Abstract
A decade of research on human embryonic stem cells (ESC) has paved the way for the discovery of alternative approaches to generating pluripotent stem cells.Combinatorial overexpression of a limited number of proteins linked to pluripotency in ESC was recently found to reprogram differentiated somatic cells back to a pluripotent state, enabling the derivation of isogenic (patient-specific) pluripotent stem cell lines. Current research is focusing on improving reprogramming protocols (e.g. circumventing the use of retroviral technology and oncoproteins), and on methods for differentiation into transplantable tissues of interest. In mouse ESC, we have previously shown that the embryonic morphogens BMP4 and Wnt3a direct blood formation via activation of Cdx and Hox genes. Ectopic expression of Cdx4 and HoxB4 enables the generation of mouse ESC-derived hematopoietic stem cells (HSC) capable of multilineage reconstitution of lethally irradiated adult mice. Here, we explore hematopoietic development from human induced pluripotent stem (iPS) cells generated in our laboratory. Our data show robust differentiation of iPS cells to mesoderm and to blood lineages, as shown by generation of CD34+CD45+ cells, hematopoietic colony activity and gene expression data, and suggest conservation of blood patterning pathways between mouse and human hematopoietic development.
Keywords: human induced pluripotent stem cells, differentiation, hematopoiesis, BMP4
INTRODUCTION
The isolation of human embryonic stem cells (hESC) in 1998 has provided a novel system for the study of early human development, and raised hopes for using ESC as a universal resource for cellular replacement therapies in the treatment of disease. In vitro, ESC can extensively proliferate, maintain an undifferentiated state, and differentiate into cystic structures termed embryoid bodies (EB), which comprise tissues of all three germ layers.1 From a developmental standpoint, ESC are equivalent to the early epiblast.2,3 When reintroduced to the embryo in blastocyst chimerization or tetraploid complementation assays, these pluripotent stem cells will contribute to all tissues of the embryo proper, including the germ line.4 Insights from developmental biology and conservation of embryonic genetic pathways from model systems such as Xenopus, zebrafish, and mouse can inform the directed in vitro differentiation of ESC towards tissues of interest.5,6 Following these principles, we have demonstrated that Cdx and Hox genes are essential regulators of embryonic blood formation in zebrafish,7,8 and can promote hematopoiesis from mouse ESC.5,9–12 The embryonic morphogens Bone Morphogenetic Protein 4 (BMP4) and Wnt3a activate the Cdx-Hox pathway, determining the transition from mesoderm to blood fate in differentiating mouse EB.5 Furthermore, ectopic expression of Cdx4 and HoxB4 enables the derivation of murine ESC-derived hematopoietic stem cells (HSC) with multilineage reconstitution potential in transplant assays.9 Given the conservation of genetic pathways between zebrafish and mouse ESC, we reasoned that similar blood patterning mechanisms are involved in human hematopoietic development.
Differentiated progeny of ESC, including hematopoietic stem and progenitor cells, express human leucocyte antigens and would thus face immunological barriers upon transplantation in an allogeneic setting.13 The ultimate goal of regenerative medicine is the development of (isogenic) cell replacement therapies that will not be rejected. By using knowledge from ESC, researchers have recently discovered an alternative way of creating pluripotent stem cells: exposure to embryonic proteins delivered by retroviral transduction14–18 reprograms adult somatic cells into a pluripotent ESC-like state. Induced pluripotent stem (iPS) cells were first generated in mice,14 and by analogous protocols, shortly after succesfully derived from human cells.15–18 When reintroduced to the embryo, mouse iPS cells chimerize all tissues, including the germ line.19,20 Human iPS cells robustly form teratomas when injected into immunodeficient mice,17 the chief assay reflecting pluripotency of human cells. In this study, we explore the in vitro hematopoietic differentiation capacity of human iPS cells derived in our laboratory17 and survey conservation of genetic pathways during blood development.5,11,12
MATERIAL AND METHODS
iPS Cell Maintainance and Differentiation
hFib2-iPS517 cells were cultured on irradiated mouse embryonic fibroblasts in serum-free medium containing basic fibroblast growth factor, as previously described.17 iPS cells were collected and differentiated as previously described.17 Confluent cultures were harvested by mechanical (cell lifter) and enzymatic dissociation (collagenase IV, Invitrogen, Karlsruhe, Germany) and differentiated for one day in basic embryoid body (EB) medium, containing Knockout DMEM (Invitrogen, Karlsruhe, Germany) supplemented with 20% fetal calf serum (Stem Cell Technologies, Grenoble, France), 0.1mM nonessential aminoacids (Invitrogen, Karlsruhe, Germany), 0.1 mM β-mercaptoethanol (Sigma-Aldrich, Taufkirch, Germany), 1mM L-Glutamine (Invitrogen, Karlsruhe, Germany), 50 µg/ml Ascorbic Acid (Sigma-Aldrich, Taufkirch, Germany), 201µg/ml human holo-transferrin (Sigma-Aldrich, Taufkirch, Germany). At day two, human stem cell factor (300 ng/ml, PeproTech, Hamburg, Germany), human Flt3-ligand (300 ng/ml, PeproTech, Hamburg, Germany), human interleukin-3 (10 ng/ml, PeproTech, Hamburg, Germany), human interleukin-6 (10 ng/ml, PeproTech, Hamburg, Germany), G-CSF (50 ng/ml, R&D Systems, Wiesbaden, Germany) and human bone morphogenetic protein 4 (50 ng/ml, R&D Systems, Wiesbaden, Germany) were added to the cultures. EB were collected at different timepoints as mentioned, dissociated into single cells, and colony-forming unit assays were performed in semi-solid medium supplemented with hematopoietic cytokines, as previously described.21 Briefly, 12.500 cells were plated in 1.5 ml MethoCult® GF H4434 (Stem Cell Technologies, Grenoble, France), incubated at 37°C and 5% CO2 for 14 days and then scored based on morphological criteria.
Antibodies and Flow Cytometry Analysis
FACS staining was performed using CD45-FITC and CD34-PECy5 antibodies (BD Pharmingen, San Jose, CA, USA). For intracellular flow, cells were fixed and permeabilized using Phosflow Lyse/Fix and Perm/Wash buffers (both from BD Biosciences, Heidelberg, Germany) according to manufacturer’s instructions, and subsequently stained with phospho-Smad1/5/8 antibody (Cell Signaling, Danvers, MA, USA) and Alexa Fluor 488 F(ab’) fragment of goat anti-rabbit IgG (Invitrogen, Karlsruhe, Germany). All samples were analysed on a FACS Canto II flow cytometer (BD Biosciences, Heidelberg, Germany).
Gene Expression Analysis
RNA was isolated with RNeasy Mini-Kit (Qiagen, Hilden, Germany) and cDNA was produced using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Real-time PCR was performed with qPCR mastermix Plus for SYBRGreen I (Eurogentec, Seraing, Belgium) on a ABI Prism 7000 qPCR machine (Applied Biosystems, Foster City, CA, USA). Annealing temperature was 60°C. Primer sequences were designed using the idT-DNA Technologies software and used as shown in Table 1.
TABLE 1.
Primer sequences
| forward | reverse | |
|---|---|---|
| CDX1 | GTA AGA CTC GGA CCA AGG ACA AGT | CTG ATT TCC GCC GGA TTG TGA TGT |
| CDX2 | GAA CCT GTG CGA GTG GAT G | ACC ACT CGA TAT TTG TCT TTC GTC |
| CDX4 | CCC TAT GCA TGG ATG CGC AAG | CCA GCT CCA ATC TTT GAT GAT CAG TGT |
| SCL | ATG CCT TCC CTA TGT TCA CCA CCA | TGA AGA TAC GCC GCA CAA CTT TGG |
| BrACHYURY | ACA AAG AGA TGA TGG AGG AAC CCG | AGG ATG AGG ATT TGC AGG TGG ACA |
| HOXA9 | TGC TTG TGG TTC TCC TCC AGT TGA | AAG CCA GTT GGC TGC TGG GTT ATT |
RESULTS AND DISCUSSION
Generation of Human iPS Cells
In a recent study, we reported generation of induced pluripotent stem cells (hFib2-iPS5) from dermal fibroblasts of an adult volunteer donor using retroviral infection with OCT4, SOX2, KLF4, MYC, hTERT and SV40 large T, followed by culture under human ESC maintainance conditions and selection for colonies displaying ESC-like morphology (Figure 1).17 hTERT and SV40 large T retroviruses were detected in early post-infection cultures, but no integration could be found in the ESC-like colonies. Presumably, hTERT and SV40 large T indirectly enhance the efficiency of the reprogramming process, for example by acting on supportive cells in the culture.17 Indeed, human iPS cells have been generated without the use of hTERT and SV40 large T, and with other combinations of embryonic proteins.15,18 Recently, addition of histone deacetylase inhibitors (e.g. valproic acid) enabled reprogramming of human cells with only two retrovirally delivered factors (OCT4 and SOX2),22,23 underscoring the epigenetic nature of the reprogramming process. Many efforts are focused on the development of reprogramming techniques based on small molecules that could bring iPS technology closer to clinical applications, by circumventing the need for viral transduction, which carries the risk of insertional mutagenesis and activation of potential oncoproteins.24
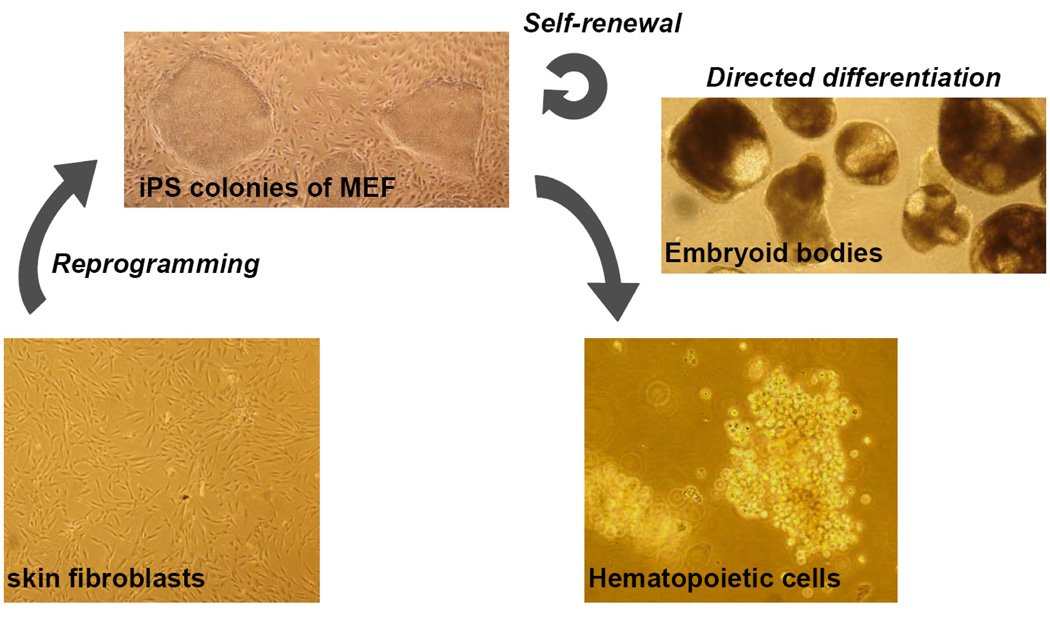
FIGURE 1. Generation of blood cells from adult skin fibroblasts.
For direct reprogramming of somatic cells, primary fibroblasts (shown here at day 28 of culture) undergo retroviral infection with embryonic genes (e.g. OCT4, SOX2, KLF4, MYC) and are afterwards grown on mouse embryonic feeder cells (MEF) in ESC growth medium. After several weeks of culture, iPS colonies displaying ESC-like morphology appear (shown here: hFib2-iPS5 colonies). iPS cells can be maintained in an undifferentiated state as a cell line or differentiated in embryoid bodies (EB, shown here hFib2-iPS5 at day 10 of differentiation) to tissues of interest, e.g. blood (shown here, myeloid colony at day 10 after plating day 17 EB-derived cells into methylcellulose supplemented with hematopoietic cytokines). Pictures were taken at 40× magnification.
hFib2-iPS5 cells closely resemble human ESC. hFib2-iPS5 colonies express immunohistochemical markers specific to ESC (alkaline phosphatase, Tra-1–81, Tra-1–60, SSEA3, SSEA4, OCT4 and NANOG) and display a molecular signature similar to undifferentiated human ESC at the transcriptional level.17 Analysis of the promotor region of genes involved in ESC pluripotency (e.g., OCT4 and NANOG) showed prominent demethylation in both human ESC and hFib2-iPS5 cells, as opposed to the methylated state documented in the starting population of dermal fibroblasts.17 Functionally, hFib2-iPS5 cells robustly form teratomas when injected subcutaneously in immunodeficient mice,17 thus meeting the ultimate criterion of pluripotency in human cells. The hFib2-iPS5 cell line has been now succesfully maintained in culture and extensively passaged over 12 months.
Hematopoietic Development from Human hFib2-iPS5 Cells
In both mice and human, primitive hematopoiesis is initiated in the yolk sac blood islands derived from extraembryonic mesoderm. Shortly after, hematopoietic stem cells (definitive, adult-type hematopoiesis) are found in the in the aorta-gonado-mesonephros (AGM) region of the embryo proper.25 Both yolk sac and AGM hematopoiesis originate from mesodermal cells displaying dual hematopoietic and endothelial potential.25 In the mouse, these cells termed hemangioblasts have been isolated as Flk1+ cells in the posterior primitive streak of mouse embryos26, and around day 3 of murine ESC differentiated in vitro.27,28 Human hemangioblasts have been demonstrated in differentiating human ESC.29 They are phenotypically less well described than in the mouse, yet are found in the CD34+CD45− cellular population,25 and express KDR (VEGF receptor 2),29 CD31,30 and angiotensin-converting-enzyme (ACE/CD143).31 With maturation along the blood lineage, blood progenitors start expressing the hematopoietic antigen CD45.21 Emergence of CD34+CD45+ cells correlates with derivation of hematopoietic progenitor cells that generate colony forming units (CFU) in functional methycellulose assays, and sorting for CD34+CD45+ cells enriches for cells with hematopoietic colony forming unit (CFU) potential.30
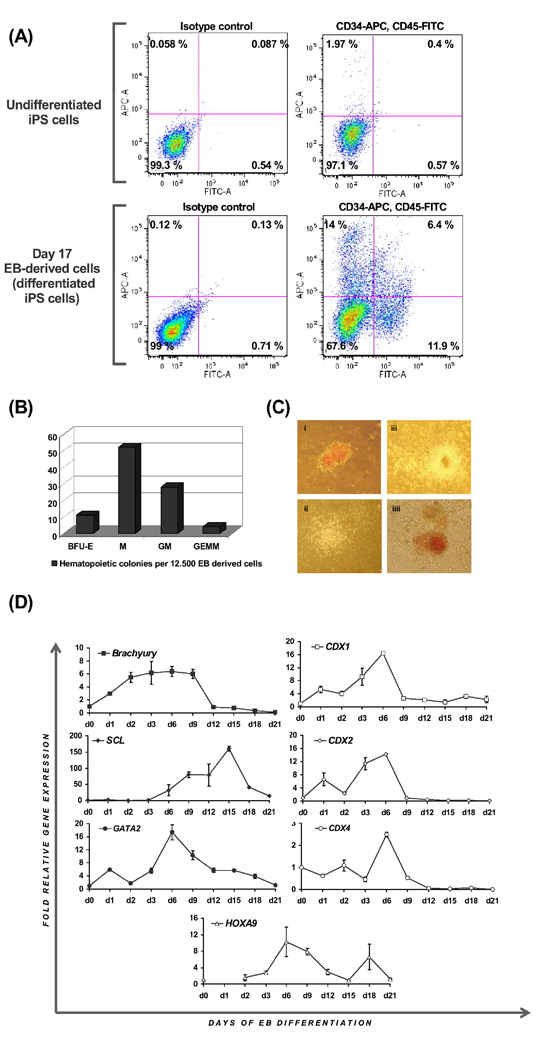
Here, we analysed whether similar hematopoietic differentiation can be achieved from human iPS cells. We differentiated hFib2-iPS cells in serum-containing media supplemented with BMP4 (50 ng/ml) and hematopoietic cytokines. Embryoid bodies (EB) formed spontaneously, starting at day 1 of differentiation (Figure 1). At day 17 of differentiation, EB were dissociated to single cells and analysed for CD34 and CD45 expression by flow cytometry, and plated into methylcellulose assays to assess CFU potential. We detected a high degree of variability among experiments, but consistently robust formation of CD34+ (28.9±12), CD45+ (26.8±13.4) and CD34+CD45+ (16.1±13.7) cells, comparable to reports of the differentiation of human hESC in this system (Figure 2A).21 Hematopoietic activity was verified in functional assays, where we documented high incidence of CFU-initiating cells (95 colonies per 12500 EB-derived cells, Figure 2B–C). Most of the colonies were of myeloid type, but robust formation of mixed colonies (CFU-GEMM) was also observed (ca. 4% of total colony number, Figure 2B–C).
FIGURE 2. Blood development from iPS cells.
(A) Day 17 EB-derived, differentiated hFib2-iPS5 cells show robust formation of CD34+ and CD45+ and CD34+CD45+ cells. (B–C) Hematopoietic colony formation upon plating in methylcellulose CFU assays. (B) Shown are colony numbers per 12.500 plated day 17 EB-derived cells and (C) pictures of different colony types (erythroid, macrophage, granulocyte-macrophage, granulocyte-erythroid-macrophage-megakaryocyte) taken at 100× magnification. (D) Time course of quantitative RT-PCR analysis of differentiating hFib2-iPS5 shows expression of mesodermal (BRACHYURY) and hematopoietic (SCL, GATA2) gene expression, as well as expression of embryonic homeobox genes previously implicated in murine blood development (CDX1, CD2, CDX4 and HOXA9). Numbers represent fold relative gene expression, as compared to the expression in undifferentiated iPS5 cells (day 0).
Furthermore, we performed a time-course of quantitative gene expression analysis in differentiating EB, and collected samples daily until day 3 of differentiation, and every third day thereafter until day 21, to survey expression of genes indicating mesodermal (BRACHYURY) and hematopoietic (SCL, GATA2) commitment. In mouse ESC, blood cells originate from Brachyury-positive mesodermal cells28. In differentiating human iPS, BRACHYURY expression was upregulated at day 2, and declined after day 9, when SCL expression appeared, indicating conversion of mesoderm to progenitors of the blood lineage (Figure 2D). As previously reported in mouse ESC, CDX-HOX genes were expressed in waves,5,11 reinforcing the notion that similar embryonic processes take place during differentiation of human iPS cells (Figure 2D). Expression of all three human CDX genes (CDX1, CDX2 and CDX4) peaked at day 6 (Figure 2D), suggesting that the function of CDX genes to pattern preformed mesoderm to blood fate may be conserved in human embryogenesis.
BMP4 Supports Mesoderm Induction and Hematopoietic Development from Human iPS Cells Cultured in Serum-Containing Medium
Previously, we showed that bone morphogenetic protein 4 (BMP4) supports hematopoietic development from mouse ESC in two distinct phases: (1) formation of ventro-posterior mesoderm and (2) specification of preformed mesodermal cells to blood via the Wnt-Cdx-Hox pathway.5 In human ESC, BMP4 together with cytokines has been shown to induce mesoderm32 and hemangioblast formation under serum-free differentiation conditions,29 and to augment formation of CD34+CD45+ cells and CFU numbers when continuously added to cultures in serum-containing medium.21
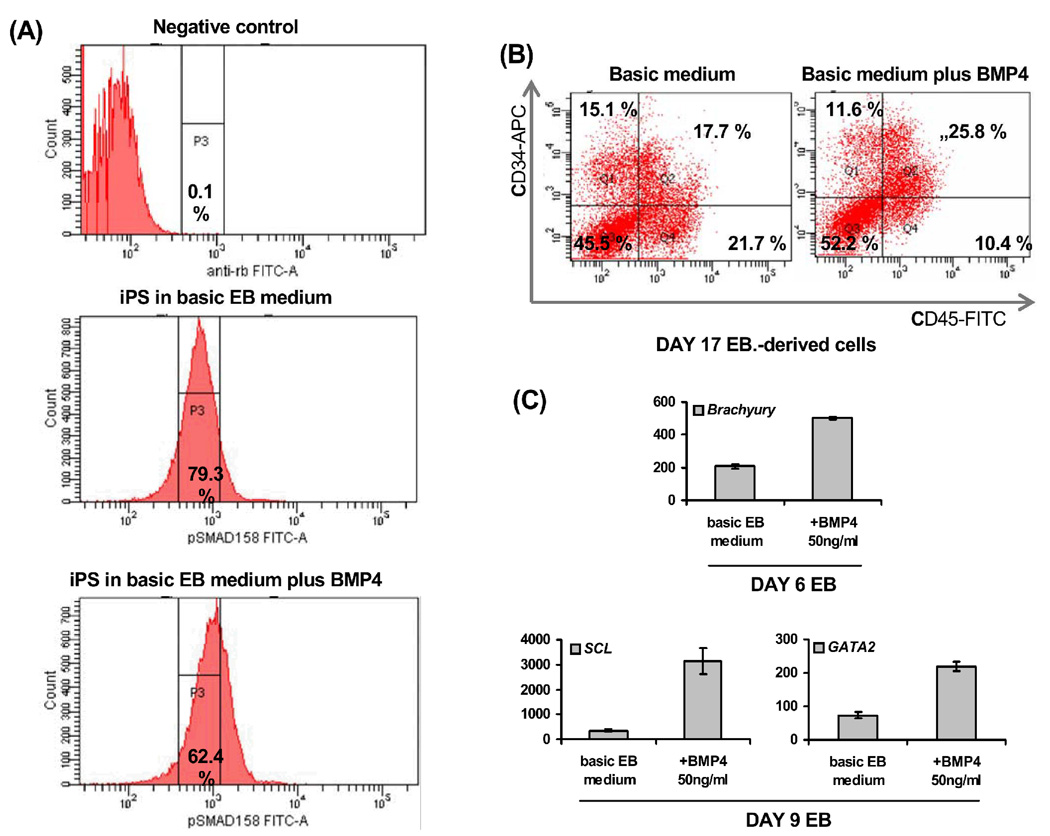
By intracellular flow cytometry, we detected strong phosphorylation of the SMAD 1/5/8 proteins, downstream mediators of BMP signaling, in hFib2-iPS5 cells cultured in basic EB differentiation medium (containing serum, but not supplemented with BMP4). Supplementation with recombinant human BMP4 (50 ng/ml) further enhanced this signal (Figure 3A). These data demonstrate that BMP4 activates SMAD1/5/8 in human iPS cells, and suggests that serum-related or endogenous BMP activity accounts for the phosporylation signal detected in serum-containing cultures. We further assessed the effect of BMP4 addition on hematopoietic development. As demonstrated in human and mouse ESC,5,32 BMP4 augmented mesoderm formation from iPS cells, as shown by analysis of BRACHYURY expression in day 6 EB, and enhanced hematopoietic activity, inducing formation of CD34+CD45+ cells (Figure 3B) and upregulating SCL and GATA2 (Figure 3C). However, consistent with results reported in human ESC,21 total CD34+ and CD45+ numbers were not significantly upregulated by BMP4 addition (Figure 3B). These data suggest that the role of BMP4 as a hematopoietic morphogen is conserved in human iPS cells differentiating along the blood lineage: stimulation of BMP4 signaling enhances mesoderm formation and directs differentiation towards blood lineages. Whether, as documented in mouse ESC, BMP4 specifically acts on preformed mesodermal cells to pattern blood fate remains to be investigated in human iPS cells. However, such a hypothesis is supported by expression data showing strong BMP4 expression in the human AGM, in close vicinity to emerging blood islands and coincident to emergence of hematopoietic cells.33
FIGURE 3. BMP4 promotes hematopoiesis from human iPS cells.
(A) The BMP downstream signaling components SMAD1/5/8 show phosphorylation in iPS5 cells cultured in basic differentiation medium;addition of BMP4 enhances this signal as measured by intracellular flow cytometry; (B) Addition of BMP4 to cultures in basic differentiation medium enhances the formation of CD34+CD45+ cells and (C) upregulates mesodermal (BRACHYURY, as measured in day 6 EB) and hematopoietic genes (SCL, GATA2, as measured in day 9 EB).
CONCLUSIONS
Transduction of four transcription factors linked to pluripotency in ESCs can reprogram differentiated adult somatic cells back to a pluripotent ESC-like state, enabling the creation of patient-specific isogenic pluripotent stem cell lines.16 iPS cells can be maintained in culture and extensively expanded in vitro, following protocols established for human ESC. Here we show that in vitro differentiated iPS cells can effectively generate blood cells. Moreover, our data indicate that in vitro human iPS differentiation recapitulates aspects of early embryonic development and suggests conservation of pathways active in mouse developmental hematopoiesis (e.g. BMP4 signaling and the CDX-HOX pathway).
ACKNOWLEDGMENTS
This study was supported by grants from the DFG SFB773, the Deutsche Krebshilfe Max-Eder-Program and the Fortune Program of the University of Tuebingen for CL. G.Q.D. was supported by grants from the United States National Institutes of Health, the NIH Director's Pioneer Award of the NIH Roadmap for Medical Research, Clinical Scientist Awards in Translational Research from the Burroughs Wellcome Fund and the Leukemia and Lymphoma Society, and the Howard Hughes Medical Institute.
REFERENCES
- 1.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 2.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 3.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley A, et al. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- 5.Lengerke C, et al. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell. 2008;2:72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Lengerke C, Daley GQ. Patterning definitive hematopoietic stem cells from embryonic stem cells. Exp Hematol. 2005;33:971–979. doi: 10.1016/j.exphem.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Davidson AJ, et al. cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature. 2003;425:300–306. doi: 10.1038/nature01973. [DOI] [PubMed] [Google Scholar]
- 8.Davidson AJ, Zon LI. The caudal-related homeobox genes cdx1a and cdx4 act redundantly to regulate hox gene expression and the formation of putative hematopoietic stem cells during zebrafish embryogenesis. Dev Biol. 2006;292:506–518. doi: 10.1016/j.ydbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, et al. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci U S A. 2005;102:19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, et al. Cdx gene deficiency compromises embryonic hematopoiesis in the mouse. Proc Natl Acad Sci U S A. 2008;105:7756–7761. doi: 10.1073/pnas.0708951105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKinney-Freeman SL, et al. Modulation of murine embryonic stem cellderived CD41+c-kit+ hematopoietic progenitors by ectopic expression of Cdx genes. Blood. 2008;111:4944–4953. doi: 10.1182/blood-2007-11-124644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lengerke C, et al. The cdx-hox pathway in hematopoietic stem cell formation from embryonic stem cells. Ann N Y Acad Sci. 2007;1106:197–208. doi: 10.1196/annals.1392.006. [DOI] [PubMed] [Google Scholar]
- 13.Drukker M, et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Park IH, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 19.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 20.Wernig M, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 21.Chadwick K, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 22.Huangfu D, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 23.Huangfu D, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gidekel S, et al. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–370. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 25.Zambidis ET, et al. Blood-forming endothelium in human ontogeny: lessons from in utero development and embryonic stem cell culture. Trends Cardiovasc Med. 2006;16:95–101. doi: 10.1016/j.tcm.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber TL, et al. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 27.Choi K, et al. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 28.Fehling HJ, et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy M, et al. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian X, et al. Hematopoietic engraftment of human embryonic stem cell-derived cells is regulated by recipient innate immunity. Stem Cells. 2006;24:1370–1380. doi: 10.1634/stemcells.2005-0340. [DOI] [PubMed] [Google Scholar]
- 31.Zambidis ET, et al. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008;112:3601–3614. doi: 10.1182/blood-2008-03-144766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang P, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- 33.Marshall CJ, Kinnon C, Thrasher AJ. Polarized expression of bone morphogenetic protein-4 in the human aorta-gonad-mesonephros region. Blood. 2000;96:1591–1593. [PubMed] [Google Scholar]