Abstract
Study Objectives:
To examine whether monetary incentives attenuate the negative effects of sleep deprivation on cognitive performance in a flanker task that requires higher-level cognitive-control processes, including error monitoring.
Design:
Twenty-four healthy adults aged 18 to 23 years were randomly divided into 2 subject groups: one received and the other did not receive monetary incentives for performance accuracy. Both subject groups performed a flanker task and underwent electroencephalographic recordings for event-related brain potentials after normal sleep and after 1 night of total sleep deprivation in a within-subject, counterbalanced, repeated-measures study design.
Results:
Monetary incentives significantly enhanced the response accuracy and reaction time variability under both normal sleep and sleep-deprived conditions, and they reduced the effects of sleep deprivation on the subjective effort level, the amplitude of the error-related negativity (an error-related event-related potential component), and the latency of the P300 (an event-related potential variable related to attention processes). However, monetary incentives could not attenuate the effects of sleep deprivation on any measures of behavior performance, such as the response accuracy, reaction time variability, or posterror accuracy adjustments; nor could they reduce the effects of sleep deprivation on the amplitude of the Pe, another error-related event-related potential component.
Conclusions:
This study shows that motivation incentives selectively reduce the effects of total sleep deprivation on some brain activities, but they cannot attenuate the effects of sleep deprivation on performance decrements in tasks that require high-level cognitive-control processes. Thus, monetary incentives and sleep deprivation may act through both common and different mechanisms to affect cognitive performance.
Citation:
Hsieh S; Li TH; Tsai LL. Impact of monetary incentives on cognitive performance and error monitoring following sleep deprivation. SLEEP 2010;33(4):499-507.
Keywords: Rewards and punishments, motivation, sleep loss, action monitoring
SLEEP LOSS DUE TO SLEEP DEPRIVATION OR FRAGMENTATION AND ITS CONSEQUENCE, I.E., SLEEPINESS, ARE COMMON IN MODERN SOCIETY. SLEEP loss and sleepiness contribute significantly to performance decrements and accidents, such as motor vehicle crashes and work error-related damages.1 Performance decrements after sleep loss are generally attributed to attention deficit, and this proposition is supported by several behavior,2,3 electrophysiologic,4,5 and functional brain imaging studies.6 Attention is a multidimensional cognitive process, and the results of several studies have suggested that sleep loss induces selective impairment of attention processes, particularly the top-down attention control processes that rely on the frontal lobe.2,3,6 This corresponds with the view of Horne7 that frontal lobe functions are sensitive to total sleep loss. The frontal lobe is critically involved in executive function, including the ability to plan or set goals, supervisory attention processes, response inhibition, and action monitoring, such as error detection and conflict processing. A series of recent studies conducted by us8–10 and others11,12 have shown that all components of error monitoring, including error detection, error correction, and posterror speed and accuracy adjustments, are impaired following sleep deprivation. Thus, sleep deprivation-related performance decrements and accidents appear to result from deficits in both attention processes and performance monitoring, particularly error monitoring in the latter.
The results of previous studies have suggested that the decline in vigilance task performance during sleep deprivation is in part related to reduced motivation.13–15 The feedback of the knowledge of results14,15 and monetary rewards13 reduce some negative effects of sleep loss on vigilance task performance. Vigilance is commonly defined as sustained attention or tonic alertness.1 Given that the reduction of vigilance related to a low arousal level gives rise to performance decrements following sleep deprivation, monetary incentives are thought to have general effects on the performance of vigilance tasks, such as effects related to arousal, alertness, or mental effort.14–16 Additionally, monetary incentives have recently been proposed to selectively influence the top-down attention mechanisms in the performance of tasks that require a high degree of top-down attention control processes.17–20 A previous study used a cued attention task in which subjects responded to targets preceded by spatially valid, invalid, or neutral (noninformative) cues and illustrated that monetary incentives enhanced the response speed in the case of the trials with the valid and invalid cues but not those with the neutral cues.19 Furthermore, a recent study has shown that a continuous 2-hour performance of a task that requires a high degree of cognitive control results in performance reduction in terms of the response speed, accuracy, and stability.21 Performance decrements due to prolonged task performance are accompanied by a decrease in the brain activity, including the amplitude of the contingent negative variation component of the event-related potential (ERP) that indexes the preparatory attention22,23 and the amplitude of the P300 component that is related to the deployment of attention resources.24 Increasing the motivation level by financial rewards attenuates the changes in behavior performance and brain activity during prolonged task performance.21 However, whether motivation incentives attenuate the negative effects of sleep deprivation on the performance of tasks that require a high degree of cognitive control needs to be studied, despite the many studies that have used vigilance tasks to investigate this.13–15 Thus, the first objective of this study was to examine whether motivation incentives reduce the effects of sleep deprivation while subjects perform tasks that require higher cognitive control processes. We used a flanker task,25 which requires a high degree of top-down attention control processing and action monitoring, with concurrent electroencephalographic (EEG) recordings for behavior and electrophysiologic examination of the effects of monetary incentives on attention processes and action monitoring after 1 night of total sleep deprivation, which was similarly applied in our previous studies.8–10
A previous study26 has shown that financial penalties for errors not only increase the performance accuracy, but also enhance error detection, as reflected by the increase in the amplitude of event-related negativity (ERN), which is a negative component of the response-locked ERPs and is frontocentrally maximized.27 As mentioned above, continuous 2-hour task performance also results in a reduction in error monitoring, such as in error detection (as reflected by the reduced ERN amplitude), error correction, and posterror slowing.21 In addition, monetary rewards lead to attenuation of the changes in error monitoring during prolonged task performance. It is proposed that the anterior cingulate cortex, located in the medial frontal cortex, is the generator of ERN (reviewed in28,29). Considering that monetary incentives can facilitate the activation of the medial frontal cortex in putatively well-rested subjects17,19 and can improve the reductions in error monitoring (ERN amplitude, error correction, and posterror slowing) during prolonged task performance,21 it is likely that monetary incentives can also reduce, if not completely block, the effect of sleep deprivation on error monitoring. Since the flanker task has been frequently used to study error monitoring and was used in this study, we also examined whether motivation incentives interacted with sleep deprivation to influence error monitoring.
METHODS
Participants
Sixty-seven university students responded to a recruitment advertisement for this study posted on the campus bulletin board system of the National Chung Cheng University or by word of mouth and underwent questionnaire interviews. Of them, 45 fulfilled all inclusion criteria, which were similar to those used in our previous studies.8,10 In brief, the participants did not have any history of medical, psychiatric, and sleep-related disorders; drug use; irregular sleep patterns; or excessive daytime sleepiness. Their anxiety and depression levels were within the normal range, and they had an intermediate type of preference for a particular time of the day. Only 24 of the 45 students (12 men; age range, 18-23 years; mean age, 20.6 years and SD, 1.1 years) consented to participate in the study as scheduled by the experimenter (THL). These participants had normal or corrected-to-normal vision. All participants provided informed written consent after being introduced to the study procedure and laboratory facilities. They were provided remuneration for their participation in the experiment. The study protocol was approved by the Institutional Human Subjects Ethics Committee of National Chung Cheng University.
Procedure
The participants performed a letter version of the flanker task25 on 2 mornings, i.e., after a normal night's sleep and a night of sleep deprivation, with a 2-week interval between and a counterbalanced sequence of the 2 sleep conditions. The participants were instructed to maintain their normal sleep schedules for at least 1 week before the day of the task or before sleep deprivation and to record their daily bedtime, wake-up time, and sleep quality in sleep logs. In addition, they wore an activity monitor on their dominant arm (AW64; Mini-Mitter, Bend, OR) during the same period. The participants were instructed to avoid alcohol or caffeine intake 1 day before the task. For the sleep-deprivation condition, the participants were instructed to wake up in the morning at their regular time on the day before the test, to not take naps during the day, and to arrive at the laboratory by 22:00. The participants were kept awake through the night under continuous monitoring. They were required to complete the Stanford Sleepiness Scale30 every hour starting at 23:00. During sleep deprivation, they were allowed to read books, listen to music, play computer games, or complete their own paperwork. For the normal sleep condition, the experimenter made telephone calls to the participants on the night before the task, reminded them to go to bed at their regular bedtime, and instructed them to arrive at the laboratory by 09:00 on the day of the test. For both sleep conditions, the flanker task was started immediately after the preparation of the scalp and eye electrodes (see below), which started at 10:00 and generally required approximately an hour. The participants performed the task individually in a sound-attenuated room with the lights switched off.
The participants were randomly divided into 2 groups: one received monetary incentives for performance accuracy and the other did not. In case of the former, the participants were informed that each trial would be followed by a “+4” (gain) and “−4” (loss) signal indicating a correct response and an error or omission, respectively; that the points would be converted into monetary payment; and that the more points they earned, the greater would be the amount of money they received. However, the conversion equation for the points or the maximum payment that could be earned was not disclosed to them. At the end of the experiment, the participants of the group that did not receive monetary incentives were given TWD1200 and those of the group that received monetary incentives were given between TWD1200 and TWD1400.
Flanker Task
The stimuli were generated by using the E-Prime software (Psychology Software Tools, Pittsburgh, PA) on a desktop personal computer. The letter stimuli (visual angle for each letter, 0.5°) were presented on a 15-inch computer screen with a dark background at a viewing distance of 80 cm, and the participant was required to press a designated key on the computer keyboard in response to the target stimulus. The participants were required to focus on the letter in the center of a visual array of 5 letters on the computer screen, which was designated as the target, and respond using either the right or left hand depending on the stimulus-response rules; for example, if the target letter was “H,” then they were asked to press the “Z” key on the keyboard using the left index finger, and if the target letter was “S,” they were asked to press the “/” key on the keyboard using the right index finger. The stimulus-response rules were counterbalanced across participants. The target letter was flanked on each side by 2 letters that were either identical to the target (congruent; e.g., HHHHH) or different from the target (incongruent; e.g, SSHSS). Within each block, 50% of the trials were congruent, and 50% were incongruent.
Each trial started with the presentation of a central fixation white cross “+” for 500 milliseconds. The flanker letters appeared 200 milliseconds after the offset of the fixation point and remained on for 50 milliseconds. The central target letter appeared 50 milliseconds after the onset of the flanker letters and remained on for another 50 milliseconds. For the group that did not receive the incentives, the next trial started 2 seconds after a key press or 4 seconds after the onset of the target letter if there was no key press within 2 seconds (maximum time interval for a response). For the group that received the incentives, an additional “+4” or “−4” incentive signal was displayed 500 milliseconds after the participant's response (the maximum time allowed for a response was 2 seconds), and it remained on for 500 milliseconds. The next trial started 2 seconds after the display of the incentive signal.
The participants were initially provided 16 practice trials to familiarize them with the task procedure and were instructed to respond to the target with maximum speed and accuracy. At the end of each practice trial, performance feedback on the correctness of the response was provided. If the reaction time was longer than 350 milliseconds, an additional instruction of “Speed up, please” was displayed on the computer screen. Subsequently, the participants completed 16 blocks of 64 trials each. The feedback instruction of “Speed up, please” was presented at the end of the last trial of each block if the mean reaction time of the block was longer than 350 milliseconds, and the incentive points were computed on the screen in case of the group that received the incentives. Between the blocks, the participants were allowed 1 minute or longer to close their eyes and relax.
Electrophysiologic Recording
During the task, the EEG was recorded from 6 scalp electrodes located at the midline sites, namely, Fz, FCz, Cz, CPz, Pz, and Oz, using Ag/AgCl electrodes mounted in an elastic cap (Quick-Cap; Neuroscan, Inc., Charlotte, NC). The EEG activity was referenced to the average of the 2 mastoids (M1 and M2). Two Ag/AgCl electrodes placed 2 centimeters above and below the left eye recorded the vertical electrooculogram, and 2 electrodes placed 1 centimeter lateral to the outer canthus of each eye recorded the horizontal electrooculogram. A ground electrode was placed on the forehead. The electrode impedances were maintained below 5 kΩ. The EEG and electrooculogram were amplified using SYNAMPS amplifiers (Neuroscan, Inc.) with a bandpass filter of 0.05 to 50 Hz and were digitized at 500 Hz.
Data Analysis
The daily bedtime, wake-up time, and time in bed recorded in the sleep log were individually averaged over the entire week before the task and were cross-examined with the activity data recorded in the wrist activity monitor. The daily activity data were scored using Actiware-Sleep v. 3.3 (Mini-Mitter).
The reaction time was measured as the latency of the first key press relative to the onset of the target letter. The intraindividual coefficient of variation (SD of reaction time/mean reaction time) was calculated to represent the intraindividual variability. The response accuracy, error rate, and omission rate were calculated as a percentage of the correct, erroneous, and omitted responses, respectively.
The EEG data were further digitally high-pass filtered at 0.1 Hz (-12 dB/octave) before subsequent analyses. The data were then segmented into stimulus-locked EEG epochs of 100 milliseconds before to 600 milliseconds after the onset of the target letter stimulus, response-locked epochs of 150 milliseconds before to 600 milliseconds after key press, and feedback-locked epochs of 100 milliseconds before to 500 milliseconds after incentive signal (“+4” or “−4”) presentation. The stimulus-, response-, and feedback-locked EEG signals were baseline corrected between −100 and 0 milliseconds, between −150 and −50 milliseconds, and between −100 and 0 milliseconds, respectively. EEG epochs containing baseline-to-peak electrooculographic amplitudes of larger than 50 μV or EEG drifts of larger than 50 μV from the baseline were excluded from the analysis. The averaged waveforms of the response-locked EEG epochs were further low-pass filtered at 10 Hz (-12 dB/octave) prior to subsequent analyses. In case of the stimulus-locked ERPs, P300 was defined as the most positive peak at Pz in the time window from 300 to 600 milliseconds. Two components of the response-locked ERPs—ERN and error positivity (Pe)—were defined as the most negative peak value in the time window from 0 to 150 milliseconds at FCz and the mean amplitude in the time window from 200 to 400 milliseconds at Cz, respectively.27,31 In the case of the feedback-locked ERPs, the feedback-related negativity (FRN) was defined as the most negative peak at FCz in the time window from 200 to 300 milliseconds.32
Two-factor mixed-design analyses of variance were performed to assess how monetary incentives (the group that received incentives vs the group that did not receive incentives; between-subject factor) and sleep conditions (normal sleep vs sleep deprivation; within-subject factor) affected subjective measures, task performance, and ERP components. Two-factor repeated-measures analyses of variance were performed to examine the effects of sleep deprivation and incentive valence (gain vs loss) on FRN latency and amplitude. Three-factor mixed-design analyses of variance were used to examine how incentives and sleep conditions influence the trial congruence effect (congruent vs incongruent trials) and posterror behavior adjustments (trials following correct responses vs trials following committed errors). Four-factor mixed-design analyses of variance were used to examine how incentives and sleep conditions affect the postconflict behavior adjustments.10 All multiple comparisons following the analyses of variance were performed using the Tukey test.
RESULTS
The behavior data reported in this study were collected from 24 participants; however, the ERP data were collected from only 20 participants (10 in the group that received incentives and 10 in the group that did not receive incentives) because of an EEG artifact rejection rate of more than 50% in 4 participants. To ensure a fair comparison between the behavior and ERP data, we reanalyzed the behavior data collected from the remaining 20 participants and found that the patterns of the statistical results were similar to those obtained from the 24 participants (see below).
Subjective and Objective Behavior Data
The participants' characteristics (sex, age, Epworth Sleepiness Scale score, Beck Anxiety Inventory score, and Beck Depression Inventory-II score [Table 1]) and sleep log data (Table 2) did not significantly differ between the subject groups that received and did not receive incentives. The maintenance of sleep schedules by the participants for 1 week before the task was comparable between the 2 sleep conditions. However, the participants woke up earlier on the day of the task under the normal sleep condition, probably because they had to consider the travel time in the morning to arrive at the laboratory on time. All but 2 of the participants had a mean daily time in bed longer than 7 hours in the week before the test day under the normal sleep conditions. We did not exclude the data from these 2 participants because their behavior performance was better than the group mean and they maintained posterror accuracy adjustments under the normal sleep conditions. Neither the subject-group differences (P = 0.392) nor the interaction effects between the subject groups and sleep conditions (P = 0.515) were significant in the Stanford Sleepiness Scale30 score. Both subject groups showed progressively higher scores (sleepier) on the Stanford Sleepiness Scale over time on the night of sleep deprivation (F10,220 = 24.82, P < 0.001).
Table 1.
Participant characteristics
| Variable | No incentives, n = 12 | Incentives, n = 12 |
|---|---|---|
| Men, no. | 5 | 7 |
| Age, y | 20.3 (1.6) | 20.1 (0.9) |
| ESS score33 | 8.6 (2.8) | 6.7 (3.8) |
| MEQ score34 | 49.4 (5.0) | 51.1 (5.4) |
| BAI score35 | 2.7 (2.1) | 2.3 (2.3) |
| BDI-II score36 | 4.3 (4.4) | 4.5 (4.6) |
Data are presented as mean (SD), except sex, which is a number. ESS refers to Epworth Sleepiness Scale; MEQ, Morning-Eveningness Questionnaire; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory.
Table 2.
Participants' sleep-log data before the flanker task test
| Condition | No incentive |
Incentive |
||
|---|---|---|---|---|
| Normal sleep | Sleep deprivation | Normal sleep | Sleep deprivation | |
| One week before the task test | ||||
| Bedtime | 0:42 (0:42) | 0:51 (0:45) | 0:26 (1:01)b | 0:26 (0:48)d |
| Wake-up time | 08:21 (0:59) | 08:40 (0:50) | 08:31 (1:00)b | 08:11 (0:48)d |
| TIB | 7:38 (1:01) | 7:49 (0:35) | 8:04 (0:20)b | 7:44 (0:34)d |
| The day before the task test or the day of sleep deprivation | ||||
| Bedtime | 0:30 (1:06) | 0:45 (1:05) | 23:56 (0:38)c | 0:15 (0:51)d |
| Wake-up time | 07:50 (0:33)ae | 08:26 (0:39) | 07:10 (0:54)ce | 08:07 (0:47)d |
| TIB | 7:22 (1:15)a | 7:40 (0:48) | 07:13 (1:00)c | 07:52 (0:40)d |
Data are presented as mean (SD) and are depicted as clock time for wake-up time and bedtime and hours and minutes for time in bed (TIB).
n = 11 because 1 participant did not record the wake-up time.
n = 11 because 1 participant misplaced his sleep data.
n = 9 because 2 participants did not record the sleep data.
n = 10 because 2 participants misplaced their sleep data.
P < 0.05 vs the same incentive group under the sleep deprivation condition by paired t test.
In contrast with the participant characteristics and sleep data, all subjective measures evaluated after the task differed significantly between the 2 subject groups as well as between the 2 sleep conditions (Table 3). Incentives lead to a higher subjective estimation of the correct response rate and greater confidence; however, sleep deprivation reduced both estimated correct response rate and confidence. The effects of the interaction of the subject groups with the sleep conditions on the estimated correct response rate or confidence were not significant but were near significant in terms of the claimed effort level (P = 0.095). The claimed effort level was similar between the 2 groups under the normal sleep condition (P > 0.05, Tukey test) but was significantly higher in the group that received incentives under the sleep deprivation condition (P < 0.01, Tukey test). Sleep deprivation attenuated the effort level in the group that did not receive incentives (P < 0.05, Tukey test) but had no effect on the group that received incentives (P > 0.05, Tukey test).
Table 3.
Participants' subjective measures
| Condition | No incentive |
Incentive |
||
|---|---|---|---|---|
| Normal sleep | TSD | Normal sleep | TSD | |
| Estimated % of correct responsesI**S** | 86.1 (6.9) | 68.1 (20.8) | 94.7 (4.4) | 85.6 (11.8) |
| Certainty of the % of estimated correct responsesI*S** | 8.0 (1.3) | 6.8 (1.7) | 8.9 (1.0) | 7.9 (1.4) |
| Effort for task performanceI*S*I×S# | 8.5 (1.4) | 7.3 (1.7) | 8.9 (0.8) | 8.8 (0.8) |
| (Estimated – real) % of correct responses | −4.4 (7.5) | −15.0 (21.5) | −2.7 (4.3) | −9.0 (10.3) |
Data are presented as mean (SD). TSD refers to total sleep deprivation; I, incentives; S, sleep condition.
P < 0.01 main and interactive effects of 2-factor (I × S) analysis of variances;
P < 0.05;
P < 0.10
Flanker Task Performance
As shown in Table 4, the 2 subject groups maintained comparable mean reaction time but differed with regard to the reaction time variability and accuracy. The participants in the group that received the monetary incentives responded more accurately, but more variably, in terms of speed. The omission rate was lower but not significant in the group that received the monetary incentives (P = 0.064). On the other hand, sleep deprivation was associated with a longer reaction time, higher reaction time variability, and lower response accuracy. Omission rate was lower but not significant following sleep deprivation (P = 0.075). The effect of the interaction between the subject groups and sleep conditions on the mean reaction time, reaction time variability, and accuracy was not significant.
Table 4.
Flanker task performance
| Variable | No incentives, n = 12 |
Incentives, n = 12 |
Significant effect | ||
|---|---|---|---|---|---|
| Sleep | Deprivation | Sleep | Deprivation | ||
| Reaction time, ms | |||||
| Total | 406 (45) | 458 (114) | 428 (57) | 468 (87) | S** |
| Correct | 411 (45) | 460 (107) | 429 (56) | 463 (83) | S** |
| Error | 359 (55) | 450 (175) | 411 (83) | 552 (186) | S** |
| Intraindividual reaction time variability | |||||
| Total | 0.18 (0.04) | 0.25 (0.08) | 0.24 (0.04) | 0.33 (0.07) | I** S** |
| Correct | 0.18 (0.04) | 0.24 (0.08) | 0.24 (0.04) | 0.32 (0.06) | I** S** |
| Error | 0.19 (0.08) | 0.31 (0.12) | 0.20 (0.12) | 0.36 (0.16) | S** |
| Response percentage, % | |||||
| Correct | 90.4 (3.6) | 83.1 (10.7) | 97.5 (1.2) | 94.5 (3.4) | I****S** |
| Error | 8.9 (3.6) | 11.1 (5.1) | 2.5 (1.2) | 4.8 (2.6) | I** S** |
| Omission | 0.7 (0.8) | 5.8 (10.5) | 0.0 (0.0) | 0.7 (1.0) | I#S# |
The data are depicted as the mean (SD). The intraindividual reaction time variability equals the SD of reaction time/mean reaction time. Two-factor (incentives [I] × sleep condition [S]) analyses of variances were applied to each behavior variable.
P < 0.10;
P < 0.01
With regard to the trial congruence effects in case of trials with correct responses, the responses to the incongruent stimuli were slower (F1,22 = 242, P < 0.001), more variable (F1,22 = 4.96, P = 0.036), and less accurate (F1,22 = 52.69, P < 0.001 for correct response rate; F1,22 = 51.53, P < 0.001 for error rate) than were the responses to the congruent stimuli. Neither incentives nor sleep conditions altered the effects of trial congruence on the mean and variability of the reaction time for the correct trials. However, as compared with the group that did not receive incentives, the group that received incentives showed reduced congruence effects on the correct response (F1,22 = 14.27, P = 0.001) and error rates (F1,22 = 12.96, P = 0.002). The effects of postconflict adjustment on the mean reaction time for the correct trials (F1,22 = 20.31, P < 0.001), correct response rate (F1,22 = 21.98, P < 0.001), and error rate (F1,22 = 26.72, P < 0.001) were significant. Neither incentives nor sleep conditions altered the effects of postconflict adjustment on the mean reaction time for the correct trials. As compared with the group that did not receive incentives, the group that received incentives showed reduced postconflict effects on the correct response (F1,22 = 4.92, P = 0.037) and error rates (F1,22 = 4.92, P = 0.037).
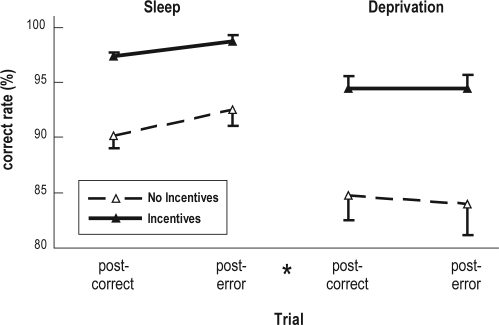
We used a priori 2-factor mixed-design analyses of variance to examine whether monetary incentives altered posterror behavior adjustments (task performance in the trial following the errors vs task performance in the trial following the correct responses) under the normal sleep condition. Significant posterror behavior adjustments were observed in the correct response (F1,22 = 7.02, P = 0.015) and error rates (F1,22 = 5.62, P = 0.027) but not in the omission rate (P = 0.112) or in the mean reaction time in the case of the correct trials (P = 0.608). Monetary incentives did not alter any posterror behavior adjustments. To further investigate the effect of the interaction between the incentives and sleep conditions on posterror behavior adjustments, we performed 3-factor analyses of variance and found that incentives did not directly affect or interact with the sleep conditions to alter posterror accuracy adjustments. However, sleep deprivation did impair posterror adjustments in the correct response (F1,22 = 7.44, P = 0.012; Figure 1) and error rates (F1,22 = 6.59, P = 0.018).
Figure 1.
Posterror accuracy adjustments. The error bars depict the SEM. The effect of the interaction between the trial types and sleep conditions was significant. *P < 0.05 vs the postcorrect trials under the normal sleep condition as evaluated by a posthoc Tukey test.
Electrophysiologic Data
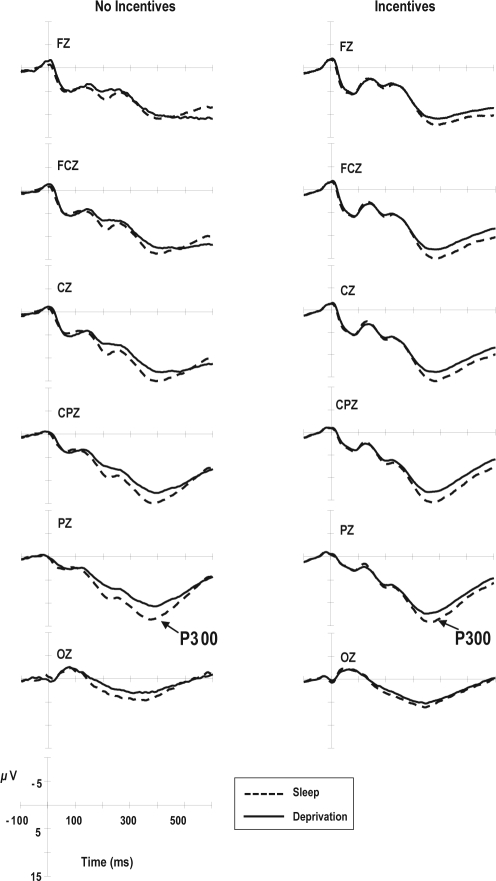
Figure 2 shows the average stimulus-locked ERP waveforms at channels Fz, FCz, Cz, CPz, Pz, and Oz for only the correct trials. As shown in Figure 2 and Table 5, subject-group differences in both the latency (P = 0.269) and the amplitude of P300 at Pz (P = 0.738) were not significant. Sleep deprivation did not alter the latency of P300 (P = 0.695) but did reduce its amplitude (F1,18 = 11.47, P = 0.003). The effect of the interaction between the subject groups and sleep conditions on the latency of P300 tended to be significant (F1,18 = 4.06, P = 0.059). Incentives reduced the mean P300 latency only under the sleep deprivation condition (P < 0.005, Tukey test).
Figure 2.
Averaged stimulus-locked event-related potential (ERP) waveforms for trials with correct responses. The P300 is a component of stimulus-locked ERP recorded at Pz.
Table 5.
Components of event-related potentials
| Variable | No incentives, n = 10 |
Incentives, n = 10 |
Significant effect | ||
|---|---|---|---|---|---|
| Sleep | Deprivation | Sleep | Deprivation | ||
| Latency, ms | |||||
| P300 | 375 (44) | 392 (62) | 376 (46) | 350 (31) | I×S# |
| ERN | 67 (17) | 70 (10) | 58 (14) | 70 (11) | S* |
| FRN for gain | NA | NA | 279 (19)a | 263 (22)a | |
| FRN for loss | NA | NA | 267 (30)a | 261 (27)a | |
| Amplitude, μV | |||||
| P300 | 15.0 (4.2) | 12.1 (4.9) | 15.1 (3.6) | 13.2 (3.8) | S* |
| ERN | −6.7 (3.4) | −0.23 (2.0) | −5.4 (3.6) | −6.5 (4.1) | I#S** I×S** |
| Pe | 7.8 (3.8) | 5.7 (3.2) | 8.1 (4.7) | 4.9 (3.9) | S** |
| FRN for gain | NA | NA | 3.4 (2.3)a | 3.4 (2.1)a | |
| FRN for loss | NA | NA | −4.5 (5.6)a | −5.0 (4.1)a | F** |
The data are depicted as the mean (SD). The P300 is a component of stimulus-locked event-related potentials (ERPs) recorded at Pz. The event-related negativity (ERN) and Pe are components of response-locked ERPs recorded at FCz and Cz, respectively. The feedback-related negativity (FRN) is a component of feedback-locked ERPs recorded at FCz. Two-factor (incentives [I] × sleep condition [S]) analysis of variance was applied to each stimulus-locked and response-locked ERP variable. The main effects of the feedback type (F, gain vs loss) and sleep condition were examined for each feedback-locked ERP variable. NA, conditions are not applicable.
n = 9;
P < 0.10;
P < 0.05;
P < 0.01
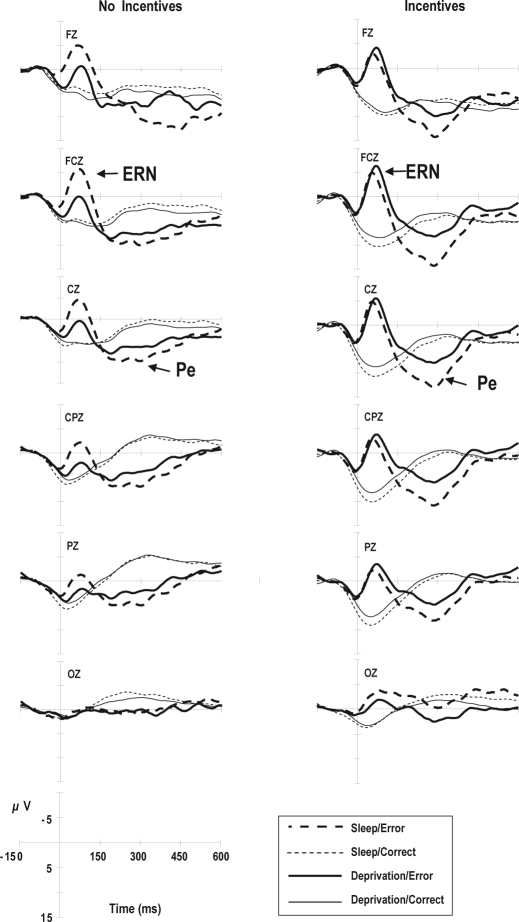
Figure 3 shows the average response-locked ERP waveforms. Two components of the response-locked ERPs, i.e., ERN at FCz and Pe at Cz, were examined for incentive and sleep effects. As shown in Figure 3 and Table 5, incentives only affected the ERN amplitude. In contrast, sleep deprivation increased the ERN latency (F1,18 = 5.98, P = 0.025) and decreased both the ERN (F1,18 = 11.33, P = 0.003) and Pe (F1,18 = 9.36, P = 0.007) amplitudes. The effect of the interaction between the subject groups and sleep conditions was significant in terms of the ERN amplitude (F1,18 = 22.59.06, P < 0.001). Sleep deprivation reduced the ERN amplitude (P < 0.01, Tukey test) in the group that did not receive incentives but did not affect the ERN amplitude in the group that received incentives (P > 0.05, Tukey test).
Figure 3.
Averaged response-locked event-related potential (ERP) waveforms for trials with correct and erroneous responses, respectively. The error-related negativity (ERN) is a component of response-locked ERPs recorded at FCz. Pe refers to error positivity.
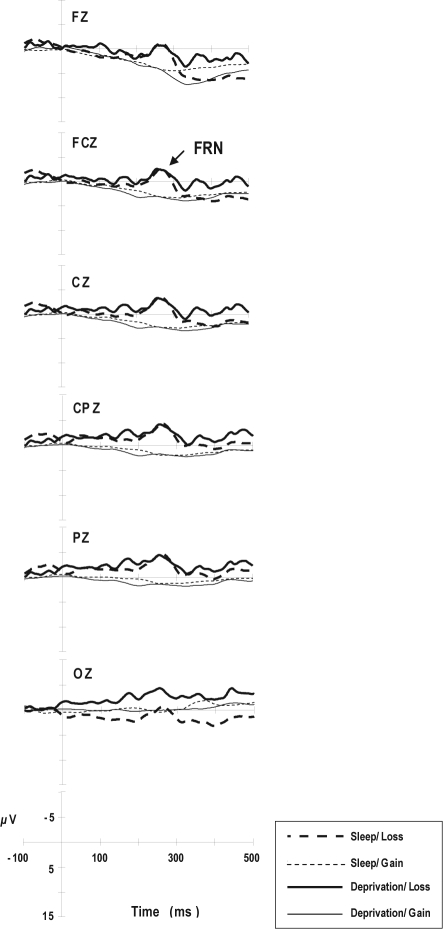
Figure 4 shows the average feedback-locked ERP waveforms. As shown in Figure 4 and Table 5, the FRN amplitude was much greater with loss feedback than with gain feedback (F1,8 = 102, P < 0.001). Sleep deprivation did not affect the FRN latency or amplitude.
Figure 4.
Averaged feedback-locked event-related potential (ERP) waveforms for loss and gain feedback, respectively. The feedback-related negativity (FRN) is a component of feedback-locked ERPs recorded at FCz.
DISCUSSION
The findings of this study are consistent with those of a previous study, which had used a flanker task and financial incentives,26 in that significant main effects of monetary incentives on the response accuracy and ERN amplitude were observed. It was noted that the group that received incentives under the sleep deprivation condition showed greater response accuracy than the group that did not receive incentives under the normal sleep condition (see Table 4). Furthermore, consistent with the findings of 2 previous studies that had used flanker tasks,10,11 sleep deprivation was shown to (1) increase the subjective sleepiness and reaction time variability; (2) reduce the response speed, response accuracy, and the P300, ERN, and Pe amplitudes; and (3) impair posterror accuracy adjustments. Thus, the experiment paradigm used in this study was valid in terms of the motivation and sleep effects on flanker task performance and ERP components. However, the results of the test for the effect of the interaction between monetary incentives and sleep deprivation failed to support the hypothesis that motivation incentives attenuate the negative effects of sleep deprivation on the performance of tasks requiring higher cognitive control processes.
The interaction effects between the incentives and sleep conditions were significant or showed a trend toward significance for 3 variables: the subjective measure of effort level, P300 latency, and ERN amplitude. The effects of sleep deprivation on the effort level and the ERN amplitude were significant in the group that did not receive incentives but were not significant in the group that received incentives. For P300 latency, the effects of sleep deprivation were not significant in the group that did not receive incentives; the effects of incentives were significant only under the sleep deprivation condition. Since effort and motivation are conceptually related,1 the loss of the effects of sleep deprivation on the effort level further supports the view that the offer of the incentives was strong enough to subjectively inhibit sleep deprivation-related reductions in motivation. Furthermore, consistent with several previous studies on motivation and brain activity,21–4 this study showed that monetary incentives completely removed the effects of sleep deprivation on the ERN amplitude and facilitated P300 onset under the sleep deprivation condition. Although monetary incentives compensated for some of the negative effects of sleep deprivation, such as error detection (ERN amplitude), and boosted the attention processes for stimulus categorization and evaluation (P300 latency),37,38 they could not alleviate sleep deprivation-induced attenuation of the accuracy of the response or compensate for sleep deprivation-induced impairment in the posterror accuracy adjustments. Thus, by comparing our results to those of Wilkinson's study,15 we arrived at the conclusion that the reduction of performance decrements induced by 1 night of sleep deprivation when motivation incentives were provided may be confined to vigilance task performance. Considering that monetary incentives effectively enhanced cognitive task performance under both normal sleep and sleep-deprivation conditions and reduced the effects of sleep deprivation on some attention and action-monitoring processes, but could not alter the effects of sleep deprivation on performance decrements, motivation incentives and sleep deprivation may act through both common and different mechanisms to affect cognitive performance.
On the other hand, this study also found that, in addition to the ERN amplitude, the FRN amplitude following sleep deprivation in the group that received monetary incentives was maintained at the same level under the normal sleep condition. The ERN observed after the performance errors and the FRN observed after negative feedback share some features in terms of the functional role in performance monitoring and neuronal sources of both, which are located in or near the anterior cingulate cortex.32,39–41 Several other theories have been developed regarding the functional significance of the ERN amplitude in addition to error detection (reviewed in27,29). Some propose that the ERN reflects the emotion/motivation significance of errors.41–45 The FRN amplitude is also correlated negatively with negative outcome expectancy.39,46,47 Thus, monetary incentives appeared to help maintain the emotion/motivation evaluation and expectancy on errors during sleep deprivation.
Although monetary incentives effectively maintained the error detection processes in terms of the ERN and FRN amplitudes under the sleep deprivation condition, they could not attenuate the reduction in the mean Pe amplitude and the impairment in the posterror accuracy adjustments following sleep deprivation. The neuronal source and functional significance of ERN and Pe have been shown to be different.27,40,41,48,49 Topographic mapping analyses using dipole source localization have shown the existence of intracranial generators in the anterior cingulate cortex and the more posterior cingulate regions that contribute to the ERN and Pe, respectively.40,41 The Pe has been claimed to be associated with a later aspect of error processing or posterror processing, including error awareness, emotional reaction to errors,49 and performance adjustments following an error (reviewed in27). Although the ERN amplitude has been shown to correlate positively with the probability of error correction and with the posterror slowing of the response speed,26 there are inconsistent findings showing no correlation between the ERN amplitude and posterror slowing.49,50 In contrast, several studies have consistently shown that the Pe amplitude correlates positively with post-error slowing,31,49 and the response speed that progressively improves over the test period.41 Very few studies on the post-error compensatory behavior have shown data on posterror accuracy; however, 1 study49 has shown a significant positive correlation between posterror slowing and post-error accuracy. The data from recent sleep deprivation studies and this study further support the relationship between the Pe amplitude and posterror behavior adjustments. Sleep deprivation results in reductions in the Pe amplitude concomitant with impairments in posterror slowing11 and posterror accuracy adjustments.10 Furthermore, when an explicit instruction to perform immediate error corrections is provided, subjects have been found to recover sleep deprivation-induced impairment in posterror accuracy adjustments concomitant with the maintenance of the Pe amplitude in similar manners between the normal sleep and sleep deprivation conditions.8,9 Thus, this study showing the dissociated effects of monetary incentives on sleep deprivation-induced impairment in error detection (ERN and FRN amplitudes) and posterror compensatory processes (Pe amplitude and posterror behavior adjustments) suggest that the ERN and Pe are involved in different processes of error monitoring.
Two other findings of this study warrant discussion. First, monetary incentives were unexpectedly found to be associated with more variable response speed. This could be related to a response strategy that focuses more on the accuracy of the response, which was presumed to have been used by the participants in the group that received incentives, because incentives were paid entirely on the basis of the response accuracy. Furthermore, in a recent study, a visual discrimination task was assigned and the probability of the reward set was manipulated for controlling reaction time variability; the study showed that, even though the median of reaction time distribution was maintained at the baseline level (no rewards were paid), the standard deviation of the reaction time distributions became reduced and the value returned to or remained higher than the baseline level if a low reward probability (0.35) and a high probability (0.8) were considered, respectively.51 In the present study, for the group that received incentives, the probability of a reward to each correct response was equivalent to 1.0; it thus appears reasonable to obtain a higher reaction time variability in the group that received incentives than in the group that did not receive incentives. Second, monetary incentives reduced the trial congruence and the effects of postconflict adjustments on response accuracy. This could be related to a ceiling limitation for the response accuracy because the correct rate for congruent trials in the group that received incentives was almost 100%.
In all, our data failed to support the hypothesis that motivation incentives could reduce the effects of sleep deprivation on performance decrements in tasks that require high-level cognitive control processes. Furthermore, we found that monetary incentives differentially compensate for the sleep deprivation-induced impairment in error detection but not for that in posterror accuracy adjustments. Thus, motivation incentives and sleep deprivation may act through both common and different mechanisms to affect error monitoring.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by the National Science Council of the Republic of China, Taiwan (NSC-94-2413-H-194-018).
REFERENCES
- 1.Oken BS, Salinsky MC, Elsas SM. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin Neurophysiol. 2006;117:1885–01. doi: 10.1016/j.clinph.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennings JR, Monk TH, van der Molen MW. Sleep deprivation influences some but not all processes of supervisory attention. Psychol Sci. 2003;14:473–9. doi: 10.1111/1467-9280.02456. [DOI] [PubMed] [Google Scholar]
- 3.Versace F, Cavallero C, De Min Tona G, Mozzato M, Stegagno L. Effects of sleep reduction on spatial attention. Biol Psychol. 2006;71:248–55. doi: 10.1016/j.biopsycho.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Humphrey DG, Kramer AF, Stanny RR. Influence of extended wakefulness on automatic and nonautomatic processing. Hum Factors. 1994;36:652–69. doi: 10.1177/001872089403600407. [DOI] [PubMed] [Google Scholar]
- 5.Morris AM, So Y, Lee KA, Lash AA, Becker CE. The P300 event-related potential. The effects of sleep deprivation. J Occup Med. 1992;34:1143–52. [PubMed] [Google Scholar]
- 6.Chee MW, Tan JC, Zheng H, et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci. 2008;28:5519–28. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horne JA. Why we sleep: The functions of sleep in humans and other mammals. Oxford: Oxford University Press, ; 1988. [Google Scholar]
- 8.Hsieh S, Cheng IC, Tsai LL. Immediate error correction process following sleep deprivation. J Sleep Res. 2007;16:137–47. doi: 10.1111/j.1365-2869.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh S, Tsai CY, Tsai LL. Error correction maintains posterror adjustments after one night of total sleep deprivation. J Sleep Res. 2009;18:159–66. doi: 10.1111/j.1365-2869.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsai LL, Young HY, Hsieh S, Lee CS. Impairment of error monitoring following sleep deprivation. Sleep. 2005;28:707–13. doi: 10.1093/sleep/28.6.707. [DOI] [PubMed] [Google Scholar]
- 11.Murphy TI, Richard M, Masaki H, Segalowitz SJ. The effect of sleepiness on performance monitoring: I know what I am doing, but do I care? J Sleep Res. 2006;15:15–21. doi: 10.1111/j.1365-2869.2006.00503.x. [DOI] [PubMed] [Google Scholar]
- 12.Scheffers MK, Humphrey DG, Stanny RR, Kramer AF, Coles MG. Error-related processing during a period of extended wakefulness. Psychophysiology. 1999;36:149–57. [PubMed] [Google Scholar]
- 13.Horne JA, Pettitt AN. High incentive effects on vigilance performance during 72 hours of total sleep deprivation. Acta Psychol. 1985;58:123–39. doi: 10.1016/0001-6918(85)90003-4. [DOI] [PubMed] [Google Scholar]
- 14.Steyvers FJ, Gaillard AW. The effects of sleep deprivation and incentives on human performance. Psychol Res. 1993;55:64–70. doi: 10.1007/BF00419894. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson RT. Interaction of lack of sleep with knowledge of results, repeated testing, and individual differences. J Exp Psychol. 1961;62:263–71. doi: 10.1037/h0048787. [DOI] [PubMed] [Google Scholar]
- 16.Hull JT, Wright KP, Jr, Czeisler CA. The influence of subjective alertness and motivation on human performance independent of circadian and homeostatic regulation. J Biol Rhythms. 2003;18:329–38. doi: 10.1177/0748730403253584. [DOI] [PubMed] [Google Scholar]
- 17.Engelmann JB, Damaraju E, Padmala S, Pessoa L. Combined effects of attention and motivation on visual task performance: Transient and sustained motivational effects. Front Hum Neurosci. 2009;3:4. doi: 10.3389/neuro.09.004.2009. doi: 10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelmann JB, Pessoa L. Motivation sharpens exogenous spatial attention. Emotion. 2007;7:668–74. doi: 10.1037/1528-3542.7.3.668. [DOI] [PubMed] [Google Scholar]
- 19.Small DM, Gitelman D, Simmons K, Bloise SM, Parrish T, Mesulam MM. Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cereb Cortex. 2005;15:1855–65. doi: 10.1093/cercor/bhi063. [DOI] [PubMed] [Google Scholar]
- 20.Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13:160–6. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boksem MA, Meijman TF, Lorist MM. Mental fatigue, motivation and action monitoring. Biol Psychol. 2006;72:123–32. doi: 10.1016/j.biopsycho.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Tecce JJ. Contingent negative variation (CNV) and psychological processes in man. Psychol Bull. 1972;77:73–108. doi: 10.1037/h0032177. [DOI] [PubMed] [Google Scholar]
- 23.Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent negative variation: An electric sign of sensorimotor association and expectancy in the human brain. Nature. 1964;203:380–4. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- 24.Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9:456–79. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophysiol. 1974;16:143–9. [Google Scholar]
- 26.Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–90. [Google Scholar]
- 27.Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- 28.Jocham G, Ullsperger M. Neuropharmacology of performance monitoring. Neurosci Biobehav Rev. 2009;33:48–60. doi: 10.1016/j.neubiorev.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clin Psychol Rev. 2008;28:1343–54. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 31.Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38:752–60. [PubMed] [Google Scholar]
- 32.Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: evidence for a generic neural system for error detection. J Cogn Neurosci. 1997;9:788–98. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- 33.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 34.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 35.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1998;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 37.Gaillard AW. Problems and paradigms in ERP research. Biol Psychol. 1988;26:91–109. doi: 10.1016/0301-0511(88)90015-4. [DOI] [PubMed] [Google Scholar]
- 38.Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: the P300 as a measure of stimulus evaluation time. Science. 1977;197:792–5. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- 39.Bellebaum C, Daum I. Learning-related changes in reward expectancy are reflected in the feedback-related negativity. Eur J Neurosci. 2008;27:1823–35. doi: 10.1111/j.1460-9568.2008.06138.x. [DOI] [PubMed] [Google Scholar]
- 40.Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- 41.Vocat R, Pourtois G, Vuilleumier P. Unavoidable errors: a spatio-temporal analysis of time-course and neural sources of evoked potentials associated with error processing in a speeded task. Neuropsychologia. 2008;46:2545–55. doi: 10.1016/j.neuropsychologia.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Ganushchak LY, Schiller NO. Motivation and semantic context affect brain error-monitoring activity: an event-related brain potentials study. Neuroimage. 2008;39:395–405. doi: 10.1016/j.neuroimage.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–60. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- 44.Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. J Exp Psychol Gen. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- 45.Pailing PE, Segalowitz SJ. The error-related negativity as a state and trait measure: motivation, personality, and ERPs in response to errors. Psychophysiology. 2004;41:84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- 46.Hajcak G, Moser JS, Holroyd CB, Simons RF. It's worse than you thought: the feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology. 2007;44:905–12. doi: 10.1111/j.1469-8986.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 47.Holroyd CB, Krigolson OE. Reward prediction error signals associated with a modified time estimation task. Psychophysiology. 2007;44:913–7. doi: 10.1111/j.1469-8986.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 48.Boksem MA, Tops M, Wester AE, Meijman TF, Lorist MM. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Res. 2006;1101:92–101. doi: 10.1016/j.brainres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Hajcak G, McDonald N, Simons RF. To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003;40:895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- 50.Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. J Neurosci. 2001;21:9430–7. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madelain L, Champrenaut L, Chauvin A. Control of sensorimotor variability by consequences. J Neurophysiol. 2007;98:2255–65. doi: 10.1152/jn.01286.2006. [DOI] [PubMed] [Google Scholar]