Abstract
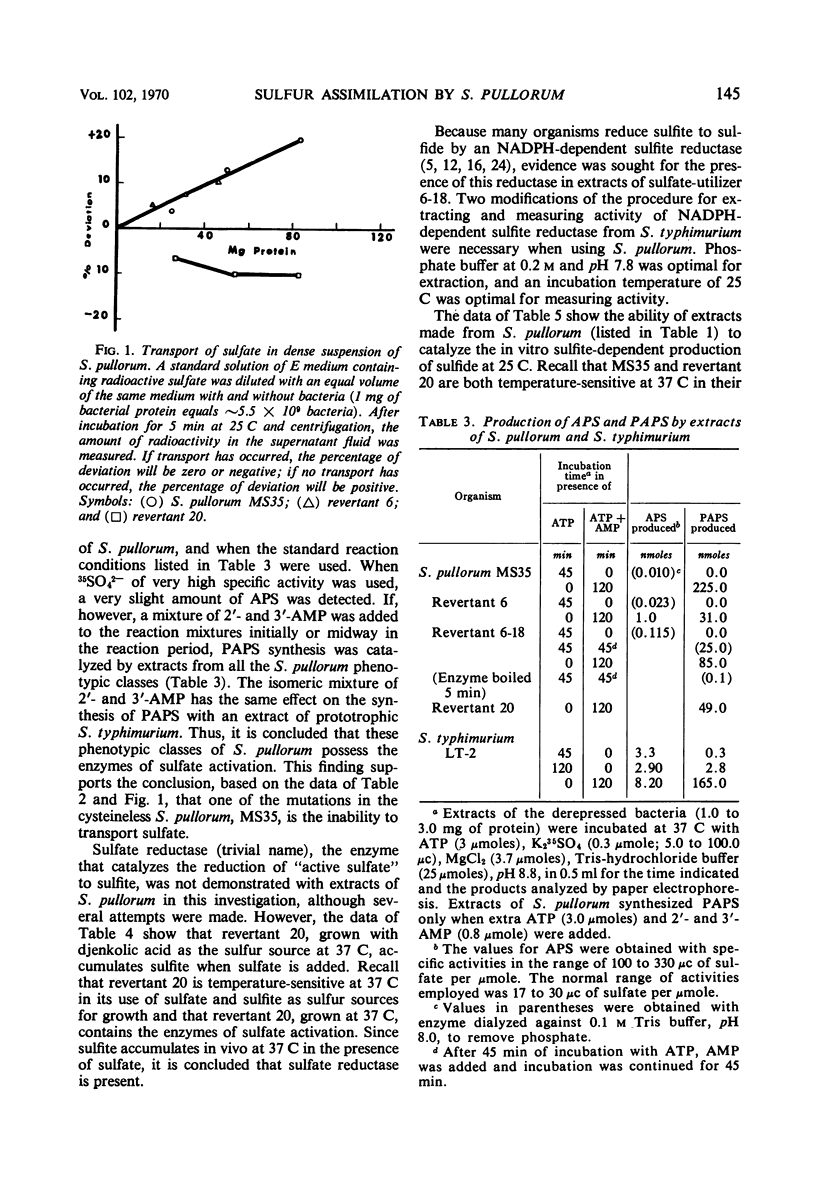
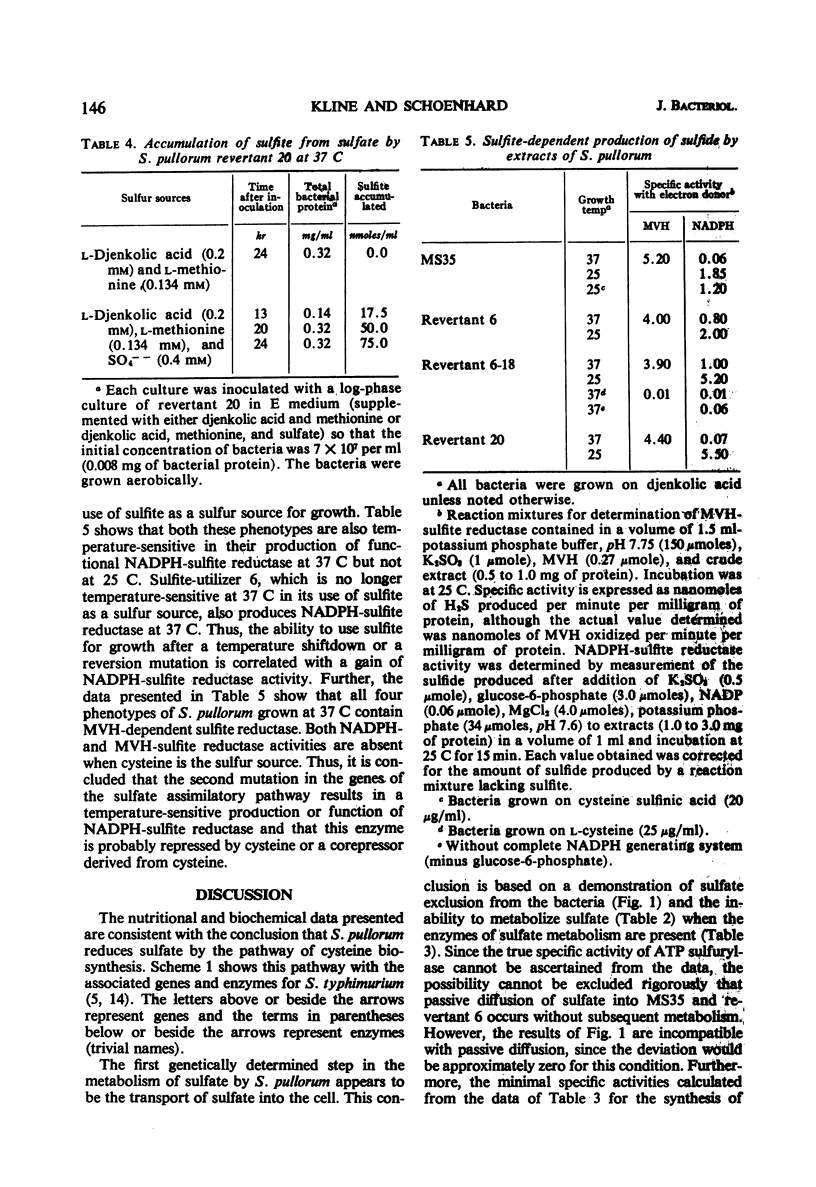
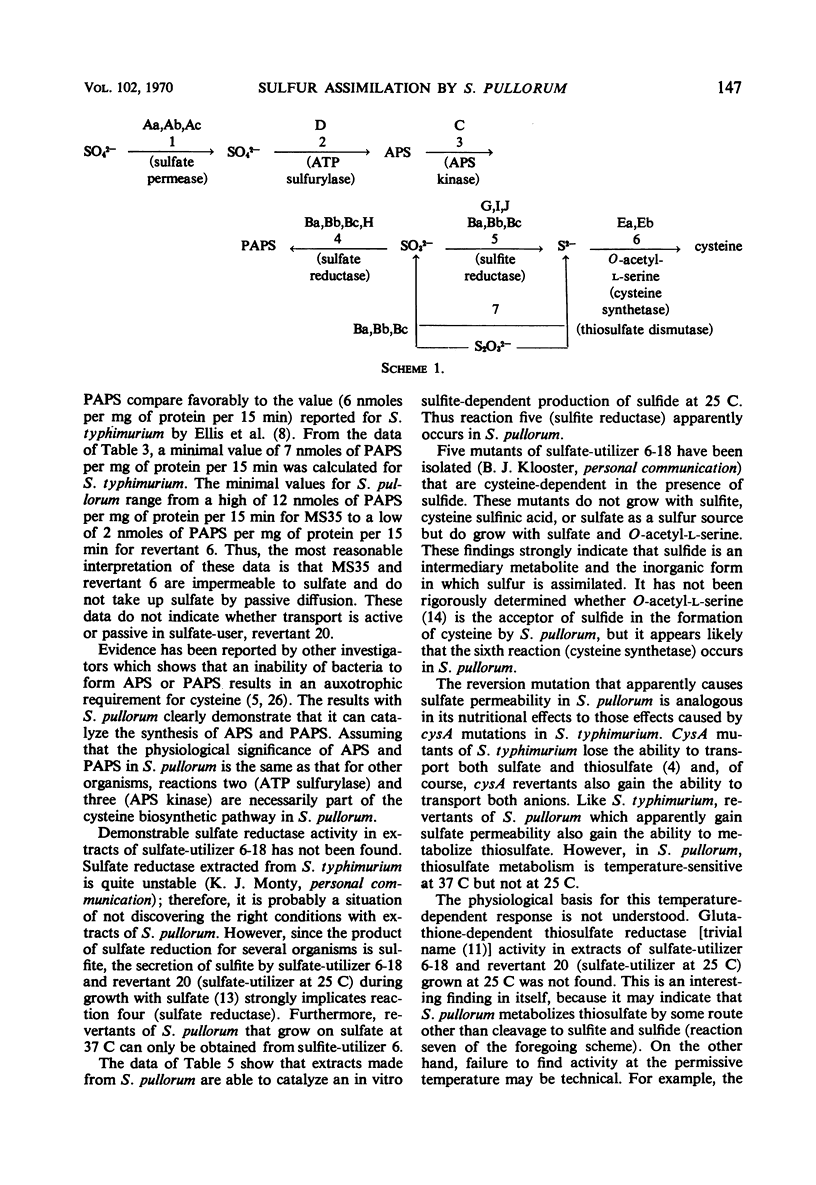
The biochemical basis for a cysteine requirement in Salmonella pullorum strain MS35 is presented. Before determining the missing biochemical functions, it was established that the assimilatory sulfate-reducing pathway for this species is an inorganic one in which 3′-phosphoadenylylsulfate (PAPS), sulfite, and sulfide are intermediates. A requirement for 2′- and 3′-adenosine monophosphate was found for in vitro synthesis of PAPS, possibly because 2′- and 3′-adenosine monophosphate inhibits endogenous nucleases that destroy PAPS. The cysteine requirement of strain MS35 was attributed to a defect at 37 C in sulfate permeation and temperature sensitivity in sulfite reduction. At 25 C, sulfite was metabolized to sulfide. A novel property of sulfate-utilizing revertants was their unselected ability to assimilate thiosulfate sulfur at 25 C but not at 37 C.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asada K. Purification and properties of a sulfite reductase from leaf tissue. J Biol Chem. 1967 Aug 25;242(16):3646–3654. [PubMed] [Google Scholar]
- DREYFUSS J. CHARACTERIZATION OF A SULFATE- AND THIOSULFATE-TRANSPORTING SYSTEM IN SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jul;239:2292–2297. [PubMed] [Google Scholar]
- Dreyfuss J., Pardee A. B. Regulation of sulfate transport in Salmonella typhimurium. J Bacteriol. 1966 Jun;91(6):2275–2280. doi: 10.1128/jb.91.6.2275-2280.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J., Humphries S. K., Pasternak C. A. Repressors of sulphate activation in Escherichia coli. Biochem J. 1964 Jul;92(1):167–172. doi: 10.1042/bj0920167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilz H., Lipmann F. THE ENZYMATIC ACTIVATION OF SULFATE. Proc Natl Acad Sci U S A. 1955 Nov 15;41(11):880–890. doi: 10.1073/pnas.41.11.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Wheldrake J. F., Pasternak C. A. The control of sulphate reduction in Escherichia coli by O-acetyl-L-serine. Biochem J. 1968 Mar;107(1):51–53. doi: 10.1042/bj1070051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMP J. D., ATKINSON D. E., EHRET A., LAZZARINI R. A. EVIDENCE FOR THE IDENTITY OF THE NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE-SPECIFIC SULFITE AND NITRITE REDUCTASES OF ESCHERICHIA COLI. J Biol Chem. 1963 Oct;238:3466–3471. [PubMed] [Google Scholar]
- Kline B. C., Schoenhard D. E. Accumulation of sulfite by a sulfate-using revertant of Salmonella pullorum. J Bacteriol. 1969 Oct;100(1):365–369. doi: 10.1128/jb.100.1.365-369.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- LEINWEBER F. J., MONTY K. J. CYSTEINE BIOSYNTHESIS IN NEUROSPORA CRASSA. I. THE METABOLISM OF SULFITE, SULFIDE, AND CYSTEINESULFINIC ACID. J Biol Chem. 1965 Feb;240:782–787. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidases and cyclic phosphodiesterases (3'-nucleotidases) of the Enterobacteriaceae. J Bacteriol. 1968 May;95(5):1732–1737. doi: 10.1128/jb.95.5.1732-1737.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASTERNAK C. A., ELLIS R. J., JONES-MORTIMER M. C., CRICHTON C. E. THE CONTROL OF SULPHATE REDUCTION IN BACTERIA. Biochem J. 1965 Jul;96:270–275. doi: 10.1042/bj0960270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R. The examination of sulphur auxotrophs: a warning. J Gen Microbiol. 1963 Mar;30:481–484. doi: 10.1099/00221287-30-3-481. [DOI] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Separation of the two enzymatic phases in active sulfate synthesis. J Biol Chem. 1958 Sep;233(3):681–685. [PubMed] [Google Scholar]
- SIEGEL L. M. A DIRECT MICRODETERMINATION FOR SULFIDE. Anal Biochem. 1965 Apr;11:126–132. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
- WHELDRAKE J. F., PASTERNAK C. A. THE CONTROL OF SULPHATE ACTIVATION IN BACTERIA. Biochem J. 1965 Jul;96:276–280. doi: 10.1042/bj0960276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON L. G., ASAHI T., BANDURSKI R. S. Yeast sulfate-reducing system. I. Reduction of sulfate to sulfite. J Biol Chem. 1961 Jun;236:1822–1829. [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]



