Abstract
Introduction:
Among the common problems related to aging is sleep quality; over half of older adults suffer from symptoms of insomnia. Age-related changes in circadian sleep/wake regulation constitute a major underlying factor. Constant and organized lifestyle may moderate the effects of circadian rhythm changes on sleep. Preliminary findings have linked daily regularity to sleep quality among healthy adults and in patients with Parkinson disease. The current study investigated the relationships between daily routines and sleep quality among community dwelling elderly.
Methods:
Ninety-six Israeli Russian-speaking elderly living in a retirement community (mean age 75 ±13.88, 72% women, 82% living alone) participated. Routines were assessed with the Scale of Older Adults Routine (SOAR) by a trained interviewer at 3 time points 2 weeks apart. A subsample (n = 33) completed the Social Rhythm Metric (SRM) 2-week diary. Sleep quality was evaluated using the Pittsburg Sleep Quality Index (PSQI). Daily Functional status was assessed with the Lawton Instrumental Activities of Daily Living (IADL).
Results:
Mean sleep efficiency was 78%, functional status was fairly good (mean IADL 45 of 50 [SD = 6.12]). Regression analyses indicated that increased stability in daily routine, as measured by the SOAR for the entire sample, predicted shorter sleep latency, higher sleep efficiency and improved sleep quality, beyond functional status, comorbidities, and age. Similar associations were found for the subsample using the SRM.
Conclusion:
Maintenance of daily routines is associated with a reduced rate of insomnia in the elderly. Further studies should examine these relations in broader populations with regard to health, functional status, and cultural background.
Citation:
Zisberg A; Gur-Yaish N; Shochat T. Contribution of routine to sleep quality in community elderly. SLEEP 2010;33(4):509-514.
Keywords: Subjective sleep quality, daily routines, life style regularity, older adults
COMPLAINTS OF SLEEP DISTURBANCE ARE COMMON IN OLDER ADULTS, WITH PREVALENCE RATES OF OVER 50% IN THE COMMUNITY, AND 70% IN THE assisted living setting.1,2 Some of the consequences of poor sleep quality in the elderly include cognitive decline,3,4 increased risk of falls,5 daytime fatigue,6 and reduced physical and mental health and health-related quality of life status.1,7
Factors that are associated with poor sleep in the elderly include physical and mental comorbidity, polypharmacy, functional status, primary sleep disorders, and changes in circadian sleep-wake patterns.8 Circadian rhythm changes which underlie common sleep complaints in older adults include an advanced sleep phase, reduced sleep consolidation and duration and early morning awakenings.9,10
Although light exposure has been demonstrated as the most powerful zeitgeber (time-cue) contributing to human entrainment to the 24-h day,11 there is evidence suggesting that social and activity rhythms also contribute to circadian entrainment in humans.12 To capture and to quantify such social and behavioral rhythms in daily life behaviors, Monk et al13 developed and validated the Social Rhythm Metric (SRM). The SRM is a 2-week diary in which subjects prospectively record the timing of a list of various daily routine events, such getting out of bed and making first daily contact with another person. Based on these reports, an average regularity score is computed, which represents personal level of daily routinization, with increasing routinization indicating a more stable lifestyle rhythm. Using this tool, they discovered that lifestyle rhythm stabilizes with age.14 Furthermore, increased lifestyle regularity was associated with fewer sleep problems15 and with a tendency towards morning types.16
Social and activity rhythms may be viewed as part of the daily life routine. Routine is a concept pertaining to strategically designed behavioral patterns used to organize and coordinate activities along the axes of time, duration, social and physical contexts, and order.17 Young18 suggested 4 functional advantages of routines and habits, including skill and performance enhancement and energy and resource conservation.
Maintenance of routine may be particularly adaptive in older individuals. Thus, routine has been associated with lower disruptive behavior and higher functional status in Alzheimer patients.19 In various studies with community dwelling older adults, researchers have found that daily routine facilitates functional status and well-being.20,21 Clark et al.22,23 showed improvement in functional, mental and physical health status, life satisfaction, social functioning, body pain, and emotional problems following a routine activity enhancement intervention among older adults.
As changes in the circadian system are considered a hallmark of aging, and are implicated as underlying factors of reduced sleep quality in the elderly, it is plausible to assume that routine lifestyle rhythms may serve as a protective mechanism contributing to the maintenance of high quality of sleep. However, in a comparison of sleep quality and daily social rhythms measured by the SRM in young and older adults, healthy 70-year-old individuals exhibited poor sleep compared to younger counterparts in their twenties, despite significantly greater lifestyle regularity in the older adults.24 It is yet to be determined whether differences in lifestyle regularity predict sleep quality within the elderly population.
Routine lifestyle rhythms may be characterized by stability in the timing, the frequency and the duration of performed activities, such as the daily timing, the number of times per day and the duration of time spent eating meals, watching television, or reading a book. Moreover, other than daily regularity, it is possible to identify weekly patterns of regularity, for example, shopping, cleaning, exercise, and social engagements. Previously, Monk et al15 have assessed the relationships between the timing of daily activities and measures of sleep quality. To date, the role of routine, as a concept related to a wide range of activities measured by their frequency and duration, has not been investigated in relation to sleep quality.
In the current prospective study, we investigated the relationship between routine and sleep quality in the elderly, using multiple indicators of routine, and controlling for risk factors for poor sleep quality. We hypothesize, that in older adults residing in a retirement community, daily routine is associated with good sleep quality, beyond functional status, comorbidities, and age.
METHODS
Participants
Ninety-six older adults living in a retirement community constituting primarily of Russian speakers participated in the study. Each apartment was fully equipped as an independent functional unit including a kitchenette. The retirement community association supplied maintenance and organized social events on occasion; however, there was no communal dining area and meals were not provided. Participants' mean age was 74.89 (SD = 13.88, range 58 to 89); 72% were female, and 82% lived alone. Participants had on average 13.9 years of education (SD = 4.2); 67% reported that their economic status was similar to other people their age; and 75% reported fair or good health. Mean IADL score was 45 (SD = 6.12) and mean Charlson's Comorbidity Index30 was 1.93 (SD = 2.23), indicating a relatively low comorbidity burden. Thirty-four percent had cardiovascular disease, 15% had lung disease, 19% had diabetes, 15% had cancer, and 6% had depression. Sleep medication was used less than once a week in 5% of the sample, between once to twice a week in 7% of the sample, and ≥ 3 times/week in 23% of the sample. Other participants (65%) reported not using any type of prescription or nonprescription sleep medication.
MEASURES
All questionnaires were translated from the original English versions to Russian, and then back-translated to English by 2 independent native Russian speakers with a background in Health Sciences. Items which were phrased differently in the back-translation were discussed in a focus group of 8 Russian speakers proficient in the English language. The final versions were approved by the primary author (A.Z.), who is fluent in both English and Russian.
Dependent Variables
The Pittsburgh Sleep Quality Index
The Pittsburgh Sleep Quality Index (PSQI)25 was used for the subjective assessment of sleep quality. The PSQI is a questionnaire consisting of 19 items which are coded on a 4-point scale (0-3) to obtain 7 subcategories, including sleep duration, sleep disturbances, sleep latency, daytime dysfunction, sleep efficiency, sleep quality and medication use. The sum of all subscores represents the total sleep quality score, ranging between 0–21, with higher scores representing lower sleep quality. A cutoff score of > 5 is used, with scores exceeding threshold indicating poor sleep quality. Respondents are asked to rate their sleep reflecting on the past month. Psychometric properties have demonstrated good reliability (internal consistency: 0.89; test retest reliability: 0.85) and good construct validity for the English language version.25 The PSQI is a widely used tool in research studies and clinical trials, and has been translated to several languages including German, Spanish, Chinese, and Hebrew, with comparable reliability and validity values.26 Internal consistency in the present study was α = 0.69. When excluding the medication use subscale due to a low rate of medication users in this sample, internal consistency increased to α = 0.76. Outcome measures included the total PSQI score as well as self report of sleep latency (SL) (question 2: how long (in minutes) has it usually taken you to fall asleep each night?), and sleep efficiency percentage (SE), computed as the ratio between the hours of actual sleep (question 4: how many hours of actual sleep did you get at night?) and total time in bed (hours computed based on reported bedtime in question 1: what time have you usually gone to bed at night? and reported wake-time in question 3: what time have you usually gotten up in the morning?), multiplied by 100.
Independent Variables
Routine
Routine was assessed for the entire sample using subscales of the Scale of Older Adults' Routine (SOAR),27 and for a subsample with the Social Rhythm Metric (SRM).13
SOAR:
SOAR is a measure of stability in activities on a daily and weekly basis. The instrument includes 3 consecutive assessments of 42 routine activities covering 5 domains of activity (basic, instrumental, leisure, social, and rest) and measured on 4 dimensions (frequency, timing, duration, and sequence). In the present study, we used a modified version of the SOAR, focusing only on the basic (8 items) and instrumental (12 items) domains, and on the frequency and duration dimensions. For each item, participants reported the frequency the activity was performed either daily or weekly (depending on the type of activity and on individual habits). The frequency of activities performed on a weekly basis was divided by 7 to provide a score of frequency on a daily basis. In addition, participants reported the amount of time (duration) spent performing each activity. For example, for some participants, bathing was performed twice a day, 5 minutes in the morning and half an hour in the evening; while others reported taking a bath twice a week for half an hour each. Scores are calculated as the standard deviation across the 3 time points for each activity for frequency and duration, and then summarized to obtain basic and instrumental frequency and duration scores, with lower scores representing more regularity. In a previous study, test-retest reliability indices ranged from 0.56 to 0.90 for the dimensions; and validity tests were moderate to good with the trait of routinization.27 Interclass correlations in the current study were r = 0.92 and r = 0.88 for the frequency of basic and instrumental activities respectively, and r = 0.95 and r = 0.86 for the duration of basic and instrumental activities respectively. For a complete description of the measure see Zisberg et al.27
SRM:
The SRM13 quantifies an individual's typical daily social rhythm patterns. This self-report instrument records the timing of specific activities and events that occur on a regular daily basis, quantifying the stability of an individual's daily routine. Scores represent level of regularity ranging from 0: least regular to 7: most regular. In the current study we used the short 5-item SRM that is highly correlated with the original 17-item instrument (r > 0.80).28 SRM stability measured by the correlation between week 1 and week 2 was r = 0.67, and the mean score was remarkably similar to that obtained on a comparable sample of healthy older adults by Monk and colleagues (4.75 and 4.40, respectively).24
Control Variables
Functional status
Functional status was measured with the Modified Lawton scale of Instrumental Activities of Daily Living (IADL).29 The IADL 10-item scale measures performance of certain instrumental tasks and the amount of assistance needed to perform each of the activities (shopping, housekeeping, etc.). The measure has demonstrated high reliability and validity through many years of research.
Comorbidities
Comorbidities were assessed using Charlson's Comorbidity Index,30 an index weighing the number and severity of health conditions among patients. The index relies on information from respondent's reports assessing 20 health conditions, each carrying a weighted score ranging from 1-6. Predictive validity of the index was shown to be high using criteria such as likelihood of death and correlations with other established predictive systems and measures.
PROCEDURE
The current study was approved by the University of Haifa IRB committee. It was conducted in 2 retirement communities in the northern part of Israel between August 2007 and September 2008. Fliers were handed out in the facility, and people who were interested enrolled in the managerial office. Interviews were conducted on site, usually in the participants' apartments by a trained interviewer, who was a registered nurse. Participants met with the interviewer 3 times at their convenience, either in the morning or the evening hours, each meeting 2 weeks apart. Demographic, functional, and comorbidities assessments were administrated during the first meeting. The SOAR was completed during each of the 3 meetings. The PSQI was administrated during the last meeting.
During the second meeting, participants were asked whether they agreed to fill in the 2-week SRM diary. Thirty-five agreed; 2 dropped out within the first few days, thus leaving 33 who completed the diary. To facilitate the diary completion process, phone call interviews were performed by the interviewer every evening for the entire 2-week period. The subsample that completed the SRM was not significantly different from the whole sample in all the characteristics mentioned above besides age; the subsample was younger with a mean age of 69 (SD = 6.9, t94 = 6.49, P < 0.001).
DATA ANALYSIS
Descriptive analyses were performed to assure the assumption of normality. Participants who did not complete all three interviews were excluded from further analyses, and demographic characteristics were compared between those with complete data versus those with missing data to preclude the possibility of bias.
Pearson correlations were performed to assess relationships between all independent and control variables with the sleep dependant variables, using Bonferroni correction for multiple correlations. To assess significant predictors of sleep quality for the entire sample for each of the 3 dependent variables (SE, SL, and PSQI total score), block multiple regression analyses were performed, using only those predictive variables that correlated significantly with the dependent variables. Analyses were performed separately for SOAR Basic and SOAR Instrumental domains. The first block in both models included the control variables IADL, comorbidity, and age. The second block included the independent variables SOAR Basic scores in the first model, and SOAR Instrumental scores in the second model. For each block, the explained variance (R2) and the standardized slope of each variable (β) were presented. For the second blocks the added explained variance (R2 change) was also presented. For the subsample that completed the SRM, partial correlations were performed between SRM and SE, SL, and PSQI total score, controlling for IADL, comorbidity, and age. All statistical analyses were performed with the SPSS 15 software package.
RESULTS
Eighty-nine of 96 participants completed all 3 time points and were included in the final analyses. The 7 participants who did not complete the study dropped out for the following reasons: 2 temporarily left the retirement community to assist caring for a newborn grandchild, one travelled overseas due to the death of a brother, one was hosting family members visiting from abroad, one was hospitalized after a fall and fracture, and 2 opted to dropout as they were displeased with the questionnaires and interviews. Of all sociodemographic variables assessed, only IADL was significantly different between those who did not complete the study (mean 48.38, SD = 1.85) and those who completed the study (mean 44.91, SD = 6.14), indicating higher functional status for those who dropped out (P < 0.05).
Descriptive statistics including means and standard deviations of all relevant variables are presented in Table 1. Mean PSQI total score was 9.54 (SD = 4.14); mean total sleep time was 6.00 h (SD = 1.06); mean SE percentage was 77.90% (SD = 13.64), and mean SL was 37.53 min (SD = 39.35). Based on the SOAR, mean standard deviation for duration of basic activities was 14.92 min (SD = 8.45), and for duration of instrumental activities was 41.37 min (SD = 36.13). Mean standard deviation for frequency of basic activities was 0.23 (SD = 0.15) and for frequency of instrumental activities was 0.12 (SD = 0.09). Mean SRM score was 4.75 (SD = 0.93).
Table 1.
Descriptives of dependent and independent variables in the study (n = 89 unless otherwise stated)
| Mean | StD | Minimum | Maximum | |
|---|---|---|---|---|
| Age | 74.89 | 13.88 | 58.00 | 89.00 |
| Comorbidities | 1.96 | 2.27 | 0.00 | 10.00 |
| IADL Score | 45.00 | 6.13 | 29 | 50 |
| PSQI (total) | 9.37 | 4.04 | 1.00 | 18.00 |
| SE (%) | 78.16 | 13.68 | 44.44 | 100.00 |
| SL (min) | 35.94 | 38.23 | 5.00 | 210.00 |
| TST | 5.99 | 1.08 | 4.00 | 9.00 |
| SOAR Frequencies Basic | 0.23 | 0.15 | 0.00 | 0.61 |
| SOAR Frequencies Instrumental | 0.13 | 0.12 | 0.00 | 0.42 |
| SOAR Duration Basic (min) | 15.06 | 8.39 | 0.00 | 49.60 |
| SOAR Duration Instrumental (min) | 37.29 | 35.41 | 0.00 | 139.18 |
| SRM (n = 33) | 4.75 | 0.93 | 3.00 | 6.20 |
IADL, Instrumental Activities of Daily Living; PSQI, Pittsburgh Sleep Quality Index; SE, sleep efficiency; SL, sleep latency; TST, total sleep time; SOAR, Scale of Older Adults Routine; SRM, Social Rhythm Metrics
Correlations of all independent and control variables with dependant sleep variables are presented in Table 2. SL was significantly correlated with duration of basic activities (r = 0.41), duration of instrumental activities (r = 0.33), and SRM (r = −0.54). Shorter SL was associated with a more stable duration of basic and instrumental activities and with more stable lifestyle regularity. SE was significantly correlated with duration of basic activities (r = −0.39), duration of instrumental activities (r = −0.33), and SRM (r = 0.76). Higher SE was associated with a more stable duration of basic and instrumental activities and with more stable lifestyle regularity. PSQI total score was significantly related to SRM (r = −0.74) and comorbidities (r = 0.32). Poorer sleep quality was associated with less lifestyle regularity and more comorbidities. All correlations were significant at P ≤ 0.002, after Bonferroni correction.
Table 2.
Pearson correlations between all variables in the study
| PSQI Total | Sleep Efficiency | Sleep Latency | |
|---|---|---|---|
| SOAR Basic Duration | 0.24 | −0.39* | 0.41* |
| SOAR Instrumental Duration | 0.207 | −0.334* | 0.330* |
| SOAR Basic Frequency | −0.159 | 0.028 | 0.123 |
| SOAR Instrumental Frequency | 0.009 | −0.090 | 0.217 |
| SRM | −0.740* | 0.763* | −0.540* |
| Age | 0.149 | −0.015 | −0.108 |
| IADL | −0.256 | 0.051 | 0.046 |
| Comorbidities | 0.316* | −0.277 | 0.217 |
P ≤ 0.002 with Bonferroni correction
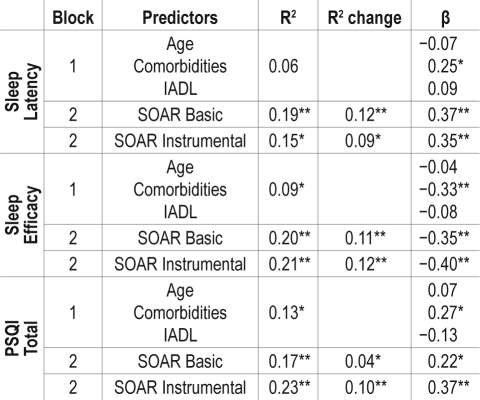
Results of regression analyses are presented in Table 3. Only the duration dimension of the SOAR was entered into the models. For SL, control variables (block 1) accounted for 6% of the variance (F3,88 = 1.93, P = 0.31); SOAR Basic (block 2) added an additional 12% (together 19%) of the variance in the first model (F4,88 = 4.83, P < 0.001), and SOAR Instrumental added an additional 9% (together 15%) of the variance in the second model (F4,88 = 3.80, P = 0.007). For SE, control variables (block 1) accounted for 9% of the variance (F3,88 = 2.90, P = 0.04); SOAR Basic (block 2) added an additional 11% (together 20%) of the variance in the first model (F4,88 = 5.37, P = 0.001), and SOAR Instrumental added an additional 12% (together 21%) of the variance in the second model (F4,88 = 5.48, P = 0.001). For total PSQI, control variables (block 1) accounted for 13% of the variance (F3,88 = 4.20, P = 0.008); SOAR Basic (block 2) added an additional 4% (together 17%) of the variance in the first model (F4,88 = 4.41, P = 0.003), and SOAR Instrumental added an additional 10% (together 23%) of the variance in the second model (F4,88 = 6.34, P < 0.001).
Table 3.
Sleep indicators by routine maintenance in basic and instrumental activities controlling for age, comorbidities and functional status (N = 89)
Correlation is significant at the 0.05 level (2-tailed)
Correlation is significant at the 0.01 level (2-tailed)
Partial correlations controlling for age, comorbidity and functional status were performed between the SRM and sleep variables. Increased lifestyle regularity was associated with shorter SL (r = −0.43, P < 0.05); higher SE (r = 0.74, P < 0.001); and lower PSQI total score (r = −0.67, P < 0.001).
DISCUSSION
Our findings support strong associations between daily routine, as measured by two instruments, and subjective sleep quality. Routine of both basic and instrumental daily activities based on the SOAR instrument, as well as increased stability in daily routine based on the SRM, were related to positive sleep outcomes based on subjective estimates of sleep latency, sleep efficiency, and overall sleep quality. Both instruments were associated with sleep quality beyond the effects of age, comorbidity, and functional status, which are well-known contributing factors to sleep quality.3,31
Based on the SOAR, stability in basic activities was more strongly associated with sleep quality than stability of instrumental activities. One possible explanation for these findings may be that basic activities refer to habitual physiological activities, whereas instrumental activities are more socially oriented. It is no wonder that basic activities such as bathing, dressing, and eating are more closely linked to sleep quality than activities such as shopping, public transportation use, and medical appointments. Whereas the former represent activities that apply to basic individual needs, the latter depend not only on the individual's routines, but also on other people's schedules and priorities. Furthermore, several basic activities are often part of the individual's sleep ritual, particularly those occurring in the evening hours.
These findings have important implications for community-dwelling older adults. Interventions aimed at improving sleep quality and associated health outcomes in community elderly have implemented structured social and physical activities on a regular daily or weekly basis.32–35 Given the strong associations between routine and sleep quality in the present study, it may be assumed that the structure and regularity of these interventions serve as underlying mechanisms promoting enhanced sleep quality.
Findings may also be relevant for elderly individuals experiencing stressful life events related to institutionalization. Findings from studies on patients residing in nursing homes and in short-term rehabilitation settings indicate that sleep and circadian rhythm disruptions are associated with functional impairment.36,37 Furthermore, these investigators have demonstrated the efficacy of interventions designed to enhance daily activities and routines in order to improve sleep/wake patterns in institutionalized patients.38,39 Future investigations may assess the effectiveness of these and other routine based interventions in enhancing functional status and clinical health profile.
This study may serve to increase awareness regarding the importance of the maintenance of basic routines in older adults. In fact, current nursing home evaluations in the United States use the Minimum Data Set,40 which includes several items related to basic routine activities. Our findings increase the importance of assessing such information, and highlight the usefulness of implementing this knowledge more suitably to tailoring towards individual's daily activities and lifestyle.
Results from the subsample that completed a 2-week SRM diary further validate the relationship between routine and sleep quality. Greater regularity of activities was strongly related to higher sleep quality. It is noteworthy that we obtained somewhat higher correlations between the SRM and the overall sleep quality score compared to a younger sample (mean age around 30) in a study conducted by Monk et al,15 even after controlling for age, comorbidity, and functional status. This difference is likely attributed to increased variation in sleep disturbances in older adults; an assumption supported by the increased variance in total PSQI scores in the current study compared to the study by Monk and colleagues. Based on the observed differences between these two studies, it is possible to hypothesize that lifestyle regularity is a more important factor in the sleep quality of older adults than younger adults.
The study should be replicated on a larger more representative sample of older community dwelling adults of more diverse ethnic backgrounds and varied living arrangements. Furthermore, these findings may indicate associations, but cannot assume a causal link between routine lifestyle and sleep quality because of the cross-sectional nature of the study. Future longitudinal studies may assess whether lifestyle regularity constitutes a cause or a consequence of quality sleep patterns.
Two possible limitations in this study are the small number of participants who completed the SRM, and the use of the short SRM version. Only 33 participants agreed to take part in an additional, labor-intensive 2-week diary arm of the study. However, the present study obtained a similar mean SRM score as that obtained in a study of a comparable age group.24 Also, we have noted that when comparing the demographic characteristics of this subsample with the entire sample, only age was found to be significantly different. Not surprisingly, the subsample was younger; nevertheless, associations between SRM and sleep variables were robust well beyond the effects of age and other control variables.
As for the use of the short SRM version, it is noteworthy that two out of five items refer to activities that reflect sleep hygiene principles, i.e., time out of bed and time going to bed. As sleep hygiene principles have been shown to improve sleep quality,41 it is not surprising that high correlations were obtained between the SRM and measures of overall sleep quality. Nevertheless, in a previous investigation, similar relationships were found between sleep quality based on the PSQI and both the short and long versions of the SRM,15 supporting the validity of the short version.
An additional study limitation was that interviews were conducted in the Russian language, and all translated questionnaires and diaries used in this study have not previously undergone formal psychometric validation. Nevertheless, we have reported acceptable psychometric properties for the present sample. Furthermore, it may be argued, that the SOAR activity domains (ADL, IADL) are widely used in elderly populations worldwide, and that the dimensions of frequency and duration may be considered both basic and universal. Thus it is unlikely that cultural factors significantly alter the psychometric properties of the original versions. In summary, our findings suggest that maintenance of daily routines in community dwelling older adults may be associated with a reduced rate of insomnia, beyond other health factors such as comorbidity and functional status. These finding bear important implications for preserving lifestyle regularity as a means for maintaining good sleep quality. Further studies should examine these relations in broader populations with regard to cultural background and living arrangements
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by grants from Center of Absorption for scientists in Israel and The Research Authority of the University of Haifa.
Footnotes
A commentary on this paper appears in this issue on page 421.
REFERENCES
- 1.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 2.Rao V, Spiro JR, Samus QM, et al. Sleep disturbances in the elderly residing in assisted living: findings from the Maryland Assisted Living Study. Int J Geriatr Psychiatry. 2005;20:956–66. doi: 10.1002/gps.1380. [DOI] [PubMed] [Google Scholar]
- 3.Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. II. Sleep. 1999;22:354–8. [PubMed] [Google Scholar]
- 4.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 5.Stone KL, Ancoli-Israel S, Blackwell T, et al. Actigraphy-measured sleep characteristics and risk of falls in older women. Arch Intern Med. 2008;168:1768–75. doi: 10.1001/archinte.168.16.1768. [DOI] [PubMed] [Google Scholar]
- 6.Goldman SE, Ancoli-Israel S, Boudreau R, et al. Sleep problems and associated daytime fatigue in community-dwelling older individuals. J Gerontol A Biol Sci Med Sci. 2008;63:1069–75. doi: 10.1093/gerona/63.10.1069. [DOI] [PubMed] [Google Scholar]
- 7.Reid KJ, Martinovich Z, Finkel S, et al. Sleep: a marker of physical and mental health in the elderly. Am J Geriatr Psychiatry. 2006;14:860–6. doi: 10.1097/01.JGP.0000206164.56404.ba. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Cooke JR. Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. J Am Geriatr Soc. 2005;53:S264–71. doi: 10.1111/j.1532-5415.2005.53392.x. [DOI] [PubMed] [Google Scholar]
- 9.Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol Regul Integr Comp Physiol. 1998;275:1478–87. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 10.Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol Int. 2000;17:285–311. doi: 10.1081/cbi-100101049. [DOI] [PubMed] [Google Scholar]
- 11.Duffy JF, Kronauer RE, Czeisler CA. Phase-shifting human circadian rhythms: influence of sleep timing, social contact and light exposure. J Physiol (Lond ) 1996;495(Pt 1):289–97. doi: 10.1113/jphysiol.1996.sp021593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mistlberger RE, Skene DJ. Social influences on mammalian circadian rhythms: animal and human studies. Biol Rev Camb Philos Soc. 2004;79:533–56. doi: 10.1017/s1464793103006353. [DOI] [PubMed] [Google Scholar]
- 13.Monk TH, Flaherty JF, Frank E, Hoskinson K, Kupfer DJ. The social rhythm metric an instrument to quantify the daily rhythms of life. J Nerv Ment Dis. 1990;178:120–6. doi: 10.1097/00005053-199002000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Monk TH, Reynolds CF, Kupfer DJ, Hoch CC, Carrier J, Houck PR. Differences over the life span in daily life-style regularity. Chronobiol Int. 1997;14:295–306. doi: 10.3109/07420529709001421. [DOI] [PubMed] [Google Scholar]
- 15.Monk TH, Reynolds CF, Buysse DJ, DeGrazia JM, Kupfer DJ. The relationship between lifestyle regularity and subjective sleep quality. Chronobiol Int. 2003;20:97–107. doi: 10.1081/cbi-120017812. [DOI] [PubMed] [Google Scholar]
- 16.Monk TH, Buysse DJ, Potts JM, DeGrazia JM, Kupfer DJ. Morningness-eveningness and lifestyle regularity. Chronobiol Int. 2004;21:435–43. doi: 10.1081/cbi-120038614. [DOI] [PubMed] [Google Scholar]
- 17.Zisberg A, Young HM, Schepp K, Zysberg L. A concept analysis of routine: relevance to nursing. J Adv Nurs. 2007;57:442–53. doi: 10.1111/j.1365-2648.2007.04103.x. [DOI] [PubMed] [Google Scholar]
- 18.Young M. The metronomic society. Harvard University Press: Natural rhythms and human timetables; 1988. [Google Scholar]
- 19.Baum CM. The contribution of occupation to function in persons with Alzheimer's disease. J Occup Sci. 1995;2:59–67. [Google Scholar]
- 20.Foldvari M, Clark M, Laviolette LC, et al. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55:192–9. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig FM. How routine facilitates wellbeing in older women. Occup Ther Int. 1997;4(213–):228. [Google Scholar]
- 22.Clark F, Azen SP, Zemke R, et al. Occupational therapy for independent-living older adults. A randomized controlled trial. JAMA. 1997;278:1321–6. [PubMed] [Google Scholar]
- 23.Clark F, Azen SP, Carlson M, et al. Embedding health-promoting changes into the daily lives of independent-living older adults long-term follow-up of occupational therapy intervention. J Gerontol B Psychol Sci Soc Sci. 2001;56:60–3. doi: 10.1093/geronb/56.1.p60. [DOI] [PubMed] [Google Scholar]
- 24.Monk TH, Reynolds CF, 3rd, Machen MA, Kupfer DJ. Daily social rhythms in the elderly and their relation to objectively recorded sleep. Sleep. 1992;15:322–9. doi: 10.1093/sleep/15.4.322. [DOI] [PubMed] [Google Scholar]
- 25.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Shochat T, Tzischinsky O, Oksenberg A, Peled R. Validation of the Pittsburgh Sleep Quality Index Hebrew translation (PSQI-H) in a sleep clinic sample. Isr Med Assoc J. 2007;9:853–6. [PubMed] [Google Scholar]
- 27.Zisberg A, Young HM, Schepp K. Development and psychometric testing of the Scale of Older Adults' Routine. J Adv Nurs. 2009;65:672–83. doi: 10.1111/j.1365-2648.2008.04901.x. [DOI] [PubMed] [Google Scholar]
- 28.Monk TH, Frank E, Potts JM, Kupfer DJ. A simple way to measure daily lifestyle regularity. J Sleep Res. 2002;11:183–90. doi: 10.1046/j.1365-2869.2002.00300.x. [DOI] [PubMed] [Google Scholar]
- 29.Lawton MP. The functional assessment of elderly people. J Am Geriatr Soc. 1971;19:465–81. doi: 10.1111/j.1532-5415.1971.tb01206.x. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Ancoli-Israel S, Ayalon L, Salzman C. Sleep in the elderly: normal variations and common sleep disorders. Harv Rev Psychiatry. 2008;16:279–86. doi: 10.1080/10673220802432210. [DOI] [PubMed] [Google Scholar]
- 32.Li F, Fisher KJ, Harmer P, Irbe D, Tearse RG, Weimer C. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults: a randomized controlled trial. J Am Geriatr Soc. 2004;52:892–900. doi: 10.1111/j.1532-5415.2004.52255.x. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka H, Shirakawa S. Sleep health, lifestyle and mental health in the Japanese elderly Ensuring sleep to promote a healthy brain and mind. J Psychosom Res. 2004;56:465–77. doi: 10.1016/j.jpsychores.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Benloucif S, Orbeta L, Ortiz R, et al. Morning or evening activity improves neuropsychological performance and subjective sleep quality in older adults. Sleep. 2004;27:1542–51. doi: 10.1093/sleep/27.8.1542. [DOI] [PubMed] [Google Scholar]
- 35.Chen KM, Chen MH, Chao HC, Hung HM, Lin HS, Li CH. Sleep quality, depression state, and health status of older adults after silver yoga exercises: Cluster randomized trial. Int J Nurs Stud. 2009;46:154–63. doi: 10.1016/j.ijnurstu.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Alessi CA, Martin JL, Webber AP, et al. More daytime sleeping predicts less functional recovery among older people undergoing inpatient post-acute rehabilitation. Sleep. 2008;31:1291. [PMC free article] [PubMed] [Google Scholar]
- 37.Martin JL, Webber AP, Alam T, Harker JO, Josephson KR, Alessi CA. Daytime sleeping, sleep disturbance, and circadian rhythms in the nursing home. Am J Geriatr Pharmacother. 2006;14:121–9. doi: 10.1097/01.JGP.0000192483.35555.a3. [DOI] [PubMed] [Google Scholar]
- 38.Martin JL, Marler MR, Harker JO, Josephson KR, Alessi CA. A multicomponent nonpharmacological intervention improves activity rhythms among nursing home residents with disrupted sleep/wake patterns. J Gerontol A Biol Sci Med Sci. 2007;62:67. doi: 10.1093/gerona/62.1.67. [DOI] [PubMed] [Google Scholar]
- 39.Alessi CA, Martin JL, Webber AP, Cynthia Kim E, Harker JO, Josephson KR. Randomized, controlled trial of a nonpharmacological intervention to improve abnormal sleep/wake patterns in nursing home residents. J Am Geriatr Soc. 2005;53:803–10. doi: 10.1111/j.1532-5415.2005.53251.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang NJ, Paek SC, Wan TT. Reliability estimates of clinical measures between minimum data set and online survey certification and reporting data of US nursing homes. Med Care. 2009;47:492–5. doi: 10.1097/mlr.0b013e31818c014b. [DOI] [PubMed] [Google Scholar]
- 41.Hoch CC, Reynolds CF, 3rd, Buysse DJ, et al. Protecting sleep quality in later life a pilot study of bed restriction and sleep hygiene. J Gerontol B Psychol Sci Soc Sci. 2001;56:52–9. doi: 10.1093/geronb/56.1.p52. [DOI] [PubMed] [Google Scholar]