Abstract
Study Objectives:
To assess circadian and homeostatic influences on subjective sleepiness and cognitive performance in older adults when sleep and waking are scheduled at different times of day; to assess changes in subjective sleepiness and cognitive performance across several weeks of an inpatient study; and to compare these findings with results from younger adults.
Design:
Three 24-h baseline days consisting of 16 h of wakefulness and an 8-h sleep opportunity followed by 3-beat cycles of a 20-h forced desynchrony (FD) condition; 18 20-h “days,” each consisting of 13.33 h of scheduled wakefulness and 6.67 h of scheduled sleep opportunity.
Setting:
Intensive Physiological Monitoring Unit of the Brigham and Women's Hospital General Clinical Research Center.
Participants:
10 healthy older adults (age 64.00 ± 5.98 y, 5 females) and 10 healthy younger adults (age 24.50 ± 3.54 y, 5 females).
Interventions:
Wake episodes during FD scheduled to begin 4 h earlier each day allowing for data collection at a full range of circadian phases.
Measurements and Results:
Subjective sleepiness, cognitive throughput, and psychomotor vigilance assessed every 2 h throughout the study. Core body temperature (CBT) data collected throughout to assess circadian phase. Older subjects were less sleepy and performed significantly better on reaction time (RT) measures than younger subjects. Decrements among younger subjects increased in magnitude further into the experiment, while the performance of older subjects remained stable.
Conclusions:
Our findings demonstrate that the waking performance and alertness of healthy older subjects are less impacted by the cumulative effects of repeated exposure to adverse circadian phase than that of young adults. This suggests that there are age-related changes in the circadian promotion of alertness, in the wake-dependent decline of alertness, and/or in how these 2 regulatory systems interact in healthy aging.
Citation:
Silva EJ; Wang W; Ronda JM; Wyatt JK; Duffy JF. Circadian and wake-dependent influences on subjective sleepiness, cognitive throughput, and reaction time performance in older and young adults. SLEEP 2010;33(4):481-490.
Keywords: Aging, age differences, circadian, sleep-wake homeostat, forced desynchrony
ACROSS A TYPICAL WAKING DAY, PERFORMANCE AND ALERTNESS ARE QUITE STABLE IN WELL-RESTED ADULTS.1 NEUROBEHAVIORAL PERFORMANCE has been shown to be influenced by both sleep-wake homeostatic (duration of prior wakefulness) and circadian (phase of the endogenous biological clock) influences. Achievement of stable performance and alertness across a normal ∼16-h waking day is hypothesized to result from the wake-dependent decline in alertness and performance being counterbalanced by an increasing wake-promoting signal from the circadian timing system that peaks towards the latter part of the habitual wake episode.2 Previous studies using designs that disrupt, restrict, and/or deprive subjects of sleep have all reported that subjective sleepiness and performance, including cognitive throughput, sustained vigilance, visual search tasks, and memory tasks, worsen with longer durations of time awake.1,3–5
Data from constant routine protocols in which subjects remain awake but in constant environmental and behavioral conditions have also shown that as time awake increases, performance decreases. These findings have been reported across a range of wake durations,1 including extreme conditions of up to 88 h of sleep deprivation.6 However, even under conditions of extreme sleep deprivation, progressive impairments in performance are reduced during the biological day compared to the biological night,1,6 an indication of the influence of the circadian timing system. This influence is such that for most measures, performance is best during the biological daytime and worst in the late biological nighttime, at or just after the circadian phase of the core body temperature nadir.1,6
The limitation of sleep deprivation protocols is that there is a confound between duration of prior wakefulness and circadian phase, and hence the independent contributions of each of these sleep-wake regulatory factors on performance cannot be estimated. The forced desynchrony protocol is a paradigm through which wake-dependent and circadian influences on performance in humans can be separated and quantified, and their interaction can be assessed. This protocol involves scheduling the subject to a rest-activity cycle duration much shorter or much longer than 24 h, beyond the range of entrainment of the circadian pacemaker.7 Using the forced desynchrony paradigm, we have reported previously that, in young adults, subjective alertness and neurobehavioral performance are affected by the duration of sustained wakefulness (a wake-dependent homeostatic process) as well as by a circadian process, and that these two processes interact such that the circadian influence on alertness and performance is stronger with greater durations of prior wakefulness.1,4,8 Similar results were achieved using imposed day lengths shorter (20 h, with 13.3 h wake),4 longer (28 h, with 18.7 h wake),1 and significantly longer than normal (42.85 h with 28.57 h wake).8
How these wake-dependent and circadian influences on performance may change with age has not been studied systematically. There are suggestions from sleep fragmentation and sleep deprivation studies that there may be age-related changes in both of these systems. An assessment of the effect of sleep fragmentation on performance in young and older subjects, in which subjects were aroused periodically throughout the sleep episode, found the performance of older subjects to be less sensitive to sleep disturbance than younger subjects,9 suggesting an age-related attenuation of the wake-dependent homeostatic influence on performance. Assessment of the response of young and older subjects to varying durations of sleep deprivation has also been performed. In a study conducted in our laboratory, we found that older adults were better able than young adults to maintain alertness and sustained attention across 26 h.10 Similar results were obtained from a study of 40 h of sleep deprivation in young and older subjects, in which the authors reported that older subjects were able to maintain virtually stable reaction time performance while younger subjects showed greater performance impairments.11 In addition to sleep fragmentation and sleep deprivation studies, results from sleep restriction studies have also suggested that older adults are less susceptible to circadian and wake-dependent performance decrements.12,13
While there is evidence from those prior studies that there may be modifications in both the circadian and wake-dependent homeostatic influences on performance with aging, the designs used in those studies did not allow for the separation of circadian and homeostatic influences, and therefore in most cases, circadian and wake-dependent influences were confounded. Therefore, one purpose of our present analysis was to separate the wake-dependent and circadian influences on performance in older adults to quantify their relative influences, and to compare those findings with results from young adults. In addition, most prior reports on performance in young adults from forced desynchrony studies averaged data across the entire forced desynchrony segment (typically 3-4 weeks), leaving open the possibility that the circadian and/or wake-dependent influence on performance might change across such a long experiment. A second purpose of the present analysis was therefore to examine subjective alertness and performance with respect to how long subjects were exposed to the repeated circadian phase misalignment inherent in forced desynchrony, and to determine whether this differed between young and older subjects. We selected subjective alertness and performance data from older subjects who participated in a 20-h forced desynchrony study and compared them with data previously-reported from young subjects in a 20-h forced desynchrony study.4
METHODS
Participants
Included in these analyses are data from ten healthy older volunteers (age 64.0 ± 5.98 years, range 55–72 years; 5 men, 5 women) and 10 healthy younger volunteers (age 24.5 ± 3.54 years, range 19–29 years; 5 men, 5 women). The subjects were medically and psychologically healthy as assessed during a screening evaluation prior to study, which included a physical examination, clinical biochemical tests on blood and urine, an electrocardiogram, psychological questionnaires (the MMPI-2,14 the Folstein Mini-Mental State Exam (older subjects only),15 and Beck Depression Inventory [younger subjects]16 or the Geriatric Depression Scale [older subjects]17), and a screening interview with a clinical psychologist or psychiatrist to rule out current or past psychopathology. None were under the care of a physician for any chronic medical condition, and none were regularly taking medications. All subjects reported having no major sleep complaints. Older subjects were evaluated for sleep disorders prior to admission via overnight polysomnography, and were screened to omit those with clinically significant sleep apnea (apnea hypopnea index [AHI] > 20; or AHI > 10 and daytime sleepiness as evidenced by an Epworth Sleepiness Scale18 score > 10) or periodic limb movement disorder (periodic limb movements with arousal index ≥ 15). All subjects were instructed to maintain a regular (± 30 min) sleep-wake schedule with 8 h in bed at their habitual times for the 3 weeks prior to study. During the week immediately prior to the study, compliance with this regular schedule was verified with a wrist activity monitor (AMI-32, Ambulatory Monitoring, Ardsley, NY; or Actiwatch-L, Philips Respironics, Murrysville, PA). For at least one week prior to the study, subjects were instructed to abstain from caffeine, nicotine, alcohol, and all prescription and over-the-counter medications. Compliance was verified upon admission by comprehensive toxicological analysis of their urine. Younger female subjects had to report regular menstrual cycles, and were tested during screening and upon admission to verify that they were not pregnant.
The studies were conducted in accordance with the principles outlined in the Declaration of Helsinki and were reviewed and approved by the Human Research Committee of the Partners HealthCare System. Each subject provided written informed consent prior to starting the study.
Experimental Procedure
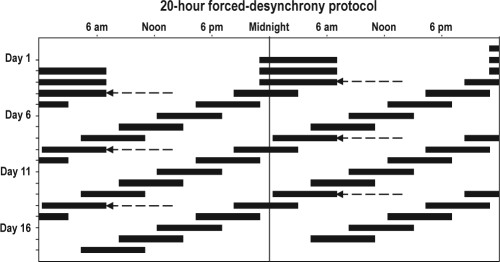
Each study began with three 24-h baseline days consisting of 16 h of wakefulness and an 8-h sleep opportunity, scheduled according to each subject's average bed and wake times from the week prior to study. These 3 baseline days were followed by a 20-h forced desynchrony condition. The 20-h forced desynchrony “days” consisted of 13.33 h of scheduled wakefulness and 6.67 h of scheduled sleep opportunity, resulting in wake episodes that were scheduled to begin 4 h earlier each day and occurring at a full range of circadian phases (Figure 1). Each subject had ≥ 18 such consecutive 20-h days (equivalent to 15 calendar days), which are reported here.
Figure 1.
Scheduled sleep-wake cycle of the study protocol plotted in double raster format, with successive days plotted to the right of, and beneath one another. Reference clock hour is indicated along the top axis. Scheduled sleep episodes are represented by the black bars. The first 3 baseline sleep episodes were scheduled to occur at each subject's habitual bedtime and to last for 8 h. Thus, in this example the subject had a habitual bedtime of 23:00 and a habitual wake time of 07:00. Beginning on Day 4, subjects began the forced desynchrony (FD) segment of the study, during which they were scheduled to sleep for 6.67 h, with sleep scheduled to occur 4 h earlier each day. The arrows mark the beginning of each FD cycle, each beginning at the same clock hour.
All data reported here were part of 2 larger studies designed to test the effects of pre-sleep melatonin administration on sleep quality. The older subjects were in a study in which they received placebo prior to each of the first 18 forced desynchrony sleep episodes and melatonin prior to subsequent sleep episodes; only data from the placebo condition are reported here. The younger subjects were in a between-subjects design study, and only those subjects randomized to the placebo condition are reported here.4,19 We included only the first 18 forced desynchrony days (equivalent to 15 calendar days) from the young subjects so as to have an equal number of study days in both age groups.
Each subject lived in a private study room in the Intensive Physiological Monitoring Unit of the Brigham and Women's Hospital General Clinical Research Center for the duration of their study. The study room was shielded from external time cues, and staff members were trained to avoid any discussion of time of day or day of protocol. Ambient light intensity during all scheduled wake episodes was approximately 0.0087 W/m2 (∼3.3 lux) at 137 cm from the floor facing toward the walls and had a maximum of 0.048 W/m2 (15 lux) at 187 cm from the floor facing toward the ceiling (dim indoor room light). Light levels were kept dim throughout the study so as to minimize the ability of the circadian system to entrain to the imposed sleep-wake schedule and to minimize the alerting effects of light. During scheduled sleep episodes, all lights were turned off. During their free time between tests, subjects were allowed to pursue sedentary activities in their study room, which typically included reading, listening to music, watching videos, or pursuing hobbies.
Data Collection
Core body temperature was recorded each minute using a rectal temperature sensor (Temperature Probe #20463, Measurement Specialties, Hampton, VA) worn throughout the study. These data were used to assess circadian period and phase (see below).
In both studies, subjects were asked to assess their subjective sleepiness with the Karolinska Sleepiness Scale (KSS)20 at 30-min intervals beginning approximately 30 min after scheduled waketime. To account for possible effects of sleep inertia,21 all assessments of subjective sleepiness from the first 2 h after scheduled waketime were excluded from the present analysis. Beginning approximately 2 h after scheduled waketime, subjects were administered a computerized neurobehavioral performance battery at 2-h intervals. Subjects were oriented to study procedures and were trained on this neurobehavioral performance battery on the admission day by an investigator (JFD or JKW). Each test battery included a KSS, a 10-min simple reaction time and visual vigilance task (Psychomotor Vigilance Test [PVT]),22 and a 4-min addition test (referred to here as ADD).23 The test battery given to the older subjects had the ADD task administered prior to the PVT, while younger subjects took the ADD task after the PVT. The protocol for younger subjects included one more neurobehavioral performance battery at the end of each wake episode than the protocol for older subjects. Tests from this additional battery have been excluded from analysis. The test battery given to younger subjects also included 2 additional short (< 2 min each) performance tests, data which were reported elsewhere.4
Data Analysis
Of 6,154 KSS tests, 11 (range of 1–3 each from 5 subjects) were omitted from our analysis because they were collected less than half of the scheduled interval (< 15 min) between observations and had a difference in scale value greater than one. Of 1,764 PVT tests, 3 from one subject were excluded from analysis because the incorrect response button was pressed in > 25% of the trials during the session. Two additional PVT observations, one each from 2 subjects, were excluded from analysis because they were collected less than half of the scheduled interval (< 1 h) between observations. A total of 1,727 ADD observations are included in this analysis, none of which were excluded for reasons of compliance or time between observations.
Observations from baseline day 1 were excluded from analysis in order to account for an adjustment period to laboratory conditions and to omit the maximum time of practice effects on the first few administrations of the cognitive performance tasks. Observations from baseline days 2 and 3 were averaged and assessed to determine if there were differences between young and older subjects under baseline entrained conditions.
To assign each data point a value for homeostatic wake-dependent influence, all observations were coded with an elapsed time since scheduled wake time. Observations were then binned into 2-h TIME AWAKE bins. In some cases, scheduled tests were delayed because of technical problems; in such cases we used in our analyses the actual time that each test was taken rather than the scheduled test time.
All data collected during the forced desynchrony segment of the study were coded to denote the circadian phase at the time of measurement. To do this, the core body temperature data from the forced desynchrony segment were assessed for intrinsic circadian period using non-orthogonal spectral analysis.7 This method takes into account the imposed 20-h rest-activity schedule and searches for an unknown periodicity in the circadian range (15 to 30 h). From this estimate of intrinsic circadian period and the program's projection of the core body temperature nadir at the start of the study segment, a circadian phase (between 0° and 359°) was assigned to each test administered during the forced desynchrony condition, with 0° representing the circadian phase of the core body temperature minimum. The data were then binned in 60° CIRCADIAN PHASE bins, equivalent to ∼4 h and centered on the assigned bin (e.g., the “0°” circadian phase bin covers the range of 330° to 30°).
To account for duration of time into the forced desynchrony protocol, each observation collected during the forced desynchrony condition was also assigned to a forced desynchrony cycle (FD CYCLE). FD CYCLES consist of six 20-h “days” (with each cycle = 120 h), with each FD cycle beginning at the same clock hour (see Figure 1).
Unless otherwise noted, all statistical analyses were performed using mixed-model analysis (SAS 9.1; SAS Institute, Cary, NC) on raw data, incorporating into the model a random intercept statement allowing for means to vary between subjects.24 Three measures were assessed from PVT data: mean reaction time (RT), mean of the fastest 10% of RT from each trial, and number of RT lapses (defined as RTs > 500 msec). For statistical analyses, mean RT and mean of the fastest 10% RT were transformed (reciprocal transformation) to better approximate a normal distribution. The analysis of RT lapses was conducted using generalized linear mixed model analysis (SAS 9.1; SAS Institute, Cary, NC) and assumed a Poisson distribution. The number of correct calculations completed, a measure of cognitive throughput25 was used as the performance measure on the ADD test.
Subjective sleepiness and all performance measures were first assessed by testing for the main effects of AGE (age group; “older” or “young”), TIME AWAKE (homeostatic process), CIRCADIAN PHASE (circadian process), and FD CYCLE, all of which were treated as categorical, rather than continuous, variables. We next tested all possible 2-way interactions, and proceeded to drop all nonsignificant 2-way interactions from further analysis. Where there were significant 2-way interactions among the factors TIME AWAKE, CIRCADIAN PHASE, and FD CYCLE, we also tested 3-way interactions by adding AGE as a factor. For all significant interactions with AGE as a factor, post hoc analyses were conducted by testing the simple main effect of AGE (between-subjects factor) at each specific level of TIME AWAKE, CIRCADIAN PHASE, and/or FD CYCLE. In this manner, we were able to determine if there were differences between “older” and “young” subjects at a distinct level of the within-subjects factor by testing the hypothesis that the two levels of AGE were equal.
Results are reported as mean ± standard deviation unless otherwise noted. Results in all figures are presented as mean ± standard error, with all observations first averaged within, and then across subjects. For all statistical tests, the critical significance level is defined as α = 0.05. All reported degrees of freedom and P-values are from the final statistical model for each measure. Final statistical models include AGE as the primary variable of interest, all significant main effects, and all significant interactions, but exclude nonsignificant interactions. Note that because significant interactions vary between measures, degrees of freedom are not uniform.
RESULTS
Baseline
During the baseline condition, mixed-model analysis revealed no significant main effect of TIME AWAKE or AGE on the ADD test or on any PVT reaction time measure. There was a significant main effect of TIME AWAKE on subjective sleepiness (KSS) during baseline (F 4, 645 = 12.88, P < 0.0001), with subjects feeling sleepier the longer they were awake. There was also a significant main effect of AGE on subjective sleepiness (KSS) during baseline (F (1, 645 = 4.86, P = 0.0278), with older subjects reporting that they were significantly less sleepy than younger subjects. Because baseline data were collected while subjects were under normally entrained conditions, an effect of CIRCADIAN PHASE was not tested on baseline observations. Baseline group means for all measures are provided in Table 1.
Table 1.
Baseline group mean values for each measure
| KSS: subjective sleepiness | ADD: # of correct calculations | PVT: mean RT (msec) | PVT: fastest 10% RTs (msec) | PVT: # of lapses (RTs > 500 msec) | |
|---|---|---|---|---|---|
| Age Group | |||||
| Younger subjects | 3.43 ± 0.07 | 41.19 ± 0.93 | 288.80 ± 4.33 | 217.26 ± 2.67 | 2.67 ± 0.29 |
| Older subjects | 2.59 ± 0.07 | 44.94 ± 0.96 | 296.59 ± 4.80 | 217.17 ± 2.94 | 2.65 ± 0.38 |
Group mean ± standard error are presented, with data first averaged within, and then across subjects in each age group. For subjective sleepiness (KSS), higher values indicate greater self-assessed sleepiness.
Forced desynchrony
Subjective sleepiness
Mixed-model analysis revealed a significant main effect of TIME AWAKE on subjective sleepiness (F 4, 6061 = 73.09, P < 0.0001), with subjects reporting increased sleepiness the longer they were awake. There was a significant main effect of CIRCADIAN PHASE on subjective sleepiness (F 5, 6061 = 164.43, P < 0.0001), with the greatest levels of sleepiness reported near the time of the core body temperature minimum. There was also a significant main effect of FD CYCLE on subjective sleepiness (F 2, 6061 = 3.33, P = 0.0357), with subjects feeling more sleepy in each successive FD cycle as the study progressed. There was an overall main effect of AGE on subjective sleepiness (F 1, 6061 = 4.35, P = 0.0372), with older subjects reporting themselves to be less sleepy than younger subjects.
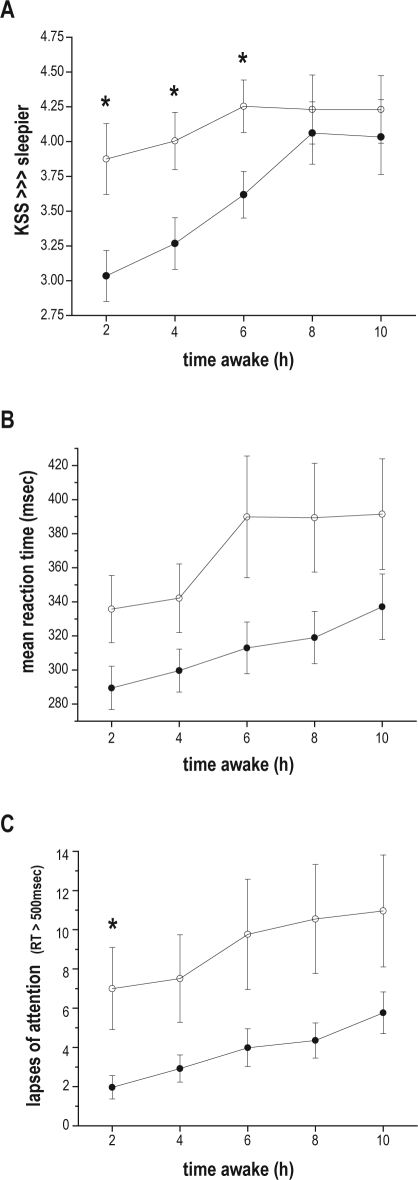
There was a significant interaction of TIME AWAKE and AGE on subjective sleepiness (F 4, 6061 = 17.38, P < 0.0001). Older subjects began the wake episode significantly less sleepy than younger subjects, but as the wake episode continued the increase in sleepiness of younger subjects was less pronounced than that of older subjects. Post hoc tests revealed that there were significant age differences in the 2-h (F 1, 6061 = 11.07, P = 0.0009), 4-h (F (1, 6061 = 8.33, P = 0.0039), and 6-h (F 1, 6061 = 6.44, P = 0.0112) TIME AWAKE bins, with older subjects feeling significantly less sleepy than younger subjects in each of these bins; but no significant AGE difference in the 8-h or 10-h TIME AWAKE bins (Figure 2, panel A).
Figure 2.
Subjective sleepiness and cognitive performance plotted with respect to time awake. Data are plotted with respect to elapsed time into the wake episode (in hours) along the x-axis. (A) subjective sleepiness as assessed by the Karolinska Sleepiness Scale (KSS), with higher numbers indicating greater sleepiness; (B) mean reaction time (RT) on the Psychomotor Vigilance Task (PVT); (C) number of lapses of attention (PVT RTs > 500 msec). Data are presented as mean ± standard error, with all observations first averaged within, and then across subjects in each age group (older subjects filled circles; younger subjects hollow circles). Asterisks (*) represent those TIME AWAKE bins where post hoc tests indicated a significant effect of AGE.
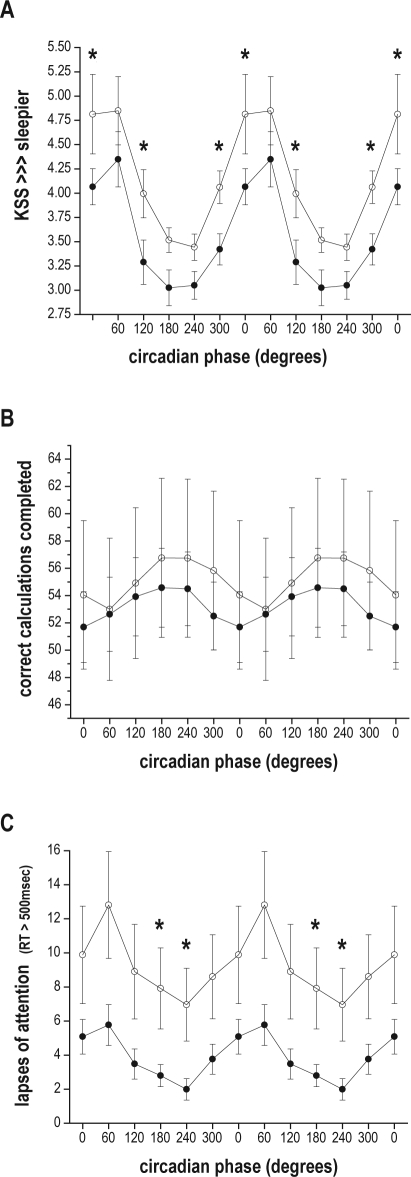
There was also a significant CIRCADIAN PHASE and AGE interaction on subjective sleepiness (F 5, 6061 = 3.26, P = 0.0061). Post hoc tests revealed that these significant age differences were in the 0° (F 1, 6061 = 7.39, P = 0.0066), 120° (F 1, 6061 = 6.01, P = 0.0143), and 300° (F 1, 6061 = 4.93, P = 0.0264) CIRCADIAN PHASE bins, with older subjects feeling significantly less sleepy than younger subjects at these circadian phases (see Figure 3, panel A).
Figure 3.
Subjective sleepiness and cognitive performance plotted with respect to circadian phase. Data are double-plotted with respect to circadian phase along the x-axis, with 0° representing the core body temperature minimum. (A) subjective sleepiness as assessed by the Karolinska Sleepiness Scale (KSS), with higher numbers indicating greater sleepiness; (B) number of correctly-completed calculations on the ADD test; (C) number of lapses of attention (reaction times > 500 msec). Data are presented as mean ± standard error, with all observations first averaged within, and then across subjects in each age group (older subjects filled circles; younger subjects hollow circles). Asterisks (*) represent those CIRCADIAN PHASE bins where post hoc tests indicated a significant effect of AGE.
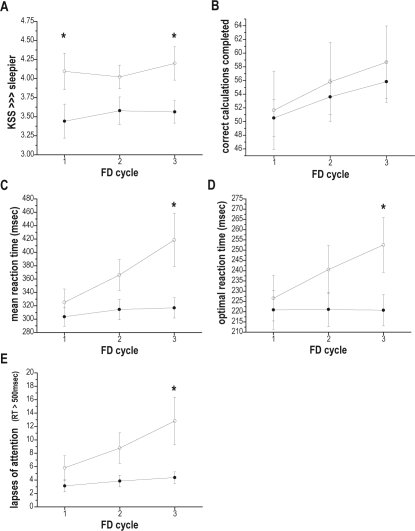
There was also a significant FD CYCLE and AGE interaction on subjective sleepiness (F 2, 6061 = 3.51, P = 0.0299) with older subjects reporting less sleepiness than younger subjects (see Figure 4, panel A). Post hoc tests revealed that there were significant age differences during the first (F 1, 6061 = 5.36, P = 0.0206) and the third (F 1, 6061 = 5.11, P = 0.0239) cycle of forced desynchrony, with older subjects feeling significantly less sleepy than younger subjects.
Figure 4.
Subjective sleepiness and cognitive performance plotted with respect to time within the experiment (forced desynchrony cycle). Data from each forced desynchrony (FD) cycle, which lasted for 120 h and began at the same clock hour, are plotted along the x-axis. (A) subjective sleepiness as assessed by the Karolinska Sleepiness Scale (KSS), with higher numbers indicating greater sleepiness; (B) number of correctly-completed calculations on the ADD test; (C) mean reaction time (RT) on the Psychomotor Vigilance Task (PVT); (D) optimal reaction times (mean of fastest 10% RT on the PVT); (E) number of lapses of attention (PVT RTs > 500 msec). Data are presented as mean ± standard error, with all observations first averaged within, and then across subjects in each age group (older subjects filled circles; younger subjects hollow circles). Asterisks (*) represent those FD CYCLES where post hoc tests indicated a significant effect of AGE.
Neurobehavioral performance – cognitive throughput
There was a significant main effect of TIME AWAKE on performance on the calculation (ADD) test (F 4, 1689 = 24.08, P < 0.0001), with subjects completing fewer calculations the longer they were awake. CIRCADIAN PHASE was also a significant main effect on the number of correct calculations completed (F 5, 1689 = 17.96, P < 0.0001), with the fewest correct calculations completed around the phase of the core body temperature minimum. FD CYCLE was a main effect on performance on the ADD test (F 2, 1689 = 213.15, P < 0.0001), with the number of completed correct calculations increasing with each successive FD cycle. There was no significant main effect of AGE on the ADD test. However, there was a significant 2-way interaction between CIRCADIAN PHASE and AGE on ADD test performance (F 5, 1689 = 2.44, P = 0.0328). Older subjects reached the nadir of their calculation performance around time of the core body temperature minimum (0°), while younger subjects reached their performance nadir in the next (60°) circadian phase bin (Figure 3, panel B). There was also a significant 2-way interaction between FD CYCLE and AGE (F 2, 1689 = 4.83, P = 0.0081). The average number of calculations completed by younger subjects was greater than the older subjects, and the degree of improvement in younger subjects was greater in each FD CYCLE (Figure 4, panel B).
Neurobehavioral performance - reaction time measures
There was a significant main effect of TIME AWAKE on all 3 PVT reaction time measures: mean RT (F (4, 1702 = 53.10, P < 0.0001); mean of the fastest 10% RTs (F 4, 1726 = 13.56, P < 0.0001); and number of lapses (RTs > 500 msec; F 4, 1651 = 125.79, P < 0.0001), with RTs and the number of lapses increasing as time awake increased. There was also a significant main effect of CIRCADIAN PHASE on all 3 reaction time measures: mean RT (F 5, 1702 = 46.13, P < 0.0001); the fastest 10% RTs (F (5, 1726 = 25.32, P < 0.0001); and number of lapses (RTs > 500 msec; F 5, 1651 = 103.76, P < 0.0001), with RTs and the number of lapses greatest around the phase of the core body temperature minimum (0°). There was also a significant main effect of FD CYCLE on all 3 reaction time measures: mean RT (F 2, 1702 = 127.28, P < 0.0001); the fastest 10% RTs (F (2, 1726 = 88.13, P < 0.0001); and the number of lapses (RTs > 500 msec; F 2, 1651 = 212.65, P < 0.0001), with RTs and the number of lapses increasing with each successive FD cycle. There was no significant main effect of AGE on any reaction time measure, although there were several significant interactions involving AGE.
There was a significant 2-way interaction between TIME AWAKE and AGE on mean RT (F 4, 1702 = 2.38, P = 0.0495) and on the number of lapses (F 4, 1651 = 12.36, P < 0.0001), with older subjects having faster RTs and fewer lapses than younger subjects as time awake increased (see Figure 2, panels B and C). Post hoc analyses revealed that older subjects had significantly fewer lapses than young subjects in the 2-h TIME AWAKE bin (F 1, 1651 = 5.48, P = 0.0193).
There was a significant CIRCADIAN PHASE and AGE interaction on the number of lapses (F 5, 1651 = 11.83, P < 0.0001), with older subjects having fewer lapses across circadian phases. Post hoc tests revealed that older subjects had significantly fewer lapses in the 180° phase bin (F 1, 1651 = 4.19, P = 0.0409) and the 240° phase bin (F 1, 1651 = 5.98, P = 0.0146; see Figure 3, panel C), times equivalent to the late biological day/early biological evening under entrained conditions.
There were significant 2-way FD CYCLE and AGE interactions on all 3 reaction time measures: mean RT (F 2, 1702 = 54.69, P < 0.0001); the fastest 10% RTs (F 2, 1726 = 77.63, P < 0.0001); and the number of lapses (RTs > 500 msec; F 2, 1651 = 37.27, P < 0.0001). RTs and the number of lapses increased with each successive FD cycle for younger subjects, whereas they increased only modestly, if at all, with successive FD cycles for older subjects (see Figure 4, panels C, D, and E). Post hoc tests revealed significant age differences during the third FD cycle for all 3 RT measures: mean RT (F 1, 1702 = 6.90, P = 0.0087), fastest 10% RTs (F 1, 1726 = 3.96, P 0.0467), number of lapses (F 1, 1651 = 5.52, P = 0.0189), with RTs and the number of lapses of younger subjects significantly greater than those of older subjects.
There was also a significant 3-way interaction between TIME AWAKE, CIRCADIAN PHASE, and AGE on the number of lapses (F 20, 1651 = 2.74, P < 0.0001). Post hoc tests revealed significant age differences in all TIME AWAKE bins at the 240° CIRCADIAN PHASE bin (2-h bin [F 1, 1651 = 5.01, P = 0.0253]; 4-h bin [F 1, 1651 = 7.95, P = 0.0049]; 6-h bin [F 1, 1651 = 6.01, P = 0.0143]; 8-h bin [F 1, 1651 = 4.28, P = 0.0386]; 10-h bin [F 1, 1651 = 4.41, P = 0.0360]), with older subjects having significantly fewer lapses than young subjects at this circadian time corresponding to the early biological evening or “wake maintenance zone.”26,27
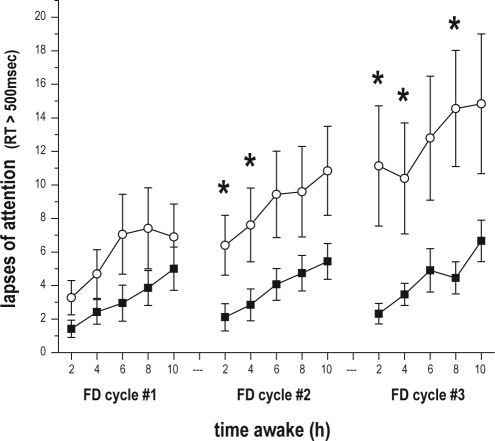
There was a significant 3-way interaction between TIME AWAKE, FD CYCLE, and AGE on the number of lapses (F8, 1651 = 3.26, P = 0.0011). While in both age groups the number of lapses increased as time awake increased, the increase in lapses with time awake was gradual and consistent across FD cycles for older subjects, but for younger subjects the magnitude of the increase in lapses with time awake was greater during each successive FD cycle (Figure 5). Post hoc tests revealed younger subjects to have significantly more lapses than older subjects in the 2-h time awake bin during both the second (F 1, 1651 = 4.65, P = 0.0312) and third (F 1, 1651 = 10.31, P = 0.0014) FD cycles; in the 4-h time awake bin during both the second (F 1, 1651 = 4.20, P = 0.0406) and third (F 1, 1651 = 5.52, P = 0.0189) FD cycles; and in the 8-h time awake bin during the third FD cycle (F 1, 1651 = 6.16, P = 0.0132).
Figure 5.
Plot of significant 3-way interaction between AGE, TIME AWAKE, and FD CYCLE on number of lapses of attention. Data from each FD cycle, all of which begin at the same clock hour, were averaged with respect to time awake (in hours) and then plotted along x-axis. Lapses of attention were defined as reaction times > 500 msec on the Psychomotor Vigilance Task (PVT). Data are presented as mean ± standard error, with all observations first averaged within, and then across subjects in each age group (older subjects filled circles; younger subjects hollow circles). Asterisks (*) indicate those TIME AWAKE × FD CYCLE bins, where post hoc tests indicated a significant effect of AGE.
DISCUSSION
We have described here data from young and older adults who participated in a forced desynchrony study, where 13.3-h waking episodes were scheduled across a full range of circadian phases for 3 cycles of forced desynchrony, equivalent to 2 calendar weeks. This protocol allowed us to separate the wake-dependent and circadian influences in order to determine whether the impact of these two sleep-wake regulatory systems on subjective sleepiness and cognitive performance changes with age. Age was a significant main effect on subjective sleepiness, and when age was included as a factor in our statistical models it was also a significant influence on cognitive throughput, reaction time, and lapses in attention in interaction with other independent variables.
As we expected based on the findings from other forced desynchrony studies in young adults,1,4,8 the duration of prior wakefulness (representing a wake-dependent homeostatic regulatory process) was a significant main effect on subjective sleepiness and cognitive performance in older subjects, with impairment building across hours of continuous wakefulness. We also found significant interactions between time awake and age on subjective sleepiness, reaction time, and the number of lapses of attention, with younger subjects performing worse overall and reporting being sleepier overall than older adults. Specifically, younger subjects showed greater levels of subjective sleepiness than older subjects, had longer RTs, and had a greater number of lapses of attention throughout the waking day. This finding is in agreement with our recent report on attention, vigilance, and objective sleepiness in a different group of older and young subjects under 26 hours of acute sleep deprivation.10
Our finding that circadian phase had a significant main effect on subjective sleepiness and neurobehavioral performance in older adults was also expected based on prior reports from forced desynchrony studies in young adults.1,4,8 As in those prior reports, we found that performance was best during the biological day and worst during the biological night, reaching a nadir around the circadian phase of the core body temperature minimum. Our finding of a significant interaction between circadian phase and age on subjective sleepiness, calculation test performance, and lapses of attention revealed that for these measures, the circadian process has a greater adverse effect on younger subjects than older subjects. We should note that our analysis of optimal reaction time performance (the fastest 10% RTs) revealed that neither the wake-dependent homeostatic nor the circadian influence showed a significant interaction with age, suggesting that for both age groups optimal RT performance is affected similarly by wake-dependent and circadian influences.
Unlike most of our prior reports from forced desynchrony studies, here we also included in our statistical model the effect of elapsed time into the forced desynchrony condition. Using this approach, we could examine whether there were changes in homeostatic and/or circadian influences on sleepiness and performance from one FD cycle to the next. Our analysis revealed that with each successive FD cycle, subjective sleepiness increased and there was a slowing of reaction time. In contrast, performance on the ADD test improved with each successive FD cycle, with this improvement most likely being due to long-term practice effects which are common on cognitive throughput tasks.28,29 The fact that sleepiness and RT performance worsened across the experiment suggest that the improvement in ADD performance (due to a presumed practice effect) would have been even greater were it not for the adverse homeostatic and/or circadian influences imposed under conditions of forced desynchrony.
In addition to a main effect of FD cycle, we also observed a significant FD cycle and age interaction on all measures. Younger subjects rated themselves as more sleepy than older subjects in all FD cycles, and their self-rated sleepiness continued to increase between the second and third FD cycles, while the subjective sleepiness of older subjects remained stable between the second and third FD cycles. The RT performance of older subjects also remained relatively stable from one cycle to the next, in contrast to that of the younger subjects whose reaction times and lapses increased with each successive FD cycle.
There are many possible explanations that could account for our finding that older adults had better-preserved RT performance, fewer lapses of attention, and lower subjective sleepiness across this study. Perhaps there are age-related changes in the circadian and/or the homeostatic processes that control sleep-wake functions in humans. There are multiple reports of an age related reduction in the amplitude of the core body temperature rhythm30–33 and plasma melatonin34,35 rhythms. This suggests that there could also be a weakening of the circadian signal that promotes sleep in the late biological night,36 which could in turn lead to older subjects being more able to remain awake and perform well at those circadian phases. In a previous forced desynchrony study comparing objective and subjective sleep quality in young and older subjects living on a 28-h day, we found evidence for a reduced circadian drive for sleep in the late biological night.37,38 Reports of difficulty maintaining sleep and early morning awakenings with aging,39 as well as reports of reduced sleepiness in older subjects compared to younger subjects during morning hours40 are consistent with this hypothesis, as are our current results, in which the older subjects reported feeling significantly less sleepy around the nadir of core body temperature minimum (0°, corresponding to the late biological night/early biological day) than the younger subjects. If there is an age-related reduction of the circadian output signal, this could also lead to a weakening of the circadian signal promoting wakefulness in the late biological day or “wake maintenance zone.”26 There is some evidence in support of this from studies using subjective40 and objective sleepiness measures40,41 although our current results do not support this. Instead, in the present study we found that older subjects had fewer lapses of attention in the circadian phase bins corresponding to the late biological day/early biological evening. This apparent discrepancy may be due to methodological differences between our study and those two prior studies, the most significant of which are day length and duration of study. Findings from the two prior reports were from ultra-short sleep wake cycles (with either 13 min41 or 150 min40 of scheduled wake) distributed across only one41 or 2 days,40 whereas in our study subjects were on a 20-h day (with 800-min scheduled wake episodes) for 2 calendar weeks. Both factors (length of each study “day” and duration of study) would have led to differences in the level of sleep pressure between the studies, both at the wake maintenance zone and at other phases. Such differences in sleep pressure could in turn have then affected sleepiness at the wake maintenance zone. Additionally, those 2 other studies did not assess performance during waking, but instead focused on subjective sleepiness and EEG-assessed sleep.40,41
An age-related change in the homeostatic process controlling sleep and wakefulness has also been suggested, due to the marked age-related changes in sleep. This is based on reports that slow wave sleep42 and slow wave activity43 decline with age, that the number of awakenings increase with age, and that there appears to be a reduction in the consolidation of NREM sleep with age,44 leading to overall reductions in sleep efficiency. If the sleep-wake homeostatic process is changed with aging, such a change might not only affect sleep but could also lead to a reduced buildup of sleepiness during waking. Our present results are consistent with this idea, because the older subjects in our study had poorer sleep at baseline and during the forced desynchrony portion of the study than the young subjects, yet reported feeling less sleepy and were better able to pay attention and respond throughout the study. We have also hypothesized10 that healthy older adults may have both a reduced sleep need as well as a reduced ability to sleep, and that the interaction between circadian and homeostatic sleep regulatory processes may change with aging.36
One slight limitation of our analysis was the difference in the test batteries taken by young and older subjects in these two experiments. Older subjects took the reaction time test (PVT) after the ADD test, while young subjects took the reaction time test first. If the order of tests within the battery had an effect on performance, one would expect performance on tests later in the battery to be worse. Our results were that older subjects, who took the PVT later in the battery showed more stable RT performance across the waking day, while younger subjects, who took the PVT earlier in the battery, showed larger declines in performance. This suggests that the order of tests within the battery had minimal impact on our findings. Another factor which might have influenced our findings is that the performance battery taken by the young subjects included additional tests following the PVT and ADD tests reported here. We therefore must consider the possibility that a greater cognitive load over the course of the study may have contributed to the increase in subjective sleepiness and decline in RT performance from one FD cycle to the next in the young group. However, two factors argue against this as a significant factor in our findings. First, the additional tests taken by the young subjects were short (less than 5 min); and in both groups subjects had at least 1.5 h “off” between each test battery, and overall test batteries took less than one-fourth of each waking episode. Second, young subjects showed improvements in their performance on the ADD test across the experiment, suggesting that at least for this performance measure, cognitive load was not a major problem. It should also be noted that there was no significant main effect of age on the ADD test or on any reaction time (PVT) measure under baseline conditions.
In summary, our findings indicate that under these laboratory conditions, healthy older subjects perceive themselves to be less sleepy during scheduled wake episodes than younger subjects and their neurobehavioral performance on reaction time measures is significantly better than that of younger subjects. By including FD cycle (or week) as a factor in our statistical analysis, we were able to determine that the overall decrement in subjective alertness and neurobehavioral performance in the younger subjects resulted from week-by-week decrements that increased across the three cycles of forced desynchrony. While there were also increases in subjective sleepiness and decrements in RT performance in the older subjects, it was significantly less than what was observed in the younger subjects. Our finding of a 3-way interaction between age, time awake and FD cycle on the number of lapses of attention may reflect the influence of a longer-term (longer than within a single wake episode) homeostatic process, and that older subjects are less vulnerable to its adverse influences on RT performance.
Together, these findings suggest that daytime sleepiness is not a normal consequence of healthy aging, but instead may result from medication side effects, medical conditions that interfere with sleep, or undiagnosed sleep disorders.45 Our findings further suggest that healthy older adults may be less vulnerable to sleepiness and performance impairments associated with night work and transmeridian travel (jet lag) than are young adults. Whether our findings apply to more typical older adults or to different types of cognitive performance are unknown. Therefore, additional studies extending our current findings should be conducted.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Wyatt has participated in speaking engagements for Vital Issues in Medicine. Dr. Duffy has received research support from Philips-Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank the subject volunteers for their participation; C. O'Brien, D. McCarthy, W. Campanella and S. Denbesten for subject recruitment; the nursing, dietary, and technical staff of the General Clinical Research Center; the Division of Sleep Medicine Chronobiology Core; and Dr. C.A. Czeisler for overall support. These studies were supported by NIH grants AG12642 and AG09975, and by NASA grant NAS9-1435 (to CAC), and were conducted in the Brigham and Women's Hospital General Clinical Research Center supported by NIH grant RR02635; data analysis was also supported by NIH grant AG06072 (to JFD).
REFERENCES
- 1.Johnson MP, Duffy JF, Dijk DJ, Ronda JM, Dyal CM, Czeisler CA. Short-term memory, alertness and performance: a reappraisal of their relationship to body temperature. J Sleep Res. 1992;1:24–9. doi: 10.1111/j.1365-2869.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 2.Achermann P, Borbély AA. Simulation of daytime vigilance by the additive interaction of a homeostatic and a circadian process. Biol Cybern. 1994;71:115–21. doi: 10.1007/BF00197314. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet MH. Performance and sleepiness following moderate sleep disruption and slow wave sleep deprivation. Physiol Behav. 1986;37:915–8. [PubMed] [Google Scholar]
- 4.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 5.Horowitz TS, Cade, BE, Wolfe JM, Czeisler CA. Searching night and day: a dissociation of effects of circadian phase and time awake on visual selective attention and vigilance. Psychol Sci. 2003;14:549–57. doi: 10.1046/j.0956-7976.2003.psci_1464.x. [DOI] [PubMed] [Google Scholar]
- 6.Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 7.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 8.Wyatt JK, Cajochen C, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep. 2004;27:374–81. doi: 10.1093/sleep/27.3.374. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet MH. The effect of sleep fragmentation on sleep and performance in younger and older subjects. Neurobiol Aging. 1989;10:21–5. doi: 10.1016/s0197-4580(89)80006-5. [DOI] [PubMed] [Google Scholar]
- 10.Duffy JF, Willson HJ, Wang W, Czeisler CA. Healthy older adults better tolerate sleep deprivation than young adults. J Am Geriatr Soc. 2009 doi: 10.1111/j.1532-5415.2009.02303.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adam M, Rétey JV, Khatami R, Landolt H-P. Age-related changes in the time course of vigilant attention during 40 hours without sleep in men. Sleep. 2006;29:55–7. doi: 10.1093/sleep/29.1.55. [DOI] [PubMed] [Google Scholar]
- 12.Blatter K, Graw P, Münch M, Knoblauch V, Wirz-Justice A, Cajochen C. Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Behav Brain Res. 2006;168:312–7. doi: 10.1016/j.bbr.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Bliese PD, Wesensten NJ, Balkin TJ. Age and individual variability in performance during sleep restriction. J Sleep Res. 2006;15:376–85. doi: 10.1111/j.1365-2869.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- 14.Hathaway SR, McKinley JC. Minnesota Multiphasic Personality Inventory-2. University of Minnesota Press; 1989. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–298. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 17.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Wyatt JK, Dijk DJ, Ritz-De Cecco A, Ronda JM, Czeisler CA. Sleep facilitating effect of exogenous melatonin in healthy young men and women is circadian-phase dependent. Sleep. 2006;29:609–18. doi: 10.1093/sleep/29.5.609. [DOI] [PubMed] [Google Scholar]
- 20.Gillberg M, Kecklund G, Åkerstedt T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep. 1994;17:236–41. doi: 10.1093/sleep/17.3.236. [DOI] [PubMed] [Google Scholar]
- 21.Silva EJ, Duffy JF. Sleep inertia varies with circadian phase and sleep stage in older adults. Behav Neurosci. 2008;122:928–35. doi: 10.1037/0735-7044.122.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods. 1985;17:652–5. [Google Scholar]
- 23.Klein KE, Wegmann HM, Athanassenas G, Hohlweck H, Kuklinski P. Air operations and circadian performance rhythms. Aviat Space Environ Med. 1976;47:221–30. [PubMed] [Google Scholar]
- 24.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 25.Gillooly PB, Smolensky MH, Albright DL, Hsi B, Thorne DR. Circadian variation in human performance evaluated by the Walter Reed performance assessment battery. Chronobiol Int. 1990;7:143–53. doi: 10.3109/07420529009056966. [DOI] [PubMed] [Google Scholar]
- 26.Lavie P. Ultrashort sleep-waking schedule III. “Gates” and “forbidden zones” for sleep. Electroenceph Clin Neurophysiol. 1986;63:414–25. doi: 10.1016/0013-4694(86)90123-9. [DOI] [PubMed] [Google Scholar]
- 27.Strogatz SH, Kronauer RE, Czeisler CA. Circadian regulation dominates homeostatic control of sleep length and prior wake length in humans. Sleep. 1986;9:353–64. doi: 10.1093/sleep/9.2.353. [DOI] [PubMed] [Google Scholar]
- 28.Mazur JE, Hastie R. Learning as accumulation: a reexamination of the learning curve. Psychol Bull. 1978;85:1256–74. [PubMed] [Google Scholar]
- 29.Wright KP, Jr, Hull JT, Hughes RJ, Ronda JM, Czeisler CA. Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cognitive Neurosci. 2006;18:508–21. doi: 10.1162/jocn.2006.18.4.508. [DOI] [PubMed] [Google Scholar]
- 30.Carrier J, Monk TH, Buysse DJ, Kupfer D. Amplitude reduction of the circadian temperature and sleep rhythms in the elderly. Chronobiol Int. 1996;13:373–86. doi: 10.3109/07420529609012661. [DOI] [PubMed] [Google Scholar]
- 31.Czeisler CA, Dumont M, Duffy JF, et al. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–6. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- 32.Vitiello MV, Smallwood RG, Avery DH, Pascualy RA, Martin DC, Prinz PN. Circadian temperature rhythms in young adult and aged men. Neurobiol Aging. 1986;7:97–100. doi: 10.1016/0197-4580(86)90146-6. [DOI] [PubMed] [Google Scholar]
- 33.Weitzman ED, Moline ML, Czeisler CA, Zimmerman JC. Chronobiology of aging: temperature, sleep-wake rhythms and entrainment. Neurobiol Aging. 1982;3:299–309. doi: 10.1016/0197-4580(82)90018-5. [DOI] [PubMed] [Google Scholar]
- 34.Iguchi H, Kato K-I, Ibayashi H. Age-dependent reduction in serum melatonin concentrations in healthy human subjects. J Clin Endocrinol Metab. 1982;55:27–9. doi: 10.1210/jcem-55-1-27. [DOI] [PubMed] [Google Scholar]
- 35.Waldhauser F, Weiszenbacher G, Tatzer E, et al. Alterations in nocturnal serum melatonin levels in humans with growth and aging. Clin Endocrinol Metab. 1988;66:648–52. doi: 10.1210/jcem-66-3-648. [DOI] [PubMed] [Google Scholar]
- 36.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol (Lond.) 1999;516:611–27. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1478–R1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 38.Dijk DJ, Duffy JF. Circadian regulation of human sleep and age-related changes in its timing, consolidation and EEG characteristics. Ann Med. 1999;31:130–40. doi: 10.3109/07853899908998789. [DOI] [PubMed] [Google Scholar]
- 39.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Münch M, Knoblauch V, Blatter K, et al. Age-related attenuation of the evening circadian arousal signal in humans. Neurobiol Aging. 2005;26:1307–19. doi: 10.1016/j.neurobiolaging.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Haimov I, Lavie P. Circadian characteristics of sleep propensity function in healthy elderly: a comparison with young adults. Sleep. 1997;20:294–300. doi: 10.1093/sleep/20.4.294. [DOI] [PubMed] [Google Scholar]
- 42.Blois R, Feinberg I, Gaillard J-M, Kupfer DJ, Webb WB. Sleep in normal and pathological aging. Experientia. 1983;39:551–8. doi: 10.1007/BF01971096. [DOI] [PubMed] [Google Scholar]
- 43.Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20-60 years old) Psychophysiol. 2001;38:232–42. [PubMed] [Google Scholar]
- 44.Dijk DJ, Duffy JF, Czeisler CA. Age-related increase in awakenings: impaired consolidation of nonREM sleep at all circadian phases. Sleep. 2001;24:565–77. doi: 10.1093/sleep/24.5.565. [DOI] [PubMed] [Google Scholar]
- 45.Colten HR, Alteveogt BM, editors. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]