Abstract
Study Objectives:
The two-process model posits that sleep is regulated by 2 independent processes, a circadian Process C and a homeostatic Process S. EEG slow-wave activity (SWA) is a marker of NREM sleep intensity and is used as an indicator of sleep homeostasis. So far, parameters of the two-process model have been derived mainly from average data. Our aim was to quantify inter-individual differences.
Design:
Polysomnographic recordings (analysis of existing data).
Setting:
Sound attenuated sleep laboratory.
Patients or Participants:
Eight healthy young males.
Interventions:
40-h sustained wakefulness.
Measurements and Results:
Process S was modeled by a saturating exponential function during wakefulness and an exponential decline during sleep. Empirical mean SWA (derivation C3A2) per NREM sleep episode at episode midpoint were used for parameter estimation. Parameters were estimated simultaneously by minimizing the mean square error between data and simulations of Process S. This approach was satisfactory for average data and most individual data. We further improved our methodological approach by limiting the time constants to a physiologically meaningful range. This allowed a satisfactory fit also for the one individual whose parameters were beyond a physiological range. The time constants of the buildup of Process S ranged from 14.1 h to 26.4 h and those of the decline from 1.2 h to 2.9 h with similar inter-individual variability of the buildup and decline of Process S.
Conclusions:
We established a robust method for parameter estimation of Process S on an individual basis.
Citation:
Rusterholz T; Dürr R; Achermann P. Inter-individual differences in the dynamics of sleep homeostasis. SLEEP 2010;33(4):491-498.
Keywords: Sleep homeostasis, sleep regulation, Process S, time constants, slow-wave activity, exponential functions
IN VIEW OF THE COMPLEXITY OF SLEEP, MODELING ITS MAJOR REGULATORY PROCESSES WAS AN ESSENTIAL STEP TO PROVIDE A CONCEPTUAL FRAMEWORK to stimulate new experiments and interpret the results. The two-process model1,2 postulates the interaction between a homeostatic Process S and a circadian Process C that is independent of sleep and waking. The homeostatic Process S takes into account the prior history of sleep and waking.
Process S rises and declines monotonically during waking and sleep, respectively. Its increase and decline were described by exponential functions.2 The saturating exponential increase of S during waking was first derived from sleep deprivation data2 and subsequently confirmed by a multiple nap protocol.3,4 Slow-wave activity (SWA; EEG power in the 0.75–4.5 Hz range), a marker of sleep intensity, was used to determine the time course of Process S during sleep.1,2,5,6
Parameter estimation in individuals is difficult. Therefore, parameters of the decline of Process S were derived from average or pooled data across subjects. Generally, an exponential function was fitted through pooled normalized mean SWA of NREM sleep episodes plotted at episode midpoints. Our aim now was to establish a reliable and stable method of parameter estimation of Process S that can be applied to individual data. Our method is based on an elaboration of the approach used previously.7 As the dynamics of the buildup and the decline of Process S are estimated simultaneously, empirical data are required under conditions of different sleep pressure. Thus, simulations and parameter estimation were based on baseline sleep followed by 40 h of sustained wakefulness and subsequent recovery sleep.8,9
Originally parameter estimation was based on the assumption, that the lower asymptote (LA) of the exponential decline of Process S is zero.1,2 The resulting parameters were used to determine the upper and lower thresholds (circadian Process C) in the two-process model.2 In subsequent studies however, a lower asymptote larger than zero was assumed (see references in Figure 6 for an overview). A lower asymptote larger than zero seems physiologically more meaningful, as a minimal amount of SWA is needed to maintain sleep.10 Furthermore, unspecific activity (noise) may also contribute to a LA > 0. We applied our method of parameter estimation to test whether different assumptions (LA > 0 vs. LA = 0) significantly affect the estimated parameters.
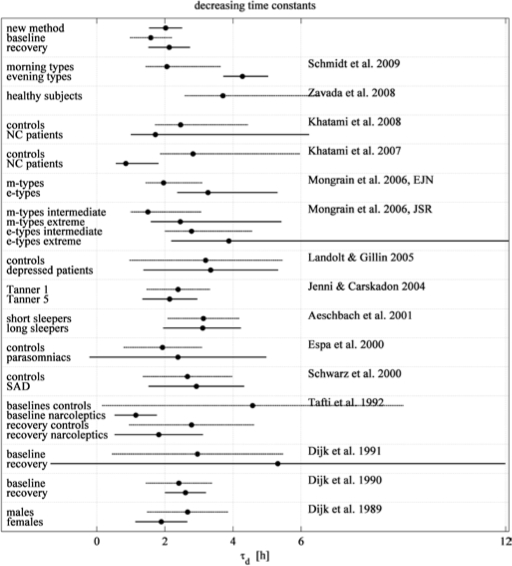
Figure 6.
Literature review of the decreasing time constant (td) of the homeostatic Process S. Mean values and 95% confidence intervals (CI) are illustrated. If the confidence interval is asymmetric, the authors estimated the decay rate (1/td). Time constants or decay rates were estimated as described in Methods and illustrated in Figure 5. Parameter estimation methods may differ somewhat. All authors assumed a lower asymptote larger than zero. New method: time constant according to Table 3, baseline and recovery: time constant according to Figure 4; morning and evening types35; healthy subjects36; NC: narcolepsy/ cataplexy, recovery sleep after 40 h of wakefulness16; NC: narcolepsy/ cataplexy, baseline sleep15; morning and evening types (CI of extreme e-types extend to 17.2 h)37,38; depressed patients: nonsuicidal outpatients with major depressive disorder39; Tanner 1 and 2: Tanner stages characterizing adolescence40; short and long sleepers22; parasomniacs41; SAD: seasonal affective disorder42; narcoleptics baseline and recovery43; baseline and recovery sleep, 24-h sleep deprivation44; baseline and recovery sleep, 36 h sleep deprivation10; males and females.45
METHODS
Participants
The analysis was performed on an existing dataset.8,9 Eight healthy young male subjects without sleep disturbances and regular bedtimes from 23:00 to 07:00 took part in this study (mean age: 23 years, range: 21–25 years). They were selected on the basis of a screening night to exclude sleep disturbances as sleep apnea, nocturnal myoclonus, prolonged sleep latency and low sleep efficiency. They all were right handed, non-smokers and moderate alcohol and caffeine consumers. The study protocol was approved by the institutional ethical committee and participants gave their written informed consent.
Study Design
Polysomnography recordings of the participants were obtained for an adaptation night, a subsequent baseline night, and a recovery night after 40 hours of sustained wakefulness. Bedtime for all 3 nights was scheduled at 23:00. Sleep was limited to 8 h for the adaptation and baseline nights and to 12 h for the recovery night. Subjects were required to abstain from alcohol or caffeine consumption 3 days prior to and during the experimental session. They had to adhere to regular bedtimes for 3 days prior to the study verified by ambulatory activity monitoring.
Data Processing
Sleep stages were visually scored for 20-s epochs (derivation C3A2) according to the criteria of Rechtschaffen and Kales.11 Power spectra were calculated for consecutive 4-s epochs (FFT, Hanning window, linear detrending) resulting in a frequency resolution of 0.25 Hz. SWA was defined as power in the 0.75–4.5 Hz range. Artifacts were excluded on a 4-s basis by visual inspection and semi automatically, whenever power in the SWA and 20–30 Hz band exceeded a threshold based on a moving average determined over 15 20-s epochs.9 Sleep stages were matched with corresponding consecutive sets of five 4-s epochs of power spectra. NREM-REM sleep cycles were determined according to the criteria of Feinberg and Floid.12 No sleep onset REM sleep episodes or skipped first REM sleep episodes were observed in our data.
Mean SWA in NREM sleep (stages 2 to 4) was calculated for each NREM sleep episode. Data were normalized within each subject with mean SWA in NREM sleep in the first 7 h 27 min of baseline sleep (minimal duration of sleep in all participants).
When data were averaged across participants, the first 4 baseline NREM sleep episodes and the first 5 episodes of recovery sleep were used (number of episodes common to all subjects). The timing of episode midpoints and the sleep length were also averaged. For the individual data, all episodes and individual sleep length entered the analysis.
Modeling Process S
Parameter estimation was based on a method developed previously7 which was elaborated for application to individual data sets. The homeostatic Process S was modeled by 2 exponential functions, one for the sleep state and one for the wake state.
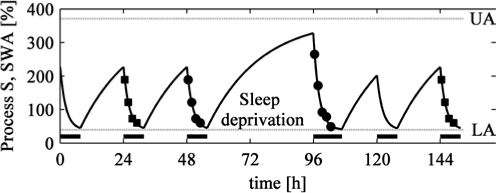
τd is the time constant of the decreasing exponential function during sleep, τi the time constant of the increasing saturating exponential function during wake, LA the lower asymptote, UA the upper asymptote, SSO the level of S at sleep onset, SWU the level of S at wake up and t is time, starting at zero with sleep onset or with wake up, respectively. Empirical mean SWA per NREM sleep episode at episode midpoints served for parameter estimation. Generally, a steady state of Process S at sleep onset is best achieved when several baseline days enter parameter estimation. In the present approach we estimated parameters by 2 means: (1) Parameter estimation based exclusively on the baseline and recovery day. (2) Computations were not merely based on the baseline and recovery day but on 4 nights by repeating single days: baseline, baseline (the single baseline data were repeated), sleep deprivation, recovery sleep, followed again by the baseline in the second night after recovery (Figure 1). Such a procedure is justified as the subjects had to adhere to regular bedtimes 3 days prior to the study and sleep deprivation effects were most probably recovered in the third night after the end of sustained wakefulness. Very similar parameters were obtained by both approaches (preliminary analyses of other derivations indicate that repeating the baseline leads to better results). We thus employed the second approach for our analysis.
Figure 1.
Illustration of the parameter estimation method. Circles: Empirical mean normalized SWA per NREM sleep episode (see Methods) plotted at NREM sleep episode midpoints for a baseline and a recovery sleep after 40 h of sustained wakefulness (sleep deprivation). Squares: Baseline data were repeated as a pre-baseline and third night after sleep deprivation. Curve: Simulation of the homeostatic Process S. Dotted horizontal lines: Upper asymptote (UA) and lower asymptote (LA) of the exponential functions. Bars: Sleep episodes.
For averaged data over participants, parameters were optimized by minimizing the mean square error (MSE) between data and simulation (Nelder-Mead simplex [direct search] method, MATLAB function “fminsearch”). To try to avoid local minima, 5 optimization runs were performed with different random initial parameter values in a given range, and they had to converge. The output with the smallest error was used as the optimal result. For parameter optimization in individuals, we multiplied 2 additional terms with the MSE (error function) to limit the 2 time constants to a physiological range.
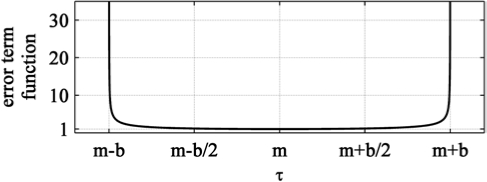
τd, τi are the time constants (see above), md (mi) is the midpoint of the corresponding symmetric interval and bd (bi) reflects its boundaries md ± bd (mi ± bi) (Figure 2). Values outside the boundaries were set to infinite. The function was derived from the Lorentz factor of special relativity theory. We used this function because of its specific jar-like shape. It is flat around the midpoint and shows a strong increase close to the boundaries (Figure 2). The values are close to 1.0 over a large range and exactly 1.0 at the midpoint. By multiplying the MSE with this function, the error is enlarged if the time constants approach the boundaries, and thus they are kept within the boundaries md ± bd (mi ± bi).
Figure 2.
Additional error term function to limit time constants t to a physiological range defined by the 2 constants m and b (m – b < t < m + b). In the parameter optimization procedure, the error term is multiplied with the mean square error (MSE) for both time constants of the buildup and decline of Process S (see equation 3).
Figure 2 illustrates these additional terms as a function of the parameter τ (τd, τi). The midpoints md and mi were set to τd and τi, respectively, obtained by the parameter estimation with average data. bd and bi were set to 2 and 20 respectively, because the time constants derived from averaged data and based on the literature were approximately 2 h and 20 h. Thus, τi was restricted to a range of approximately 0 to 40 h and τd to a range of 0 to 4 h. Additionally, LA was forced to be larger or equal zero.
Baseline and Recovery Decrease of Process S
To compare the parameters of the decline of Process S derived with our new approach with the “traditional” estimation based on pooled data, exponential functions were fitted through normalized and pooled SWA data of all subjects separately for baseline and recovery sleep. In addition, it allowed comparing the decline in baseline and recovery sleep. MATLAB functions “nlinfit” and “nlparci” were used for parameter estimation and computation of the 95% confidence intervals.
Statistics
The adjusted coefficient of determination R2 was used to evaluate the goodness of fit. Only the empirical data (baseline, recovery) were used to determine the goodness of fit.
SSerr is the residual sum of squares, SStot the total sum of squares between observations and mean value, n the number of data points and p the number of regressors (parameters). The adjusted R2 13 takes into account that r2 would increase with increasing number of regressors even without improvement of the fit and that the number of data points differ between individuals.
Parameters derived for LA > 0 and LA = 0 were compared with 2-tailed paired t-tests and the corresponding goodness of fit (R2) with 2-sided paired Wilcoxon signed-rank tests. To evaluate the correlation between restricted and unrestricted optimized parameters in individuals, Pearson linear correlation coefficients were calculated. All data processing (calculation of SWA, simulation, optimization, and statistics) was performed with MATLAB (The Mathworks, Natwick, MA, USA).
RESULTS
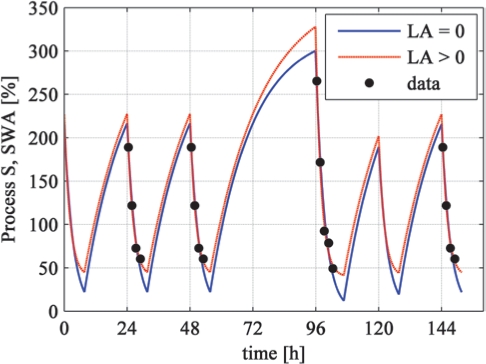
Parameters estimated with averaged data across participants are shown in Table 1 and the corresponding simulations are illustrated in Figure 3. Some early studies assumed that the lower asymptote is zero.1,2 In subsequent studies, a lower asymptote larger than zero was assumed (see references in Figure 6 for an overview). For LA > 0, the increasing time constant τi was 19.9 h and the decreasing time constant τd was 2.2 h. For LA = 0, the increasing time constant τi was smaller (15.8 h) whereas the decreasing time constant τd was larger (3.4 h). The goodness of fit (R2; Table 1) was better for LA > 0, in part due to the additional degree of freedom.
Table 1.
Estimated parameters of the homeostatic Process S based on normalized data averaged over participants (n = 8)
| ti [h] | td [h] | UA [%] | LA [%] | R2 | |
|---|---|---|---|---|---|
| LA > 0 | 19.90 | 2.16 | 371.28 | 39.58 | 0.97 |
| LA = 0 | 15.78 | 3.37 | 323.88 | 0 | 0.93 |
Optimization was performed for an estimated lower asymptote (LA > 0) and a lower asymptote set to zero (LA = 0).
ti time constant of buildup; td time constant of decrease; UA upper asymptote; LA lower asymptote; R2 goodness of fit.
Figure 3.
Simulation of Process S (curves) based on parameters determined with an estimated lower asymptote (LA > 0) and a lower asymptote set to zero (LA = 0). The dots represent empirical mean normalized SWA per NREM sleep episode plotted at episode midpoints (average data of 8 subjects). Simulations were based on the protocol illustrated in Figure 1.
Next, we aimed at parameter estimation for data of individual subjects. Our approach was successful for average data (Table 1) as well as for most individuals (Table 2, columns “unrestr.”). Subject 11 showed a very large increasing time constant (114.9 h), which is rather unphysiologic and far from published values or the values of the other subjects. Such a large time constant yields a nearly linear increase of Process S during wakefulness. In this subject, also the upper asymptote was much higher (1273%) than in the other subjects. Therefore, based on the values estimated for average data we limited the time constants to a range we considered physiologically meaningful. In addition, the lower asymptote had to be larger or equal to zero (see Methods for details).
Table 2.
Comparison of parameters of the homeostatic Process S in individuals optimized unrestricted (unrestr.) and restricted (restr.)
|
ti [h] |
td [h] |
UA [%] |
LA [%] |
|||||
|---|---|---|---|---|---|---|---|---|
| subjects | unrestr. | restr. | unrestr. | restr. | unrestr. | restr. | unrestr. | restr. |
| 2 | 37.50 | 26.43 | 1.03 | 1.15 | 717.33 | 556.60 | 62.57 | 59.70 |
| 5 | 14.35 | 16.10 | 1.91 | 1.95 | 368.01 | 382.83 | 30.66 | 30.02 |
| 7 | 33.26 | 25.34 | 3.71 | 2.91 | 362.46 | 330.36 | 37.53 | 48.05 |
| 10 | 24.94 | 21.38 | 2.24 | 2.20 | 504.76 | 470.38 | 29.60 | 29.56 |
| 11 | 114.93 | 25.49 | 1.22 | 1.49 | 1272.53 | 431.09 | 65.43 | 59.05 |
| 14 | 18.07 | 18.65 | 2.64 | 2.52 | 337.06 | 344.39 | 35.45 | 37.66 |
| 16 | 25.20 | 22.83 | 1.57 | 1.64 | 474.71 | 444.14 | 41.39 | 39.66 |
| 18 | 11.48 | 14.09 | 2.45 | 2.33 | 231.86 | 246.81 | 46.85 | 49.11 |
| MEAN | 34.97 | 21.29 | 2.10 | 2.02 | 533.59 | 400.83 | 43.68 | 44.10 |
| STD | 33.51 | 4.61 | 0.87 | 0.58 | 331.76 | 95.69 | 13.73 | 11.82 |
| CV [%] | 95.8 | 21.6 | 41.4 | 28.7 | 62.2 | 23.9 | 31.4 | 26.8 |
| CORR | 0.60 | 0.98 | 0.54 | 0.94 | ||||
Time constants were restricted to a physiological range (see Methods).
STD standard deviation; CV coefficient of variation; CORR correlation of parameters (unrestr. and restr.); ti time constant of buildup; td time constant of decrease; UA upper asymptote; LA lower asymptote.
The restricted optimization procedure not only reduced the increasing time constant, it also lowered the upper asymptote towards the values observed of the other subjects (Table 2, columns “restr.”). In general, the buildup of Process S and not its decline was mainly affected by the restricted parameter optimization procedure. This is reflected in the correlations between unrestricted and restricted parameters: for parameters of the decline the correlation was high (0.98, time constant; 0.94, lower asymptote) and low for the buildup parameters (0.60, time constant; 0.54, upper asymptote). In summary, the elaborated procedure for parameter estimation for individuals was satisfactory and was used for the following analyses.
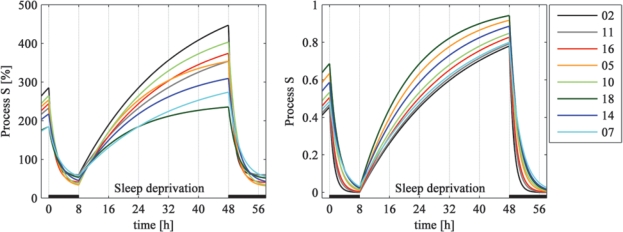
Figure 4 illustrates the impact of inter-individual variation in the parameters on the time course of Process S. The left panel shows Process S scaled in the range of normalized SWA. Both, time constants and asymptotes contribute to the inter-individual variation. In the right panel asymptotes were set to 0 and 1 in order to emphasize the impact of the time constants. One may get the impression that the decline of Process S shows less variation than the buildup. However, restricted parameters showed similar inter-individual variability as reflected in the coefficient of variation (CV; Table 2).
Figure 4.
Simulation of Process S (baseline sleep, sleep deprivation, recovery sleep) in all 8 individuals with the parameters estimated for LA > 0. Left: Process S in the value range of normalized SWA [%]. Time constants and asymptotes according to Table 3. Right: Simulation with individual time constants and asymptotes set to zero and one to emphasize the influence of the time constants. Simulations were sorted according to increasing values of the time constants (td) of the decline of Process S. Bars: Sleep episodes.
Table 3 shows the parameters of Process S estimated for individuals with the assumption of the lower asymptote LA > 0 and LA = 0. Process S with LA > 0 fitted better with the empirical data as indicated by a higher adjusted coefficient of determination (R2; Table 3). Furthermore, the 95% confidence interval of the lower asymptote (35.9%–52.3%) did not include the zero level. This confirms that the assumption of a lower asymptote larger than zero is more appropriate and statistically justified. For a LA > 0, the increasing time constants were significantly larger and the decreasing time constants smaller than for LA = 0. Also the asymptotes differed between the two assumptions, but not the difference between upper and lower asymptote (Table 3). This means that parameters derived with different assumptions, i.e., with an estimated lower asymptote or a lower asymptote of zero, cannot be compared.
Table 3.
Parameters of the homeostatic Process S determined in individual subjects for an estimated lower asymptote (LA > 0) and a lower asymptote set to zero (LA = 0)
|
LA > 0 |
LA = 0 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| subjects | ti [h] | td [h] | UA [%] | LA [%] | UA-LA [%] | R2 | ti [h] | td [h] | UA [%] | R2 |
| 2 | 26.43 | 1.15 | 556.60 | 59.70 | 496.90 | 0.88 | 14.92 | 3.43 | 320.53 | 0.37 |
| 5 | 16.10 | 1.95 | 382.83 | 30.02 | 352.81 | 0.79 | 13.54 | 2.82 | 339.86 | 0.75 |
| 7 | 25.34 | 2.91 | 330.36 | 48.05 | 282.31 | 0.74 | 20.09 | 4.69 | 306.61 | 0.57 |
| 10 | 21.38 | 2.20 | 470.38 | 29.56 | 440.82 | 0.53 | 18.50 | 3.24 | 410.82 | 0.50 |
| 11 | 25.49 | 1.49 | 431.09 | 59.05 | 372.04 | 0.73 | 18.48 | 3.74 | 325.50 | 0.38 |
| 14 | 18.65 | 2.52 | 344.39 | 37.66 | 306.73 | 0.88 | 16.09 | 4.01 | 309.53 | 0.79 |
| 16 | 22.83 | 1.64 | 444.14 | 39.66 | 404.48 | 0.92 | 16.19 | 3.12 | 328.42 | 0.82 |
| 18 | 14.09 | 2.33 | 246.81 | 49.11 | 197.70 | 0.61 | 11.43 | 4.38 | 226.38 | 0.48 |
| MEAN | 21.29 | 2.02 | 400.83 | 44.10 | 356.72 | 0.79 | 16.16 | 3.68 | 320.95 | 0.61 |
| CI | 17.44-25.14 | 1.54-2.51 | 320.83-480.82 | 34.22-53.99 | 277.68-435.77 | 0.66-0.87 | 13.77-18.54 | 3.14-4.22 | 278.81-363.10 | 0.43-0.75 |
| STD | 4.61 | 0.58 | 95.69 | 11.82 | 94.55 | 0.11 | 2.86 | 0.65 | 50.41 | 0.09 |
| P value | 2.60E-03 | 4.89E-05 | 1.70E-02 | 1.74E-01 | 7.81E-03 | |||||
Time constants were restricted to a physiologically meaningful range (see Methods).
CI, 95% confidence interval; STD, standard deviation; ti time constant of buildup; td time constant of decrease; UA, upper asymptote; LA, lower asymptote. Parameters determined for LA > 0 and LA = 0 were compared by paired t-tests (paired Wilcoxon test for R2; P values listed the columns for LA > 0; UA-LA [LA > 0] was compared to UA [LA = 0]).
Finelli et al.8 reported an association between the rise rate of θ activity in waking and the increase of SWA (first NREM sleep episode) after sleep deprivation. As τi is the parameter characterizing the buildup, we investigated its relation with afore mentioned variables. τi correlated with both, the rise rate of θ activity in waking (in %/h; r = 0.69, LA > 0; r = 0.89, LA = 0) and the increase of SWA (in %; r = 0.83, LA > 0; r = 0.72, LA = 0; one outlier was excluded as performed by Finelli et al.8).
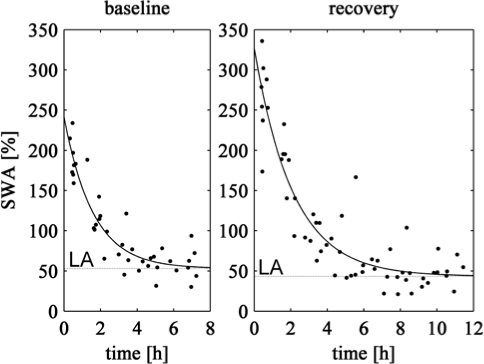
We also compared the decline of Process S between baseline and recover sleep. Figure 5 illustrates the exponential functions fitted through normalized and pooled data of all subjects. The decreasing time constants τd and their 95% confidence intervals were 1.59 (0.96–2.21) h for the baseline and 2.13 (1.52–2.73) h for recovery sleep. The resulting lower asymptotes LA were 53.1% (37.2%–68.9%) for the baseline and 43.3% (27.3%–59.3%) for recovery sleep. The parameters derived for both nights did not differ, since the corresponding confidence intervals largely overlapped, confirming a basic assumption of the two-process model.1,2 These results also match with the parameters of the decline obtained with the elaborated algorithm for individual data (Table 3; confidence intervals also overlap) and for average data over participants (Table 1).
Figure 5.
Exponential decline fitted to pooled SWA data plotted at episode midpoints. Time zero corresponds to sleep onset (stage 2). Black dots are SWA data of all subjects normalized per individual (n = 8). Baseline sleep: td = 1.59 (0.96–2.21) h; LA = 53.1% (37.2%–68.9%); 95% confidence interval in brackets. Recovery sleep: td = 2.13 (1.52–2.73) h, LA = 43.3% (27.3%–59.3%).
DISCUSSION
We have established a robust and reliable method of parameter estimation of the homeostatic Process S. Parameters of the increase and decline of S were estimated simultaneously based on data of baseline and recovery sleep after 40 h of sustained wakefulness. The approach can easily be expanded to more complex protocols, e.g., including naps. However, a sufficient experimental challenge including sleep deprivation is needed to quantify the buildup (τi, UA).
The simultaneous determination of the buildup and decline of Process S is similar to the approach of Daan et al.2 The buildup of S was derived from the level of S at wake up and the levels of S at sleep onset of baseline and recovery sleep, i.e., from 3 data points. This is the minimum number of data points needed to estimate the parameters of a saturating exponential function. Therefore, the parameters of the buildup are less stable and more sensitive to variation in empirical data than those of the decline based on more data points. Parameter estimation in individuals was especially difficult for the buildup of Process S (Table 2). Restricting the time constants to a physiological range mainly affected the buildup. Jenni et al.7 applied a similar approach, but without restriction of the time constants, and reported that in one case parameter estimation did not converge.
Average data of SWA can be in general well described by exponential functions (Figure 1). Data of individual subjects, however, do not always show a monotonic decline during sleep which renders parameter estimation on an individual basis challenging. Thus, we had to restrict the time constants to a physiologically meaningful range. This approach proved to be reliable. But it has to be mentioned that the restriction also narrowed the parameter distribution between individuals and consequently, the corresponding confidence intervals.
Fitting an exponential decline rather than a more elaborate approach14 has several limitations. (1) An exponential decline is only justified when sleep is undisturbed. For example, the fragmented baseline sleep of narcoleptic patients resulted in a faster decline constant of Process S than in control subjects.15 However, when the narcoleptics were sleep deprived the decline of S in recovery sleep after 40 h of sustained wakefulness was no longer different from controls.16 Sleep deprivation had reduced sleep fragmentation in recovery sleep considerably revealing the “true” dynamics of Process S. In our subjects, sleep was not fragmented. Waking after sleep onset was low (6.8 min in baseline sleep, 1.0 min in recovery8). (2) An exponential decline depends strongly on the definition of NREM sleep episodes. In particular, the first episode might be long due to a skipped first REM sleep episode. NREM sleep episodes need to be terminated if a drop of SWA is observed at a point where REM sleep might be expected. In our data base this was not necessary.
Absolute SWA values vary considerably. Therefore, we normalized the data to achieve a similar contribution (weight) of the individuals to the mean data. Thus, time constants derived from average data are influenced by the normalization procedure. In contrast, normalization does not affect the time constants determined on an individual basis, since it leads to a multiplication of the absolute data with a fixed factor. Only the comparisons of the asymptotes are affected by the normalization procedure and caution is needed when comparing asymptotes determined with different normalizations.
SWA is considered as a marker of sleep intensity and serves to derive the dynamics of Process S. Sleep homeostasis however, is mainly reflected in the time constants. In the original version of the two-process model2 which focused on the time constants, Process S varied in the range of 0 to 1. The longer the time constant of the buildup, the better is the capacity of an individual to cope with sleep deprivation or sleep restriction, and a short time constant of the decline indicates a fast dissipation of sleep pressure. Jenni et al.7 observed a faster increase of Process S in early pubertal children compared with mature adolescents, while the decrease of S was similar in both groups. The meaning of the standardized upper asymptote, or rather the difference between the asymptotes, is less evident. While absolute values of the asymptotes reflect SWA of an individual and are related to maturational17 and age dependent18–20 changes, normalized asymptotes might reflect the capacity of the brain to generate or produce slow waves. This view is supported by the smaller difference between normalized asymptotes in prepubertal children than in mature adolescents.7 Together with the faster buildup of S in prepubertal children, this could indicate that the young brain reaches its capacity to generate slow waves after shorter wakefulness than the mature brain. However, such an interpretation of the meaningfulness of normalized asymptotes must be further tested, e.g., by investigating sleep homeostasis longitudinally in the course of childhood and adolescence. Subject 2 has the largest difference between the asymptotes and the largest time constant of the buildup of the group (Figure 4, Table 3) pointing to a low homeostatic sleep need. In contrast, subject 18 has a high homeostatic need for sleep.
Figure 6 provides an overview of the decreasing time constant and the corresponding 95% confidence intervals (CI) reported in literature. We included only time constants that were determined for a lower asymptote larger than zero. SWA data were mainly determined for a central derivation (mostly C3A2). The overview covers different factors such as baseline data, experimental manipulations, different sex and age, morning and evening types, short and long sleepers, as well as data of patients. The size of the confidence intervals points to considerable inter-individual variation. The average time constant of the decline of Process S is in the range of 2 to 3 h, largely independent of additional factors. Also, our time constants derived with the new approach correspond well to the picture emerging from the literature. The lower asymptotes are difficult to compare due to the diversity of normalizations applied by the different researchers. In simulations of Process S, buildup parameters were not often estimated. A few attempts were based on SWA,2–4,7 More recently, the buildup parameters have been derived from EEG θ activity during waking21–24 when such data were available. Whether buildup parameters derived from waking and sleep data are equivalent needs to be established in future studies.
Determining the parameters on an individual basis allowed comparing the influence of a lower asymptote of zero with one larger than zero. Estimating the lower asymptote, resulting in a value larger than zero, led to a better fit between the simulation and empirical data, in part due to introducing an additional parameter. Most important however, parameters of the two assumptions differed significantly. This leads to the conclusion that parameters derived with different assumptions cannot be compared. This also holds for homeostatic parameters determined for animal data.25–28
There is an increasing interest in individual aspects of sleep.29 A functional relationship between distinct EEG markers of sleep regulation in waking and sleep was established based on inter-individual variation.8 Our analysis also revealed a good correlation between the time constant of the buildup of Process S and an EEG marker during waking (rise rate of θ activity). Individual differences in habitual sleep duration may be associated with accumulated sleep debt in young adults.30 Sleep need (determined as asymptotic sleep duration) appears to be 1.5 h shorter in older (60–76 years) than in younger (18–32 years) subjects.31 Individual sleep need may thus contribute to the variation observed in the response to sleep deprivation8 and present analysis. Also genetic factors may contribute to individual differences in sleep homeostasis.32–34
In summary, we established a robust method for the estimation of the parameters of the homeostatic Process S. It provides a basis for future investigations such as age dependent or sex specific differences in sleep homeostasis or to assess regional (topographic) specificity in the dynamics of sleep homeostasis.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Drs. A. Borbély and I. Tobler for comments on the manuscript. The study was supported by SNSF grant 320000-112674 and EU LSHM-CT-2005-518189.
REFERENCES
- 1.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 2.Daan S, Beersma DGM, Borbély AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–78. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 3.Beersma DGM, Daan S, Dijk DJ. Sleep intensity and timing: A model for their circadian control. In: Carpenter GA, editor. Some mathematical questions in biology - circadian rhythms. Lectures on Mathematics in the Life Sciences ed. Vol. 19. Providence, Rhode Island: The American Mathematical Society; 1987. pp. 39–62. [Google Scholar]
- 4.Dijk DJ, Beersma DGM, Daan S. EEG power density during nap sleep: Reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms. 1987;2:207–19. doi: 10.1177/074873048700200304. [DOI] [PubMed] [Google Scholar]
- 5.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: Elsevier Saunders; 2005. pp. 405–17. [Google Scholar]
- 6.Achermann P. The two-process model of sleep regulation revisited. Aviat Space Environ Med. 2004;75:A37–43. [PubMed] [Google Scholar]
- 7.Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–54. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- 8.Finelli LA, Baumann H, Borbély AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–9. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 9.Finelli LA, Achermann P, Borbély AA. Individual ‘fingerprints’ in human sleep EEG topography. Neuropsychopharmacology. 2001;25:S57–62. doi: 10.1016/S0893-133X(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 10.Dijk DJ, Brunner DP, Borbély AA. Time course of EEG power density during long sleep in humans. Am J Physiol. 1990;258:R650–61. doi: 10.1152/ajpregu.1990.258.3.R650. [DOI] [PubMed] [Google Scholar]
- 11.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda, Maryland: National Institutes of Health; 1968. [Google Scholar]
- 12.Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16:283–91. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg SL, Abramowitz SK. Data Analysis for the Behavioral Sciences Using SPSS. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 14.Achermann P, Dijk DJ, Brunner DP, Borbély AA. A model of human sleep homeostasis based on EEG slow-wave activity: Quantitative comparison of data and simulations. Brain Res Bull. 1993;31:97–113. doi: 10.1016/0361-9230(93)90016-5. [DOI] [PubMed] [Google Scholar]
- 15.Khatami R, Landolt HP, Achermann P, et al. Insufficient non-REM sleep intensity in narcolepsy-cataplexy. Sleep. 2007;30:980–9. doi: 10.1093/sleep/30.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khatami R, Landolt HP, Achermann P, et al. Challenging sleep homeostasis in narcolepsy-cataplexy: implications for non-REM and REM sleep regulation. Sleep. 2008;31:859–67. doi: 10.1093/sleep/31.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinberg I, Thode HC, Jr, Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol. 1990;142:149–61. doi: 10.1016/s0022-5193(05)80218-8. [DOI] [PubMed] [Google Scholar]
- 18.Dijk DJ, Beersma DG, van den Hoofdakker RH. All night spectral analysis of EEG sleep in young adult and middle-aged male subjects. Neurobiol Aging. 1989;10:677–82. doi: 10.1016/0197-4580(89)90004-3. [DOI] [PubMed] [Google Scholar]
- 19.Gaudreau H, Carrier J, Montplaisir J. Age-related modifications of NREM sleep EEG: from childhood to middle age. J Sleep Res. 2001;10:165–72. doi: 10.1046/j.1365-2869.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- 20.Landolt HP, Dijk DJ, Achermann P, Borbély AA. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738:205–12. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- 21.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Two circadian rhythms in the human electroencephalogram during wakefulness. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1771–9. doi: 10.1152/ajpregu.1999.277.6.R1771. [DOI] [PubMed] [Google Scholar]
- 22.Aeschbach D, Postolache TT, Sher L, Matthews JR, Jackson MA, Wehr TA. Evidence from the waking electroencephalogram that short sleepers live under higher homeostatic sleep pressure than long sleepers. Neuroscience. 2001;102:493–502. doi: 10.1016/s0306-4522(00)00518-2. [DOI] [PubMed] [Google Scholar]
- 23.Cajochen C, Brunner DP, Kräauchi K, Graw P, WirzJustice A. Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep. 1995;18:890–4. doi: 10.1093/sleep/18.10.890. [DOI] [PubMed] [Google Scholar]
- 24.Taillard J, Philip P, Coste O, Sagaspe P, Bioulac B. The circadian and homeostatic modulation of sleep pressure during wakefulness differs between morning and evening chronotypes. J Sleep Res. 2003;12:275–82. doi: 10.1046/j.0962-1105.2003.00369.x. [DOI] [PubMed] [Google Scholar]
- 25.Franken P, Tobler I, Borbély AA. Sleep homeostasis in the rat: simulation of the time course of EEG slow-wave activity [published erratum appeared in Neurosci Lett 1991;132:279] Neurosci Lett. 1991;130:141–4. doi: 10.1016/0304-3940(91)90382-4. [DOI] [PubMed] [Google Scholar]
- 26.Huber R, Deboer T, Tobler I. Effects of sleep deprivation on sleep and sleep EEG in three mouse strains: empirical data and simulations. Brain Res. 2000;857:8–19. doi: 10.1016/s0006-8993(99)02248-9. [DOI] [PubMed] [Google Scholar]
- 27.Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21:2610–21. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vyazovskiy VV, Achermann P, Tobler I. Sleep homeostasis in the rat in the light and dark period. Brain Res Bull. 2007;74:37–44. doi: 10.1016/j.brainresbull.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Van Dongen HPA, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005;28:479–96. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 30.Klerman EB, Dijk DJ. Interindividual variation in sleep duration and its association with sleep debt in young adults. Sleep. 2005;28:1253–9. doi: 10.1093/sleep/28.10.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klerman EB, Dijk DJ. Age-related reduction in the maximal capacity for sleep - Implications for insomnia. Curr Biol. 2008;18:1118–23. doi: 10.1016/j.cub.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viola AU, Archer SN, James LM, et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17:613–8. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 33.Rétey JV, Adam M, Honegger E, et al. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci U S A. 2005;102:15676–81. doi: 10.1073/pnas.0505414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landolt HP. Genotype-dependent differences in sleep, vigilance, and response to stimulants. Curr Pharm Des. 2008;14:3396–407. doi: 10.2174/138161208786549344. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt C, Collette F, Leclercq Y, et al. Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science. 2009;324:516–9. doi: 10.1126/science.1167337. [DOI] [PubMed] [Google Scholar]
- 36.Zavada A, Strijkstra AM, Boerema AS, Daan S, Beersma DGM. Evidence for differential human slow-wave activity regulation across the brain. J Sleep Res. 2009;18:3–10. doi: 10.1111/j.1365-2869.2008.00696.x. [DOI] [PubMed] [Google Scholar]
- 37.Mongrain V, Carrier J, Dumont M. Difference in sleep regulation between morning and evening circadian types as indexed by antero-posterior analyses of the sleep EEG. Eur J Neurosci. 2006;23:497–504. doi: 10.1111/j.1460-9568.2005.04561.x. [DOI] [PubMed] [Google Scholar]
- 38.Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness-eveningness. J Sleep Res. 2006;15:162–6. doi: 10.1111/j.1365-2869.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- 39.Landolt HP, Gillin JC. Similar sleep EEG topography in middle-aged depressed patients and healthy controls. Sleep. 2005;28:239–47. doi: 10.1093/sleep/28.2.239. [DOI] [PubMed] [Google Scholar]
- 40.Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–83. [PubMed] [Google Scholar]
- 41.Espa F, Ondze B, Deglise P, Billiard M, Besset A. Sleep architecture, slow wave activity, and sleep spindles in adult patients with sleepwalking and sleep terrors. Clin Neurophysiol. 2000;111:929–39. doi: 10.1016/s1388-2457(00)00249-2. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz PJ, Rosenthal NE, Kajimura N, et al. Ultradian oscillations in cranial thermoregulation and electroencephalographic slow-wave activity during sleep are abnormal in humans with annual winter depression. Brain Res. 2000;866:152–67. doi: 10.1016/s0006-8993(00)02271-x. [DOI] [PubMed] [Google Scholar]
- 43.Tafti M, Rondouin G, Besset A, Billiard M. Sleep deprivation in narcoleptic subjects: Effect of sleep stages and EEG power density. Electroencephalogr Clin Neurophysiol. 1992;83:339–49. doi: 10.1016/0013-4694(92)90069-t. [DOI] [PubMed] [Google Scholar]
- 44.Dijk DJ, Brunner DP, Borbély AA. EEG power density during recovery sleep in the morning. Electroencephalogr Clin Neurophysiol. 1991;78:203–14. doi: 10.1016/0013-4694(91)90034-2. [DOI] [PubMed] [Google Scholar]
- 45.Dijk D-J, Beersma DGM, Bloem GM. Sex differences in the sleep EEG of young adults: Visual scoring and spectral analysis. Sleep. 1989;12:500–7. doi: 10.1093/sleep/12.6.500. [DOI] [PubMed] [Google Scholar]