measurements were collected at the same time within 4 weeks of the polysomnography.
Body Surface and Upper Airway Measurements
Waist circumference was measured with the patient standing erect, at the midpoint between the costal margin and the iliac crest, at the end of normal expiration. Hip circumference was measured at the level of the greater trochanter. Neck circumference was measured with the patient seated, at the level of the anterior border of the cricoid cartilage. Mallampati score19 and pharyngeal grade were scored according to visual scales.20–23
DXA-Measured Body Mass
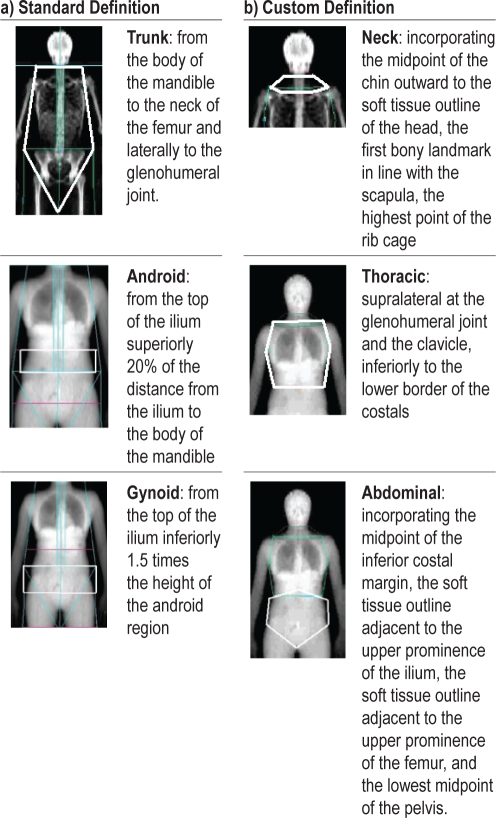
Percentage of fat and lean tissue, and bone density (g/cm2) were measured using a Lunar Prodigy DXA machine (GE Healthcare, Madison, WI). Participants were positioned centrally on the scanner bed with the mandible on a vertical plane. Hands were palm down, slightly away from the body. The EnCORE software v10.50. (General Electric Company, Madison, WI) defined standard regions: trunk, android, and gynoid (Figure 1a). Three customized regions were also analyzed (neck, abdominal and thoracic; Figure 1b). The peripheral central ratio (PCR) was calculated as fat in the peripheral areas of the body (fat in the body – fat in the trunk)/fat in the trunk.
Figure 1.
DXA region definitions
Statistical Analysis
Data were analyzed using R version 2.7.1 (2008, The R Foundation for Statistical Computing) using SimHap.24 Normally distributed continuous variables were described using mean and standard deviations, and nonparametric variables using the median and interquartile range (IQR). χ2, Students t test, and Mann-Whitney U test were used to test the assumption that the study sample was representative of the WASHS case series and investigate sex differences within the study sample. AHI was right skewed and, therefore, loge-transformed prior to analysis. Three multivariate regression models for each sex were developed. Model 1 investigated the predictive value of traditional anthropometric measures of obesity (BMI, waist and neck circumferences, WHR, and NWR) in explaining disease severity. Model 2 investigated the predictive value of DXA-measured mass in the total body, neck, thoracic, abdominal, android, and gynoid region. Fat and lean measures were expressed as a percentage of total body mass in explaining disease severity. In Model 3, the relative associations of traditional anthropometric and DXA variables with AHI were investigated in a multivariate model. In all 3 models, the predictive value of age, BMI, ESS, Mallampati score, and pharyngeal grade were investigated. Models were adjusted for these covariates only when they were significantly associated with LogeAHI. All continuous covariates were centered for (multivariate) regression analysis to reduce collinearity. This was justified during forward stepwise-regression diagnostics in ensuring Model 3a and 3b met the assumptions of normality, linearity and homoscedasticity. Statistical significance for univariate models was defined at the 5% level; however, because our initial aim was to develop and compare 3 models, we used a revised threshold of P < 0.017 to determine statistical significance in multivariate models. The study had 80% power at the α 0.05 level to detect an effect size of at least 0.15 Loge(events/h) in men and 0.23 Loge(events/h) in women for the DXA-measured mass covariates, which had a standard deviation of around 2.
RESULTS
Characteristics of Case Series
The study sample comprised 60 men and 36 women, which is similar to the sex ratio of WASHS χ2(DF 1) = 0.0117, P = 0.9). Likewise the DXA group was typical of WASHS case series for age, AHI, traditional anthropometric measures, and ESS score (P > 0.05). Within the study sample, there were no significant differences in age, ESS, or BMI between men and women (Table 1). Men were heavier, were taller, and had thicker necks than women, on average (P < 0.001). Men and women also differed significantly in measures of disease severity; men, on average, had a higher AHI, lower mean oxygen saturation, more severe lowest oxygen saturation, and higher frequency of times during the night with an oxygen saturation of less than 90% (Table 1).
Participants were mostly overweight (18%, BMI 25-29.9 kg/m2) or obese (68%, BMI > 30 kg/m2). The proportion of obese participants did not differ by sex. Men were more likely to have an AHI in the severe range (42% men vs 19% women had > 40 events/hour, χ2(DF = 1) = 4.1, P = 0.04). Oropharyngeal measurements showed 54% of participants had a Mallampati score of III or IV, and this did not differ by sex (55% men vs 50% women, χ2(DF = 1) = 0.2, P = 0.7). A greater proportion of men had a crowded oropharynx (pharyngeal grade III, IV, or not visible in 73% men vs 41% women, χ2(DF = 1) = 5.2, P = 0.02).
Body Morphology
DXA-quantified fat and lean mass in body regions for each sex are shown in Table 2. Although men and women were similar with respect to age, ESS score, and BMI, there were significant sex differences with regard to the percentage of lean and fat tissue, both in the whole body and regionally. In all areas of the body, men had a greater percentage of lean mass in comparison with women. Men and women had similar percentages of fat in the neck region, but the ratio of fat-to-lean tissue (SD) in the neck was 0.45 (0.15) in men and 0.65 (0.19) in women (P < 0.000001). In all other regions, men had a significantly lower percentage of fat. Men had a greater proportion of fat located in the central regions abdomen and neck (PCR = 0.6 in men and 0.8 in women; Table 2). Given that men and women differed both in measures of obesity and disease outcome, predictive models were generated separately for each sex.
Table 2.
Sex differences in DXA-quantified regional body mass
| Total body mass | Men | Women | P Value |
|---|---|---|---|
| % fat | 33 ± 7 | 46 ± 5 | < 0.0001 |
| % lean | 63 ± 6 | 51 ± 5 | < 0.00001 |
| PCR | 0.6 ± 0.2 | 0.8 ± 0.2 | < 0.0001 |
| BMD, g/cm3 | 1.3 ± 0.1 | 1.2 ± 0.1 | < 0.0001 |
| Thoracic mass | |||
| % fat | 10 ± 3 | 12 ± 3 | 0.0002 |
| % lean | 18 ± 2 | 16 ± 2 | < 0.00001 |
| Abdominal mass | |||
| % fat | 10 ± 2 | 12 ± 2 | < 0.00001 |
| % lean | 13 ± 1 | 12 ± 1 | < 0.00001 |
| Android mass | |||
| % fat | 4 ± 1 | 5 ± 1 | 0.01 |
| % lean | 5 ± 1 | 4 ± 1 | < 0.00001 |
| Neck mass | |||
| % fat | 0.7 ± 0.2 | 0.8 ± 0.2 | NS |
| % lean | 1.6 ± 0.3 | 1.2 ± 0.2 | < 0.00001 |
| Trunk mass | |||
| % fat | 21 ± 5 | 25 ± 4 | < 0.0001 |
| % lean | 31 ± 3 | 27 ± 3 | < 0.00001 |
| Gynoid mass | |||
| % fat | 5 ± 1 | 8 ± 1 | < 0.0001 |
| % lean | 10 ± 1 | 8 ± 1 | < 0.00001 |
Data are shown as mean ± SD. DXA refers to dual-energy absorptiometry scanning; NS, not significant at a P value of 0.05; % fat, fat in region (g)/total body mass (g) × 100; % lean, lean tissue in region (g)/total body mass (g) = 100; Ratio, fat in region (g)/lean tissue in region (g); PCR, peripheral central ratio of fat, i.e., (fat in the total body – fat in the trunk)/fat in the trunk; BMD, bone mineral density (g × 103.
Predictors of Severity of OSA in Men
Of the DXA-measured obesity variables, percentage of fat in the abdominal area was the best univariate predictor of AHI in men (Table 3) and explained the most variance in AHI (r2 = 16%). The thoracic and android region explained a similar amount of variance in AHI (r2 = 15 & 14%). Neck circumference and weight were comparable with DXA-measured fat; both explained 17% of the variance of AHI. Neither percentage of fat or lean tissue in the neck appeared to be associated with severity of OSA in men. Univariate analyses suggested that BMI was the least predictive anthropometric measurement in men (r2 = 11%). Bone mineral density was significantly associated with OSA severity in men.
Table 3.
Univariate predictors of Loge(AHI)
| DXA measurements, % | Men |
Women |
||||
|---|---|---|---|---|---|---|
| r2 | β (95% CI) | P value | r2 | β (95% CI) | P value | |
| Neck region | ||||||
| Fat | 0.06 | 0.96 (−0.01, 1.93) | NS | 0.25 | 2.51 (1.01, 4.00) | 0.002 |
| Lean | 0.02 | −0.36 (−1.10, 0.38) | NS | < 0.01 | 0.04 (−1.63, 1.71) | NS |
| Thoracic mass | ||||||
| Fat | 0.15 | 0.12 (0.05, 0.19) | 0.001 | 0.24 | 0.18 (0.07, 0.29) | 0.003 |
| Lean | 0.04 | −0.09 (−0.20, 0.03) | NS | < 0.01 | 0.01 (−0.17, 0.18) | NS |
| Abdominal mass | ||||||
| Fat | 0.16 | 0.14 (0.06, 0.23) | 0.001 | 0.17 | 0.21 (0.05, 0.37) | 0.01 |
| Lean | 0.09 | −0.17 (−0.32, −0.03) | 0.02 | 0.02 | −0.13 (−0.46, 0.20) | NS |
| Android mass | ||||||
| Fat | 0.14 | 0.31 (0.11, 0.52) | 0.003 | 0.26 | 0.63 (0.27, 0.99) | 0.001 |
| Lean | 0.11 | −0.65 (−1.14, −0.16) | 0.01 | 0.01 | 0.29 (−0.63, 1.20) | NS |
| Trunk mass | ||||||
| Fat | 0.15 | 0.07 (0.03, 0.11) | 0.002 | 0.16 | 0.11 (0.02, 0.19) | 0.02 |
| Lean | 0.12 | 0.11 (−0.18, −0.03) | 0.006 | 0.03 | −0.07 (−0.20, 0.07) | NS |
| Gynoid mass | ||||||
| Fat | 0.04 | 0.13 (−0.04, 0.31) | NS | < 0.01 | 0.02 (−0.25, 0.29) | NS |
| Lean | 0.10 | −0.27 (−0.48, −0.05) | 0.02 | 0.12 | −0.44 (−0.84, −0.04) | 0.04 |
| Total body mass | ||||||
| Fat, % | 0.12 | 0.04 (0.01, 0.07) | 0.006 | 0.16 | 0.08 (0.02, 0.14) | 0.02 |
| Lean, % | 0.12 | −0.05 (−0.08, −0.01) | 0.007 | 0.13 | −0.08 (−0.15, −0.01) | 0.03 |
| BMD, g/cm2 | 0.10 | 2.41 (0.49, 4.33) | 0.02 | 0.05 | 2.29 (−1.07, 5.65) | NS |
| PCR | 0.13 | −1.54 (−2.56, −0.51) | 0.004 | 0.04 | −1.24 (−3.20, 0.72) | NS |
| Anthropometric measures | ||||||
| BMI, kg/m2 | 0.11 | 0.05 (0.01, 0.09) | 0.01 | 0.21 | 0.06 (0.02, 0.10) | 0.005 |
| Neck circumference, cm | 0.17 | 0.08 (0.04, 0.13) | 0.001 | 0.10 | 0.10 (0.02, 0.19) | 0.02 |
| Waist circumference, cm | 0.16 | 0.10 (0.02, 0.19) | 0.02 | 0.19 | 0.03 (0.01, 0.04) | 0.008 |
| Weight, kg | 0.17 | 0.02 (0.01, 0.03) | 0.001 | 0.14 | 0.02 (0.01, 0.03) | 0.03 |
| WHR | 0.05 | 2.24 (−0.45, 4.92) | NS | 0.13 | 5.00 (0.53, 9.47) | 0.03 |
| NWR | < 0.01 | −2.06 (−9.67, 5.56) | NS | 0.11 | −9.4 (−18.35, −0.47) | 0.04 |
| Age, y | < 0.01 | 0.01 (−0.01, 0.02) | NS | < 0.01 | 0.01 (−0.02, 0.03) | NS |
| FVC, L | 0.03 | 0.17 (−0.11, 0.44) | NS | 0.04 | −0.28 (−0.85, 0.29) | NS |
| ESS, score | 0.16 | 0.07 (0.03, 0.11) | 0.001 | < 0.01 | 0.01 (−0.06, 0.07) | NS |
| Narrow airwaya | 0.05 | 0.37 (−0.06, 0.79) | NS | 0.12 | 0.79 (0.03, 1.56) | 0.05 |
| Mallampati scoreb | 0.04 | 0.08 | ||||
| II | −0.57 (−1.36, 0.23) | NS | 0.40 (−0.62, 1.43) | NS | ||
| III | −0.43 (−1.24, 0.38) | NS | 0.61 (−0.39, 1.60) | NS | ||
| IV | −0.40 (−1.22, 0.43) | NS | 0.96 (−0.29, 2.21) | NS | ||
AHI refers to apnea-hypopnea index; DXA, dual-energy absorptiometry; CI, confidence interval; Fat %, (fat (g)/total body mass (g)) × 100; Lean %, (lean mass (g)/total body mass (g)) × 100; PCR, peripheral central ratio of fat, i.e., (fat in the total body – fat in the trunk)/fat in the trunk × 100; NS, not significant at a P value of 0.05; ESS, Epworth Sleepiness Scale; BMD, bone mineral density; WHR, waist-to-hip ratio; NWR, neck-to-waist ratio
Pharyngeal grade III or IV (reference category = pharyngeal grade I or II)
The reference category for the Mallampati score is I.
Multivariate analysis using only traditional anthropometric measures to characterize obesity suggested that ESS score and neck circumference predicted 28% of the variance in AHI in men (Table 4: model 1a). When only DXA-measured mass variables were included, percentage of fat in the abdominal region was the best predictor of OSA severity (r2 = 29%; Table 4: model 2a). In Model 3, where both traditional anthropometric measures and DXA-measured mass were considered, percentage of fat in the abdominal region and NWR accounted for 37% of the variance in AHI (Table 4: model 3a).
Table 4.
Multivariate predictors of Loge(AHI) in men and women
| Model | r2 | Predictors in men | β (95% CI) | P value | Model | r2 | Predictors in women | β (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|---|
| 1a | 0.28 | ESS | 0.06 (0.02, 0.09) | 0.004 | 1b | 0.21 | BMI | 0.06 (0.02, 0.10) | 0.005 |
| Neck circumference | 0.07 (0.03, 0.12) | 0.003 | |||||||
| 2a | 0.29 | ESS | 0.06 (0.02, 0.10) | 0.003 | 2b | 0.26 | Android fat (%) | 0.63 (0.27, 0.99) | 0.001 |
| Abdominal fat (%) | 0.13 (0.05, 0.20) | 0.002 | |||||||
| 3a | 0.37 | ESS | 0.06 (0.03, 0.10) | 0.001 | 3b | 0.33 | Neck fat (%) | 1.92 (0.39, 3.45) | 0.02a |
| Abdominal fat (%) | 0.21 (0.11, 0.31) | < 0.00001 | BMI | 0.04 (0.01, 0.08) | 0.04a | ||||
| NWR | 10.91 (2.63, 19.20) | 0.01 |
Models 1a & 1b are anthropometric measures of obesity; Models 2a & 2b are derived from dual-energy absorptiometry (DXA) measures of obesity; Models 3a & 3b are anthropometric measures + DXA measures of obesity. AHI refers to apnea-hypopnea index; CI, confidence interval; ESS, Epworth Sleepiness Scale; NWR, neck-to-waist ratio.
P values not significant using a multiple correction threshold of P < 0.017.
Predictors of Severity of OSA in Women
In women, percentage of fat in the android region was the best univariate predictor of AHI (r2 = 26%); however, percentage of fat in the neck and thoracic regions explained comparable variance in AHI (Table 3). The best anthropometric measurement of obesity in women was BMI, but this measurement was a poorer predictor than DXA-measured variables (Table 3). Unlike in men, in women, the ESS score was not significantly associated with disease severity; however, a narrow airway (pharyngeal grade: ≥ III vs ≤ II) was.
Multivariate analysis suggested that this association was not independent of BMI or fat in the android region. Further, multivariate modeling of only traditional anthropometric measures and only DXA-measured mass suggested that BMI (Table 4: model 1b) and DXA-measured percentage of fat in the android region (Table 4: model 2b) were the best independent predictors of AHI in women. However, Model 3, in which the traditional anthropometric measures and DXA-measured obesity were investigated in the same multivariate model, suggested that the overall independent predictors of AHI in women were percentage of fat in the neck region and BMI, which together explained 33% of the variance in AHI, though this failed to reach statistical significance after correction for multiple comparisons (Table 4: model 3b).
DISCUSSION
This study investigated the association of traditional anthropometric measures and DXA-measured mass with each other and with OSA severity in a well-characterized case series of men and women. When considered separately, DXA measures of fat mass were more predictive of OSA severity than were anthropometric measures in women. The men and women in this series had comparable BMI, but the men had a lower ratio of fat-to-lean body mass in all body regions, and neck circumference and weight were as predictive of disease severity as DXA-measured fat mass in men. In both men and women, however, a combination of anthropometric measurements and DXA-measured mass improved this predictive capacity. DXA measures may therefore have clinical and epidemiologic utility.
Our results suggest that, in both men and women, it is centrally located rather than peripherally located fat that contributes to the pathogenesis and severity of OSA. However, there were substantial sex-based differences in the association between fat distribution and severity of OSA.
We found that neck fat was associated with disease severity in women but not in men, whereas neck circumference was associated with both sexes, but particularly in men. In the neck, the percentage of fat mass was similar between sexes, but the mean ratio of fat-to-lean tissue was 0.45 in men and 0.65 in women (P < 0.000001). This would suggest that, in men, lean tissue is a substantial contributor to neck circumference. In women, an increased neck circumference appears to be more likely to be associated with a disproportionate increase in fat, despite the tendency of women to accumulate fat more peripherally, as compared with men. Our findings question the commonly held view that the male preponderance of OSA is thought to be related to neck fat and its direct compressive effect on the airway. Enlarged neck circumference has often been attributed to increased adipose tissue in the neck; however, larger studies have not directly measured neck fat,15 and MRI-based studies have been conducted using male-biased case-control designs with relatively small sample sizes.10–12,17
Some studies have found an association between size of peripharyngeal fat pads and the presence of OSA using MRI studies exclusively from men10,11; however, this finding has not been universally replicated.17 Studies including women have found fat-pad thickness to be slightly higher in the OSA groups11,12; however these studies have had insufficient power to consider the influence of sex on fat-pad thickness. These findings conflict with those from a sophisticated mixed-sex case-control analysis of the upper airway using volumetric MRI by Schwab et al., in which adjustment for visceral fat in the neck failed to account for differences between soft tissue structure volumes in cases and control subjects.25 Although based on greater case numbers than in previous MRI studies using sex- and ethnicity-matched control subjects, the Schwab study did not investigate whether there were sex-based differences in the association between fat distribution and presence of OSA.25 The present study is unique in that we used a relatively large sample of women to investigate the mechanistic effects of total fat volume on severity of OSA within a clinical sample using DXA and found an association between the proportion of fat in the neck and severity of OSA. It should be noted that this finding failed to reach significance when corrected for multiple comparisons; nevertheless, this exploratory work suggests that the mechanism underpinning this direct relationship could be through the influence of fat on extraluminal tissue pressure and, thereby, the compressive force on the upper airway.
We found that percentage of abdominal fat was most clearly associated with OSA severity in men. CT-derived visceral adipose tissue in the abdomen has been found to correlate with AHI,26 a finding replicated in a nuclear MRI study of 60 men.26 Importantly, the MRI study, unlike the CT study, also measured subcutaneous neck fat and peripharyngeal fat in the airway and concluded that fat accumulation in the neck was not associated with OSA severity in men.17 Hence, our findings agree with an MRI-based study of similar power and design. The role of abdominal fat in upper airway instability is increasingly recognized: recumbent abdominal obesity is likely to be associated with increased craniad displacement of the diaphragm, decreasing longitudinal tracheal traction and increasing propensity for upper airway collapse.8 Accumulation of fat in the chest wall (abdominal and thoracic) also decreases functional residual capacity, particularly when the individual is recumbent and asleep, increasing intrathoracic pressure and thereby extramural tissue pressure at the thoracic inlet, further increasing upper airway collapsibility.27
Other similar studies have used MRI in a case-control design to investigate the role of visceral fat in the pathogenesis of OSA. Our study concentrated on the mechanistic effects of total fat volume on severity of OSA within a clinical sample using DXA, which cannot distinguish between subcutaneous and visceral fat, nor can it identify the anatomic distribution of the fat. Distribution of fat laterally in the neck has been purported to be important; however, this view is not substantiated by the current literature.28 CT imaging is currently considered the gold standard technology for measuring fat mass, but we did not compare DXA-measured obesity with either CT or MRI imaging studies in our sample, since it was not a viable option to screen large numbers of patients with OSA with either of these technologies due to cost and, in the case of CT, high radiation dose.16 A limitation of DXA is that it does not discriminate visceral from subcutaneous fat. However, larger subjects who may experience greater claustrophobia in CT or MRI scanners are more likely to consent to a DXA scan. The relatively small sample size of MRI and CT studies, coupled with a lower response fraction, leave these studies open to sources of bias. DXA-measured total fat volume has been found to predict visceral fat better than anthropometric measures in some studies29,30 but not others.31,32 Regardless, correlation between total fat volume measured by DXA and CT is high, particularly in people with greater abdominal fat.32
We sought to determine the predictive value of DXA measures of regional obesity on the severity of OSA, as compared wtih traditional surrogate measures of obesity, such as neck and waist circumference and BMI. We quantified total and regional measures of obesity using DXA, which has good precision (99%) for the total body; however, precision is reduced for regional definitions of mass.33 We customized 3 regions of interest: the neck, the thoracic region, and the abdominal region, based upon bony landmarks and soft tissue, which were more complex in definition than standard regions, such the trunk and android regions. This is a novel application of DXA. Previous studies investigating the predictive value of DXA-measured regional fat in comparison with anthropometric surrogates have largely concentrated on abdominal region in association with metabolic risk factors, studies in which visceral fat is considered pathogenic. Most,34–36 but not all,16,37 concluded that DXA-measured abdominal fat is a better predictor of metabolic risk factors than are anthropometric surrogates.
The pathogenesis of OSA is heterogeneous within the population, with risk factors differing among subgroups of individuals. Clinical epidemiologic studies often rely upon BMI or waist circumference to control for the confounding influence of obesity when investigating risk factors. In our study, men and women were similar with respect to both BMI and waist circumference, yet we found clear sex differences in fat and lean mass in the whole body and regionally (P < 0.0001). This finding indicates that reliance upon BMI or waist circumference alone to measure obesity, which confounds the relationship between almost all cardiovascular and metabolic risk factors, is likely to misrepresent the potentially important influence of regional obesity.
Our study showed that a combination of anthropometric and DXA measures predicted 33% to 37% of the variance associated with OSA severity. Although these results support the hypothesis that central obesity is an important mediator of severity of OSA in men and women, there are clearly other contributing factors—such as airway dimension, ethnicity, and menopause status in women—that were not considered by the scope of this study. There is increasing evidence that pathogenesis and severity of OSA is mediated by genetic components; however, these factors are likely to differ between ethnic groups,38,39 with varying environmental mediators at large across discrete populations. Intermediate phenotypes may be more informative than AHI in examining causal relationships. This study has demonstrated that DXA combined with standard anthropometry could be a valuable method for examining obesity-related genetic factors.
Limitations
Our findings are based upon relatively small numbers (60 men and 36 women) and therefore should be interpreted with caution. Nevertheless, the numbers are large relative to comparable studies. Furthermore, the allocation of patients to recruiting physicians was random, which would reduce selection bias. Formal statistical comparisons indicated that our case series was representative of the WASHS population with regard to age, sex, indexes of disordered breathing, traditional anthropometric measures, upper airway characteristics, and ESS scores. The WASHS case series is a large sample of individuals with sleep disorders, with the majority diagnosis being OSA. The physical limits of the DXA machine would have excluded 30% of all male WASHS patients, of which 26% were excluded because of height, not weight, restrictions. An independent t test of the mean LogeAHI score of WASHS men who would have exceeded the limits of the DXA system (in comparison with those who would not) was not significant. This would suggest that, although the limits of the DXA system used would have excluded a significant proportion of the total male clinical sample, the criteria for exclusion were not related to OSA severity. On this basis, we feel confident that the results from our subsample were representative of men attending a sleep clinic. In women, a small proportion of WASHS patients (7%) would have been excluded by the physical limitations of the DXA machine, largely due to obesity (∼6%), and those excluded represented women with more severe OSA (mean AHI 58 vs 25 events per hour; independent t test of the mean LogeAHI in WASHS patients excluded vs not excluded P < 0.001). However, the direction of the bias was such that inclusion of larger women and those with more severe OSA would likely strengthen the associations found in our subsample. Thus, although our sample size is modest and restricted by the physical limits of the DXA system used, the participants are characteristic of a much larger sleep clinic sample, and our results are likely to be generalizable to this and similar populations.
Implications of Findings
In summary, we found that both DXA and anthropometric measures predict a similar proportion of OSA severity; however, AHI was best predicted by a combination of DXA-measured mass and anthropometric measurements. This study has shown that a pattern of central obesity is associated with OSA severity, although the pattern of association of regional obesity with AHI is different for each sex. In women, a direct influence of neck fat on the upper airway patency is implicated. In men, abdominal obesity, which has more indirect effects on the upper airway, appears to be the predominant obesity-related influence.
This study highlights 3 issues pertinent to future research of OSA. Firstly, use of anthropometric measures such as waist circumference and BMI, which are almost universally used to characterize obesity in research studies, dilute important sex differences that influence the role of obesity in OSA. Secondly, the obesity-related sex differences we found support the already accepted hypothesis that OSA is a heterogeneous disorder. Therefore, intermediate phenotypes, like regional obesity, may represent a more informative approach in examining causal relationships in OSA populations, including genetic associations, which are an underresearched but undeniable future direction for OSA research. This study suggests that DXA combined with standard anthropometry could be a valuable method for examining obesity-related genetic factors. Thirdly, women have been underrepresented in sleep research. This has been useful to the understanding of the pathogenesis in men, but the results are not generalizable to women with OSA, who, according to the parent WASHS case series from which this study is a representative subsample, constitute approximately one third of the burden of OSA cases. Future research should seek to characterize the heterogeneous pathology of OSA using large samples that adequately represent persons who develop OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Kirkness is a co-investigator in a clinical research trial sponsored by ResMed. This trial is unrelated to this study. Dr. Eastwood and Mr. Hillman have received research support from Apnex Medical. Ms. Ward has received research support from Apnex Medical and ResMed. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge G. Love, K. Maddison, J. Walsh, T. Kuzich, and patients who have participated in WASHS. WASHS is supported by Sir Charles Gairdner Hospital Research Foundation, the Hollywood Private Hospital Research Foundation and the Western Australian Genetic Epidemiology Resource (National Health and Medical Research Council of Australia Enabling Facility). L Simpson is supported by an Australian Postgraduate Award. P Eastwood is supported by an Australian National Health and Medical Research Council Senior Research Fellowship (No. 513705).
Footnotes
A commentary on this paper appears in this issue on page 419.
REFERENCES
- 1.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 4.Lam JC, Ip MS. An update on obstructive sleep apnea and the metabolic syndrome. Curr Opin Pul Med. 2007;13:484–9. doi: 10.1097/MCP.0b013e3282efae9c. [DOI] [PubMed] [Google Scholar]
- 5.Grunstein R, Wilcox I, Yang TS, Gould Y, Hedner J. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1993;17:533–40. [PubMed] [Google Scholar]
- 6.Kairaitis K, Parikh R, Stavrinou R, et al. Upper airway extraluminal tissue pressure fluctuations during breathing in rabbits. J Appl Physiol. 2003;95:1560–6. doi: 10.1152/japplphysiol.00432.2003. [DOI] [PubMed] [Google Scholar]
- 7.Babb TG, Wyrick BL, DeLorey DS, Chase PJ, Feng MY. Fat distribution and end-expiratory lung volume in lean and obese men and women. Chest. 2008;134:704–11. doi: 10.1378/chest.07-1728. [DOI] [PubMed] [Google Scholar]
- 8.Rowley JA, Permutt S, Willey S, Smith PL, Schwartz AR. Effect of tracheal and tongue displacement on upper airway airflow dynamics. J Appl Physiol. 1996;80:2171–8. doi: 10.1152/jappl.1996.80.6.2171. [DOI] [PubMed] [Google Scholar]
- 9.Whittle AT, Marshall I, Mortimore IL, Wraith PK, Sellar RJ, Douglas NJ. Neck soft tissue and fat distribution: comparison between normal men and women by magnetic resonance imaging. Thorax. 1999;54:323–28. doi: 10.1136/thx.54.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hora F, Napolis LM, Daltro C, et al. Clinical, anthropometric and upper airway anatomic characteristics of obese patients with obstructive sleep apnea syndrome. Respiration. 2007;74:517–24. doi: 10.1159/000097790. [DOI] [PubMed] [Google Scholar]
- 11.Mortimore IL, Marshall I, Wraith PK, Sellar RJ, Douglas NJ. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am J Respir Crit Care Med. 1998;157:280–3. doi: 10.1164/ajrccm.157.1.9703018. [DOI] [PubMed] [Google Scholar]
- 12.Ciscar MA, Juan G, Martinez V, et al. Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J. 2001;17:79–86. doi: 10.1183/09031936.01.17100790. [DOI] [PubMed] [Google Scholar]
- 13.Davies RJ, Ali NJ, Stradling JR. Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnoea syndrome. Thorax. 1992;47:101–5. doi: 10.1136/thx.47.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J. Waist circumference: a simple, inexpensive, and reliable tool that should be included as part of physical examinations in the doctor's office. Am J Clin Nutr. 2003;78:902–3. doi: 10.1093/ajcn/78.5.902. [DOI] [PubMed] [Google Scholar]
- 15.Katz I, Stradling J, Slutsky AS, Zamel N, Hoffstein V. Do patients with obstructive sleep apnea have thick necks? Am Rev Respir Dis. 1990;141:1228–31. doi: 10.1164/ajrccm/141.5_Pt_1.1228. [DOI] [PubMed] [Google Scholar]
- 16.Lee K, Lee S, Kim YJ, Kim YJ. Waist circumference, dual-energy X-ray absortiometrically measured abdominal adiposity, and computed tomographically derived intra-abdominal fat area on detecting metabolic risk factors in obese women. Nutrition. 2008;24:625–31. doi: 10.1016/j.nut.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Schafer H, Pauleit D, Sudhop T, Gouni-Berthold I, Ewig S, Berthold HK. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 2002;122:829–39. doi: 10.1378/chest.122.3.829. [DOI] [PubMed] [Google Scholar]
- 18.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 19.Sampsoon G, Young J. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;1987:487–90. doi: 10.1111/j.1365-2044.1987.tb04039.x. [DOI] [PubMed] [Google Scholar]
- 20.Lam B, Ip MS, Tench E, Ryan CF. Craniofacial profile in Asian and white subjects with obstructive sleep apnoea. Thorax. 2005;60:504–10. doi: 10.1136/thx.2004.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai WH, Remmers JE, Brant R, Flemons WW, Davies J, Macarthur C. A decision rule for diagnostic testing in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167:1427–32. doi: 10.1164/rccm.200112-110OC. [DOI] [PubMed] [Google Scholar]
- 22.Nuckton TJ, Glidden DV, Browner WS, Claman DM. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep. 2006;29:903–8. doi: 10.1093/sleep/29.7.903. [DOI] [PubMed] [Google Scholar]
- 23.Thong J, Pang K. Clinical parameters in obstructive sleep apnea: are there any correlations? J Otolaryngol Head Neck Surg. 2008;37:894–900. [PubMed] [Google Scholar]
- 24.McCaskie PA, Carter KW, Hazelton M, Palmer LJ. SimHap. A comprehensive modeling framework for epidemiological outcomes and a multiple imputation approach to haplotypic analysis of population-based data. 2007. < www.genepi.org.au/simhap>.
- 25.Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–30. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 26.Shinohara E, Kihara S, Yamashita S, Yamane M, Nishida M, Arai T. Visceral fat accumulation is an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med. 1997;241:11–8. doi: 10.1046/j.1365-2796.1997.63889000.x. [DOI] [PubMed] [Google Scholar]
- 27.Stanchina ML, Malhotra A, Fogel RB, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26:851–6. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 28.Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152:1673–89. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- 29.Park YW, Heymsfield SB, Gallagher D. Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? Int J Obes Relat Metab Disord. 2002;26:978–83. doi: 10.1038/sj.ijo.0801982. [DOI] [PubMed] [Google Scholar]
- 30.Kamel EG, McNeill G, Van Wijk MC. Usefulness of anthropometry and DXA in predicting intra-abdominal fat in obese men and women. Obes Res. 2000;8:36–42. doi: 10.1038/oby.2000.6. [DOI] [PubMed] [Google Scholar]
- 31.Clasey JL, Bouchard C, Teates CD, et al. The use of anthropometric and dual-energy X-ray absorptiometry (DXA) measures to estimate total abdominal and abdominal visceral fat in men and women. Obes Res. 1999;7:256–64. doi: 10.1002/j.1550-8528.1999.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 32.Snijder MB, Visser M, Dekker JM, et al. The prediction of visceral fat by dual-energy X-ray absorptiometry in the elderly: a comparison with computed tomography and anthropometry. Int J Obes Relat Metab Disord. 2002;26:984–93. doi: 10.1038/sj.ijo.0801968. [DOI] [PubMed] [Google Scholar]
- 33.Laskey MA. Dual-energy X-ray absorptiometry and body composition. Nutrition. 1996;12:45–51. doi: 10.1016/0899-9007(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 34.Carey D, Jenkins A, Campbell L, Freund J, Chisholm D. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45:633–8. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- 35.Paradisi G, Smith L, Burtner C, et al. Dual energy X-ray absorptiometry assessment of fat mass distribution and its association with the insulin resistance syndrome. Diabetes Care. 1999;22:1310–7. doi: 10.2337/diacare.22.8.1310. [DOI] [PubMed] [Google Scholar]
- 36.Rissanen P, Hamalainen P, Vanninen E, Tenhunen-Eskelinen M, Uusitupa M. Relationship of metabolic variables to abdominal adiposity measured by different anthropometric measurements and dual-energy X-ray absorptiometry in obese middle-aged women. Int J Obes Relat Metab Disord. 1997;21:367–71. doi: 10.1038/sj.ijo.0800414. [DOI] [PubMed] [Google Scholar]
- 37.dos Santos RE, Aldrighi JM, Lanz JR, Ferezin PC, Marone MM. Relationship of body fat distribution by waist circumference, dual-energy X-ray absorptiometry and ultrasonography to insulin resistance by homeostasis model assessment and lipid profile in obese and non-obese postmenopausal women. Gynecol Endocrinol. 2005;21:295–301. doi: 10.1080/09513590500361937. [DOI] [PubMed] [Google Scholar]
- 38.Palmer LJ, Buxbaum SG, Larkin E, et al. A whole-genome scan for obstructive sleep apnea and obesity. Am J Hum Genet. 2003;72:340–50. doi: 10.1086/346064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer LJ, Buxbaum SG, Larkin EK, et al. Whole genome scan for obstructive sleep apnea and obesity in African-American families. Am J Respir Crit Care Med. 2004;169:1314–21. doi: 10.1164/rccm.200304-493OC. [DOI] [PubMed] [Google Scholar]